Abstract
Tumours evolve several mechanisms to evade apoptosis, yet many resected carcinomas show significantly elevated caspase activity. Moreover, caspase activity is positively correlated with tumour aggression and adverse patient outcome. These observations indicate that caspases might have a functional role in promoting tumour invasion and metastasis. Using a Drosophila model of invasion, we show that precise effector caspase activity drives cell invasion without initiating apoptosis. Affected cells express the matrix metalloprotinase Mmp1 and invade by activating Jnk. Our results link Jnk and effector caspase signalling during the invasive process and suggest that tumours under apoptotic stresses from treatment, immune surveillance or intrinsic signals might be induced further along the metastatic cascade.
Keywords: Drosophila , invasion, caspase, apoptosis, JNK
INTRODUCTION
Evasion of apoptosis is a hallmark of cancer [1, 2]. During apoptosis, executioner proteases known as effector caspases cleave thousands of cellular substrates to promote orderly cell disassembly [3, 4]. To avoid this, tumours use a variety of mechanisms to deflect programmed cell death signals. Many of these, such as mutated P53 and BCL-2 overexpression, involve blocking the signals that lead to caspase activation [5]. Others, such as overexpression of XIAP, block active caspases themselves [6]. Yet tumour cell lines and resected patient breast, pancreatic and colonic tumours show high levels of effector caspase activity without undergoing apoptosis [7]. Thus, tumours survive with effector caspase cleavage without undergoing cell death. Further, clinical data indicate that effector caspase levels increase in parallel with breast tumour invasiveness as well as adverse patient prognosis [8, 9]. Previous studies in cancer cell lines also demonstrate that apoptotic and invasion signals can show crosstalk [10]. These studies suggest that caspases have a functional role during tumour invasion and metastasis.
In this study, we examine the potential for a functional role of in situ caspase activation during invasion. Using Drosophila melanogaster, we show that sub-apoptotic caspase activation leads to matrix metalloprotinase (MMP) expression and cell invasion. This signalling axis requires the effector caspase Drice (Drosophila caspase 3) and the Jnk signal transduction pathway.
RESULTS AND DISCUSSION
Moderate caspase activity leads to cell invasion
High expression levels of Hid activate initiator caspases by sequestering the IAP family member Diap1 [11]. Controlled activation of caspases frequently leads to non-apoptotic phenotypes [12, 13, 14]. To investigate whether caspase activation in the absence of apoptosis leads to migratory behaviour, we co-expressed hid with p35, a baculovirus-derived suicide substrate that specifically inhibits effector caspase activity but not the initiator caspase Dronc (Drosophila caspase 9 [15]). Transgenes, including green fluorescent protein (GFP), were expressed in the ptc domain, which includes a stripe of epithelial cells at the anterior/posterior boundary of the larval wing disc (Fig 1A). We previously used this pattern to study invasion mediated by knockdown of the Src-negative regulator carboxy-terminal Src Kinase (Csk; [16]).
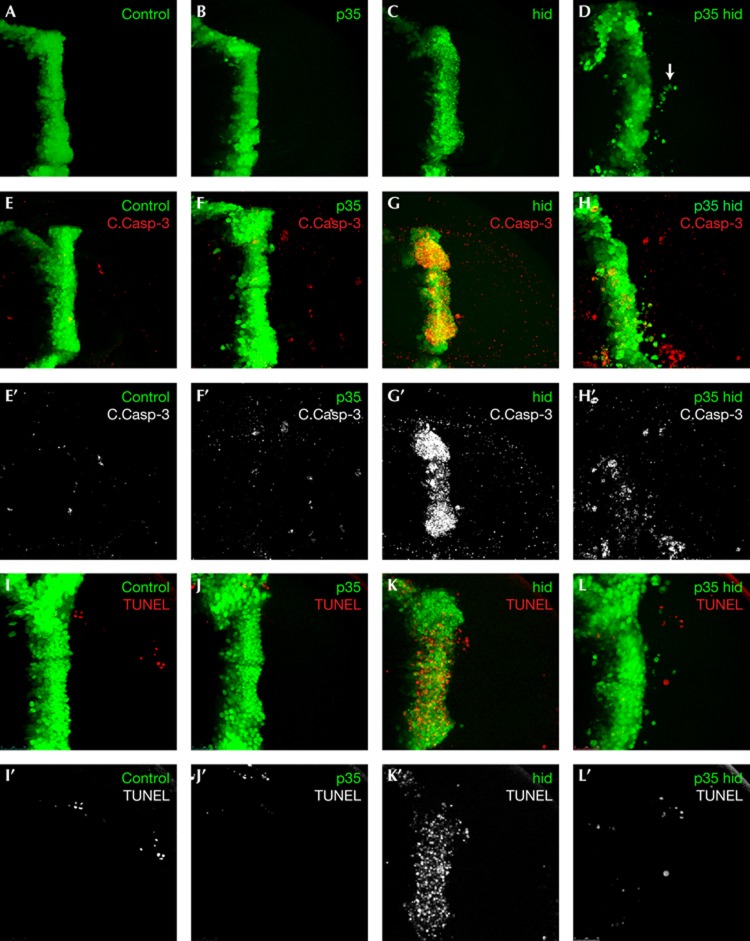
Figure 1.
