Abstract
Purpose
Incorporation of cytarabine into DNA activates checkpoint kinase 1 (Chk1), which stabilizes stalled replication forks, induces S-phase slowing, and diminishes cytarabine cytotoxicity. The selective Chk1 inhibitor SCH 900776 abrogates cytarabine-induced S-phase arrest and enhances cytarabine cytotoxicity in acute leukemia cell lines and leukemic blasts in vitro. To extend these findings to the clinical setting, we have conducted a phase I study of cytarabine and SCH 900776.
Experimental Design
Twenty-four adults with relapsed and refractory acute leukemias received timed sequential, continuous infusion cytarabine 2 g/m2 over 72 hours (667 mg/m2/24 hours) beginning on day 1 and again on day 10. SCH 900776 was administered as a 15- to 30-minute infusion on days 2, 3, 11, and 12. The starting dose of SCH 900776 was 10 mg/m2/dose.
Results
Dose-limiting toxicities consisting of corrected QT interval prolongation and grade 3 palmar-plantar erythrodysesthesia occurred at 140 mg flat dosing (dose level 5, equivalent to 80 mg/m2). Complete remissions occurred in 8 of 24 (33%) patients, with 7 of 8 at 40 mg/m2 or higher. SCH 900776 did not accumulate at any dose level. Marrow blasts obtained pretreatment and during therapy showed increased phosphorylation of H2Ax after SCH 900776 beginning at 40 mg/m2, consistent with unrepaired DNA damage.
Conclusions
These data support a randomized phase II trial of cytarabine +/− SCH 900776 at a recommended flat dose of 100 mg (equivalent to 56 mg/m2) for adults with poor-risk leukemias. The trial (SP P05247) was registered at www.clinicaltrials.gov as NCT #00907517.
Introduction
More than 40 years after its introduction, cytarabine remains one of the most effective agents for the treatment of acute myelogenous leukemia (AML; refs. 1, 2). According to current understanding, this agent enters leukemic cells on nucleoside transporters (3, 4) and is then phosphorylated to cytosine arabinoside triphosphate (5, 6), which acts as a competitive inhibitor of replicative DNA polymerases (7–9). In addition, once incorporated into DNA (9–11), cytarabine causes replication fork slowing (12). These events conspire to activate the replication checkpoint. A critical aspect of that checkpoint involves the phosphorylation and activation of checkpoint kinase 1 (Chk1), which stabilizes replication forks, activates DNA repair, and suppresses apoptosis (13–18).
We (19, 20) and others (21, 22) have shown that cytarabine induces activation of the replication checkpoint and S-phase arrest. Inhibition of Chk1 by 7-hydroxystaurosporine (UCN-01) enhances cytarabine-induced apoptosis in vitro (23). Clinical trials combining UCN-01 with S-phase damaging agents have been problematic, however, because of the drug’s long half-life (24, 25) and its diverse toxicities that may reflect inhibition of a large number of kinases (26, 27). Depletion of Chk1 either by siRNA or by tanespimycin-induced release from HSP90 and subsequent proteasomal degradation (28) also enhances the cytotoxicity of cytarabine in AML cell lines and primary AML specimens (20).
Building on these observations, we conducted a phase I trial to determine if cytarabine activates the S-phase checkpoint and tanespimycin downregulates HSP90 client proteins in vivo (29, 30). Comparison of leukemic blasts harvested before treatment and 48 hours after the start of continuous cytarabine showed cytarabine-induced Chk1 phosphorylation in the majority of paired specimens, consistent with checkpoint activation (30). However, samples harvested 24 hours after tanespimycin administration showed negligible changes in client protein levels, and tanespimycin toxicities precluded administration of higher doses (29).
SCH 900776 is a selective and potent ATP-competitive Chk1 inhibitor identified using a cell-based screen for accumulation of the phosphorylated histone γ-H2AX, a marker of DNA double-strand breaks (DSB), in the presence of the replication inhibitor hydroxyurea (31). SCH 900776 inhibits Chk1 without substantial effect on the potentially antagonistic checkpoint regulators Chk2 or CDK1. In preclinical models, SCH 900776 synergized with antimetabolites, including the nucleoside analogs cytarabine and gemcitabine, to induce apoptosis and long-lasting tumor regressions in ovarian and pancreatic tumor models, without apparent exacerbation of toxicity in normal tissues (31). Our subsequent studies showed that SCH 900776 diminished cytarabine-induced S-phase arrest and enhanced cytarabine cytotoxicity in AML lines and AML clinical specimens ex vivo (30). Because of redundant checkpoint mechanisms in normal cells, normal myeloid progenitors were sensitized much less (30).
To extend these findings to the clinical setting, we have conducted a phase I dose-escalation trial of SCH 900776 in combination with timed-sequential cytarabine in 24 adults with relapsed and refractory acute leukemias. In particular, patients were treated with cytarabine by continuous infusion on days 1 to 3 and 10 to 12, with SCH 900776 administration beginning 24 hours after the start of each cytarabine infusion to allow for checkpoint activation before administration of the CHK1 inhibitor (31).
Patients, Materials, and Methods
Patient eligibility and selection
Patients of age 18 years and older with pathologically confirmed, relapsed and refractory AML, acute lymphoblastic leukemia (ALL), or chronic myelogenous leukemia in accelerated phase (CML-AP) or blast crisis (CML-BC) were eligible provided that they had Eastern Cooperative Oncology Group (ECOG) performance status 0 to 2, serum creatinine ≤ 1.5× upper limit of normal (ULN), hepatic enzymes ≤5× ULN, bilirubin ≤1.5 mg/dL, and left ventricular ejection fraction (LVEF) 45% or more. Patients were eligible if they had undergone no more than 4 prior courses of cytotoxic induction therapies. Patients who had undergone allogeneic or autologous stem cell transplantation (SCT) and had relapsed or were refractory thereafter (i.e., had persistent leukemia) were eligible. Patients were not eligible if they had a peripheral blast count ≥50,000/mm3. Full criteria for eligibility and ineligibility were similar to those detailed for previous studies of investigational agents combined with cytarabine in adults with relapsed and refractory acute leukemias (29, 32). In agreement with the Declaration of Helsinki, all patients provided written informed consent according to The Johns Hopkins Medical Institutional Review Board guidelines.
