Abstract
Objectives
Treatment approaches for patients with Myalgic Encephalomyelitis/chronic fatigue syndrome (ME/CFS) have been controversial. This paper provides the theoretical and conceptual background for the Energy Envelope Theory to assist patients with ME/CFS and reviews evidence of its treatment efficacy.
Methods
Over a 15-year period, efforts were directed to develop a non-pharmacologic intervention that endeavored to help patients with ME/CFS self-monitor and self-regulate energy expenditures and learn to pace activities and stay within their energy envelope.
Conclusions
Studies show that the energy envelope approach, which involves rehabilitation methods, helps patients with ME/CFS pace activities and manage symptoms and can significantly improve their quality of life.
Keywords: chronic fatigue syndrome, Myalgic Encephalomyelitis, energy envelope, pacing
In 2005, 133 million people in the United States (half of whom were adults) had a chronic health condition, while 63 million people had multiple chronic conditions. [1] One of the more complicated and debilitating health conditions is referred to as Myalgic Encephalomyelitis/chronic fatigue syndrome (ME/CFS). Its most prominent symptoms are post-exertional malaise and memory and concentration problems. ME/CFS affects about one million Americans, although many of them are undiagnosed. [2]
Unfortunately, most patients with ME/CFS find the medical health care they receive for this condition insufficient. This sentiment is documented by a number of studies, including one by Green, Romei, and Natelson [3] who found that 95% of individuals seeking medical treatment for ME/CFS reported feelings of estrangement. In addition, Twemlow, Bradshaw, Coyne, and Lerma [4] found that individuals with ME/CFS reported that they were made worse by their health care workers 66% more often than general medical patients. Health care practitioners could play a key role in better helping these patients with ME/CFS. In this article, we identify reasons for discontent with current medical practices for treating ME/CFS and also review the development of alternative non-pharmacologic treatment techniques.
Cognitive Behavior Therapy
Negative reactions to health care services might in part be explained by the types of interventions offered to patients with ME/CFS. While cognitive behavior therapy (CBT) has been applied to many medical problems, from pain to fibromyalgia, its application to ME/CFS has been more controversial, perhaps due to certain components of CBT that some investigators practice (e.g., [5,6]). Typical of the psychogenic view of ME/CFS is a research group from the Netherlands, [7] who believe that these individuals attribute their symptoms to physical causes, are overly preoccupied by their physical limitations, and do not maintain regular activity. According to this model, these factors cause individuals with ME/CFS to be functionally impaired, implying that the central problem patients experience is a psychosomatic preoccupation with their fatigue. Song and Jason [8] tested this model with five groups: people who met the criteria for ME/CFS, those with psychiatrically explained chronic fatigue, people with medically explained chronic fatigue, a group with idiopathic chronic fatigue and a group with fatigue related to substance abuse. The Vercoulen et al. [7] model was replicated for the sample with psychiatric conditions causing the fatigue but not for the patients with ME/CFS. In addition, Price, Mitchell, Tidy, and Hunot [9] recently reviewed 15 studies of CBT with a total of 1,043 ME/CFS participants. At treatment’s end, 40% of people in the CBT group showed clinical improvement in contrast to only 26% in usual care, but changes were not maintained at a one- to seven-month follow-up when including patients who had dropped out.
Patient reactions to CBT and graded exercise have been mixed at best. One survey of 2,338 ME/CFS respondents found that 26% felt their ME/CFS worsened after trying CBT, and graded exercise was felt to be the treatment that made more people worse than any other. [10] Results of other surveys conducted by the ME Association showed that for patients with ME/CFS who had received graded exercise therapy, 33.1% felt “much worse” and 23.4% judged themselves to be “slightly worse”. [11] Similarly, another survey of patients with ME/CFS found that 34% of those who tried graded exercise therapy perceived themselves to be worse. [12] Of course there are limitations to patient surveys, as the fidelity of the interventions that respondents have been provided is not always clear. Still, the results of patient surveys do suggest that many patients with ME/CFS are reluctant to engage in CBT- or Graded Exercise-based interventions.
There may be physiological reasons for the somewhat negative reactions to these CBT interventions for patients with ME/CFS. Jammes, Steinberg, Mambrini, Bregeon, and Delliaux [13] found that incremental exercise among individuals with ME/CFS was associated with oxidative stress and marked alterations of muscle membrane excitability. Black, O’Connor, and McCully [14] found that when individuals with ME/CFS were asked to systematically increase their daily physical activity by 30%, their overall mood, muscle pain intensity, and time spent with fatigue each day worsened. It should be noted that systematic increases do not imply a gradual increase in activity, so this study would not replicate protocols that are based on graded approaches to activity. There is some evidence that optimism, stress, and social support may not be related to recovery from ME/CFS. For example, Camacho and Jason [15] studied patients who had achieved a form of partial remission from ME/CFS, patients who had not recovered from ME/CFS, and a non-ME/CFS control group. There were no significant differences between these groups on measures of optimism, stress, and social support. In a natural history study, Jason, Porter, Hunnell, Rademaker, and Richman [16] examined the course of ME/CFS over approximately a ten-year period of time, and they found that initial, baseline measures of stress, support or coping were not significant predictors of ME/CFS versus controls at the follow-up. These findings are consistent with what would be expected from people dealing with a chronic illness such as ME/CFS.
