Abstract
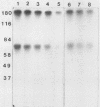
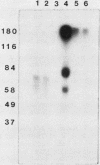
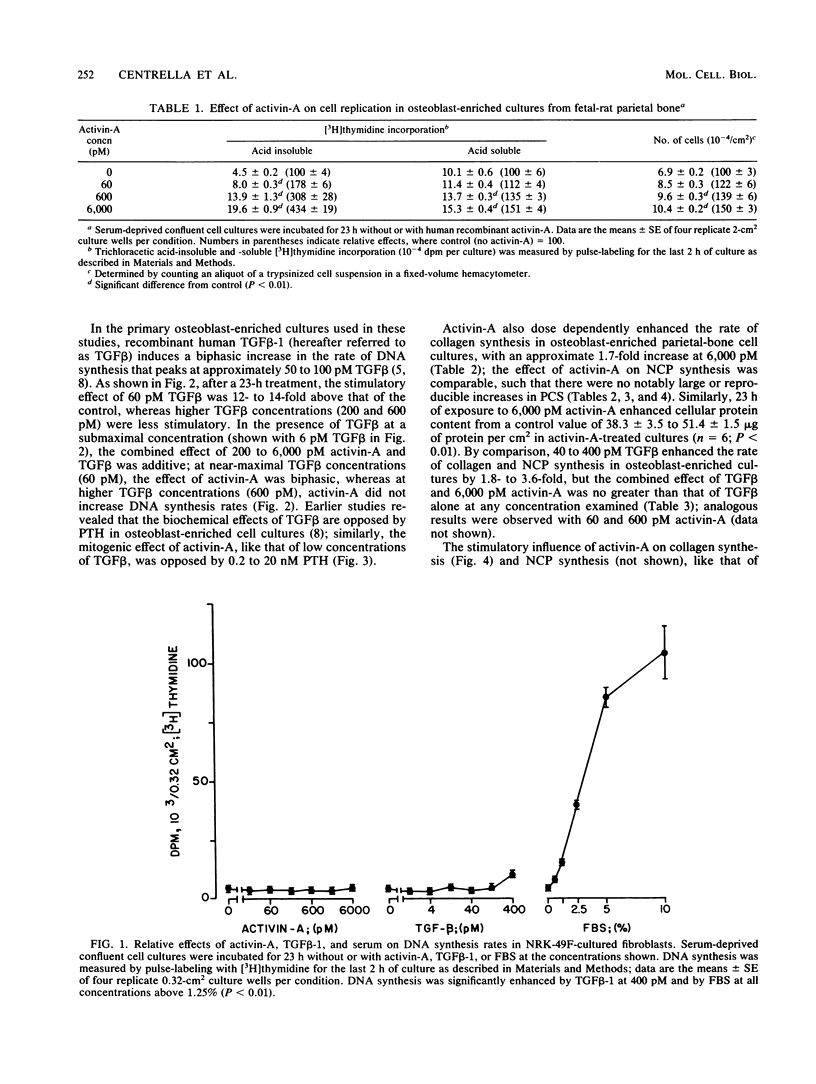
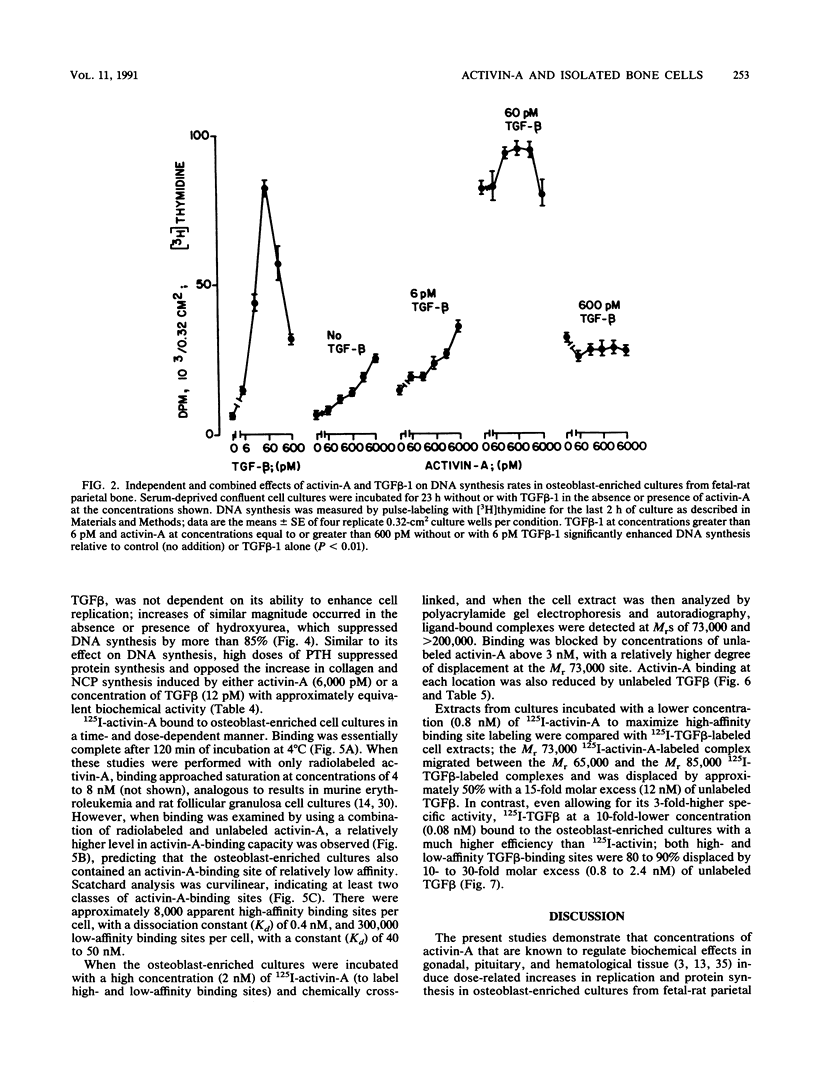
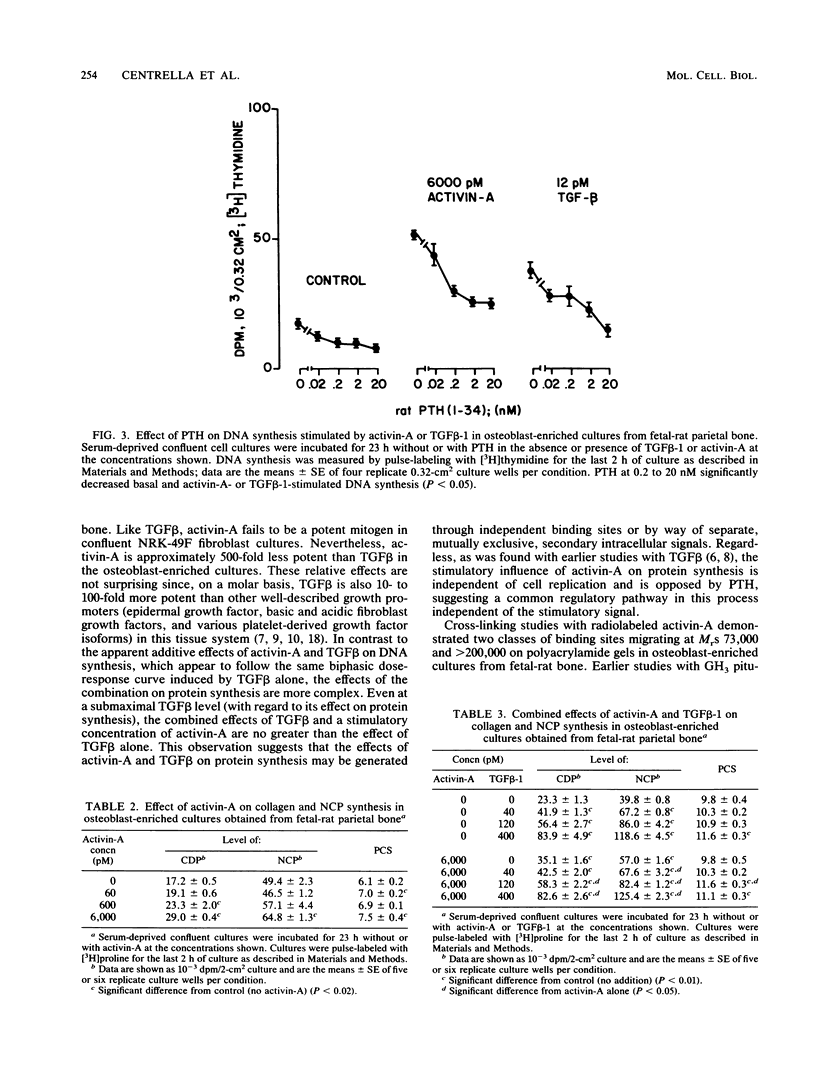
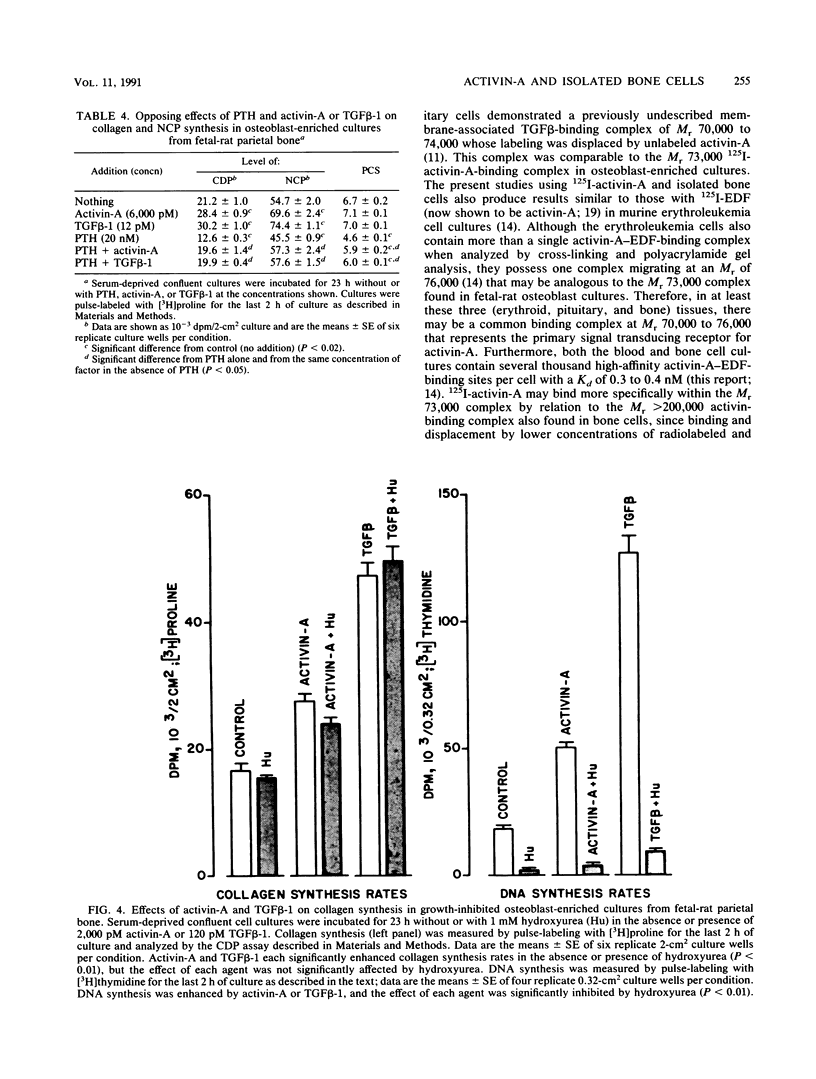
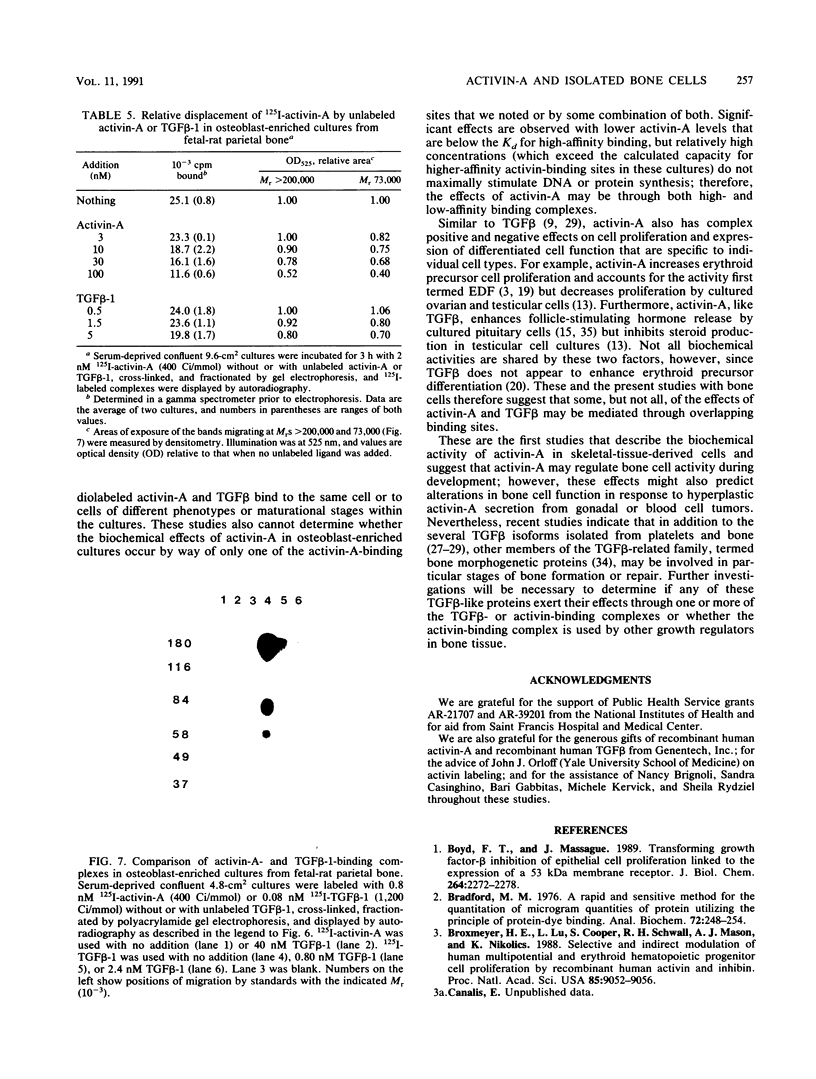
Activin, a disulfide-linked polypeptide dimer first isolated from gonadal tissue extracts, has amino acid sequence and structural homology with transforming growth factor beta (TGF beta). Along with other activities, TGF beta regulates replication and differentiation and interacts with a defined set of binding sites on isolated bone cells. To determine if activin shares these properties, recombinant human activin-A (A-chain homodimer) was examined in osteoblast-enriched cultures obtained from fetal-rat parietal bone. After 23 h of treatment, 60 to 6,000 pM activin-A increased the rate of [3H]thymidine incorporation into DNA 1.5- to 4.0-fold, and at 600 to 6,000 pM, it enhanced the rate of [3H]proline incorporation into collagen and noncollagen protein by up to 1.7-fold. Like earlier studies with TGF beta in primary osteoblast-enriched cultures, the stimulatory effects of activin-A on DNA and protein synthesis were opposed by parathyroid hormone, and the influence of activin-A on collagen synthesis was independent of cell replication. Binding studies with 125I-activin-A indicated approximately 8,000 high-affinity (Kd = 0.4 nM) and 300,000 low-affinity (Kd = 40 to 50 nM) binding sites per cell. Polyacrylamide gel analysis revealed 125I-activin-A-binding complexes of Mr greater than 200,000 and 73,000 which did not appear to correspond to primary TGF beta-binding