Treatment schema
All patients received continuous infusion cytarabine 2 g/m2 over 72 hours (667 mg/m2/24 hours) beginning on day 1 and again on day 10. SCH 900776 was administered as a 15- to 30-minute infusion on days 2, 3, 11, and 12. On the basis of animal toxicology studies, the starting dose of SCH 900776 was 10 mg/m2/dose. Using a traditional "3+3" design, the SCH 900776 dose was doubled for levels 2 and 3 (20 and 40 mg/m2/dose, respectively) and increased by 40% thereafter (level 4, 56 mg/m2/dose; level 5, 80 mg/m2). At level 5, dosing calculations were changed to flat dosing, such that the dose for level 5 was 140 mg. The occurrence of any dose-limiting toxicity (DLT) in 33% of a patient cohort defined the maximal-administered dose (MAD). Patients who achieved complete remission (CR) after cycle 1 were eligible to receive additional cycles of SCH 900776 + cytarabine beginning 30 ± 7 days following hospital discharge from the first cycle. Patients who achieved CR and had a suitably matched related or unrelated donor or a related haploidentical donor were eligible to undergo allogeneic SCT.
Supportive care
Prophylactic measures against tumor lysis syndrome (TLS), cytarabine-related adult respiratory distress syndrome (ARDS), cytokine release syndrome, and gastrointestinal-based infection were similar to those used in previous studies of cytarabine-based timed sequential therapy (32). Growth factors were not permitted.
Response and toxicity evaluations
Bone marrow aspirates and biopsies were obtained before treatment, on day 14 of treatment, and at hematologic recovery or when leukemia regrowth was suspected. Hematologic recovery was defined as absolute neutrophil count (ANC) ≥500/mm3 and a transfusion-independent platelet count of ≥50,000/mm3. The definitions of response were as follows (33): CR required normal marrow aspirate with absence of identifiable leukemia, ANC ≥1,000/mm3, platelet count ≥100,000/mm3, absence of blasts in peripheral blood, and absence of extramedullary disease; CR with incomplete recovery (CRi) required all CR criteria except for residual neutropenia or thrombocytopenia; and NR was defined as persistent leukemia in marrow and/or blood without significant decrease from pretreatment levels.
Adverse events were described and graded on the basis of NCI Common Toxicity Criteria, version 3.0, and treating physician’s assessment. DLT was assessed during cycle 1 according to CTCAE v 3.0, with the following considered to be DLTs: (i) myelosuppression with bone marrow cellularity less than 5% that occurred more than 6 weeks following the last dose of chemotherapy (day 55) without any evidence of residual leukemia; (ii) any grade 4 drug-related nonhematologic toxicity, not including alopecia, inadequately treated nausea, vomiting, diarrhea, fatigue, infection or febrile neutropenia, or clinically nonsignificant, treatable or reversible laboratory abnormalities, such as hyperuricemia associated with TLS; (iii) any grade 3 drug-related nonhematologic toxicity that did not resolve to_grade 2 within 48 hours, with the exception of alopecia, fatigue, infection or febrile neutropenia, grade 3 elevations of transaminases and/or alkaline phosphatase and/or bilirubin that improved to ≤ grade 2 in ≤ 7 days, or grade 3 mucositis, diarrhea, nausea, or vomiting that improved to ≤ grade 2 (including use of supportive care) in ≤7 days; (iv) grade 3 neurotoxicity or nephrotoxicity of any duration; and (v) inability to administer day 10 (+1 day) cytarabine therapy due to ongoing, uncontrolled serious or life-threatening toxicity, such as overwhelming infection or sepsis with hemodynamic instability, pulmonary failure, acute elevation in creatinine accompanied by oliguria or anuria.
Beginning at dose level 4 (56 mg/m2 per dose of SCH 900776), electrocardiograms (EKG) were conducted in triplicate within 72 hours of the first dose of SCH 900776 and before each subsequent dose. Single EKGs were required at the end of the SCH 900776 infusion and 15 and 60 minutes following the end of the infusion. Mean QTcF (Frederika correction) had to be 470 milliseconds or less to receive SCH 900776. EKGs were conducted on a precalibrated EKG machine and all EKGs underwent central cardiology review. Prolonged QTcF was defined as follows: grade 1, 451–480 milliseconds; grade 2, 481–500 milliseconds; grade 3, ≥501 milliseconds on at least 2 separate EKGs; grade 4, ≥501 milliseconds or >60 milliseconds change from baseline and Torsaud de pointes, polymorphic ventricular tachycardia, or signs or symptoms of a serious arrhythmia. Two additional EKGs were required if QTcF was prolonged by more than 30 milliseconds but less than 60 milliseconds, with repeats every 15 minutes until normalization. Telemetry was required for grade 3 or 4 prolongations until QTcF returned to 30 milliseconds or less of baseline.
Laboratory correlates
Pharmacokinetics
Blood samples were obtained on cycle 1 days 2 and 3 before the start of the SCH 900776 infusion and just before completion of the SCH 900776 infusion, 15 minutes after the end of the infusion, and 1, 3, 6, and 8 hours after the end of the SCH 900776 infusion. An additional sample was collected on day 4 (48 hours after the day 2 infusion). On days 11 and 12, samples were collected before the start of the SCH 900776 infusion, just before completion of the SCH 900776 infusion, and 1 hour after the end of the infusion. Plasma concentrations of SCH 900776 were determined using validated high-performance liquid chromatography/tandem mass spectroscopy (LC/MS-MS) methods (31). Pharmacokinetic data were analyzed using a noncompartmental method with the aid of WinNonLin version 5.2 software (Pharsight Corporation) for determining the following pharmacokinetic parameters. The maximum concentration (Cmax) was obtained from original data. The terminal rate constant (ke) was obtained by regression analysis of the log-linear portion of the concentration–time curve. The terminal half-life (t1/2) was calculated as 0.693/ke. The area under the plasma concentration–time curve (AUC) to the last quantifiable concentration (AUC0–t) was determined by use of the linear trapezoidal rule. AUC0–8.25 h and AUC0–8.5 h are area under the curve of plasma concentration versus time from time 0 to 8.25 and 8.5 hours, respectively, after dosing. AUC from 0 to infinity (AUC0–∞) was calculated by AUC0–t + Ct/ke, where Ct is the last measured plasma concentration. Numerical variables were summarized as mean (%CV).
