Abstract
In addition to its anti-inflammatory activity, Meriva®, a proprietary lecithin formulation of curcumin, has been anecdotally reported to decrease acute pain in patients with various chronic diseases. Given that curcumin can desensitize transient receptor potential A1, a nociceptor seemingly also mediating the analgesic effect of acetaminophen, as well as inhibiting and downregulating the expression of cyclo-oxygenase 2, the selective target of nimesulide, a nonsteroidal anti-inflammatory agent, we carried out a pilot comparative study of the acute pain-relieving properties of these three agents. At a dose of 2 g (corresponding to 400 mg of curcumin), Meriva showed clear analgesic activity, comparable with that of a standard dose (1 g) of acetaminophen, but lower than that of a therapeutic (100 mg) dose of nimesulide. The analgesic activity of lower (1.5 g) doses of Meriva was less satisfactory, and the onset of activity was longer than that of nimesulide for both doses. On the other hand, gastric tolerability was significantly better than that of nimesulide and comparable with that of acetaminophen. Taken together, our results show that the preclinical analgesic properties of curcumin have clinical relevance, at least at a dose of 2 g as the Meriva formulation. While this dose is significantly higher than that used to relieve chronic inflammatory conditions (1–1.2 g/day), its pain-relieving activity could benefit from the general downregulation of the inflammatory response induced by curcumin, considering that the transient receptor potential channel-mediated mechanisms of analgesia are magnified by attenuation of inflammation. In patients on treatment with Meriva, this would also translate into better control of acute pain, providing a rationale for the analgesic properties associated with this curcumin formulation.
Keywords: curcumin phytosome, Meriva®, acute pain, acetaminophen, nimesulide, tolerability
Introduction
With over 100 different molecular targets, curcumin is the archetypal pleiotropic dietary agent,1 and is currently at the forefront of biomedical research, as cogently testified by the over 5150 entries for curcumin in PubMed, ie, about 10% of those for aspirin, the best known drug. The redundancy of targets makes it difficult to decipher the clinical translation of the biochemical signature of curcumin, but there is general agreement that the most important clinical targets of curcumin are transcription factors (NF-kB, STAT3, Nrf2), and that curcumin can exert beneficial effects by modulating the genomic and cell-signaling pathways involved in the inflammatory response.1,2 For this reason, the effects of curcumin are not expected to be immediately measurable but rather to develop gradually.1
The dismally low oral bioavailability of curcumin has long hampered the clinical translation of its medicinal potential,3 but this issue has now been substantially improved by various formulation strategies, with dispersion in lecithin, as in Meriva® (Indena SpA, Milan, Italy) being the best documented in terms of comparative pharmacokinetics4 and clinical efficacy.5–9 During a series of recent clinical studies of Meriva for various chronic diseases,5–9 a rapid analgesic effect was observed within 1–2 hours of ingestion by some patients. Similar anecdotal observations were reported by other users, raising the issue of the significance of these findings. These reports, and the recent discovery that curcumin can desensitize or inhibit a series of transient receptor potential ion channels involved in the generation of painful stimuli, ie, transient receptor potential cation channel 1 (TRPA1) and transient receptor potential cation channel subfamily V member 1 (TRPV1),10–12 provided a rationale to investigate the activity of Meriva in the mitigation of acute pain.
Here we report a pilot comparative study of the analgesic properties of Meriva and two popular analgesic drugs, acetaminophen and nimesulide. The mechanism of action of acetaminophen has long been elusive, but has recently been related to the desensitization, by its quinone metabolite, of spinal TRPA1,13 an ion channel that is also sensitive to the desensitizing activity of curcumin.10,11 As a powerful and rapidly acting cyclo-oxygenase 2 inhibitor,14 nimesulide outperforms curcumin as a direct inhibitor of this enzyme but is nevertheless largely devoid of the genomic capacity to downregulate inflammatory cytokines, as shown by curcumin.15
Materials and methods
Fifteen subjects with acute algesic episodes were enrolled (Table 1). Patients were excluded if they had a history of heart, renal, hepatic, or autoimmune disease, cancer, or gastroduodenal pathology. The lower and upper age limits for eligibility were 18 and 70 years, respectively. All patients signed a written informed consent before entering into the study, in accordance with the Declaration of Helsinki.
Table 1.