sites. These results indicate that activin-A produces TGF beta-like effects in bone and that some of these effects may be mediated, at least in part, by distinct activin receptors on bone cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyd F. T., Massagué J. Transforming growth factor-beta inhibition of epithelial cell proliferation linked to the expression of a 53-kDa membrane receptor. J Biol Chem. 1989 Feb 5;264(4):2272–2278. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Broxmeyer H. E., Lu L., Cooper S., Schwall R. H., Mason A. J., Nikolics K. Selective and indirect modulation of human multipotential and erythroid hematopoietic progenitor cell proliferation by recombinant human activin and inhibin. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9052–9056. doi: 10.1073/pnas.85.23.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canalis E., McCarthy T., Centrella M. Growth factors and the regulation of bone remodeling. J Clin Invest. 1988 Feb;81(2):277–281. doi: 10.1172/JCI113318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centrella M., Canalis E., McCarthy T. L., Stewart A. F., Orloff J. J., Insogna K. L. Parathyroid hormone-related protein modulates the effect of transforming growth factor-beta on deoxyribonucleic acid and collagen synthesis in fetal rat bone cells. Endocrinology. 1989 Jul;125(1):199–208. doi: 10.1210/endo-125-1-199. [DOI] [PubMed] [Google Scholar]
- Centrella M., McCarthy T. L., Canalis E. Mitogenesis in fetal rat bone cells simultaneously exposed to type beta transforming growth factor and other growth regulators. FASEB J. 1987 Oct;1(4):312–317. doi: 10.1096/fasebj.1.4.3498658. [DOI] [PubMed] [Google Scholar]