Pharmacodynamics
To determine the in vivo effects of ara-C and SCH 900776 administration on leukemia blast cell DNA damage as measured by phosphorylation of histone H2Ax, bone marrow aspirates were obtained during cycle 1 pretreatment (day 0), 24 hours after initiation of the cytarabine infusion but before SCH 900776 (day 2), and 2 hours after the second SCH 900776 infusion (day 3). Blasts were isolated from fresh marrow aspirates on Ficoll-Hypaque gradients and prospectively prepared for immunoblotting as described previously (20, 29).
Statistical considerations
The primary objective was to determine the combination-recommended phase II dose (RP2D) by assessing the safety, tolerability, DLT, and antileukemic activity (CR rate) of the combination of cytarabine and SCH 900776 in adult patients with relapsed and/or refractory acute leukemias. In addition, disease-free survival (DFS) was calculated from the date of achievement of CR or CRi to last known follow-up or relapse. Survival data were analyzed as of June 1, 2012.
Results
Patient characteristics
A total of 24 adults (median age 57 years; range, 23–73) with relapsed (9/24, 37%) or refractory (15/24, 63%) acute leukemias were enrolled on study between September 2009 and January 2011 (Table 1). The majority had AML that had not responded to the most recent therapy (refractory, 13/24, 54%), previous exposure to moderately high doses of cytarabine (15/24, 63%), and/or adverse genetic features (15/24, 63%: 12/24, 50%, adverse cytogenetics; 3/24, 13%, FLT-3-internal tandem duplication). In addition, 7 of 21 (33%) patients with AML had received prior allogeneic SCT. Median time from SCT to relapse was 7 months (range, 3–16).
Table 1.
Demographic and biologic characteristics of 24 adults with relapsed or refractory acute leukemia
| AML (n = 21) |
ALL (n = 2) |
CML-BC (n = 1) |
|
|---|---|---|---|
| Male/female | 9/12 | 1/1 | 1/0 |
| Age, median (range) | 56 (23–73) | 30, 63 | 36 |
| Previous cytarabine therapy | 19 | 1 | 1 |
| 2 g/m2/72 h infusion | 7 | 0 | 1 |
| High-dose cytarabinea | 7 | 0 | 0 |
| Relapsed | 8 | 1 | 0 |
| No prior CR, median (range) | 2 (1–3) | 2 | 0 |
| Prior allogeneic SCT | 6 | 0 | 0 |
| Refractoryb | 13 | 1 | 1 |
| Prior regimens, median (range) | 2 (1–4) | 3 | 3 |
| Prior allogeneic SCT | 1 | 0 | 0 |
| Secondary AML | 8 | 0 | 0 |
| MDS/MPDc | 6 | 0 | 0 |
| Treatment related | 2 | 0 | 0 |
| Adverse genetics | 13 | 1 | 1 |
| Single cytogenetics | 3 | 1 | 0 |
| Complex cytogenetics | 7 | 0 | 1 |
| FLT3 mutation | 3 | 0 | 0 |
Two to 3 g/m2 given every 12 hours for 6 to 12 doses as consolidation therapy or reinduction therapy.
Includes primary refractory and/or refractory to multiple therapies.
Myelodysplastic syndrome/myeloproliferative disorder.
Toxicities
Table 2 describes the toxicity profile for patients receiving the timed sequential cytarabine/SCH 900776 combination. DLT occurred at dose level 5 (SCH 900776 140 mg flat dosing, equivalent to 80 mg/m2), manifested by asymptomatic but extended (45 minute) grade 3 QTcF prolongation in 1 patient, transient (<15 minute) grade 3 QTcF prolongation in 1 patient, and prolonged (>7 days) grade 3 palmar-plantar erythodysesthesia, representing an exacerbation of a known cytarabine toxicity. Two additional patients had grade 2 (483–485 milliseconds) prolongation at the end of the SCH 900776 infusion and for 15 minutes thereafter with subsequent spontaneous resolution. All episodes of QTcF prolongation occurred in the setting of electrolyte optimization and avoidance of agents that could cause or exacerbate QTcF prolongation (in particular antiemetics, antifungals, and other CYP3A4 substrates).
Table 2.
Grade 3 to 4 nonhematologic toxicities
| Category | 10 mg/m2 (n = 3) |
20 mg/m2 (n = 3) |
40 mg/m2 (n = 6) |
56 mg/m2 (n = 6) |
140 mg (n = 6) |
|---|---|---|---|---|---|
| Cardiac: QTcF prolongation | 1/6a | ||||
| Gastrointestinal: mucositis | 1/6 | ||||
| Liver: hyperbilirubinemiab | 1/3 | 2/6 | 1/6 | ||
| Skin: hand–foot syndrome | 1/3 | 1/6c |
Plus 1 additional patient with transient (<30 minute) grade 3 QTcF > 500 milliseconds.
All grade 3 and resolved within 48 hours.
Prolonged (>7 days).
Other than the single occurrence of prolonged grade 3 palmer-plantar dysesthesia, other cytarabine-related toxicities, in particular TLS, ARDS, and gastrointestinal mucositis, were not exacerbated by addition of SCH 900776. Grade 1 TLS occurred in 1 patient at dose level 5 and symptoms compatible with grade 1ARDSoccurred in 2 patients at dose level 4 with rapid reversal in each instance. While 100% of patients experienced some gastrointestinal symptoms during therapy, all were grade 1 to 2 and transient in nature with appropriate supportive care. The 4 instances of grade 3 hyperbilirubinemia were, likewise, short-lived and resolved completely within 48 hours or less. Likewise, the incidence of grade 3/4 infectious complications was similar to what would be expected with cytarabine across all dose levels: total bacteremia 4 (17%), Gram-negative bacteremia 1 (4%), Clostridium difficile colitis 1 (4%), Candidemia 1 (4%), radiographic evidence for new or reactivated fungal pneumonia 6 (25%; ref. 32). For those achieving CR/CRi, median time to recovery of ANC >100/mm3 was day 41 (range, 33–64) and platelets >20,000/mm3 was day 39 (range, 31–80), similar to other clinical trials of timed sequential cytarabine combinations (32). No patient died before day 30 and 4 patients (2 dose level 1, 2 dose level 5) died on days 38 to 54 (1 refractory fungal pneumonia, 3 persistent AML), for an overall day 60 death rate of 17%. On the basis of these observations, we concluded that the MTD and RP2D is cytarabine 667 mg/m2/d by continuous infusion on days 1 to 3 and 10 to 12 along with SCH 900776 56 mg/m2/d IV on days 2, 3, 11, and 12.
