Abstract
Human hereditary deafness at the DFNB29 autosomal locus on chromosome 21q22.1 is caused by recessive mutations of CLDN14, encoding claudin 14. This tight junction protein is tetra-membrane spanning that localizes to the apical tight junctions of organ of Corti hair cells and in many other tissues. Typically, the DFNB29 phenotype is characterized by pre-lingual, bi-lateral, sensorineural hearing loss. The goal of this study was to define the identity and frequency of CLDN14 mutations and associated inner ear phenotypes in a cohort of 800 Pakistani families segregating deafness. Hearing loss in 15 multi-generational families was found to co-segregate with CLDN14-linked STR markers. The sequence of the six exons and regions flanking the introns of CLDN14 in these 15 families revealed five likely pathogenic alleles. Two are novel missense substitutions (p.Ser87Ile and p.Ala94Val) while p.Arg81His, p.Val85Asp and p.Met133ArgfsX23 have been reported previously. Haplotype analyses indicate that p.Val85Asp and p.Met133ArgfsX23 are founder mutations. The p.Val85Asp accounts for approximately 67% of the mutant alleles of CLDN14 in our cohort. Combined with previously reported data, CLDN14 mutations were identified in 18 of 800 Pakistani families (2.25%; 95% CI, 1.4-3.5%). Hearing loss in the affected individuals homozygous for CLDN14 mutations varied from moderate to profound. This phenotypic variability may be due to environmental factors (e.g. drug and noise exposure) and/or genetic modifiers.
Keywords: CLDN14, claudin 14, DFNB29, mild hearing loss, profound deafness, Pakistan
INTRODUCTION
Mutations of CLDN14 cause autosomal recessive nonsyndromic deafness at the DFNB29 locus. To date, six different pathogenic variants of human CLDN14 have been identified in families segregating severe to profound hearing loss, but no obvious vestibular phenotype.1-4 Similarly, a Cldn14 knockout mouse is also deaf.5 Although claudin 14 is expressed in the mouse vestibular sensory epithelium, Cldn14 knockout mice appear to have no obvious vestibular disorder such as circling behavior or head-bobbing.5
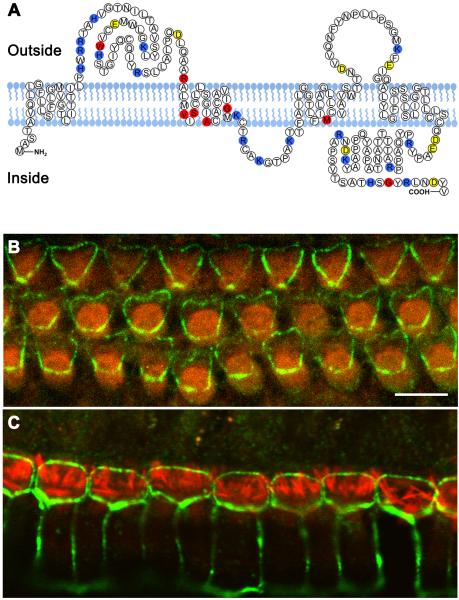
The mammalian claudin family of twenty-seven genes encodes tight junction proteins that function to maintain integrity of the apical and basolateral membrane domains and prevent diffusion of solutes and solvent molecules through intercellular spaces within epithelial sheets.6-9 The claudin proteins are predicted to have four transmembrane domains and short cytosolic amino and carboxy termini.10,11 While the first and the fourth transmembrane regions as well as the extracellular loops are highly conserved among the different claudin species, the second and the third transmembrane regions are variable.12 The first extracellular loop of these proteins has an important role in homophilic interactions (Figure 1a).13,14 To date, most of the known mutations of claudin 14 are within or close to the second or third transmembrane domains (Figure 1a) and some of them have been shown to affect membrane localization. For example, p.Val85Asp impairs the ability of claudin 14 to form tight junction strands.4
Figure 1.
Eight different pathogenic alleles of CLDN14 are associated with hearing loss in human. (a) Schematic of human claudin14. Topology of claudin14 was predicted by TMpred software. Yellow and blue amino acids indicate negatively and positively charged residues, respectively. The positions of eight residues mutated in all reported DFNB29 families are red. (b) Localization of claudin 14 in the apical bicellular tight junctions between the outer hair cells (OHCs) and Deiters’ cells (green). (c) Localization of claudin 14 (green) in the tight junctions between inner hair cells (IHCs) and pillar cells and between two adjacent pillar cells. Filamentous actin is highlighted by rhodamine-phalloidin (red). Scale bar is 5 μm.