- Centrella M., McCarthy T. L., Canalis E. Parathyroid hormone modulates transforming growth factor beta activity and binding in osteoblast-enriched cell cultures from fetal rat parietal bone. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5889–5893. doi: 10.1073/pnas.85.16.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centrella M., McCarthy T. L., Canalis E. Skeletal tissue and transforming growth factor beta. FASEB J. 1988 Dec;2(15):3066–3073. doi: 10.1096/fasebj.2.15.2903838. [DOI] [PubMed] [Google Scholar]
- Centrella M., McCarthy T. L., Canalis E. Transforming growth factor beta is a bifunctional regulator of replication and collagen synthesis in osteoblast-enriched cell cultures from fetal rat bone. J Biol Chem. 1987 Feb 25;262(6):2869–2874. [PubMed] [Google Scholar]
- Cheifetz S., Ling N., Guillemin R., Massagué J. A surface component on GH3 pituitary cells that recognizes transforming growth factor-beta, activin, and inhibin. J Biol Chem. 1988 Nov 25;263(33):17225–17228. [PubMed] [Google Scholar]
- Gonzalez-Manchon C., Vale W. Activin-A, inhibin and transforming growth factor-beta modulate growth of two gonadal cell lines. Endocrinology. 1989 Sep;125(3):1666–1672. doi: 10.1210/endo-125-3-1666. [DOI] [PubMed] [Google Scholar]
- Hino M., Tojo A., Miyazono K., Miura Y., Chiba S., Eto Y., Shibai H., Takaku F. Characterization of cellular receptors for erythroid differentiation factor on murine erythroleukemia cells. J Biol Chem. 1989 Jun 15;264(17):10309–10314. [PubMed] [Google Scholar]
- Ling N., Ying S. Y., Ueno N., Shimasaki S., Esch F., Hotta M., Guillemin R. Pituitary FSH is released by a heterodimer of the beta-subunits from the two forms of inhibin. Nature. 1986 Jun 19;321(6072):779–782. doi: 10.1038/321779a0. [DOI] [PubMed] [Google Scholar]