Characterization of Caspase-directed invasion. (A–D) Wing discs of indicated genotypes demonstrating the invasion phenotype; cells are labelled by GFP expression under control of the ptc driver. Arrow in panel D indicates a group of cells that have migrated from the ptc domain. (E–H′) Wing discs of indicated genotypes probed with an antibody to cleaved Caspase-3 (pseudocoloured red in panels E–H, grey in panels E′–H′). (I–L′) Caspase activity in p35-hid wing discs is not accompanied by apoptosis, as demonstrated by TUNEL staining (red). Anterior in all panels is to the left. GFP, green fluorescent protein; TUNEL, TdT-mediated dUTP nick end labelling.
ptc>p35,hid (p35-hid) discs contained many fully detached GFP cells in the posterior compartment away from the ptc domain (Fig 1D). p35-hid invasion was qualitatively different from the one reported by Martin et al [17] in which irradiated p35-expressing cells failed to detach from the expression domain. p35-hid invading cells were found exclusively in basal planes of the tissue; they had cleanly detached and migrated several cell diameters away from the posterior edge of the ptc expression domain. They displayed a robust, rounded morphology indicative of healthy cells. The p35-hid invasion phenotype was similar in character to but weaker than Csk-deficient invasion, consistent with Src potentiating many downstream effectors of invasion, including caspase-independent targets.
Consistent with previous work [16], expression of hid alone led to extensive apoptotic cell death but no invasion (Fig 1C), indicating that simply inducing cell death is not sufficient to cause ‘migration’. Wing discs expressing p35 alone showed no invasion (Fig 1B) but contained occasional cells with processes extended towards the posterior compartment (Fig 2B; supplementary Fig S1 online; P-values: p35-hid=1E−15 hid=0.7 p35=1), presumably reflecting a block in the normal stochastic activation of apoptosis during development.