Clinical outcome
As presented in Table 3 and detailed in Table 4 the achievement of complete tumor clearance (CTC) by day 14 of therapy occurred across all dose levels in 12 of 24 (50%) patients, including 2 of 6 (17%) at SCH 900776 doses of 20 mg/m2 and at least 3 of 6 (50%) beginning at dose level 3 (40 mg/m2). Similarly, the overall response rate (CR plus CRi) was 8 of 24 (33%), with 1 CRi (17%) of 6 patients treated with SCH 900776 doses of ≤ 20 mg/m2 and 7 (39%; 5 CR, 2 CRi) of 18 patients treated at dose level 3 (40 mg/m2) or higher, with at least 2 of 6 (33%) in each of dose levels 3 through 5. All 8 CR/CRi occurred in the 21 (38%) patients with AML. CR/CRi was achieved in 8 of the 12 who had day 14 CTC but in none who had residual leukemia on day 14. All CR/CRi were associated with cytogenetic clearance. CR/CRi occurred in 6 of 14 (43%) patients who had received prior high doses of cytarabine, 3 of 7 (43%) of those undergoing previous alloSCT, 2 of 10 (20%) patients with AML with adverse cytogenetics but none with FLT3-ITD AML, 3 of 8 (38%) with secondary AML, 4/8 (50%) with relapsed AML (2 with multiple relapses), and 4 of 13 (31%) with refractory AML, including 3 with primary refractory AML. Three patients received an additional 1 to 2 cycles of therapy in CR. Four (50%), including 2 who received additional post-CR cycles, were able to proceed to alloSCT or donor lymphocyte infusion (DLI) following achievement of CR/CRi. Median duration of response for all patients with CR/CRi was 10 months (range, 0.2–27+). As of June 1, 2012, 5 of the 8 responders remain alive at 19+ to 30.5+ months.
Table 3.
Dose escalation and clinical outcome for 24 adults with relapsed or refractory acute leukemia
| SCH 900776 dose level | Day 14 CTC | Response (CR/CRi) | Response in pts with prior cytarabine |
|---|---|---|---|
| 1. 10 mg/m2 (n = 3) | 1 | 1 (0/1): AML | 1 (high)a |
| 2. 20 mg/m2 (n = 3) | 1 | 0 (0/0) | 0 |
| 3. 40 (mg/m2 (n = 6) | 4 | 3 (2/1): all AML | 2 (both high) |
| 4. 56 (mg/m2 (n = 6) | 3 | 2 (2/0): both AML | 2 (1 high) |
| 5. 140 mg (n = 6) | 3 | 2 (1/1): both AML | 2 (both high) |
| Total | 12 | 8 (5/3): all AML | 7 (6 high) |
Cytarabine 2 g/m2/72 hours infusion or high dose (2–3 g/m2 given every 12 hours for 6–12 doses).
Table 4.
Characteristics of patients with AML responding to cytarabine/SCH 900776 combination
| Dose level |
Age/sex | Disease status |
No. of prior regimen/cytarabinea |
Prior SCT |
Cytogenetics | Response (CR/CRi) |
SCT or DLI |
DFS (mo) |
|---|---|---|---|---|---|---|---|---|
| 1 | 49 M | Refb | 4/+ | + | 46XY | CRi | SCT | 2.0 |
| 3 | 52F | 10 Refb | 1/− | − | 45XX, −5q, −7, +19q | CR | — | 2.2 |
| 3 | 55M | 10 Refb | 1/+ | − | 46XY, +20q | CR | SCT | 11.3 |
| 3 | 25 M | Relapse 7 moc | 4/+ | + | 46XY, t(8;21), −9q | CR | — | 25.2+ |
| 4 | 70M | 10 Refb | 1/+ | − | 46XY | CR | — | 9.7 |
| 4 | 60 M | Relapse 8 mo | 4/+ | + | 46XY | CR | DLI | 22.4+ |
| 5 | 73 M | Relapse 66 mod | 2/+ | − | 46XY | CRi | — | 0.2 |
| 5 | 61 F | Relapse 19 mo | 2/+ | − | 46XX, inv (16), +22 | CR | SCT | 18.1+ |
Cytarabine, 2 g/m2/72 hours infusion or high dose.
Ref, refractory.
CR duration before beginning cytarabine/SCH900776.
Possible new AML rather than relapse, refractory to reinduction.