The structure of most intercellular tight junctions in the inner ear is similar to that reported in other epithelia.15-18 However, the structure of the bicellular junctions between hair cells and supporting cells, especially between an outer hair cell and adjacent Deiter’s cell are more elaborate and highly specialized to maintain the ionic barrier between endolymph and perilymph.16-18 These tight junctions contain a high amount of claudin 14 and are prominently stained with anti-claudin 14 antibody (Figure 1b-c). The apical junctional complexes between the cells of the organ of Corti lack desmosomes and gap junctions and have a combination of tight junction and adherens junction features, and extend down the depth of the reticular lamina, a region spanning 3-5 μm.18
The goal of this study was to determine the spectrum of mutant alleles, and the frequencies of these alleles in 800 Pakistani families segregating nonsyndromic deafness, and to measure variability in the clinical phenotype of CLDN14 pathogenic variants. We found that pathogenic alleles of CLDN14 are associated with hearing loss that ranges from moderate to profound, and that mutant alleles of this gene appear to be a common cause of heritable hearing loss among Pakistanis.
METHODS
Family participation and clinical evaluation
This study was approved by IRBs at the National Centre of Excellence in Molecular Biology (NCEMB), Lahore, Pakistan (FWA00001758), at the National Institutes of Health, USA (Combined Neuroscience IRB; OH-93-N-016), and at the Cincinnati Children’s Hospital Research Foundation, USA (2009-0684; 2010-0291). Written informed consent was obtained from adult subjects and parents of minor subjects. Hearing was evaluated in audiology clinics by pure tone audiometry at octave frequencies with intensities up to 110 dBHL. Vestibular function was evaluated by tandem gait and Romberg testing.19
Genotype and mutational analysis
Genomic DNA was extracted from 10 ml of peripheral venous blood as described.20,21 Three fluorescently labeled microsatellite markers (D21S2078, D21S1252 and D21S2080) linked to CLDN14 were PCR-genotyped as described.20 Primers for polymerase chain reaction (PCR) amplification, and CLDN14 sequencing were designed using Primer3 (http://frodo.wi.mit.edu/).
Co-segregation of the mutations with hearing loss in each family was demonstrated for all subjects participating in this study. Control DNA samples from ethnically matched Pakistanis were sequenced to ascertain novel variants of CLDN14. Three prediction programs, SIFT,22 Polyphen-2,23 and MutationTaster24 were used to evaluate the potential effect of each novel missense mutation.
Immunolocalization of claudin 14 in the mouse organ of Corti
Immunolocalization of claudin 14 using tissue from C57BL/6 mouse organ of Corti was performed as described previously using a custom rabbit polyclonal PB108 anti-claudin 14 antibody with validated specificity. There was no immuno-localization signal when tissue from a Cldn14 knockout mouse was used.5
RESULTS
Pathogenic variants of CLDN14
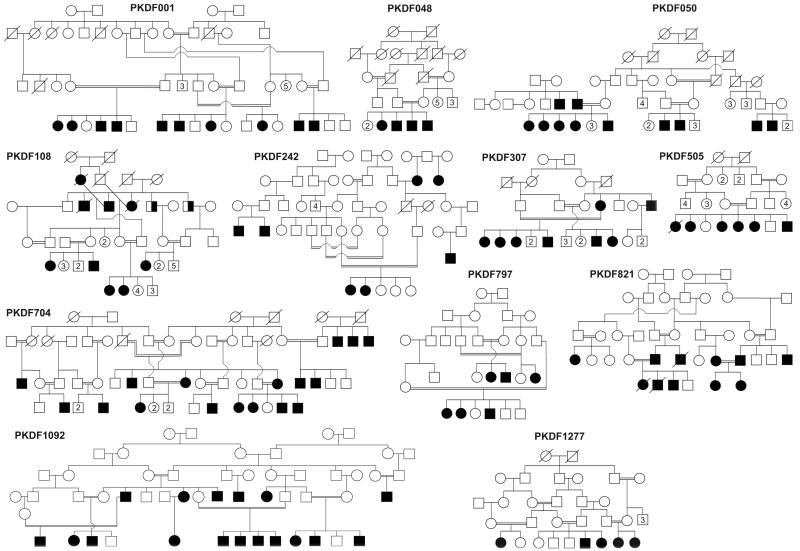
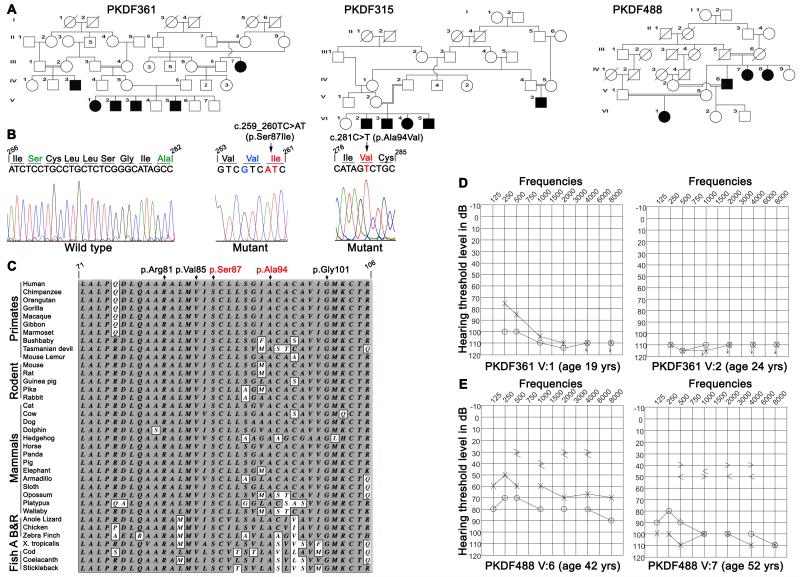