An Alternative Approach
Beginning in 1993, our research team began collecting behavioral time-series data to better understand the relationships between activity and fatigue in patients with ME/CFS. It was clear to us that some individuals had high levels of fatigue that were influenced by how much activity they engaged in, and we wanted to better understand this relationship. We hypothesized that by limiting levels of activity in order to reduce high levels of fatigue and other symptoms, positive gains may occur for the patients. This rehabilitation model was rather different from CBT approaches being evaluated for patients with ME/CFS. The following case study illustrates such an energy conservation approach. [17] Peter (this is not his real name), who had been diagnosed with ME/CFS, collected data from 6:30 a.m. until 9:30 p.m. each day. Each hour, he recorded his fatigue and activity levels. Findings indicated that as activity level increased, particularly in the morning, the intensity of fatigue increased. When activities decreased and the participant rested or relaxed at home, the fatigue tended to decrease. These findings suggested to us that interventions may be constructed in a way that focused on reducing activity and increasing resting in order to help patients with ME/CFS better cope with their illness.
Next, we collected data from Mary, a more impaired person with diagnosed ME/CFS. She had left work permanently and reported requiring about 15 hours of sleep per day. Her activities were limited to walking around the house and completing necessary errands. Mary’s fatigue was rated as severe at all time-points, even though her activity level was rather low. Mary’s data, therefore, were very distinct from Peter’s data, whose energy levels fluctuated dramatically over time and were associated with activity level. For Mary, energy levels were consistently low, but there did appear to be a marginal relationship between greater levels of activity and higher levels of fatigue.
In our next study, conducted in 1996, Jason, Tryon, et al. [18] collected behavioral data in which participants with ME/CFS were asked to rate perceived or available energy and the amount of energy expended. We found a significant relationship between current fatigue level and the amount of energy that participants perceived they had expended two days prior. In a different data set, results indicated that actigraphy data (from actigraphs that electronically monitor activity) were significantly related to self-reported indices of expended energy. [19] In another study, data collected with actigraphs showed that people with ME/CFS had fewer episodes of intense activity over short periods of time (such as exercising), whereas this type of activity was common for a healthy control subject. [20]
As we began thinking about using these data to develop interventions to help individuals with ME/CFS cope with their levels of fatigue, we decided to assess the service needs of people with ME/CFS. In 1996, our research team distributed a brief survey of open- and closed-ended items for participants with ME/CFS designed to assess their utilization of and preference for a variety of services. [21] One preferred service involved a volunteer caregiver system to provide assistance with daily chores and errands. These data helped us set priorities for the subsequent development of a service program involving volunteer buddies to examine if it helped patients expending more energy than they had available to them.
Intervention Studies
We next developed a volunteer caregiving program that provided patients help with daily chores on a regular basis, perceived as one of the higher priority needs by the national sample of patients with ME/CFS. [21] In collaboration with the Chicago Chronic Fatigue Syndrome Association, Shlaes and Jason [22] developed a program whereby people with ME/CFS received a volunteer buddy and a mentor who had ME/CFS. The buddy was an individual in the community who agreed to spend one hour a week conducting home visits with an individual with ME/CFS. Buddy-participant matches were made based upon need and interest assessments completed by the participants and buddies. Mentors were individuals with ME/CFS who were willing and able to engage in two hours of phone contact each month with the participants. The role of mentor was designed to include informational and emotional support.
We recruited twelve patients with ME/CFS; half received a buddy and mentor for four months whereas the other half were controls who received no intervention. Participants who received the buddy/mentor intervention experienced significant decreases in fatigue severity, while the control group experienced significant increases in fatigue severity. Not only did the patients with ME/CFS benefit from the buddy/mentor program, but volunteers benefited from their experiences as well. [23] By working directly with people with a disability such as ME/CFS, volunteers can learn a great deal about the obstacles patients need to overcome as well as their unique strengths.
In our work with this buddy/mentor program, we learned that by avoiding overexertion, people with ME/CFS could avoid setbacks and relapses while also increasing their tolerance for activity. We concluded that rehabilitative treatment planning and illness management programs for patients with ME/CFS could be tailored to patients’ situations. For example, patients who continually overexerted themselves were advised to cut back and conserve their energy resources so that long-term gains in their tolerance to activity could be made. Our work suggested that people with ME/CFS should not necessarily either increase or decrease their activity levels, but rather moderate activity and practice energy conservation. This strategy, which we called the “Energy Envelope Theory,” was suggested to us by a member of the buddy/mentor program.
During 1995 and 1996, we began further developing our rehabilitation ideas of energy conservation and the use of moderation for patients with ME/CFS. Our first publications on the Energy Envelope Theory appeared in 1997 and 1999. [24,25] This theory suggested that by maintaining expended energy levels within the “envelope” of perceived available energy levels, patients with ME/CFS would better be able to sustain physical and mental functioning while reducing symptom severity and the frequency of relapses. In a later correlational study, we found that the individuals with ME/CFS experienced a range of symptoms and disability when they extended beyond their energy envelopes. [26] This evolving approach suggested that patients could be taught to assess their perceived available energy levels on a daily basis and use that level to gauge their energy expenditures for each day. Appendix A provides a form that can be used for capturing some of the variables mentioned in this article, and training materials and other information about the buddy program can be obtained by writing to the first author.
Applying this approach as a ME/CFS rehabilitation management tool involves accepting and working within the limits imposed by the illness rather than fighting against them. Within the area of rehabilitation of chronic illnesses, this approach has some similarities to acceptance and commitment therapy, which emphases acceptance and commitment along with behavior-change strategies. [27] Over time, individuals with ME/CFS may find that they experience fewer crashes and decreased fatigue and symptom severity. It may even be possible that, by maintaining energy levels in this way, patients with ME/CFS might be able to expand their energy envelope. That is, their perceived energy levels may increase over time, allowing them to engage in higher levels of physical activity. This rehabilitation approach differs from graded exercise therapy, which emphasizes maintaining or increasing activity even when patients experience symptoms. In addition, the energy envelope approach does not challenge patients’ belief in a medical cause for their ME/CFS.