- Mason A. J., Hayflick J. S., Ling N., Esch F., Ueno N., Ying S. Y., Guillemin R., Niall H., Seeburg P. H. Complementary DNA sequences of ovarian follicular fluid inhibin show precursor structure and homology with transforming growth factor-beta. Nature. 1985 Dec 19;318(6047):659–663. doi: 10.1038/318659a0. [DOI] [PubMed] [Google Scholar]
- McCarthy T. L., Centrella M., Canalis E. Effects of fibroblast growth factors on deoxyribonucleic acid and collagen synthesis in rat parietal bone cells. Endocrinology. 1989 Oct;125(4):2118–2126. doi: 10.1210/endo-125-4-2118. [DOI] [PubMed] [Google Scholar]
- McCarthy T. L., Centrella M., Canalis E. Further biochemical and molecular characterization of primary rat parietal bone cell cultures. J Bone Miner Res. 1988 Aug;3(4):401–408. doi: 10.1002/jbmr.5650030406. [DOI] [PubMed] [Google Scholar]
- Murata M., Eto Y., Shibai H., Sakai M., Muramatsu M. Erythroid differentiation factor is encoded by the same mRNA as that of the inhibin beta A chain. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2434–2438. doi: 10.1073/pnas.85.8.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta M., Greenberger J. S., Anklesaria P., Bassols A., Massagué J. Two forms of transforming growth factor-beta distinguished by multipotential haematopoietic progenitor cells. Nature. 1987 Oct 8;329(6139):539–541. doi: 10.1038/329539a0. [DOI] [PubMed] [Google Scholar]