We reported that mutations of CLDN14 cause DFNB29 deafness.1 Subsequently deafness segregating in 15 additional families (Figures 2 & 3) was found to be linked to STR markers for CLDN14 (Table 1). Sequence analysis of CLDN14 revealed four likely pathogenic variants. Among the five variants of CLDN14, two were novel missense substitutions p.Ser87Ile (c.259_260TC>AT) and p.Ala94Val (c.281C>T). In family PKDF361, we detected three nearly adjacent nucleotides changes (c.256A>G and c.259_260TC>AT; Figure 3b), which are predicted to result in two substitutions (p.Ile86Val and p.Ser87Ile; Table 1). SIFT, Polyphen-2, and MutationTaster predicted that p.Ile86Val is a benign polymorphism. Furthermore, claudin 14 orthologs in Armadillo, cow and hedgehog have a valine residue at position 86. Therefore, we considered p.Ile86Val as a non-pathogenic substitution although no carriers of c.256A>G were found in our 184 control subjects or in 1000 Genome and NHLBI-ESP databases. These data suggest that c.256A>G is a rare and benign variant while p.Ser87Ile is predicted to be deleterious (Table 1).
Figure 2.
Pakistani DFNB29 families. Filled symbols represent individuals with prelingual, sensorineural hearing loss, and double horizontal lines indicate a consanguineous marriage. Half fill symbols in family PKDF108 are individuals (ages 72 and 75 years) that have age related hearing loss. Numbers represent unaffected siblings.
Figure 3.
Pedigree of three DFNB29 families, sequencing chromatograms, pure tone audiograms and ClustalW alignment of thirty six claudin14 orthologs. (a) Pedigrees of families PKDF361, PKDF315 and PKDF488. Filled symbols represent affected individuals. (b) Wild type and homozygous mutant nucleotide sequence chromatograms of exon 3 of CLDN14 illustrating homozygosity for the c.259_260TC>AT (p.Ser87Ile) and c.281C>T (p.Ala94Val) mutations (arrows). Shown in green are the amino acids that are mutated (red) in the DFNB29 families, while blue color represent the non-deleterious change found in family PKDF361. (c) ClustalW multiple sequence alignment of the 36 amino acids of claudin14 shows that p.Ser87 and p.Ala94 residues are conserved across species (shaded background). For comparison, three previously reported mutated residues, p.Arg81, p.Val85 and p.Gly101 are also shown. Amino acids are numbered with reference to GenBank Accession number NP_036262. (d) Pure tone air and bone conduction thresholds for family PKDF361 individuals V:1 (19 yo female), V:2 (24 yo male). Individual V:1 has severe to profound hearing loss in her left ear, while right ear showed profound deafness. Right ear air conduction: O; Left ear air conduction: X; Right ear bone conduction: >; Left ear bone conduction: <; ↓ indicates the threshold level beyond the measurable range. (e) Pure tone air and bone conduction thresholds for family PKDF488 individuals V:6 (42 yo male) revealed moderate sensorineural hearing loss in his left ear, while the right ear showed a severe degree of hearing loss. In contrast, individual V:7 (52 yo female) had severe to profound, bilateral, sensorineural hearing impairment.
Table 1. CLDN14 variants in Pakistani families segregating DFNB29 hearing loss#.