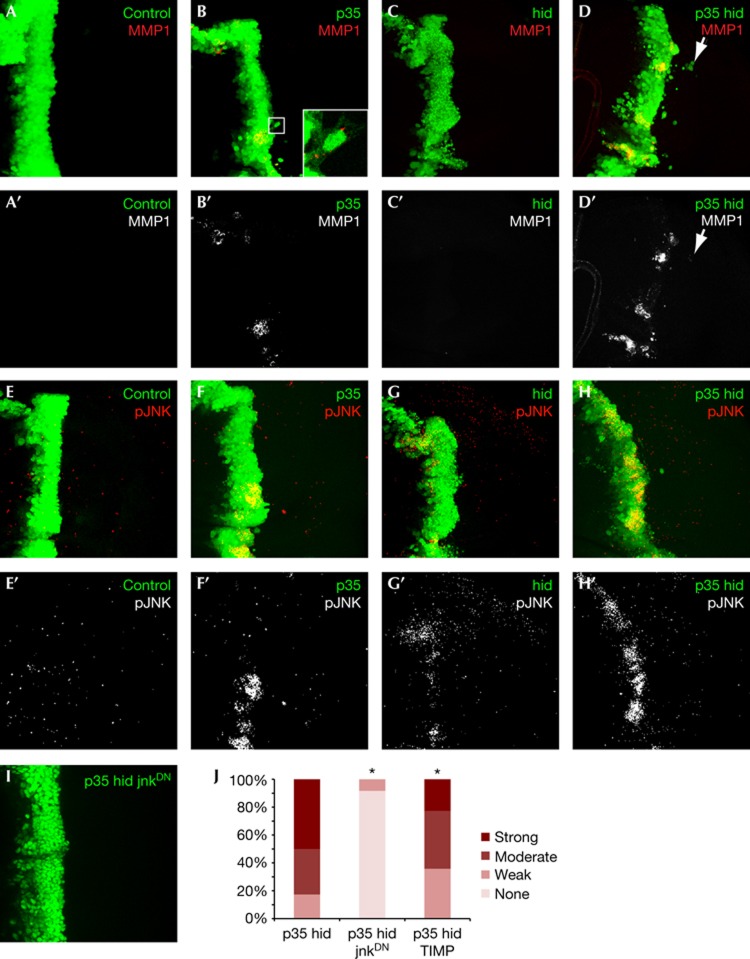
Figure 2.
p35-hid invading cells activate the Jnk pathway. (A–D) Mmp1 expression in wing discs with indicated genotypes (A′–D′: MMP1 channel only of images shown in A–D). Inset in panel B shows P35-dependent cell extensions; unlike p35-hid, p35 cells rarely detach completely from the ptc domain. (E–H′) phospho-JNK staining (red) in wing discs of indicated genotypes (E′–H′: pJNK channel only of images shown in E–H) (I) JnkDN blocks p35-hid-dependent cell migration. Anterior in all panels is to the left. (J) Quantification of invasion phenotype. Bonferroni-corrected significance (α=0.05) is indicated with asterisks. MMP, matrix metalloproteinase.
In contrast with P35, Diap1 acts as a broad inhibitor of caspase activity [11, 18, 19, 20]. When hid was co-expressed with its physiological antagonist diap1, no indicative effect on invasion was observed (supplementary Fig S1A–C online), suggesting that caspase activation is required for invasion. Overall, these results indicate the existence of a non-apoptotic caspase pathway leading to invasion and that caspase activation alone should not be used as an indicator of cell death.
p35-hid cells activate caspases but not apoptosis
We next monitored activation of apoptosis using an antibody against human cleaved Caspase 3, which measures the activity of the initiator caspase Dronc in Drosophila [21]. In control and p35 discs, cells within the ptc domain showed low Dronc activity (Fig 1E,F). By contrast, most hid-expressing cells exhibited high levels of Dronc activity (Fig 1G). Activity was observed primarily in the basal region of the ptc domain but also at intermediate confocal planes, suggesting that cells actively undergoing basal extrusion are at an intermediate stage of cell death. Confirming apoptosis, most hid cells were marked positive by TdT-mediated dUTP nick end labelling (TUNEL) in contrast to control and p35 cells (Fig 1L–K).
p35-hid cells also showed elevated Dronc activity, including those migrating from the ptc domain (Fig 1H). In contrast to hid discs, however, p35-hid discs showed a concentration of TUNEL staining indistinguishable from controls (Fig 1L). In particular, invading cells rarely marked with TUNEL, indicating that they have the characteristics of previously described ‘undead’ cells [17, 22]. We found occasional caspase- and TUNEL-marked, GFP-negative cells in the posterior compartment. Lineage tracing experiments [23] indicated that these cells were not undead cells that lost ptc or GFP expression (data not shown); rather they are likely wild-type cells that activated apoptosis as part of their normal developmental programme.