Characteristics of excluded studies
| Subjects (n) | 15 |
| Male/female ratio | 7/8 |
| Age (years, mean ± SD) | 50.07 ± 12.67 |
| Body weight (kg, mean ± SD) | 68.60 ± 8.79 |
| Diagnosis (n) | |
| Osteoarthritic pain (shoulder, knee) | 3 |
| Neuropathic pain (neuralgia, low back pain) | 6 |
| Recurrent headache | 3 |
| Muscular pain (contractions, sport injuries) | 2 |
| Dental pulpitis pain, on antibiotic therapy | 1 |
Treatment and evaluation
The subjects received four tablet containers, containing nimesulide 100 mg in the first container, acetaminophen 500 mg in the second container, and Meriva 500 mg in the third and fourth containers. The composition of Meriva is curcumin (20%), phosphatidylcholine (40%), and micro-crystalline cellulose (40%). Participants were blinded to the product contained in the pills, that were simply named as A (nimesulide), B (acetaminophen), and C and D (Meriva). Meriva was supplied by Indena SpA, and nimesulide and acetaminophen were from commercial sources. The sequence of treatment was selected accordingly to the number of tablets to be taken, ie, one for nimesulide, two for acetaminophen, and three (first cycle) or four (second cycle) for Meriva.
The subjects were instructed to take one tablet of A on the first day of pain after enrollment, followed, after 48 hours of discontinuance, by two tablets of B, and, after further 24 hours of discontinuance, by three tablets of C. This protocol comprised the first cycle. In the protocol for the second cycle, subjects took one tablet of A on day 4, followed by two tablets of B and four tablets of D, always with the same 24–48-hour discontinuance cycle between treatments. The discontinuance between treatments was included in order to allow washout of the parent compound and/or active metabolites before intake of further treatment. This was of particular importance for nimesulide, which has an half-life of 2–5 hours but generates an active metabolite, ie, 4-hydroxynimesulide. Therefore, a 48-hour washout was always observed between treatments A and B and of 24 hours between treatments B and C.
Fourteen subjects participated in the first cycle and 15 participated in the second cycle; the difference in patient numbers was due to the fact that one patient, on antibiotic therapy for dental pain caused by pulpitis, was enrolled when the first cycle of the study had already been completed. All participants were allowed to take a second dose of the same product if pain perception was still significant 12 hours after the first dose. This opportunity was not utilized in practice, except for Meriva, which has a shorter duration of action than the two other agents. After each intake of medication, the subjects completed a questionnaire, with the assistance of a clinician, canvassing the following items: pain perception, estimated according to the five-point visual analog scale devised by Scott-Huskisson (0, nil; 1, slightly perceptible; 2, mild; 3, severe; 4, intolerable); tolerability on the day of treatment (poor, fair, good, excellent); compliance (poor, fair, good, very good); and adverse events on the day of treatment and during the two following days.
Statistical analysis
Pain perception scores were analyzed according to a randomized block factorial design, and comparison between mean values were done using the Tukey-Kramer test. Correspondence analysis and the Pearson Chi-square test were used to assess tolerability, compliance, and presence of adverse events.
Results
Patient compliance with and tolerability of the treatments were analyzed using a detailed questionnaire. Compliance with treatment was very good for nimesulide and acetaminophen, good for Meriva 1.5 g (corresponding to 300 mg of curcumin) and fair to good for Meriva 2 g (corresponding to 400 mg of curcumin, Table 2). The latter observation can be explained by the fact that intake of four pills was uncomfortable for some subjects.
Table 2.
Compliance with the acute analgesic treatments
| Nimesulide 100 mg | Acetaminophen 1 g | Meriva 1.5 g | Meriva 2.0 g | |
|---|---|---|---|---|
| Cycle 1 (n = 14)* | ||||
| Good | 0 | 0 | 11 | |
| Very good | 14 | 14 | 3 | |
| Cycle 2 (n = 15)* | ||||
| Fair | 0 | 0 | 5 | |
| Good | 0 | 0 | 10 | |
| Very good | 15 | 15 | 0 | |
Notes: Data are presented as number of subjects for each score (poor, fair, good, very good). The number of subjects for each treatment cycle is reported in brackets. *P < 0.0001, correspondence analysis and Pearson Chi-square test.