| Family | Ethnicity | Haplotype | Nucleotide variation |
Predicted effect | SIFT | Polyphen 2 |
Mutation Taster | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|
| D21S2078 | D21S1252 | D21S2080 | ||||||||
|
|
||||||||||
| PKSR9a | Punjabi | 152 | 244 | 174 | c.254T>A | p.Val85Asp | Deleterious | Damaging | Disease causing | [1] |
| PKDF001 | Punjabi | 152 | 244 | 174 | c.254T>A | p.Val85Asp | this study | |||
| PKDF009 | Punjabi | 152 | 244 | 174 | c.254T>A | p.Val85Asp | [25] | |||
| PKDF048 | Punjabi | 152 | 244 | 174 | c.254T>A | p.Val85Asp | this study | |||
| PKDF050 | Punjabi | 152 | 244 | 174 | c.254T>A | p.Val85Asp | this study | |||
| PKDF108 | Punjabi | 152 | 244 | 174 | c.254T>A | p.Val85Asp | this study | |||
| PKDF242 | Punjabi | 152 | 244 | 174 | c.254T>A | p.Val85Asp | this study | |||
| PKDF307 | Punjabi | 152 | 244 | 174 | c.254T>A | p.Val85Asp | this study | |||
| PKDF505 | Punjabi | 156 | 244 | 166 | c.254T>A | p.Val85Asp | this study | |||
| PKDF704 | Punjabi | 152 | 244 | 174 | c.254T>A | p.Val85Asp | this study | |||
| PKDF797 | Punjabi | 152 | 244 | 174 | c.254T>A | p.Val85Asp | this study | |||
| PKDF821 | Punjabi | 152 | 244 | 174 | c.254T>A | p.Val85Asp | this study | |||
| PKDF1277 | Balochi | 154 | 234 | 166 | c.242G>A | p.Arg81His | [2]; this study | |||
| PKDF1092 | Punjabi | 154 | 246 | 166 | c.398delT | p.Met133ArgfsX23 | Deleterious | Damaging | Disease causing | this study |
| PKSN6 | Punjabi | 154 | 246 | 166 | c.398delT | p.Met133ArgfsX23 | [1] | |||
| PKDF361 | Sindhi | 156 | 246 | 166 | c.256A>G | p.Ile86Val | Tolerated | Benign | Polymorphism | this study |
| c.259_260TC>AT | p.Ser87Ile | Deleterious | Damaging | Disease causing | this study | |||||
| PKDF315 | Punjabi | 154 | 288 | 172 | c.281C>T | p.Ala94Val | Deleterious | Damaging | Disease causing | this study |
| PKDF488 | Punjabi | 154 | 228 | 172 | c.281C>T | p.Ala94Val | this study | |||
All variants were found in the homozygous state, and previously unreported variants are shown in bold.
In addition, we detected p.Ala94Val in families PKDF315 and PKDF488 (Figure 3a-b). Both p.Ser87Ile and p.Ala94Val mutations affect amino acid residues that are conserved among 36 claudin 14 orthologs (Figure 3c). No carriers of c.259_260TC>AT and c.281C>T were found among 384 ethnically matched control chromosomes that we Sanger sequenced, in the 1000 Genome database, or in 5,400 individuals listed in the NHLBI-ESP variant database. Our data indicate that these variants are not common polymorphisms, and in each family homozygosity for the mutant allele of CLDN14 co-segregated with deafness while carriers had normal hearing.
We observed the previously reported variants c.254T>A (p.Val85Asp) in twelve families, c.242G>A (p.Arg81His) in one family and c.398delT (p.Met133ArgfsX23) in one family. All of these mutations cosegregated with deafness. STR markers linked to CLDN14 were genotyped in unrelated affected individuals homozygous for the c.254T>A and c.398delT mutations and for both alleles the flanking haplotypes were consistent with a founder effect (Table 1).
DFNB29 hearing loss phenotype
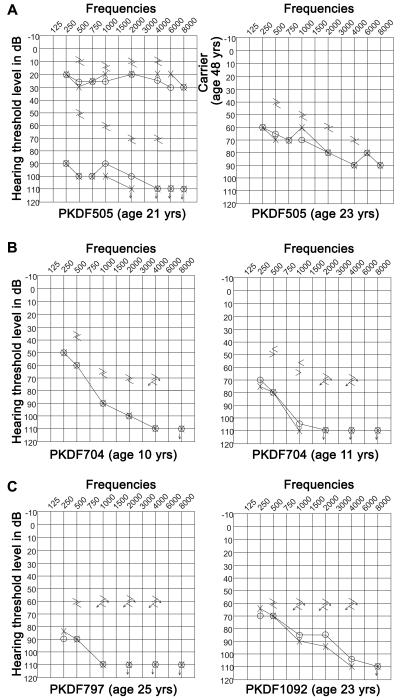
Pure tone air and bone conduction audiometry revealed inter- and intra-familial variability in the severity of hearing loss (Figures 3d-e and 4) in these families. The affected individuals of family PKDF361 had pre-lingual severe to profound hearing loss across all the tested frequencies (Figure 3d). The 42 year old affected individual (V:6) of family PKDF488 had bilateral moderate to severe, sensorineural hearing loss while his sibling (V:7 age 52 years) had profound hearing loss across almost all the frequencies (Figure 3e). Phenotypic variability was associated with the known p.Val85Asp mutation (Figure 4), an allele we reported in two large multi-generation Pakistani families segregating pre-lingual, severe to profound hearing loss.1,25 In this study, we identified eleven additional families segregating the p.Val85Asp allele of CLDN14. Hearing loss in members of these families ranged from moderate to profound with greater severity at higher frequencies (Figure 4), which is in agreement with degeneration of sensory hair cells from base to apex seen in the Cldn14 knockout mice.5
Figure 4.