Undead cells activate Jnk, express Mmp1
Invading tumour cells express MMPs to degrade the extracellular matrix and basement membrane [24]. Control and hid discs displayed undetectable levels of Mmp1 (Fig 2A,C). In contrast, p35-hid discs demonstrated high Mmp1 expression levels localized to discrete regions within the ptc domain (Fig 2D), zones of local invasion containing lines of cells migrating away. Many of the migrating cells also retained lower Mmp1 levels (supplementary Fig S1D,E online). Expression of tissue inhibitor of metalloproteinase in p35-hid discs partially suppressed invasion (Fig 2J), indicating a functional requirement for MMP expression in p35-hid-mediated invasion. Intriguingly, Mmp1 expression was also elevated in p35 discs (Fig 2B). These Mmp1-rich regions frequently associated with attached cells possessing elongated cell processes (Fig 2B) and also showed high Dronc activity (supplementary Fig S2A online), suggesting that apoptosis activated as part of the normal developmental programme in these cells is blocked by p35 expression. Consistent with this view, Mmp1 expression was strongly suppressed in p35 discs that were null for the effector caspase drice (supplementary Fig S2B online). In summary, while Mmp1 expression in both p35 and p35-hid discs corresponded with caspase activation but not apoptosis, absence of invasion despite MMP1 expression in p35 discs suggests that endogenous level of caspase activation in these cells is not high enough to initiate the entire invasion programme.
The Jnk pathway is a conserved regulator of MMP expression, and is required for invasion downstream of many oncogenes, including Src [25, 26, 27]. In Drosophila, Jnk has been reported to lie both upstream and downstream of Hid [28]. To assess the activity of the unique Drosophila ortholog bsk/jnk, we used an antibody specific to the activated Jnk isoform pJnk. pJnk staining localized to discrete patches in p35 discs (Fig 2E,F), consistent with the Mmp1 expression pattern, while staining in hid discs was extremely weak (Fig 2G). By contrast, p35-hid discs showed clear evidence of Jnk activation within the ptc domain compared with control (Fig 2H).
We confirmed these results with the Jnk pathway transcriptional reporter msn-LacZ in p35 and p35-hid discs, whereas msn-LacZ levels were undetectable in hid discs, consistent with very low pJNK levels in these discs (supplementary Fig S2C–F online). In addition to reporting Jnk activity, the ‘msn-lacZ’ transgene interrupts the msn locus[29], reducing the msn gene dosage by half. We observed a complete suppression of the p35-hid invasion phenotype in the presence of msn-lacZ (supplementary Fig S2F online) indicating a dependence on Jnk activity. Consistent with this view, co-expression of a dominant-negative Jnk transgene also led to complete suppression of the invasion phenotype (P-value=1E−12, Fig 2I,J).
JNK has previously been shown to exhibit positive feedback with caspases during apoptosis [28] and here we suggest that this loop contributes to tumour invasion. That is, the same mechanisms that direct the multistep apoptosis process—including Actin remodelling, removal from the epithelium, interaction with macrophages, and so on—are co-opted for tumour invasion (for example, van Ham et al [30]).
The effector caspase Drice is required for migration
Which caspases are responsible for Jnk activation and subsequent invasion? The initiator caspase Nc/Dronc and the effector Ice/Drice mediate virtually all apoptotic processes in Drosophila; they follow an activation pattern conserved with mammals [31, 32, 33, 34, 35]. Knockdown of Dronc with a short hairpin completely suppressed invasion in p35-hid cells (P-value=2.7E−5, Fig 3A,G), suggesting that Dronc mediates the signal leading to invasion.
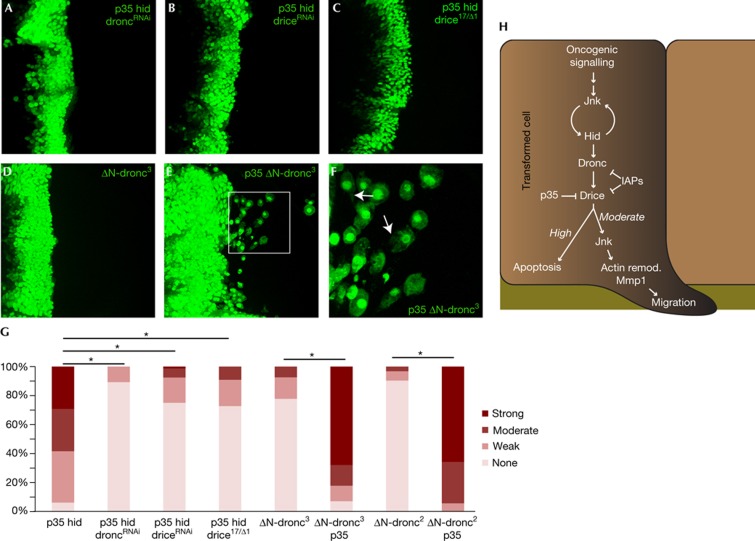
Figure 3.