Case Studies involving the Energy Envelope
Our thinking on the Energy Envelope Theory was furthered by a case study that began in the spring of 1995. [24] For a 16-month period, a participant with ME/CFS completed daily ratings of fatigue, perceived energy, and expended energy. We found this method of assessing available and expended energy achieved good inter-rater reliability. [28] Fatigue and the energy that is currently available to the person (what we call perceived energy) are different constructs, although they are often related. We often found that the patient was very fatigued and had little perceived energy; however, sometimes the patient reported he was not fatigued (for example, when resting), but still reported little perceived energy.
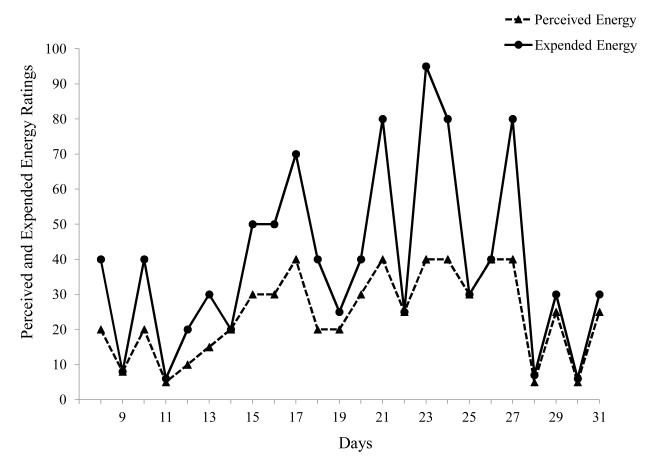
Figure 1 shows the first month of data collection in the spring of 1995 for the patient with ME/CFS. We found that the participant’s expended energy tended to greatly exceed levels of perceived or available energy. As a result, the degree of fatigue experienced by the participant remained consistently high despite periodic fluctuations. Data from the spring of 1995 demonstrated that when the participant’s energy expenditure greatly exceeded his levels of perceived energy, his fatigue levels were extremely high. In addition to exacerbating levels of fatigue, this overexertion also appeared to deplete the participant’s energy resources.
Figure 1.
Patient exceeds Energy Envelope
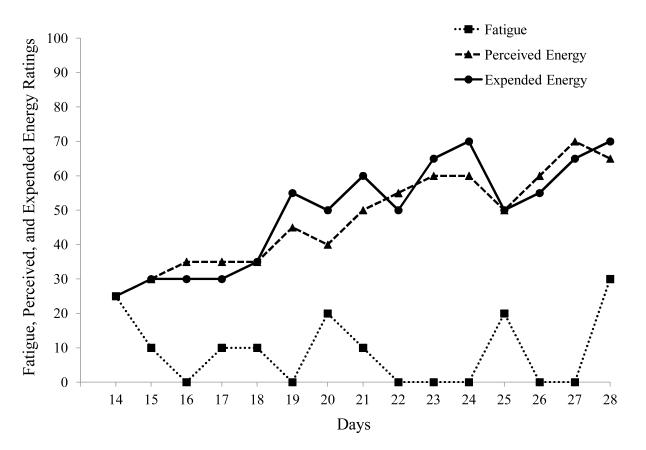
As seen in Figure 2, the data collected in the fall of 1995 painted an entirely different picture. Over the preceding months, the participant had made efforts to keep his expended energy close to his perceived energy. In Figure 2 there is a pattern of gradually increasing perceived and expended energy, with perceived and expended energy remaining relatively well matched. These findings suggest that when levels of expended energy and perceived energy are monitored and kept relatively close, people with ME/CFS may be able to increase their energy resources while containing their levels of fatigue. During the summer of 1996, 16 months after data collection had begun, the participant’s levels of fatigue were considerably lower than at the beginning of the study. Furthermore, the participant’s levels of perceived and expended energy were much more comparable than they were initially, indicating progress toward remaining within the energy envelope.
Figure 2.
Patient within Energy Envelope
In a second case study, a female patient with ME/CFS made daily ratings of perceived energy, expended energy, and fatigue level. [25] Her perceived and expended energy ratings indicated that she was considerably above her energy envelope. However, when she visited a relative, her perceived and expended energy were more in balance, as her relative could help her reduce the amount of activity she needed to carry out each day. During this period of time, her fatigue ratings decreased, demonstrating once again the importance of support in reducing the amount of activities and expended energy that patients with ME/CFS have to carry out on a daily basis.
In our next study, the Energy Envelope Theory was tested within the context of the buddy program, which had been in operation since the mid-1990s. Participants with ME/CFS were provided with a buddy to assist with household needs and were also provided education about remaining within their energy envelope. [29] During baseline conditions, two individuals had an average expended energy consistently above the average perceived energy, and they reported high levels of daily fatigue. However, with the buddy treatment intervention, expended energy was reduced so that it matched perceived energy. In addition, daily fatigue ratings dropped, and severity ratings for five of eight core ME/CFS symptoms decreased.
Randomized Clinical Trials
Taylor, Jason, Shiraishi, Schoeny, and Keller [30] provided a group of patients with ME/CFS an illness management and treatment planning intervention. The Energy Envelope Theory played a critical role in the formulation of this intervention. Forty-seven participants with ME/CFS were randomly assigned to two groups, with one group receiving the intervention in the first year, and the second group serving as a control (at the end of the study, they were also provided the intervention). The intervention began with a group-based program. The first hour-long group was devoted to goal setting, with members reporting on behaviorally-focused and attainable objectives and goals using an individualized action plan. The next hour focused on the following topics: the Energy Envelope Theory, cognitive coping skills, personal relationships, self-relaxation and coping, economic self-sufficiency, nutritional approaches, and employment issues. For the next seven-month period, case coordinators (two people who had mostly recovered from ME/CFS) assisted each participant with appropriate supportive services, such as individualized self-advocacy training, assertiveness skills, and some financial support to create their own linkages to community-based services. We found that those who were provided this comprehensive intervention, designed to provide resources to patients, had an overall significant increase in their self-esteem, well-being, mastery, work, energy, and interpersonal relationships.