Pure tone air and bone conduction measurements from DFNB29 families segregating p.Val85Asp revealed intra- and inter-familial variability in thresholds. (a) Twenty-one year old affected individual of family PKDF505 has profound, bilateral, sensorineural hearing loss, while his twenty-three years old sibling has moderate to severe, bilateral sensorineural hearing impairment. Shown also is their 48 years old mother, a carrier of p.Val85Asp allele, with normal hearing thresholds across all frequencies. (b) Ten year old affected individual of family PKDF704 has moderate to profound, bilateral, sensorineural hearing loss, while her eleven year old sibling has severe to profound, bilateral sensorineural hearing impairment. (c) Twenty-five year old affected individual of family PKDF797 has severe to profound hearing loss, while a twenty-three old affected individual of family PKDF1092 has moderate to profound, sensorineural hearing loss.
Discussion
CLDN14 mutations are a common cause of recessive hearing loss in the Pakistani population since mutations of this gene account for 2.25% (18 of 800 families; 95% CI, 1.4-3.5%) of deafness in the NCEMB Pakistani study cohort (Table 2).1-3,25 The probands of the NCEMB deafness cohort are usually students in schools for the hearing impaired and are usually profoundly deaf from birth. We may have under-estimated the contribution of mutations of CLDN14 to hearing loss by overlooking individuals with mild or delayed-onset hearing loss due to mutations of CLDN14.
Table 2.
Contribution of different genes to hearing loss in Pakistani families
| Gene | Locus | Percentage (fraction) of families |
95% CI | Reference |
|---|---|---|---|---|
| CLDN14 | DFNB29 | 2.25 (18/800) | 1.4 – 3.5 | [1, 25], this study |
| SLC26A4 | DFNB4/PDS | 7.23 (56/775) | 5.6 – 9.2 | [35, 36] |
| GJB2 | DFNB1 | 6.12 (12/196) | 3.5 – 10.4 | [37] |
| HGF | DFNB39 | 5.12 (41/800) | 3.8 – 6.8 | [38] |
| TMC1 | DFNB7/11 | 3.41 (19/557) | 2.2 – 5.3 | [39, 40] |
| MYO15A | DFNB3 | 3.33 (20/600) | 2.2 – 5.1 | [41, 42] |
| OTOF | DFNB9 | 2.33 (13/557) | 1.4 – 4.0 | [43] |
| TRIC | DFNB49 | 1.30 (11/841) | 0.7 – 2.3 | [28, 44] |
| TRIOBP | DFNB28 | 1.29 (10/775) | 0.7 – 2.3 | [45, 46] |
| ILDR1 | DFNB42 | 1.29 (11/850) | 0.7 – 2.3 | [47] |
| MYO6 | DFNB37 | 1.20 (3/250) | 0.4 – 3.4 | [48] |
| GIPC3 | DFNB72 | 0.75 (6/800) | 0.3 – 1.6 | [49] |
| TPRN | DFNB79 | 0.50 (4/800) | 0.2 – 1.2 | [50] |
| RDX | DFNB24 | 0.36 (2/557) | 0.1 – 1.2 | [51] |
CI: confidence interval.
Severity of hearing thresholds in our DFNB29 families does not seem to be directly correlated with age of the subject (Figure 4). For example, although similar in age a 23 year old affected woman of family PKDF505 has significantly better hearing, especially at low frequencies, than her 21year old sister (Figure 4). Similarly, in our previous study, audiograms from multiple affected individuals of family PKDF009 did not show any correlation between hearing thresholds and age of the affected individuals.25 It is possible that an environmental factor (e.g. drugs, noise etc.) may be the cause of this inter- and intra-familial phenotypic variability in hearing thresholds. We hypothesized that a genetic modifier is the cause of this inter- and intra-familial phenotypic variability in hearing thresholds, especially in individual harboring the same CLDN14 mutation. Similar phenotypic variability has been documented for many other deafness causing mutations in humans.26-28 The sensory epithelium of mouse inner ear expresses claudin family members 1, 2, 3, 9, 10, 12, 14 and 18,29 and variation in expression of these other claudins in the auditory system may be modulating the severity of the hearing loss phenotype.