Pharmacokinetics
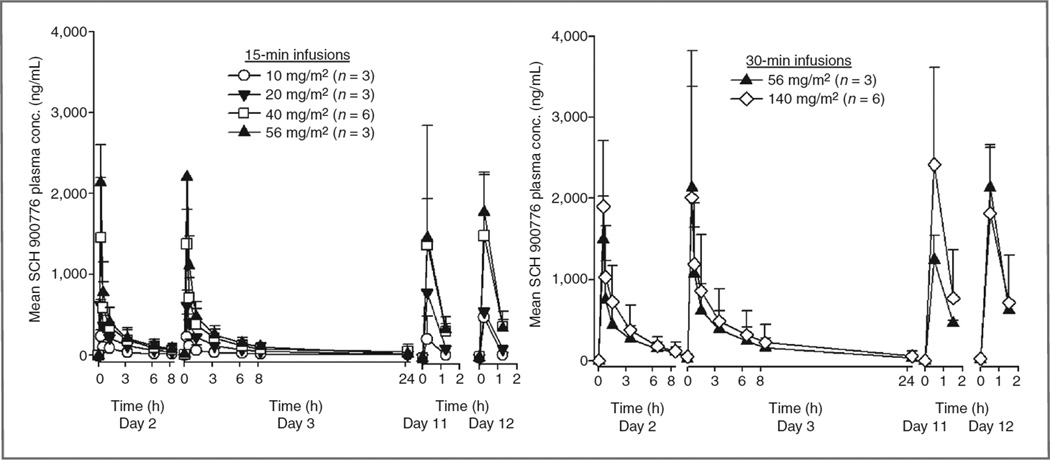
Pharmacokinetic analyses were conducted in all 24 patients, and preliminary mean SCH 900776 plasma pharmacokinetic parameters are presented in Fig. 1. Doserelated increases in SCH 900776 plasma concentrations were observed in mean Cmax and exposure (AUC0–8.25 h for 15-minute infusions, AUC0–8.5 h for 30-minute infusions, and AUC0–∞) values over the dose range evaluated. As dose increased in a ratio of 1:2:4:6 following 15-minute infusions, mean Cmax (day 2) increased in a ratio of 1:3:6:9 and exposure (AUC0–8.25 h, day 2) increased in a ratio of 1:3:5:6. At each dose level, plasma Cmax and exposure (AUC0–8.25 h for 15-minute infusion or AUC0–8.5 h for 30-minute infusion) values were comparable for study days 2 and 3. Harmonic mean half-lives ranged from 5.4 to 6.5 hours across the full dosing range.
Figure 1.
Mean (±SD) plasma concentration profiles of SCH 900776 following 15- or 30-mintue intravenous infusions of 10 (○), 20 (▼), 40 (□) or 56 mg/m2 (▲) or 140 mg SCH 900776 (◊) in combination with cytarabine.
Pharmacodynamics
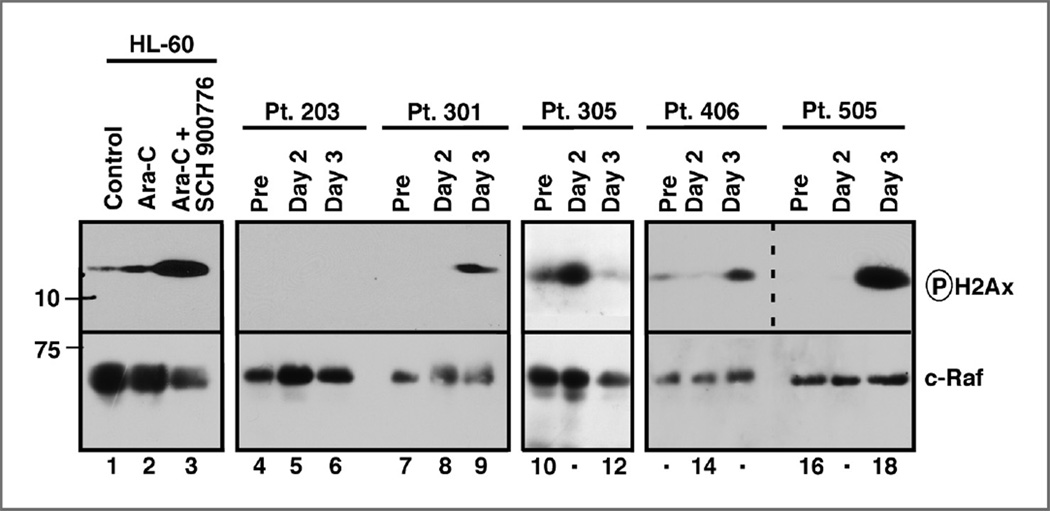
To assess the impact of SCH 900776 given during the continuous cytarabine infusion on leukemic blast cell DNA damage in vivo, we examined H2Ax phosphorylation in bone marrow aspirates harvested sequentially before (day 0), 24 hours after beginning the cytarabine infusion but before the first SCH 900776 dose (day 2), and 2 hours after the end of the second SCH 900776 infusion (day 3) in patients who consented to the extra marrow aspirates. As illustrated in Fig. 2, we observed an increase in H2Ax phosphorylation after 24 hours of continuous cytarabine infusion in 2 of 10 samples (e.g., patient 305) and a further, much more robust increase in H2Ax phosphorylation in 3 of 7 specimens 26 hours later following the second SCH 900776 dose (patients 301, 406, and 505). This marker of enhanced DNA damage, which parallels the assay used to identify SCH 900776 as an agent that interrupts the replication checkpoint (31), was observed in 3 of 5 sets of samples from patients treated with 40 mg/m2 SCH 900776 or higher, providing evidence that the action of SCH 900776 observed in preclinical studies can also be detected in the clinical setting.
Figure 2.
Assessment of H2Ax phosphorylation in sequential marrow samples from individual patients undergoing treatment with cytarabine and SCH 900776 at dose levels 2 to 5. Aliquots of whole-cell lysates containing protein from 5 × 105 marrow mononuclear cells (median 80% blasts; range, 64%–98%) were subjected to SDS-PAGE and immunoblotting for phosphorylated H2Ax or, as a loading control, c-Raf (lanes 4–18). Duplicate blots probed for lamin B1 and histone H1 yielded similar results. Each blot also contained whole-cell lysates from 3 × 105 HL-60 cells treated for 24 hours with diluent, 1 µmol/L cytarabine, or 1 µmol/L cytarabine + 1 µmol/L SCH 900776 (lanes 1–3, respectively). Dashed line indicates 2 different exposure times for the phospho-H2Ax blot from patients 406 and 505.