In general, all treatments were well tolerated (Table 3), with tolerability being excellent for acetaminophen and Meriva 1.5 g, poor to fair for nimesulide, and fair to good for Meriva 2 g. No side effects were noticed for acetaminophen and Meriva 1.5 g, but a few subjects reported gastrointestinal discomfort (strong heartburn and gastroesophageal reflux, requiring antacid therapy) with nimesulide. Meriva 2 g also induced stomach heaviness, nausea, and heartburn in some participants, mostly those who had previously reported these side effects with nimesulide (Table 4). However, unlike the gastric discomfort associated with nimesulide, that induced by Meriva had an early onset, so was possibly nonspecific, being related, apart from an increased sensitivity to gastric injury, to the sheer challenge of ingestion of four pills. To study the time course of analgesic activity, pain intensity was evaluated by the visual analog scale before and 0.5, 2, 3, 4, 6, 8, 12, and 24 hours after administration. Mean pain perception scores are reported in Figure 1.
Table 3.
Comparative tolerability of Meriva and other analgesics
| Cycle 1 (n = 14)*,** | Nimesulide 100 mg | Acetaminophen 1 g | Meriva 1.5 g | |||
|---|---|---|---|---|---|---|
| Poor | 2 | 0 | 0 | |||
| Fair | 4 | 0 | 0 | |||
| Good | 0 | 0 | 1 | |||
| Excellent | 8 | 14 | 13 | |||
| Cycle 2 (n = 15)*** | Nimesulide 100 mg | Acetaminophen 1 g | Meriva 2.0 g | |||
|
| ||||||
| Poor | 6 | 0 | 0 | |||
| Fair | 3 | 0 | 8 | |||
| Good | 1 | 0 | 1 | |||
| Excellent | 5 | 15 | 6 | |||
Notes: After each medication intake, subjects completed a questionnaire with the following tolerability scores: poor, fair, good, excellent. Data are presented as the number of subjects with each score. The number of subjects for each treatment cycle is reported in brackets. *P < 0.0084, correspondence analysis; **P < 0.0150 Pearson Chi-square test; ***P < 0.0001, correspondence analysis and Pearson Chi-square test.
Table 4.
Incidence of side effects after acute analgesic treatment
| Nimesulide 100 mg | Acetaminophen 1 g | Meriva 1.5 g | Meriva 2.0 g | |
|---|---|---|---|---|
| Cycle 1 (n = 14)*,** | ||||
| None | 8 | 14 | 14 | |
| Gastric symptoms* | 6 | 0 | 0 | |
| Cycle 2 (n = 15)***,§ | ||||
| None | 6 | 15 | 6 | |
| Gastric symptoms* | 9 | 0 | 9 | |
Notes: Data are presented as the number of subjects reporting side effects after acute analgesic treatment. The number of subjects for each treatment cycle is reported in brackets. Gastric symptoms were stomach heaviness, nausea, and heartburn for Meriva 2 g, and strong heartburn and gastroesophageal reflux (requiring antacid therapy) for nimesulide 100 mg. *P < 0.0005, correspondence analysis; **P < 0.0009, Pearson Chi-square test; ***P < 0.0001, correspondence analysis; §P < 0.0006 Pearson Chi-square test.
Figure 1.
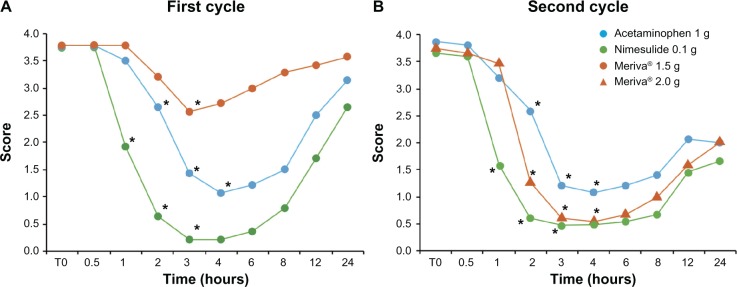
Analgesic effect of Meriva, nimesulide, and acetaminophen.
Notes: Data are presented as the mean pain perception scores at different times after acute analgesic treatment. After each intake of medication, subjects completed a questionnaire using the following pain perception scores: 0, absent; 1, slightly perceptible; 2, mild; 3, severe; 4, intolerable pain. Statistical analysis was done according to a randomized block factorial design, and comparisons between mean values were done using the Tukey-Kramer test. See Results for statistical details. *P < 0.001.
The efficacy and safety results obtained for nimesulide and acetaminophen are consistent with those reported in the literature. A significant reduction in pain perception was observed 1–3 hours after taking nimesulide and 2–4 hours after taking acetaminophen. Conversely, Meriva 1.5 g had a statistically significant effect only after 3 hours, with an overall analgesic effect significantly lower than that of the other two drugs. In this cycle, nimesulide showed the highest and long-lasting analgesic effect, followed by acetaminophen.