Mutations in other tight junction proteins are also known to cause deafness in humans and mice.28,30,31 Claudin 11-deficient mice are deaf demonstrating that this tight junction protein is also necessary for maintenance of the intra-strial compartment and generation of the endocochlear potential.30 Both claudin 14 and claudin 9 mutant mice also display deafness with no vestibular defects.5,31 Claudin 14 is expressed specifically by the cells forming the reticular lamina (Figure 1b-c) and the vestibular sensory epithelia, while claudin 9 is present in nearly all of the epithelia lining the scala media and the vestibular organs.5,31,32 Mouse mutants of Cldn14 and Cldn9 both display cochlear hair cell loss by the second week of life, which progresses rapidly to include the entire cochlea within the next few weeks.5,31 Loss of either of these claudins results in increased paracellular permeability of K+ in the reticular lamina and an elevation in the K+ concentration around the basolateral regions of hair cells, which is toxic.5,31,33,34 Thus, both claudins are required to form a permeability barrier against cations.5,31 The two novel mutations identified in this study, p.Ser87Ile and p.Ala94Val, are within the second transmembrane domain and in the vicinity of p.Val85, which has been shown to affect the membrane localization of claudin 14.4 Therefore, these two new mutations might also impair the trafficking of claudin 14 to the plasma membrane.
Two of the mutations (p.Arg81His; p.Ser87Ile) of CLDN14 identified in this study were found only once. However, unlike these two rare mutations, three other mutations (p.Val85Asp; p.Met133ArgfsX23; p.Ala94Val) account for approximately 89% of the CLDN14 alleles we found in our cohort of Pakistani families segregating deafness (Table 1). In conclusion, there is considerable genetic and allelic heterogeneity that accounts for recessively inherited deafness in Pakistan (Table 2). CLDN14 mutations are a frequent cause of genetic deafness in this population and are associated with marked inter- and intra-familial variability in hearing thresholds.
Acknowledgments
We thank the families for the participation and cooperation, and R. Bhatti and T. Kausar for technical assistance and Drs. D. Drayna, G. Nayak and K. Kurima for critiques of the manuscript. This work was also supported by the Action on Hearing Loss grant and National Institute on Deafness and Other Communication Disorders (NIDCD/NIH) research grants R01 DC011803 and R01 DC011748 to S.R. and intramural funds from NIDCD DC000039-15 to T.B.F.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Electronic database information
NHLBI-ESP variant database, http://evs.gs.washington.edu/EVS/
Primer3, http://frodo.wi.mit.edu/
1000 Genome, http://browser.1000genomes.org/
TMpred, http://www.ch.embnet.org/software/TMPRED_form.html
SIFT, http://sift.jcvi.org/
Polyphen-2, http://genetics.bwh.harvard.edu/pph2/
MutationTaster, http://www.mutationtaster.org/index.html
References
- 1.Wilcox ER, Burton QL, Naz S, et al. Mutations in the gene encoding tight junction claudin-14 cause autosomal recessive deafness DFNB29. Cell. 2001;104:165–172. doi: 10.1016/s0092-8674(01)00200-8. [DOI] [PubMed] [Google Scholar]
- 2.Lee K, Ansar M, Andrade PB, et al. Novel CLDN14 mutations in Pakistani families with autosomal recessive non-syndromic hearing loss. Am J Med Genet A. 2012;158A:315–321. doi: 10.1002/ajmg.a.34407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bashir R, Fatima A, Naz S. Mutations in CLDN14 are associated with different hearing thresholds. J Hum Genet. 2010;55:767–770. doi: 10.1038/jhg.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wattenhofer M, Reymond A, Falciola V, et al. Different mechanisms preclude mutant CLDN14 proteins from forming tight junctions in vitro. Hum Mutat. 2005;25:543–549. doi: 10.1002/humu.20172. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Yosef T, Belyantseva IA, Saunders TL, et al. Claudin 14 knockout mice, a model for autosomal recessive deafness DFNB29, are deaf due to cochlear hair cell degeneration. Hum Mol Genet. 2003;12:2049–2061. doi: 10.1093/hmg/ddg210. [DOI] [PubMed] [Google Scholar]
- 6.Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 2009;1:a002584. doi: 10.1101/cshperspect.a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulzke JD, Fromm M. Tight junctions: molecular structure meets function. Ann N Y Acad Sci. 2009;1165:1–6. doi: 10.1111/j.1749-6632.2009.04925.x. [DOI] [PubMed] [Google Scholar]
- 8.Tsukita S, Yamazaki Y, Katsuno T, Tamura A. Tight junction-based epithelial microenvironment and cell proliferation. Oncogene. 2008;27:6930–6938. doi: 10.1038/onc.2008.344. [DOI] [PubMed] [Google Scholar]