- Orloff J. J., Wu T. L., Heath H. W., Brady T. G., Brines M. L., Stewart A. F. Characterization of canine renal receptors for the parathyroid hormone-like protein associated with humoral hypercalcemia of malignancy. J Biol Chem. 1989 Apr 15;264(11):6097–6103. [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Raisz L. G. Local and systemic factors in the pathogenesis of osteoporosis. N Engl J Med. 1988 Mar 31;318(13):818–828. doi: 10.1056/NEJM198803313181305. [DOI] [PubMed] [Google Scholar]
- Rosenblatt M., Callahan E. N., Mahaffey J. E., Pont A., Potts J. T., Jr Parathyroid hormone inhibitors. Design, synthesis, and biologic evaluation of hormone analogues. J Biol Chem. 1977 Aug 25;252(16):5847–5851. [PubMed] [Google Scholar]
- Schwall R. H., Nikolics K., Szonyi E., Gorman C., Mason A. J. Recombinant expression and characterization of human activin A. Mol Endocrinol. 1988 Dec;2(12):1237–1242. doi: 10.1210/mend-2-12-1237. [DOI] [PubMed] [Google Scholar]
- Segarini P. R., Rosen D. M., Seyedin S. M. Binding of transforming growth factor-beta to cell surface proteins varies with cell type. Mol Endocrinol. 1989 Feb;3(2):261–272. doi: 10.1210/mend-3-2-261. [DOI] [PubMed] [Google Scholar]
- Seyedin S. M., Segarini P. R., Rosen D. M., Thompson A. Y., Bentz H., Graycar J. Cartilage-inducing factor-B is a unique protein structurally and functionally related to transforming growth factor-beta. J Biol Chem. 1987 Feb 15;262(5):1946–1949. [PubMed] [Google Scholar]
- Seyedin S. M., Thompson A. Y., Bentz H., Rosen D. M., McPherson J. M., Conti A., Siegel N. R., Galluppi G. R., Piez K. A. Cartilage-inducing factor-A. Apparent identity to transforming growth factor-beta. J Biol Chem. 1986 May 5;261(13):5693–5695. [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-beta. J Cell Biol. 1987 Sep;105(3):1039–1045. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino H., Nakamura T., Hasegawa Y., Miyamoto K., Igarashi M., Eto Y., Shibai H., Titani K. Identification of a specific receptor for erythroid differentiation factor on follicular granulosa cell. J Biol Chem. 1988 Oct 25;263(30):15249–15252. [PubMed] [Google Scholar]
- Wakefield L. M., Smith D. M., Masui T., Harris C. C., Sporn M. B. Distribution and modulation of the cellular receptor for transforming growth factor-beta. J Cell Biol. 1987 Aug;105(2):965–975. doi: 10.1083/jcb.105.2.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozney J. M., Rosen V., Celeste A. J., Mitsock L. M., Whitters M. J., Kriz R. W., Hewick R. M., Wang E. A. Novel regulators of bone formation: molecular clones and activities. Science. 1988 Dec 16;242(4885):1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- Ying S. Y. Inhibins, activins, and follistatins: gonadal proteins modulating the secretion of follicle-stimulating hormone. Endocr Rev. 1988 May;9(2):267–293. doi: 10.1210/edrv-9-2-267. [DOI] [PubMed] [Google Scholar]
- ten Dijke P., Hansen P., Iwata K. K., Pieler C., Foulkes J. G. Identification of another member of the transforming growth factor type beta gene family. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4715–4719. doi: 10.1073/pnas.85.13.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Dijke P., Iwata K. K., Goddard C., Pieler C., Canalis E., McCarthy T. L., Centrella M. Recombinant transforming growth factor type beta 3: biological activities and receptor-binding properties in isolated bone cells. Mol Cell Biol. 1990 Sep;10(9):4473–4479. doi: 10.1128/mcb.10.9.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]