While the results for nimesulide and acetaminophen were not markedly different from those observed in the first cycle, the higher dose of Meriva (2 g) reduced pain perception after 2 hours. The analgesic effect of Meriva 2 g lasted 4 hours, and a second dose was then necessary in some cases (after 6–8 hours for headache, 8 hours for neuropathic pain, and 12 hours for neuralgia and pain from osteoarthritis).
The analgesic effect of this Meriva dose was lower than that of nimesulide, but higher than that associated with acetaminophen. For all three agents, the effect was still significant 4 hours after administration.
Discussion
Analgesic properties have been reported for curcumin in preclinical studies. Curcumin can attenuate thermal hyperalgesia associated with diabetic neuropathic pain by inhibition of tumor necrosis alpha and nitric oxide release,16 have an antihyperalgesic effect in a formalin-induced orofacial pain model in rats,17 and decrease TRPV1-mediated pain hypersensitivity.12 Further, intrathecal administration of curcumin significantly decreased the sensitivity of rats in the formalin test.18 The relatively quick onset of these activities is at odds with the mainly genomic anti-inflammatory mechanism(s) of curcumin, suggesting a direct activity on the mechanism of translation of inflammatory stress into a painful sensation, a process where thermal transient receptor potential play a critical role.19 Curcumin behaves as a combined TRPV1 inhibitor12 and TRPA1 desensitizer,10,11 and this direct action is complemented by that mediated via inflammatory mediators, a major class of thermal transient receptor potential modulators.19 However, the clinical relevance of these observations has not yet been evaluated.
To fill this gap in our knowledge, we carried out a pilot comparative study of curcumin (formulated as Meriva to improve its poor oral absorption)4 and two popular analgesic drugs (acetaminophen and nimesulide). At a dose of 2 g (corresponding to 400 mg of curcumin), Meriva had a clear analgesic effect in subjects affected by acute pain, confirming anecdotal reports of the pain-relieving properties of this curcumin formulation. At this dose, the activity was higher than that associated with 500 mg of acetaminophen, while a lower dose of Meriva (1.5 g, corresponding to 300 mg of curcumin) gave only transient and often unsatisfactory relief of pain, indicative of suboptimal therapeutic plasma concentrations. The analgesic effect of Meriva achieved significance only 2 hours after administration, as observed for acetaminophen. Conversely, nimesulide was more rapidly acting, with strongest pain-relieving properties being reported one hour after administration.
At both doses used, the tolerability of Meriva was substantially better than that of nimesulide, which often requires concomitant administration of antacids to alleviate the gastric irritation associated with its use. The similarity of action of curcumin and acetaminophen supports the view that these compounds share the same mechanism of action, while nimesulide is a powerful inhibitor of cyclo-oxygenases,14 a class of enzymes only modestly inhibited by curcumin in a direct way.
Taken together, our results show that preclinical data on the analgesic properties of curcumin have a clinical translation, at least at a dose of 2 g as in the Meriva formulation. While this dose of Meriva is significantly higher than that used to relief chronic inflammatory conditions (1–1.2 g/day),8 its pain-relieving activity could benefit from general down-regulation of the inflammatory response induced by curcumin, given that transient receptor potential-mediated mechanisms of analgesia are magnified by attenuation of inflammation. Overall, this would translate into better control of chronic pain, providing a rationale for the inclusion of Meriva in protocols of treatment for acute pain of various origins.
Acknowledgments
The authors thank Martino Recchia for the statistical analysis.
Footnotes
Disclosure
GA is a consultant to Indena SpA, manufacturer of Meriva. FF and ST are employees of Indena SpA. The other authors have no conflicts of interest in this work.