- 9.Steed E, Rodrigues NT, Balda MS, Matter K. Identification of MarvelD3 as a tight junction-associated transmembrane protein of the occludin family. BMC Cell Biol. 2009;10:95. doi: 10.1186/1471-2121-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsukita S, Furuse M. Overcoming barriers in the study of tight junction functions: from occludin to claudin. Genes Cells. 1998;3:569–573. doi: 10.1046/j.1365-2443.1998.00212.x. [DOI] [PubMed] [Google Scholar]
- 11.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morita K, Furuse M, Fujimoto K, Tsukita S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci U S A. 1999;96:511–516. doi: 10.1073/pnas.96.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim TS, Vedula SR, Hunziker W, Lim CT. Kinetics of adhesion mediated by extracellular loops of claudin-2 as revealed by single-molecule force spectroscopy. J Mol Biol. 2008;381:681–691. doi: 10.1016/j.jmb.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Lim TS, Vedula SR, Kausalya PJ, Hunziker W, Lim CT. Single-molecular-level study of claudin-1-mediated adhesion. Langmuir. 2008;24:490–495. doi: 10.1021/la702436x. [DOI] [PubMed] [Google Scholar]
- 15.Jahnke K. [Intercellular junctions in the guinea pig stria vascularis as shown by freeze-etching (author’s transl)] Anat Embryol (Berl) 1975;147:189–201. doi: 10.1007/BF00306733. [DOI] [PubMed] [Google Scholar]
- 16.Jahnke K. The fine structure of freeze-fractured intercellular junctions in the guinea pig inner ear. Acta Otolaryngol Suppl. 1975;336:1–40. [PubMed] [Google Scholar]
- 17.Gulley RL, Reese TS. Intercellular junctions in the reticular lamina of the organ of Corti. J Neurocytol. 1976;5:479–507. doi: 10.1007/BF01181652. [DOI] [PubMed] [Google Scholar]
- 18.Nunes FD, Lopez LN, Lin HW, et al. Distinct subdomain organization and molecular composition of a tight junction with adherens junction features. J Cell Sci. 2006;119:4819–4827. doi: 10.1242/jcs.03233. [DOI] [PubMed] [Google Scholar]
- 19.Khasnis A, Gokula RM. Romberg’s test. J Postgrad Med. 2003;49:169–172. [PubMed] [Google Scholar]
- 20.Ahmed ZM, Riazuddin S, Bernstein SL, et al. Mutations of the protocadherin gene PCDH15 cause Usher syndrome type 1F. Am J Hum Genet. 2001;69:25–34. doi: 10.1086/321277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimberg J, Nawoschik S, Belluscio L, McKee R, Turck A, Eisenberg A. A simple and efficient non-organic procedure for the isolation of genomic DNA from blood. Nucleic Acids Res. 1989;17:8390. doi: 10.1093/nar/17.20.8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4:1073–1081. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 23.Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarz JM, Rodelsperger C, Schuelke M, Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods. 2010;7:575–576. doi: 10.1038/nmeth0810-575. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed ZM, Riazuddin S, Friedman TB, Wilcox ER, Griffith AJ. Clinical manifestations of DFNB29 deafness. Adv Otorhinolaryngol. 2002;61:156–160. doi: 10.1159/000066828. [DOI] [PubMed] [Google Scholar]
- 26.Schultz JM, Yang Y, Caride AJ, et al. Modification of human hearing loss by plasma-membrane calcium pump PMCA2. N Engl J Med. 2005;352:1557–1564. doi: 10.1056/NEJMoa043899. [DOI] [PubMed] [Google Scholar]
- 27.Riazuddin S, Castelein CM, Ahmed ZM, et al. Dominant modifier DFNM1 suppresses recessive deafness DFNB26. Nat Genet. 2000;26:431–434. doi: 10.1038/82558. [DOI] [PubMed] [Google Scholar]
- 28.Riazuddin S, Ahmed ZM, Fanning AS, et al. Tricellulin is a tight-junction protein necessary for hearing. Am J Hum Genet. 2006;79:1040–1051. doi: 10.1086/510022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitajiri S, Miyamoto T, Mineharu A, et al. Compartmentalization established by claudin-11-based tight junctions in stria vascularis is required for hearing through generation of endocochlear potential. J Cell Sci. 2004;117:5087–5096. doi: 10.1242/jcs.01393. [DOI] [PubMed] [Google Scholar]
- 30.Gow A, Davies C, Southwood CM, et al. Deafness in Claudin 11-null mice reveals the critical contribution of basal cell tight junctions to stria vascularis function. J Neurosci. 2004;24:7051–7062. doi: 10.1523/JNEUROSCI.1640-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakano Y, Kim SH, Kim HM, et al. A claudin-9-based ion permeability barrier is essential for hearing. PLoS Genet. 2009;5:e1000610. doi: 10.1371/journal.pgen.1000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitajiri SI, Furuse M, Morita K, et al. Expression patterns of claudins, tight junction adhesion molecules, in the inner ear. Hear Res. 2004;187:25–34. doi: 10.1016/s0378-5955(03)00338-1. [DOI] [PubMed] [Google Scholar]