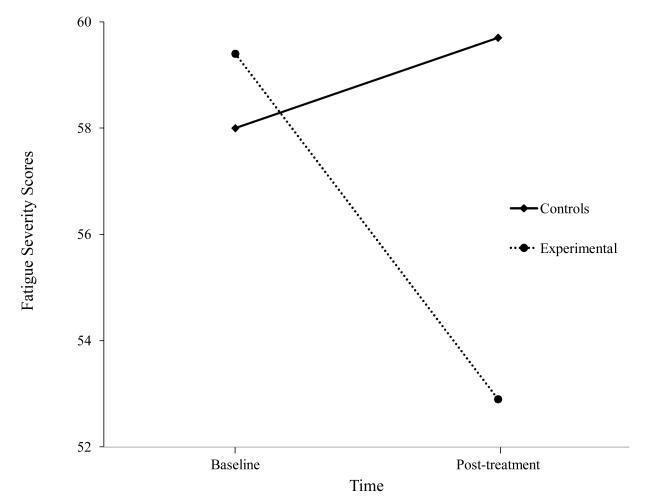
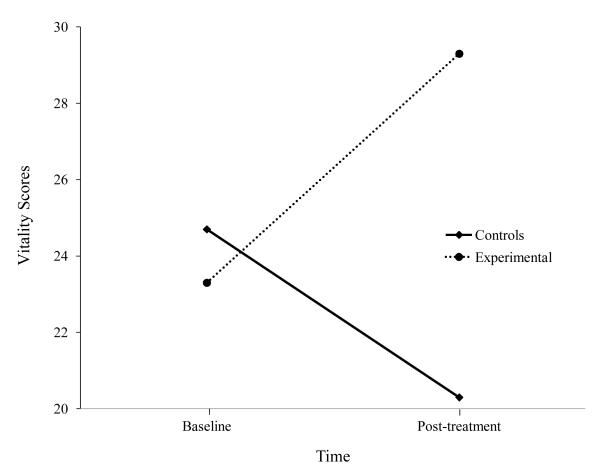
Our next Energy Envelope study was an evaluation of our buddy program with a larger sample of patients with ME/CFS. [31] In this study, buddies were students at DePaul University. Thirty participants with ME/CFS were randomly assigned to either a four-month buddy intervention or a control condition. Those who received the buddy intervention had significantly greater reductions in fatigue severity and increases in vitality as shown in Figures 3 and 4. Once again, we found that helping individuals with ME/CFS both monitor and stay within their energy boundaries led to important improvements.
Figure 3.
Fatigue scores for those provided buddies and Controls
Figure 4.
Vitality scores for those provided buddies and Controls
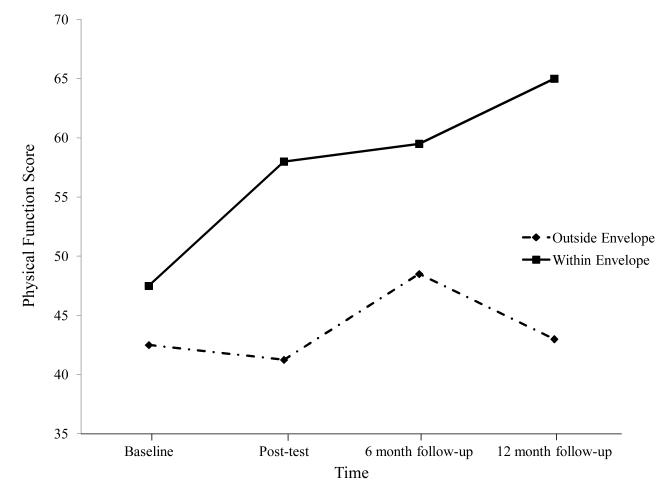
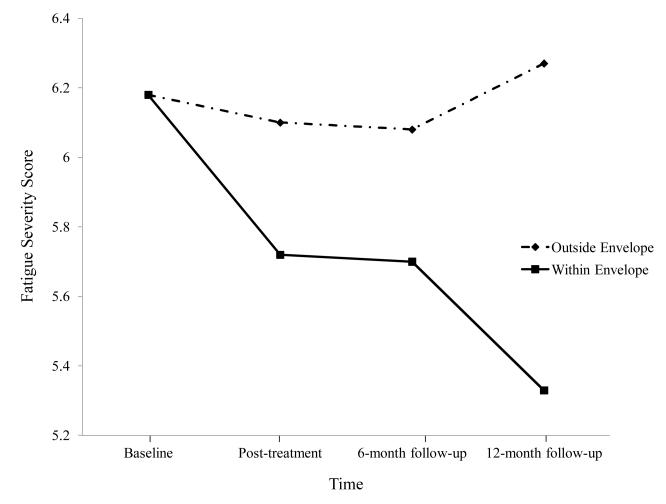
A subsequent intervention involved a randomized trial of non-pharmacologic interventions for ME/CFS. One hundred and fourteen patients with ME/CFS were randomly assigned to four interventions in which they were provided 13 biweekly sessions with a trained nurse therapist. We collected baseline, post-treatment, and six- and twelve-month follow-up data. Although there were some differences among the four types of interventions, overall, we saw general improvement from all interventions. Jason, Benton, et al. [32] divided this entire sample of patients with ME/CFS into two groups: those who were able to keep expended energy close to available energy and those who were not successful at this task. Those who were able to stay within their energy envelopes had significant improvements in physical functioning and fatigue severity (see Figures 5 and 6). Findings suggest that helping patients with ME/CFS maintain appropriate energy expenditures in coordination with available energy reserves can help improve functioning over time. In addition, using the same data set, Brown, Khorana, and Jason [33] found that patients with ME/CFS who stayed within their energy envelopes before treatment showed more improvement in physical functioning and fatigue compared with those who were outside of their energy envelopes. These findings suggest that an assessment of perceived available and expended energy could help guide the development of individualized, non- pharmacologic interventions for people with ME/CFS.