Moderate effector caspase signalling is required for invasion. (A–E) Representative images of wing discs with indicated genotypes. F shows a magnified image of boxed region in E: migrating cells show lamellipodia-like structures labelled by arrows. Anterior in all panels is to the left. (G) Quantification of invasion phenotypes. Bonferroni-corrected significance (α=0.05) is indicated with asterisks. (H) Working model: a precise calibration of caspase activators and inhibitors is required to direct migration. Mmp1, matrix metalloproteinase; remod, remodelling.
Previous work has demonstrated that Dronc activation cleaves and activates the pro-Drice dimer, permitting Drice to cleave a variety of cellular substrates [35]. Interestingly, in p35-hid wing discs, reducing Drice levels also led to suppression of invasion (P-value=2.3E−14; Fig 3B,C). The suppression of migration indicates that when apoptosis is blocked, precise levels of Drice activity are required to promote invasion. To test this prediction, we asked whether reducing Dronc or Drice levels in otherwise apoptotic hid cells would be sufficient to induce migration in the absence of p35. We found that while hid-droncRNAi cells failed to migrate (P-value=0.55, supplementary Fig S3A,C online), hid-driceRNAi discs showed an intermediate migration phenotype (P-value=2.6E−5, supplementary Fig S3B,C online). In summary, Hid-induced caspase activity can be reduced to a level that suppresses apoptosis but is still sufficient to direct migration by either blocking Drice activity (p35-hid) or by reducing total Drice levels (hid-driceRNAi). Reducing Drice levels plus inhibiting Drice function (p35-hid-driceRNAi) brings Drice activity to a level too low to induce invasion. Overall, these experiments indicate that caspase activation must be precisely controlled to promote invasion.
To further test this model, we generated ‘undead cells’ by expressing the activated Dronc isoform ΔN-dronc3, which lacks its amino-terminal domain; this alteration frees ΔN-dronc3 from Diap1 inhibition to direct apoptosis in vivo [36, 37, 38]. We found that ΔN-dronc3 alone did not promote migration (P-value=1, Fig 3D). However, co-expression of ΔN-dronc3 with p35 led to an aggressive invasion phenotype (P-value=3.9E−9) with extensive lamellipodia-like cellular processes (Fig 3E,F); cells were observed at a significant distance from the ptc domain. These results were confirmed using a second transgenic insertion (P-value=2.6E−13, Fig 3G).
Overall, these results indicate that effector caspase activity below levels sufficient to direct cell death might be optimal for migration of transformed cells (Fig 3H). This signalling promotes migration through Jnk, consistent with previous studies showing that Jnk lies downstream of Dronc [39]. Caspase activation of Jnk frequently leads to compensatory proliferation, a homeostatic programme of cell replacement after apoptosis. Compensatory proliferation studies of ‘undead cells’ have come to opposite conclusions concerning the role of Drice [40, 41]. Our work is consistent with the mammalian literature placing the JNK pathway as a caspase target.
Effector caspases are active in tumours in situ and are associated with metastasis; our results indicate that cells with moderate caspase activity that are protected from apoptosis are prone to migration. In this view, therapeutic interventions proposed to increase tumour apoptosis might paradoxically exacerbate malignancy, as has been previously suggested [42, 43]. Tumour inflammation has also been suggested to promote metastasis [44] and might do so via stimulation of the extrinsic apoptosis pathway. Tumour cells commonly contain high levels of XIAP, which blocks caspases’ active site in a manner similar to P35 [45, 46, 47, 48, 49]. This might provide an important mechanism directing tumours to metastasize, though our experiments (for example, supplementary Fig 1 online) emphasize the importance of precise caspase activity. A better understanding of caspases’ role in tumour progression might enhance our ability to predict a tumour’s progression and the impact of treatments designed to promote the apoptosis process.