References
- 1.Esatbeyoglu T, Huebbe P, Ernst IM, Chin D, Wagner AE, Rimbach G.Curcumin – from molecule to biological function. Angew Chem Int Ed Engl 201251225308–5332. [DOI] [PubMed] [Google Scholar]
- 2.Zhou H, Beevers CS, Huang S. The targets of curcumin. Curr Drug Targets. 2011;12(3):332–347. doi: 10.2174/138945011794815356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 4.Cuomo J, Appendino G, Dern AS, et al. Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation. J Nat Prod. 2011;74(4):664–669. doi: 10.1021/np1007262. [DOI] [PubMed] [Google Scholar]
- 5.Allegri P, Rissotto R, Herbort CP, Murialdo U. CNS diseases and uveitis. J Ophthalmic Vis Res. 2011;6(4):284–308. [PMC free article] [PubMed] [Google Scholar]
- 6.Appendino G, Belcaro G, Cornelli U, et al. Potential role of curcumin phytosome (Meriva) in controlling the evolution of diabetic microangiopathy. A pilot study. Panminerva Med. 2011;53(3 Suppl 1):43–49. [PubMed] [Google Scholar]
- 7.Belcaro G, Cesarone MR, Dugall M, et al. Product-evaluation registry of Meriva(R), a curcumin-phosphatidylcholine complex, for the complementary management of osteoarthritis. Panminerva Med. 2010;52(2 Suppl 1):55–62. [PubMed] [Google Scholar]
- 8.Belcaro G, Cesarone MR, Dugall M, et al. Efficacy and safety of Meriva(R), a curcumin-phosphatidylcholine complex, during extended administration in osteoarthritis patients. Altern Med Rev. 2010;15(4):337–344. [PubMed] [Google Scholar]
- 9.Mazzolani F. Pilot study of oral administration of a curcumin-phospholipid formulation for treatment of central serous chorioretinopathy. Clin Ophthalmol. 2012;6:801–806. doi: 10.2147/OPTH.S31859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avonto C, Taglialatela-Scafati O, Pollastro F, et al. An NMR spectroscopic method to identify and classify thiol-trapping agents: revival of Michael acceptors for drug discovery? Angew Chem Int Ed Engl. 2011;50(2):467–471. doi: 10.1002/anie.201005959. [DOI] [PubMed] [Google Scholar]
- 11.Leamy AW, Shukla P, McAlexander MA, Carr MJ, Ghatta S. Curcumin ((E,E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) activates and desensitizes the nociceptor ion channel TRPA1. Neurosci Lett. 2011;503(3):157–162. doi: 10.1016/j.neulet.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 12.Yeon KY, Kim SA, Kim YH, et al. Curcumin produces an antihype-ralgesic effect via antagonism of TRPV1. J Dent Res. 2010;89(2):170–174. doi: 10.1177/0022034509356169. [DOI] [PubMed] [Google Scholar]
- 13.Andersson DA, Gentry C, Alenmyr L, et al. TRPA1 mediates spinal antinociception induced by acetaminophen and the cannabinoid delta(9)-tetrahydrocannabinol. Nat Commun. 2011;2:551. doi: 10.1038/ncomms1559. [DOI] [PubMed] [Google Scholar]
- 14.Prevot A, Mosig D, Martini S, Guignard JP. Nimesulide, a cyclooxygenase-2 preferential inhibitor, impairs renal function in the newborn rabbit. Pediatr Res. 2004;55(2):254–260. doi: 10.1203/01.PDR.0000100904.17064.47. [DOI] [PubMed] [Google Scholar]
- 15.Colitti M, Gaspardo B, Della Pria A, Scaini C, Stefanon B. Transcriptome modification of white blood cells after dietary administration of curcumin and non-steroidal anti-inflammatory drug in osteoarthritic affected dogs. Vet Immunol Immunopathol. 2012;147(3–4):136–146. doi: 10.1016/j.vetimm.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Sharma S, Kulkarni SK, Agrewala JN, Chopra K. Curcumin attenuates thermal hyperalgesia in a diabetic mouse model of neuropathic pain. Eur J Pharmacol. 2006;536(3):256–261. doi: 10.1016/j.ejphar.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Mittal N, Joshi R, Hota D, Chakrabarti A. Evaluation of antihyperalgesic effect of curcumin on formalin-induced orofacial pain in rat. Phytother Res. 2009;23(4):507–512. doi: 10.1002/ptr.2662. [DOI] [PubMed] [Google Scholar]
- 18.Han YK, Lee SH, Jeong HJ, Kim MS, Yoon MH, Kim WM. Analgesic effects of intrathecal curcumin in the rat formalin test. Korean J Pain. 2012;25(1):1–6. doi: 10.3344/kjp.2012.25.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vay L, Gu C, McNaughton PA. The thermo-TRP ion channel family: properties and therapeutic implications. Br J Pharmacol. 2012;165(4):787–801. doi: 10.1111/j.1476-5381.2011.01601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