Figure 5.
Physical Functioning for those within and outside energy envelope.
Figure 6.
Fatigue severity for those within and outside energy envelope.
We have used data from this same study to identify predictors of improvement after treatment. We combined the four treatment groups of patients with ME/CFS into two groups, improvers and non-improvers, based on a measure of physical functioning. About half the participants in each group were improvers and the other half were non-improvers. [34] We found important baseline differences between those who improved versus did not improve over time. Improvers, at baseline, had decreased T and B cells and elevated NK percentage numbers, whereas non-improvers had an elevated humoral immune response (a dominance of the Type 2 over the Type 1 immune response). In other words, those with the most severe immune baseline characteristics tended to be non-improvers. As ME/CFS is associated with a shift toward a Type 2 immune response, those with this pattern at baseline tended to not improve over the course of the trial. [35] We also categorized these patients into abnormal versus normal baseline cortisol levels. We categorized the readings as abnormal if cortisol levels from five tests during one day continued to rise, were flat, or were abnormally low over time. [36] We found that patients with ME/CFS with normal cortisol at baseline had the most improvement over time for activity levels, fatigue severity, depression, anxiety, and immune system markers. Patients with normal baseline cortisol evidenced improvements on a number of immunologic and self-report measures, whereas patients most impaired on hypothalamic-pituitary-adrenal (HPA) functioning at baseline were least able to improve when provided rehabilitation interventions.
‘Pacing’ Approaches
The ‘pacing’ approach is very similar to the Energy Envelope Theory. Pacing for patients with ME/CFS involves encouraging them to be as active as possible within the limits imposed by the illness. [37,38] Patients with ME/CFS are instructed to ignore symptoms that do not make them feel unwell. However, patients are instructed to either rest or change to an activity involving different muscles when more serious symptoms occur, indicating that their ‘limits’ have been exceeded (e.g., onset of muscle weakness, dizziness, or a flu-like malaise). [39] Goudsmit, Ho-Yen, and Dancey [40] used pacing as part of a multi-component ME/CFS program with very low attrition rates. Their physician-led program found significant differences between the treated patients and controls for fatigue, somatic symptoms, and self-efficacy. In addition, using this approach, improvements were maintained at the 12-month follow-up.
Other investigators have also found support for the principles discussed in this article. For example, a 12-week program by Wallman, Morton, Goodman, Grove, and Guilfoyle [41] provided support for remaining as active as possible within the limits imposed by ME/CFS. Using symptom-contingent graded exercise, increases in exercise were advised only when patients felt they were coping with their current activity levels as determined by average scores on a perception of effort scale. Most importantly, and in contrast to graded exercise approaches, patients could stop if they experienced a relapse or if symptoms became worse. None felt that the exercise/pacing program made them worse, and 91% of the participants rated themselves as ‘better’ in respect to their overall health.
Nijs, Paul, and Wallman [42] have used pacing in the following way. To establish a baseline, patients with ME/CFS were initially asked to estimate their physical capabilities during their normal daily activity, and then they were instructed to reduce their activities by 25%. So patients who were able to walk 20 minutes without exacerbating their symptoms were instructed to walk for no longer than 15 minutes, followed by a 15-minute break. If no symptoms occurred, they were permitted to walk for an additional 15 minutes. However, on bad days, they were instructed to walk for only 50% of baseline. Activity was always interspersed with breaks in which the patient with ME/CFS relaxed or engaged in light activity (e.g., reading). The intervention was tailored according to each individual’s capabilities. Patients were encouraged to use a pedometer to provide reliable information on the activities undertaken.
Staci Stevens and other exercise physiologists have cited evidence that the aerobic system of energy production is damaged in patients with ME/CFS. While aerobic exercise causes post-exertional malaise in patients with ME/CFS, the patients’ anaerobic systems are not damaged. After testing to establish a patient’s anaerobic threshold, Stevens and colleagues have established a tailored anaerobic program. [43] In this program, the pacing component is introduced first. An initial program may involve four repetitions of four exercises, using two-pound weights and a rubber exercise band. If there is no exacerbation of symptoms after two weeks, an additional repetition is added.
There are a number of other health care practitioners suggesting approaches that focus on energy conservation for patients with ME/CFS. For example, an excellent source of information on the energy envelope is available at Campbell’s [44] CFIDS and Fibromyalgia website. He covers many techniques such as energy envelope, activity forms, logs and diaries, setting limits, activity plans, etc. These strategies are implementable by patients of varying levels of illness severity.