METHODS
Fly genetics. Experimental genotypes were generated by standard crossing and were cultured at 18 °C on Bloomington Semi-defined Media except for crosses involving droncΔ.3a/ΔN-dronc3 (25 °C).
Immunohistochemistry. Antibodies targeted Mmp1 (mouse, 1:50, DSHB), cleaved Caspase-3 (rabbit, 1:200, Cell Signaling Technology), β-galactosidase (mouse, 1:100, DSHB), and phospho-SAPK/JNK (G9; mouse, 1:100, Cell Signaling), Alexa Fluor 568- or Cy5-conjugated secondary antibodies (1:100, Invitrogen, Jackson). TUNEL assay was performed using the In Situ Cell Death Detection Kit, TMR Red (Roche).
Statistical procedures. More than 20 wing discs were dissected for each genotype. Each disc was binned to one of the following phenotypic categories based on the number of migrating cells within the posterior compartment: weak: 1–5 cells; moderate: 6–15 cells; and strong: >15 cells. To establish suppressor and enhancer genotypes, the categories were defined to be centred around the ‘moderate’ class. This procedure was performed at least twice and results pooled. The Wilcoxon rank sum test was used to compute P-values with respect to controls and deemed significant on the basis of an α=0.05 threshold (R Language: wilcox.test function). We report Bonferroni-adjusted P-values—with the comparison genotype noted when unclear—on the basis of several statistical comparisons made with each genotype.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank members of the Cagan Laboratory for important discussions, and especially Jay Pendse for statistical assistance. V.A.R. was supported by 5T32CA078207 and 5T32GM007280. This work was supported by National Institutes of Health grants NCI R01 CA109730 and 5R01EY011495.
Author contributions: V.A.R., E.B. and R.L.C. designed experiments. V.A.R. performed most experiments with help from E.B. V.A.R., E.B. and R.L.C. all contributed to writing of manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646–674 [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100: 57–70 [DOI] [PubMed] [Google Scholar]
- Susin SA, Zamzami N, Castedo M, Daugas E, Wang HG, Geley S, Fassy F, Reed JC, Kroemer G (1997) The central executioner of apoptosis: multiple connections between protease activation and mitochondria in Fas/APO-1/CD95- and ceramide-induced apoptosis. J Exp Med 186: 25–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnaiyan AM, Dixit VM (1997) Portrait of an executioner: the molecular mechanism of FAS/APO-1-induced apoptosis. Semin Immunol 9: 69–76 [DOI] [PubMed] [Google Scholar]
- Igney FH, Krammer PH (2002) Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer 2: 277–288 [DOI] [PubMed] [Google Scholar]
- Chai J, Shiozaki E, Srinivasula SM, Wu Q, Datta P, Alnemri ES, Shi Y (2001) Structural basis of caspase-7 inhibition by XIAP. Cell 104: 769–780 [DOI] [PubMed] [Google Scholar]
- Yang L, Cao Z, Yan H, Wood WC (2003) Coexistence of high levels of apoptotic signaling and inhibitor of apoptosis proteins in human tumor cells: implication for cancer specific therapy. Cancer Res 63: 6815–6824 [PubMed] [Google Scholar]
- Nakopoulou L, Alexandrou P, Stefanaki K, Panayotopoulou E, Lazaris AC, Davaris PS (2001) Immunohistochemical expression of caspase-3 as an adverse indicator of the clinical outcome in human breast cancer. Pathobiology 69: 266–273 [DOI] [PubMed] [Google Scholar]
- Vakkala M, Paakko P, Soini Y (1999) Expression of caspases 3, 6 and 8 is increased in parallel with apoptosis and histological aggressiveness of the breast lesion. Br J Cancer 81: 592–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai M, Kusama T, Hamanaka Y, Koga T, Endo H, Tatsuta M, Inoue M (2005) Cross talk between apoptosis and invasion signaling in cancer cells through caspase-3 activation. Cancer Res 65: 9121–9125 [DOI] [PubMed] [Google Scholar]