Discussion
This phase I trial shows that administration of the selective Chk1 inhibitor SCH 900776 in combination with the prototypic antileukemic nucleoside analog cytarabine is feasible and tolerable. In addition, there is preliminary evidence of clinical activity in this small group of adults with relapsed and/or refractory acute leukemias, including those who have had previous exposure to moderate- to high-dose cytarabine and those who have undergone previous allogeneic SCT. The notion that part of the responses might relate to adding SCH 900776 to the timed sequential cytarabine infusions is supported by the presence of a doseresponse curve, with clinical responses to SCH 900776 beginning at 40 mg/m2, in conjunction with Cmax exceeding 1,000 ng/mL. Indeed, the CR/CRi rate was 1 of 6 (17%) for patients who received <40 mg/m2 SCH 900776 versus 7 of 18 (39%) for those receiving ≥ 40 mg/m2 (P = 0.0005, Fisher exact test). Of the 8 who responded, 6 had previously received moderate- to high-dose cytarabine, 2 had undergone prior alloSCT and 2 had primary refractory AML following moderate- to high-dose cytarabine. Nonetheless, because many patients who are deemed refractory to cytarabine do not receive subsequent cytarabine or, alternatively, receive higher doses of cytarabine that lead to responses in a small subset of such patients, we cannot exclude the possibility that some or all of the responders might have responded to timed sequential cytarabine without SCH 900776. Moreover, the small sample size and heterogeneous nature of this patient population preclude any ability to draw accurate conclusions about additive or synergistic clinical effects of adding SCH 900776 to cytarabine, and we do not know if the overall response rate seen in our study is different from that seen with high-dose cytarabine alone. To address these questions will require a larger study with randomization between the SCH 900776/cytarabine combination versus cytarabine alone.
Importantly, there seemed to be no enhancement of cytarabine-related marrow or mucosal toxicities over the dose range tested. This finding stands in distinction to our previous study of cytarabine plus tanespimycin (29), where DLT was reached before significant blast cell Chk1 downregulation was achieved. This difference likely relates to the more selective targeting of Chk1 by SCH 900776 (31), with few if any of the off-target effects on other critical pathways that are seen with tanespimycin.
The DLT of the cytarabine/SCH 900776 combination occurred at the SCH 900776 dose of 140 mg (equivalent to 80 mg/m2) and was manifested by asymptomatic prolongation of the QTcF interval. This effect on cardiac conduction was likely related directly to SCH 900776, without contribution from cytarabine. On the other hand, the significant "hand-foot syndrome" (palmar-plantar erythrodysesthesia) in 1 patient at that dose level could be attributed to the addition of SCH 900776 to cytarabine and likely represents exacerbation of a known cytarabine toxicity. Nonetheless, at the dose levels of SCH 900776 tested in this study, there was no consistent enhancement of timed sequential cytarabine-related marrow aplasia, mucosal toxicities or related consequences such as gastrointestinal-based infections or other manifestations of gastrointestinal barrier breakdown. On the basis of these findings, the RP2D for SCH 900776 is 100 mg/dose (equivalent to 56 mg/m2/dose) given by 30-minute infusion on days 2, 3 11, and 12 in combination with cytarabine 2 g/m2/72 hours given in a timed sequential fashion on days 1 to 3 and 10 to 12.
The clinical trial drug schedule was based on preclinical in vitro studies of AML cell lines and primary AML marrow cells showing that (i) cytarabine induces checkpoint activation and S-phase arrest via Chk1 activation (20, 30), (ii) SCH 900776 effectively enhances cytarabine-induced apoptosis by abrogating S-phase arrest (30), and (iii) SCH 900776 augments the suppressive effects of cytarabine on leukemia cell colony formation (30). Importantly, SCH 900776 has been shown to override the replication checkpoint when administered briefly after a prolonged antimetabolite exposure (31). Although the in vivo correlates obtained longitudinally during cytarabine plus SCH 900776 treatment were limited by the ability of patients to accept no more than 1 bone marrow aspirate each day, our results substantiated the in vitro findings and suggest that SCH 900776 administration following and during infusional cytarabine may have led to an increase in unrepaired DNA DSBs in some leukemias, as measured by H2Ax phosphorylation. Because the ficoll gradient sedimentation used to isolate blasts removes apoptotic cells, it is unlikely that the increased H2Ax phosphorylation reported here reflects secondary DNA fragmentation due to apoptosis. Accordingly, H2Ax phosphorylation or related markers of ongoing, unrepaired DNA damage could be investigated further as a potential predictor of response and should be incorporated into subsequent clinical trials of this and other nucleoside-SCH 900776 regimens.
It would be useful to identify potential responders to the cytarabine/SCH 900776 combination based on pretreatment characteristics. At least in this limited patient cohort, prior treatment with cytarabine did not preclude a response (Table 4). Previous studies have suggested that AMLs with a complex karyotype exhibit higher levels of constitutive H2Ax phosphorylation (34) and increased sensitivity to checkpoint interruption with the Chk1/Chk2 inhibitor AZD 7762 (35). In the present study, only 2 of 10 (20%) patients with a complex karyotype achieved CR/CRi versus 5 of 14 (36%) patients without complex cytogenetics. Given the small sample size of this dose-finding trial, further study in a larger cohort is required to determine whether there truly is any relationship between prior cytarabine treatment, the presence of a complex karyotype, or any other pretreatment parameter and response to this therapy.
In summary, based on the role of Chk1 in protecting leukemia cells from cytarabine-induced DNA damage, the ability of SCH 900776 to impede this protection in preclinical studies, and the preliminary suggestion of antileukemia activity in the present phase I trial, further study of the cytarabine/SCH 900776 combination in a larger group of relapsed and refractory patients with AML seems warranted.
Translational Relevance.
Cytarabine incorporation into DNA activates checkpoint kinase 1 (Chk1), which induces S-phase slowing and leads to cytarabine resistance. Previous studies have shown that the selective Chk1 inhibitor SCH900776 can overcome S-phase checkpoint activation and enhance cytarabine cytotoxicity in acute leukemia cell lines and primary leukemic blasts in vitro. To build on these results, here we report the first clinical trial of a selective Chk1 inhibitor in acute leukemias. This phase I study of cytarabine and SCH 900776 in adults with resistant leukemias shows that the combination of SCH 900776 and cytarabine has tolerable side effects in normal tissues, enhances the DNA damaging effects of cytarabine in at least some clinical leukemias in vivo, and induces remission in both relapsed and refractory acute myelogenous leukemia (AML). Accordingly, further study of the cytarabine/SCH 900776 combination in a larger group of relapsed and refractory patients with AML seems warranted.