Cox, Ludlam, Mason, Wagner, and Sharpe [45] have described adaptive pacing therapy (APT), which involves engaging in activities that do not produce an increase in symptoms. In addition to instructions involving activity and rest, treatment also includes information on relaxation techniques, improving sleep, and posture. It is unclear, however, how these researchers developed the rule of never going beyond 70% of a person’s perceived energy limit, which would not be compatible with the Energy Envelope Theory, described earlier by Jason and colleagues. [19,25] A recent, well publicized British study by White et al. about patients with ME/CFS compared APT with other interventions, including CBT, graded exercise therapy, and specialist medical care. [6] The results indicated that APT had little effect. In evaluating this APT intervention, it is certainly possible that additional periods of pre-emptive rest might have provided patients with ME/CFS more time to focus on symptoms, which is a maladaptive coping strategy. It does not appear that encouraging patients with ME/CFS to do less than they can promotes positive outcomes. The Envelope Theory suggests that there needs to be a balance between perceived and expended energy, and if expended energy is consistently lower than perceived energy, the patient with ME/CFS may be too inactive. [24,25,26] In other words, pacing that involves patients doing less than they can and resting more frequently could be maladaptive.
Conclusion
The series of studies summarized in this article provide support for the Energy Envelope Theory as an approach to the rehabilitation management of ME/CFS. This theory would recommend that health care professionals who treat patients with ME/CFS incorporate strategies that help patients self-monitor and self-regulate energy expenditures. Learning to pace activities and stay within the energy envelope appears to have favorable outcomes for patients with ME/CFS. Non-pharmacologic rehabilitative interventions are used for people with cancer and heart disease, but they are only one part of the treatment plan, and, when used by themselves, they are not curative. Similarly, helping patients with ME/CFS remain within their energy envelopes is only one part of a rehabilitation plan.
Much attention of researchers has focused on the potential benefits of cognitive behavioral and graded exercise interventions. For example, in a review paper, Van Cauwenbergh, De Kooning, Ickmans, and Nijs [46] concluded that randomized studies support exercise treatment using a time-contingent approach, yet this is contradicted by patient surveys. Kindlon [11] suggests that this discrepancy may be due to the heterogeneity of patients in the different trials and the way in which the harms and treatment compliance have been reported in the randomized trials. As mentioned earlier, the long-term outcomes of this type of intervention are still unclear, but interventions that challenge basic patient illness beliefs may solidify already negative attitudes of medical personnel toward people with ME/CFS. The Energy Envelope Theory and pacing represent alternative approaches for helping patients with ME/CFS. These approaches involve helping patients better monitor energy levels, stay within their energy envelopes, sustain lifestyle changes that involve reprioritizing activities, and possibly rebalance their lifestyles between work and leisure.
Being overextended and exceeding energy reserves can be an impediment to improving functionality and reducing fatigue levels. Kindling is an explanation of what might occur when patients with ME/CFS overexert themselves and deplete energy reserves. [47] The kindling hypothesis suggests that once a patient’s system is charged, either by high-intensity stimulation or by chronically repeated low-intensity stimulation, activities that involve going beyond energy reserves may enhance an already high level of arousal. In a sense, patients with ME/CFS might have this type of cortical excitability that may be due to kindling. When they go beyond their energy reserves; the kindling results in high arousal, which has implications for the hypothalamus, the autonomic nervous system, and the immune system. Within the brain, areas of the prefrontal cortex and anterior cingulate influence the amygdala, and kindling in these areas could cause continuous sympathetic nervous stimulation that would eventually lead to glandular depletion. [48] Other ME/CFS research suggests that long-term sensory receptor activation may lead to sensitization of the spinal cord and brain systems that transmit fatigue signals, causing long-term fatigue enhancement within the central nervous system. [49-53] Interventions that focus on energy balance and pacing might reduce the kindling and sensitization that could be occurring among patients with ME/CFS. This understanding of ME/CFS symptoms suggests potential difficulties using graded activity approaches, which encourage higher levels of activity regardless of symptoms.
The Energy Envelope approach to ME/CFS symptom management and rehabilitation has important implications for health care practitioners who see individuals with ME/CFS. Although this approach is not curative, it may provide this patient population with strategies to aid in symptom management, which can significantly improve the quality of life for these individuals. There certainly is a need to include biological measures within future clinical trials with these types of approaches so that we can learn about who may profit most from these non-pharmacologic rehabilitation approaches, using outcomes beyond self-report measures. [34,36]
Acknowledgments
Funding was provided by NIAID (grant numbers AI 49720 and AI 055735).
Appendix A
Directions for ME/CFS Self-Monitoring Questionnaire
At the extreme left, note the day of the observation. Please fill out the form for a one week period at the same time each day (e.g., in the morning for the previous day).
For the first column, on a 100 point scale, rate the perceived energy that you have (0=no energy; 100=abundant energy, like when you were completely well). Next rate the expended energy for that day, on a similar 100 point scale (0=no energy; 100=abundant energy, like when you were completely well). For the next column, rate your level of fatigue on a 100 point scale (0=no fatigue; 100=extreme fatigue).
The next three columns involve items tapping negative and positive emotions. For negative affect, rate the amount you were upset, nervous, and irritated on a 100 point scale, with 0=none, 50=moderate, and 100=extreme. For positive affect, rate the amount you were happy and delighted, with the same 100 point scale.
For the next columns, you will rate somatic and cognitive difficulties, and you will use the same 100 point scale. We will select these somatic and cognitive problems, to be monitored, together.