- Wang SL, Hawkins CJ, Yoo SJ, Muller HA, Hay BA (1999) The Drosophila caspase inhibitor DIAP1 is essential for cell survival and is negatively regulated by HID. Cell 98: 453–463 [DOI] [PubMed] [Google Scholar]
- Koto A, Kuranaga E, Miura M (2009) Temporal regulation of Drosophila IAP1 determines caspase functions in sensory organ development. J Cell Biol 187: 219–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan Y, Gibbs-Bar L, Kalifa Y, Feinstein-Rotkopf Y, Arama E (2010) Gradients of a ubiquitin E3 ligase inhibitor and a caspase inhibitor determine differentiation or death in spermatids. Dev Cell 19: 160–173 [DOI] [PubMed] [Google Scholar]
- Li Z, Jo J, Jia JM, Lo SC, Whitcomb DJ, Jiao S, Cho K, Sheng M (2010) Caspase-3 activation via mitochondria is required for long-term depression and AMPA receptor internalization. Cell 141: 859–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannan E, Vandergaast R, Friesen PD (2007) Baculovirus caspase inhibitors P49 and P35 block virus-induced apoptosis downstream of effector caspase DrICE activation in Drosophila melanogaster cells. J Virol 81: 9319–9330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal M, Larson DE, Cagan RL (2006) Csk-deficient boundary cells are eliminated from normal Drosophila epithelia by exclusion, migration, and apoptosis. Dev Cell 10: 33–44 [DOI] [PubMed] [Google Scholar]
- Martin FA, Perez-Garijo A, Morata G (2009) Apoptosis in Drosophila: compensatory proliferation and undead cells. Int J Dev Biol 53: 1341–1347 [DOI] [PubMed] [Google Scholar]
- O’Riordan MX, Bauler LD, Scott FL, Duckett CS (2008) Inhibitor of apoptosis proteins in eukaryotic evolution and development: a model of thematic conservation. Dev Cell 15: 497–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal L, McCall K, Agapite J, Hartwieg E, Steller H (2000) Induction of apoptosis by Drosophila reaper, hid and grim through inhibition of IAP function. EMBO J 19: 589–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisi S, Mazzon I, White K (2000) Diverse domains of THREAD/DIAP1 are required to inhibit apoptosis induced by REAPER and HID in Drosophila. Genetics 154: 669–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Bergmann A (2010) The cleaved-Caspase-3 antibody is a marker of Caspase-9-like DRONC activity in Drosophila. Cell Death Differ 17: 534–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay BA, Wassarman DA, Rubin GM (1995) Drosophila homologs of baculovirus inhibitor of apoptosis proteins function to block cell death. Cell 83: 1253–1262 [DOI] [PubMed] [Google Scholar]
- Evans CJ et al. (2009) G-TRACE: rapid Gal4-based cell lineage analysis in Drosophila. Nat Methods 6: 603–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua H, Li M, Luo T, Yin Y, Jiang Y (2011) Matrix metalloproteinases in tumorigenesis: an evolving paradigm. Cell Mol Life Sci 68: 3853–3868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino M (2010) Src signaling in cancer invasion. J Cell Physiol 223: 14–26 [DOI] [PubMed] [Google Scholar]
- Uhlirova M, Bohmann D (2006) JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. EMBO J 25: 5294–5304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Rajfur Z, Borchers C, Schaller MD, Jacobson K (2003) JNK phosphorylates paxillin and regulates cell migration. Nature 424: 219–223 [DOI] [PubMed] [Google Scholar]
- Shlevkov E, Morata G (2012) A dp53/JNK-dependant feedback amplification loop is essential for the apoptotic response to stress in Drosophila. Cell Death Differ 19: 451–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling AC, Stern D, Beaton A, Rhem EJ, Laverty T, Mozden N, Misra S, Rubin GM (1999) The Berkeley Drosophila Genome Project gene disruption project: single P-element insertions mutating 25% of vital Drosophila genes. Genetics 153: 135–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ham TJ, Kokel D, Peterson RT (2012) Apoptotic cells are cleared by directional migration and elmo1- dependent macrophage engulfment. Curr Biol 22: 830–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DM, Granville DJ, Lowenberger C (2009) The insect caspases. Apoptosis 14: 247–256 [DOI] [PubMed] [Google Scholar]