- 33.Hibino H, Kurachi Y. Molecular and physiological bases of the K+ circulation in the mammalian inner ear. Physiology (Bethesda) 2006;21:336–345. doi: 10.1152/physiol.00023.2006. [DOI] [PubMed] [Google Scholar]
- 34.Wangemann P. Supporting sensory transduction: cochlear fluid homeostasis and the endocochlear potential. J Physiol. 2006;576:11–21. doi: 10.1113/jphysiol.2006.112888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anwar S, Riazuddin S, Ahmed ZM, et al. SLC26A4 mutation spectrum associated with DFNB4 deafness and Pendred’s syndrome in Pakistanis. J Hum Genet. 2009;54:266–270. doi: 10.1038/jhg.2009.21. [DOI] [PubMed] [Google Scholar]
- 36.Park HJ, Shaukat S, Liu XZ, et al. Origins and frequencies of SLC26A4 (PDS) mutations in east and south Asians: global implications for the epidemiology of deafness. J Med Genet. 2003;40:242–248. doi: 10.1136/jmg.40.4.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santos RL, Wajid M, Khan MN, et al. Novel sequence variants in the TMC1 gene in Pakistani families with autosomal recessive hearing impairment. Hum Mutat. 2005;26:396. doi: 10.1002/humu.9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schultz JM, Khan SN, Ahmed ZM, et al. Noncoding mutations of HGF are associated with nonsyndromic hearing loss, DFNB39. Am J Hum Genet. 2009;85:25–39. doi: 10.1016/j.ajhg.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurima K, Peters LM, Yang Y, et al. Dominant and recessive deafness caused by mutations of a novel gene, TMC1, required for cochlear hair-cell function. Nat Genet. 2002;30:277–284. doi: 10.1038/ng842. [DOI] [PubMed] [Google Scholar]
- 40.Kitajiri SI, McNamara R, Makishima T, et al. Identities, frequencies and origins of TMC1 mutations causing DFNB7/B11 deafness in Pakistan. Clin Genet. 2007;72:546–550. doi: 10.1111/j.1399-0004.2007.00895.x. [DOI] [PubMed] [Google Scholar]
- 41.Nal N, Ahmed ZM, Erkal E, et al. Mutational spectrum of MYO15A: the large N-terminal extension of myosin XVA is required for hearing. Hum Mutat. 2007;28:1014–1019. doi: 10.1002/humu.20556. [DOI] [PubMed] [Google Scholar]
- 42.Liburd N, Ghosh M, Riazuddin S, et al. Novel mutations of MYO15A associated with profound deafness in consanguineous families and moderately severe hearing loss in a patient with Smith-Magenis syndrome. Hum Genet. 2001;109:535–541. doi: 10.1007/s004390100604. [DOI] [PubMed] [Google Scholar]
- 43.Choi BY, Ahmed ZM, Riazuddin S, et al. Identities and frequencies of mutations of the otoferlin gene (OTOF) causing DFNB9 deafness in Pakistan. Clin Genet. 2009;75:237–243. doi: 10.1111/j.1399-0004.2008.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chishti MS, Bhatti A, Tamim S, et al. Splice-site mutations in the TRIC gene underlie autosomal recessive nonsyndromic hearing impairment in Pakistani families. J Hum Genet. 2008;53:101–105. doi: 10.1007/s10038-007-0209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kitajiri S, Sakamoto T, Belyantseva IA, et al. Actin-bundling protein TRIOBP forms resilient rootlets of hair cell stereocilia essential for hearing. Cell. 2010;141:786–798. doi: 10.1016/j.cell.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riazuddin S, Khan SN, Ahmed ZM, et al. Mutations in TRIOBP, which encodes a putative cytoskeletal-organizing protein, are associated with nonsyndromic recessive deafness. Am J Hum Genet. 2006;78:137–143. doi: 10.1086/499164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borck G, Ur Rehman A, Lee K, et al. Loss-of-function mutations of ILDR1 cause autosomal-recessive hearing impairment DFNB42. Am J Hum Genet. 2011;88:127–137. doi: 10.1016/j.ajhg.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahmed ZM, Morell RJ, Riazuddin S, et al. Mutations of MYO6 are associated with recessive deafness, DFNB37. Am J Hum Genet. 2003;72:1315–1322. doi: 10.1086/375122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rehman AU, Gul K, Morell RJ, et al. Mutations of GIPC3 cause nonsyndromic hearing loss DFNB72 but not DFNB81 that also maps to chromosome 19p. Hum Genet. 2011;130:759–765. doi: 10.1007/s00439-011-1018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rehman AU, Morell RJ, Belyantseva IA, et al. Targeted capture and next-generation sequencing identifies C9orf75, encoding taperin, as the mutated gene in nonsyndromic deafness DFNB79. Am J Hum Genet. 2010;86:378–388. doi: 10.1016/j.ajhg.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khan SY, Ahmed ZM, Shabbir MI, et al. Mutations of the RDX gene cause nonsyndromic hearing loss at the DFNB24 locus. Hum Mutat. 2007;28:417–423. doi: 10.1002/humu.20469. [DOI] [PubMed] [Google Scholar]