References
- [1].Wu S, Green A. Projection of Chronic Illness Prevalence and Cost Inflation. RAND Health; Santa Monica, CA: 2000. [Google Scholar]
- [2].Jason LA, Richman JA, Rademaker AW, Jordan KM, Plioplys AV, Taylor RR, McCready W, Huang CF, Plioplys S. A community-based study of chronic fatigue syndrome. Archives of Internal Medicine. 1999;159:2129–2137. doi: 10.1001/archinte.159.18.2129. [DOI] [PubMed] [Google Scholar]
- [3].Green J, Romei J, Natelson BJ. Stigma and chronic fatigue syndrome. Journal of Chronic Fatigue Syndrome. 1999;5:63–75. [Google Scholar]
- [4].Twemlow SW, Bradshaw SL, Jr, Coyne L, Lerma BH. Patterns of utilization of medical care and perceptions of the relationship between doctor and patient with chronic illness including chronic fatigue syndrome. Psychological Reports. 1997;80:643–659. doi: 10.2466/pr0.1997.80.2.643. [DOI] [PubMed] [Google Scholar]
- [5].Prins JB, Bleijenberg G, Bazelmans E, Elving LD, de Boo THM, Severens JL, van der Wilt G, Spinhoven P, van der Meer JW. Cognitive behavior therapy for chronic fatigue syndrome: A multicenter randomized controlled trial. The Lancet. 2001;357:841–847. doi: 10.1016/S0140-6736(00)04198-2. [DOI] [PubMed] [Google Scholar]
- [6].White PD, Goldsmith KA, Johnson AL, Potts L, Walwyn R, DeCesare JC, Baber HL, Burgess M, Clark LV, Cox DL, Bavinton J, Angus BJ, Murphy G, Murphy M, O’Dowd H, Wilks D, McCrone P, Chalter T, Sharpe M, on behalf of the PACE trial management group Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): A randomised trial. The Lancet. 2011;377:823–36. doi: 10.1016/S0140-6736(11)60096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vercoulen JH, Swanink CM, Galama JM, Fennis JF, Jongen PJ, Hommes OR, van der Meer JW, Bleijenberg G. The persistence of fatigue in chronic fatigue syndrome and multiple clerosis: Development of a model. Journal of Psychosomatic Research. 1998;45:507–517. doi: 10.1016/s0022-3999(98)00023-3. [DOI] [PubMed] [Google Scholar]
- [8].Song S, Jason LA. [cited 2012 Aug];A population-based study of chronic fatigue syndrome (CFS) experienced in differing patient groups: An effort to replicate Vercoulen et al.’s model of CFS. Journal of Mental Health [Internet] 2005 14:277–289. Available from: http://www.cfids-cab.org/cfs-inform/Subgroups/song.jason05.pdf. [Google Scholar]
- [9].Price JR, Mitchell E, Tidy E, Hunot V. [cited 2012 Aug];Cognitive behaviour therapy for chronic fatigue syndrome in adults. Cochrane Database of Systematic Reviews [Internet] 2008 Apr;3:1–55. doi: 10.1002/14651858.CD001027.pub2. Available from: http://www.cfids-cab.org/rc/Price.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. [cited 2012 Aug];Action for M.E. Severely neglected: M.E. in the U.K.—membership survey. London Action for M.E. 2001 Mar;2:1–8. Available from: http://www.actionforme.org.uk/Resources/Action%20for%20ME/Documents/get-informed/Severely%20Neglected%202001. [Google Scholar]
- [11].Kindlon T. Reporting of harms associated with graded exercise therapy and cognitive behavioural therapy in myalgic encephalomyelitis/chronic fatigue syndrome. Bulletin of IACFS/ME. 2011;9(2):59–111. [Google Scholar]
- [12]. [cited 2012 Aug];Action for ME and Association of Young People with ME. ME 2008: What progress? 2008 May; Available at: http://www.actionforme.org.uk/Resources/Action%20for%20ME/Documents/get-informed/ME%202008%20%20What%20progress.pdf.
- [13].Jammes Y, Steinberg JG, Mambrini O, Bregeon F, Delliaux S. Chronic fatigue syndrome: Assessment of increased oxidative stress and altered muscle excitability in response to incremental exercise. Journal of Internal Medicine. 2005;257:299–310. doi: 10.1111/j.1365-2796.2005.01452.x. [DOI] [PubMed] [Google Scholar]
- [14].Black CD, O’Connor PJ, McCully KK. Increased daily physical activity and fatigue symptoms in chronic fatigue syndrome. Dynamic Medicine. 2005;4(3) doi: 10.1186/1476-5918-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Camacho J, Jason LA. Psychosocial factors show little relationship to chronic fatigue syndrome recovery. Journal of Psychology and the Behavioral Sciences. 1998;12:60–70. Available on the Intenet: http://view.fdu.edu/default.aspx?id=794. [Google Scholar]
- [16].Jason LA, Porter N, Hunnell J, Rademaker A, Richman JA. CFS prevalence and risk factors over time. Journal of Health Psychology. 2011;16:445–456. doi: 10.1177/1359105310383603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jason LA, Holden JG, Taylor SL, Melrose H. Monitoring energy levels in Chronic Fatigue Syndrome. The Psychological Record. 1995;45:643–654. [Google Scholar]
- [18].Jason LA, Tryon W, Taylor R, King K, Frankenberry E, Jordan K. Monitoring and assessing symptoms of chronic fatigue syndrome: Use of time series regression. Psychological Reports. 1999;85:121–130. doi: 10.2466/pr0.1999.85.1.121. [DOI] [PubMed] [Google Scholar]
- [19].Jason LA, Tryon W, Frankenberry E, King C. Chronic Fatigue Syndrome: Relationships of self-ratings and actigraphy. Psychological Reports. 1997;81:1223–1226. doi: 10.2466/pr0.1997.81.3f.1223. [DOI] [PubMed] [Google Scholar]