- Kumar S (2007) Caspase function in programmed cell death. Cell Death Differ 14: 32–43 [DOI] [PubMed] [Google Scholar]
- Xu D, Woodfield SE, Lee TV, Fan Y, Antonio C, Bergmann A (2009) Genetic control of programmed cell death (apoptosis) in Drosophila. Fly 3: 78–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florentin A, Arama E (2012) Caspase levels and execution efficiencies determine the apoptotic potential of the cell. J Cell Biol 196: 513–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Yu X, Topf M, Dorstyn L, Kumar S, Ludtke SJ, Akey CW (2011) Structure of the Drosophila apoptosome at 6.9 a resolution. Structure 19: 128–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai J, Yan N, Huh JR, Wu JW, Li W, Hay BA, Shi Y (2003) Molecular mechanism of Reaper-Grim-Hid-mediated suppression of DIAP1-dependent Dronc ubiquitination. Nat Struct Biol 10: 892–898 [DOI] [PubMed] [Google Scholar]
- Zachariou A, Tenev T, Goyal L, Agapite J, Steller H, Meier P (2003) IAP-antagonists exhibit non-redundant modes of action through differential DIAP1 binding. EMBO J 22: 6642–6652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier P, Silke J, Leevers SJ, Evan GI (2000) The Drosophila caspase DRONC is regulated by DIAP1. EMBO J 19: 598–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryoo HD, Gorenc T, Steller H (2004) Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev Cell 7: 491–501 [DOI] [PubMed] [Google Scholar]
- Kondo S, Senoo-Matsuda N, Hiromi Y, Miura M (2006) DRONC coordinates cell death and compensatory proliferation. Mol Cell Biol 26: 7258–7268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Bergmann A (2008) Distinct mechanisms of apoptosis-induced compensatory proliferation in proliferating and differentiating tissues in the Drosophila eye. Dev Cell 14: 399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild-Bode C, Weller M, Rimner A, Dichgans J, Wick W (2001) Sublethal irradiation promotes migration and invasiveness of glioma cells: implications for radiotherapy of human glioblastoma. Cancer Res 61: 2744–2750 [PubMed] [Google Scholar]
- Imamura F, Horai T, Mukai M, Shinkai K, Akedo H (1990) Potentiation of invasive capacity of rat ascites hepatoma cells by adriamycin. Cancer Res 50: 2018–2021 [PubMed] [Google Scholar]
- Qian BZ, Pollard JW (2010) Macrophage diversity enhances tumor progression and metastasis. Cell 141: 39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Nakabayashi Y, Nakata K, Reed JC, Takahashi R (2001) X-linked inhibitor of apoptosis protein (XIAP) inhibits caspase-3 and -7 in distinct modes. J Biol Chem 276: 27058–27063 [DOI] [PubMed] [Google Scholar]
- Huang Y, Park YC, Rich RL, Segal D, Myszka DG, Wu H (2001) Structural basis of caspase inhibition by XIAP: differential roles of the linker versus the BIR domain. Cell 104: 781–790 [PubMed] [Google Scholar]
- Riedl SJ, Renatus M, Schwarzenbacher R, Zhou Q, Sun C, Fesik SW, Liddington RC, Salvesen GS (2001) Structural basis for the inhibition of caspase-3 by XIAP. Cell 104: 791–800 [DOI] [PubMed] [Google Scholar]
- Tenev T, Zachariou A, Wilson R, Ditzel M, Meier P (2005) IAPs are functionally non-equivalent and regulate effector caspases through distinct mechanisms. Nat Cell Biol 7: 70–77 [DOI] [PubMed] [Google Scholar]
- Xu G, Cirilli M, Huang Y, Rich RL, Myszka DG, Wu H (2001) Covalent inhibition revealed by the crystal structure of the caspase-8/p35 complex. Nature 410: 494–497 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.