Acknowledgments
The authors thank the Johns Hopkins Sidney Kimmel Cancer Center nursing staff for superb medical care, and the patients and their families, without whose partnership we could never have conducted the trial and from whom we learned critical information that will help us to improve the treatment of these diseases.
Grant Support
This work was supported by research funding from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. (Whitehouse Station, NJ), National Cancer Institute Cancer Center Support Grant (2P30 CA06973-47), National Center for Research Resources Grant (UL1 RR025005), and philanthropic funds from Dr. Robert E. Fischell in memory of his late wife Marian.
S. Loechner is employed (other than primary affiliation; e.g., consulting) by Merck as CPM at time of study conduct. D.A. Parry is employed (other than primary affiliation; e.g., consulting) by Schering Plough/Merck as Senior Research Fellow. J.A. Horowitz is employed (other than primary affiliation; e.g., consulting) by Merck/Schering Plough as Section Head, has a commercial research grant and other commercial research support from Merck, and has other support (e.g., expert testimony) from Merck.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed by the other authors.
Authors' Contributions
Conception and design: J.E. Karp, S.D. Gore, D.A. Parry, R. Isaacs, S.H. Kaufmann
Development of methodology: J.E. Karp, S.D. Gore, K. Peterson, P. Schneider, R. Isaacs
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): J.E. Karp, J.M. Greer, S.D. Gore, K.W. Pratz, B.D. Smith, K. Mackey, M.A. McDevitt, H.E. Carraway, D.E. Gladstone, M.M. Showel, S.H. Kaufmann
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): J.E. Karp, B.M. Thomas, C. Sorge, T. Freshwater, R. Isaacs
Writing, review, and/or revision of the manuscript: J.E. Karp, B.M. Thomas, S.D. Gore, K.W. Pratz, B.D. Smith, M.J. Levis, H.E. Carraway, D. E. Gladstone, S. Loechner, D.A. Parry, J.A. Horowitz, R. Isaacs, S.H. Kaufmann
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): J.E. Karp, J.M. Greer, K.S. Flatten, K. Mackey, H.E. Carraway, J.A. Horowitz, R. Isaacs
Study supervision: J.E. Karp, K. Mackey, R. Isaacs
Oversight of study conduct, data management: S. Loechner
References
- 1.Lowenberg B, Pabst T, Vellenga E, van Putten W, Schouten HC, Graux C, et al. Cytarabine dose for acute myeloid leukemia. New Engl J Med. 2011;364:1027–1036. doi: 10.1056/NEJMoa1010222. [DOI] [PubMed] [Google Scholar]
- 2.Tallman MS, Gilliland DG, Rowe JM. Drug therapy for acute myeloid leukemia. Blood. 2005;106:1154–1163. doi: 10.1182/blood-2005-01-0178. [DOI] [PubMed] [Google Scholar]
- 3.Mackey JR, Mani RS, Selner M, Mowles D, Young JD, Belt JA, et al. Functional nucleoside transporters are required for gemcitabine influx and manifestation of toxicity in cancer cell lines. Cancer Res. 1998;58:4349–4357. [PubMed] [Google Scholar]
- 4.Huang M, Wang Y, Collins M, Gu JJ, Mitchell BS, Graves LM. Inhibition of nucleoside transport by p38 MAPK inhbiitiors. J Biol Chem. 2002;277:28364–28367. doi: 10.1074/jbc.C200321200. [DOI] [PubMed] [Google Scholar]
- 5.Liliemark JO, Plunkett W, Dikxon DO. Relationship of 1-beta-D-arabinofuranosylcytosine in plasma to 1-beta-D-arabinofuranosylcytosine 50-triphosphate levels in leukemic cells during treatment with high-dose 1-beta-D-arabinofuranosylcytosine. Cancer Res. 1985;45:5952–5957. [PubMed] [Google Scholar]
- 6.Karp JE, Donehower RC, Dole GB, Burke PJ. Correlation of drugperturbed marrow cell growth kinetics and intracellular 1-beta-D-arabinofuranosylcytosine with clinical response in adult acute myelogenous leukemia. Blood. 1987;69:1134–1140. [PubMed] [Google Scholar]
- 7.Kimball AP, Wilson MJ. Inhibition of DNA polymerase by beta-Darabinosylcytosine and reversal of inhibition by deoxycytidine-50-triphosphate. Proc Soc Exp Biol Med. 1968;127:429–432. doi: 10.3181/00379727-127-32708. [DOI] [PubMed] [Google Scholar]
- 8.Momparler RL. Effect of cytosine arabinoside 50-triphosphate onmammalian DNA polymerase. Biochem Biophys Res Commun. 1969;34:464–471. doi: 10.1016/0006-291x(69)90405-7. [DOI] [PubMed] [Google Scholar]
- 9.Graham FL, Whitmore GF. Studies in mouse L-cells on the incorporation of 1-beta-D arabinofuranosylcytosine 50-triphosphatase. Cancer Res. 1970;30:2636–2644. [PubMed] [Google Scholar]
- 10.Kufe DW, Major PP, Egan EM, Beardsley GP. Correlation of cytotoxicity with incorporation of ara-C in DNA. J Biol Chem. 1980;225:8897–8900. [PubMed] [Google Scholar]
- 11.Major PP, Egan EM, Beardsley GP, Minden MD, Kufe DW. Lethality of human myeloblasts correlates with the incorporation of arabinofuranosylcytosine into DNA. Proc Natl Acad Sci U S A. 1981;78:3235–3239. doi: 10.1073/pnas.78.5.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ewald B, Sampath D, Plunkett W. ATM and the Mre11-Rad50-Nbs1 complex respond to nucleoside analogue-induced stalled replication forks and contribute to drug resistance. Cancer Res. 2008;68:7947–7955. doi: 10.1158/0008-5472.CAN-08-0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science. 2003;300:1542–1548. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
- 14.Dai Y, Grant S. New insights into checkpoint kinase 1 in the DNA damage response signaling network. Clin Cancer Res. 2010;16:376–383. doi: 10.1158/1078-0432.CCR-09-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feijoo C, Hall-Jackson C, Wu R, Jenkins D, Leitch J, Gilbert DM, et al. Activation ofmammalian Chk1 during DNA replication arrest: a role for Chk1 in the intra-S phase checkpoint monitoring replication origin firing. J Cell Biol. 2001;154:913–923. doi: 10.1083/jcb.200104099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zachos G, Rainey MD, Gillespie DAF. Chk1-deficient tumour cells are viable but exhibit multiple checkpoint and survival defects. EMBO J. 2003;22:713–723. doi: 10.1093/emboj/cdg060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimuta K, Nakajo N, Uto K, Hayano Y, Okazaki K, Sagata N. Chk1 is activated transiently and targets Cdc25A fior degradation at the Xenopus midblastula transition. EMBO J. 2002;21:3694–3703. doi: 10.1093/emboj/cdf357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao H, Watkins JL, Piwnica-Worms H. Disruption of the checkpoint kinase 1/cell division cycle 25A pathway abrogates ionizing radiationinduced S and G2 checkpoints. Proc Natl Acad Sci U S A. 2002;99:14795–14800. doi: 10.1073/pnas.182557299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loegering D, Arlander SAH, Hackbarth J, Vroman B, Lieberman HB, Karnitz LM, et al. Rad9 protects cells from topoisomerase poisoninduced cell death. J Biol Chem. 2004;279:18641–18647. doi: 10.1074/jbc.M313536200. [DOI] [PubMed] [Google Scholar]
- 20.Mesa RA, Loegering D, Powell HL, Flatten K, Arlander SAH, Dai NT, et al. Heat shock protein 90 inhibition sensitizes acute myelogenous leukemia cells to cytarabine. Blood. 2005;106:318–327. doi: 10.1182/blood-2004-09-3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao H, Piwnica-Worms H. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol Cell Biol. 2001;21:4129–4139. doi: 10.1128/MCB.21.13.4129-4139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho SH, Toouli CD, Fuji GH, Crain C, Parry D. Chk1 is essential for tumor cell viability following activation of the replication checkpoint. Cell cycle. 2005;4:131–139. doi: 10.4161/cc.4.1.1299. [DOI] [PubMed] [Google Scholar]
- 23.Wang S, Wang Z, Grant S. Bryostatin 1 and UCN-01 potentiate 1-B-Darabinofuranosylcytosine- induced apoptosis in human myeloid leukemia cells through disparate mechanisms. Mol Pharmacol. 2003;63:232–242. doi: 10.1124/mol.63.1.232. [DOI] [PubMed] [Google Scholar]
- 24.Fuse E, Tanii H, Kurata N, Kobayashi H, Shimada Y, Tamura T, et al. Unpredicted clinical pharmacology of UCN-01 caused by specific binding to human alpha 1-acid glycoprotein. Cancer Res. 1998;58:3248–3252. [PubMed] [Google Scholar]
- 25.Sausville EA, Arbuck SG, Messmann R, Headlee D, Bauer KS, Lush RM, et al. Phase I trial of 72-hour continuous infusion UCN-01in patients with refractory neoplasms. J Clin Oncol. 2001;19:2319–2333. doi: 10.1200/JCO.2001.19.8.2319. [DOI] [PubMed] [Google Scholar]
- 26.Ruegg UT, Burgess GM. Staurosporine, K252 and UCN-01: potent but nonspecific inhibitors of protein kinases. Trends Pharmacol Sci. 1989;10:218–220. doi: 10.1016/0165-6147(89)90263-0. [DOI] [PubMed] [Google Scholar]
- 27.Senderowicz AM. Small-molecule cyclin-dependent kinase modulators. Oncogene. 2003;22:6609–6620. doi: 10.1038/sj.onc.1206954. [DOI] [PubMed] [Google Scholar]
- 28.Arlander SJ, Eapen AK, Vroman BT, McDonald RJ, Toft DO, Karnitz LM. Hsp90 inhibition depletes Chk1 and sensitizes tumor cells to replication stress. J Biol Chem. 2003;278:52572–52577. doi: 10.1074/jbc.M309054200. [DOI] [PubMed] [Google Scholar]
- 29.Kaufmann SH, Karp JE, Litzow MR, Mesa RA, Hogan W, Steensma DP, et al. Phase I and pharmacological study of cytarabine and tanespimycin in relapsed and refractory acute leukemia. Haematologica. 2011;96:1619–1626. doi: 10.3324/haematol.2011.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schenk EL, Koh BD, Flatten KS, Peterson KL, Parry D, Hess AD, et al. Effects of selective checkpoint kinase I inhibition on cytarabine cytotoxicity in acute myelogenous leukemia. Clin Cancer Res. 2012;18:5364–5373. doi: 10.1158/1078-0432.CCR-12-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guzi TJ, Paruch K, Dwyer MP, Labroli M, Shanahan F, Davis N, et al. Targeting the replication checkpoint using SCH 900776, a potent and functionally selectiveCHK1 inhibitor identified via high content screening. Mol Cancer Ther. 2011;10:591–602. doi: 10.1158/1535-7163.MCT-10-0928. [DOI] [PubMed] [Google Scholar]
- 32.Karp JE, Smith BD, Resar LS, Greer JM, Blackford A, Zhao M, et al. Phase I and pharmacokinetic study of bolus-infusion flavopiridol follwed by cytosine arabinoside and mitoxantrone for acute leukemia. Blood. 2011;117:3302–3310. doi: 10.1182/blood-2010-09-310862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 34.Cavelier C, Didier C, Prade N, Mansat-De Mas V, Manenti S, Recher C, et al. Constitutive activation of the DNA damage signaling pathway in acute myeloid leukemia with complex karyotype: potential importance for checkpoint targeting therapy. Cancer Res. 2009;69:8652–8661. doi: 10.1158/0008-5472.CAN-09-0939. [DOI] [PubMed] [Google Scholar]
- 35.Didier C, Demur C, Grimal F, Jullien D, Manenti S, Ducommon B. Evaluation of checkpoint kinase targeting therapy in acute myeloid leukemia with complex karyotype. Cancer Biol Ther. 2012;13:307–313. doi: 10.4161/cbt.13.5.19074. [DOI] [PMC free article] [PubMed] [Google Scholar]