- [20].Jason LA, King CP, Frankenberry EL, Jordan KM, Tryon WW, Rademaker F, Huang CF. Chronic fatigue syndrome: Assessing symptoms and activity level. Journal of Clinical Psychology. 1999;55:411–424. doi: 10.1002/(sici)1097-4679(199904)55:4<411::aid-jclp6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- [21].Jason LA, Ferrari JR, Taylor RR, Slavich SP, Stenzel CL. A national assessment of the service, support, and housing preferences by persons with chronic fatigue syndrome: Toward a comprehensive rehabilitation program. Evaluation and the Health Professions. 1996;19:194–207. doi: 10.1177/016327879601900204. [DOI] [PubMed] [Google Scholar]
- [22].Shlaes JL, Jason LA. A buddy/mentor program for people with Chronic Fatigue Syndrome. The CFIDS Chronicle. 1996;9:21–25. [Google Scholar]
- [23].Ferrari JR, Jason LA. Caring for people with chronic fatigue syndrome: Perceived stress versus satisfaction. Rehabilitation Counseling Bulletin. 1997;40(4):240. [Google Scholar]
- [24].King CP, Jason LA, Frankenberry EL, Jordan KM, Tryon W. Think inside the envelope. The CFIDS Chronicle. 1997;10:10–14. [Google Scholar]
- [25].Jason L, Melrose H, Lerman A, Burroughs V, Lewis K, King C, Frankenberry E. Managing chronic fatigue syndrome: Overview and case study. American Association of Occupational Health Nurses Journal. 1999;47:17–21. [PubMed] [Google Scholar]
- [26].Jason LA, Muldowney K, Torres-Harding S. The energy envelope theory and myalgic encephalomyelitis/chronic fatigue syndrome. American Association of Occupational Health Nurses Journal. 2008;56:189–195. doi: 10.3928/08910162-20080501-06. [DOI] [PubMed] [Google Scholar]
- [27].Hayes SC, Luoma J, Bond F, Masuda A, Lillis J. Acceptance and Commitment Therapy: Model, processes, and outcomes. Behaviour Research and Therapy. 2006;44(1):1–25. doi: 10.1016/j.brat.2005.06.006. [DOI] [PubMed] [Google Scholar]
- [28].Hawk C, Jason LA, Torres-Harding S. Reliability of a chronic fatigue syndrome questionnaire. Journal of Chronic Fatigue Syndrome. 2007;13:41–66. [Google Scholar]
- [29].Pesek J, Jason L, Taylor R. An empirical investigation of the envelope theory. Journal of Human Behavior in the Social Environment. 2000;3:59–77. [Google Scholar]
- [30].Taylor RR, Jason LA, Shiraishi Y, Schoeny ME, Keller J. Conservation of resources theory, perceived stress, and chronic fatigue syndrome: Outcomes of a consumer-driven rehabilitation program. Rehabilitation Psychology. 2006;51:157–65. [Google Scholar]
- [31].Jason LA, Roesner N, Porter N, Parenti B, Mortensen J, Till L. Provision of social support to individuals with CFS. Journal of Clinical Psychology. 2010;66:249–258. doi: 10.1002/jclp.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jason LA, Benton M, Torres-Harding S, Muldowney K. The impact of energy modulation on physical functioning and fatigue severity among patients with ME/CFS. Patient Education and Counseling. 2009;77:237–241. doi: 10.1016/j.pec.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Brown M, Khorana N, Jason LA. The role of changes in activity as a function of perceived available and expended energy in nonpharmacological treatment outcomes for ME/CFS. Journal of Clinical Psychology. 2011;3:253–60. doi: 10.1002/jclp.20744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jason LA, Torres-Harding S, Brown M, Sorenson M, Donalek J, Corradi K, Maher K, Fletcher MA. Predictors of change following participation in non-pharmacologic interventions for CFS. Tropical Medicine and Health. 2008;36:23–32. Available on the Internet: http://www.jstage.jst.go.jp/article/tmh/36/1/23/_pdf. [Google Scholar]
- [35].Hanson SJ, Gause W, Natelson B. Detection of immunologically significant factors for chronic fatigue syndrome using neural-network classifiers. Clinical and Diagnostic Laboratory Immunology. 2001;8:658–662. doi: 10.1128/CDLI.8.3.658-662.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Jason LA, Torres-Harding S, Maher K, Reynolds N, Brown M, Sorenson M, Donalek J, Corradi K, Fletcher MA, Lu T. Baseline cortisol levels predict treatment outcomes in chronic fatigue syndrome non-pharmacologic clinical trial. Journal of Chronic Fatigue Syndrome. 2007;14:39–59. [Google Scholar]
- [37].Goudsmit EM. The psychological aspects and management of chronic fatigue syndrome[PhD thesis] Brunel University, UK; London: 1996. Available on the Internet from Ethos: http://ethos.bl.uk/Home.do. [Google Scholar]
- [38].Goudsmit EM, Nijs J, Jason LA, Wallman KE. Pacing as a strategy to improve energy management in myalgic encephalomyelitis/chronic fatigue syndrome: a consensus document. Disability and Rehabilitation. 2011 doi: 10.3109/09638288.2011.635746. [DOI] [PubMed] [Google Scholar]
- [39].Goudsmit EM, Howes S. Pacing: A strategy to improve energy management in chronic fatigue syndrome. Health Psychology Update. 2008;17(1):46–52. [Google Scholar]
- [40].Goudsmit EM, Ho-Yen DO, Dancey CP. Learning to cope with chronic illness. Efficacy of a multi-component treatment for people with chronic fatigue syndrome. Patient Education and Counseling. 2009;77:231–36. doi: 10.1016/j.pec.2009.05.015. [DOI] [PubMed] [Google Scholar]
- [41].Wallman KE, Morton AR, Goodman C, Grove R, Guilfoyle AM. Randomised controlled trial of graded exercise in chronic fatigue syndrome. Medical Journal of Australia. 2004;180:444–448. doi: 10.5694/j.1326-5377.2004.tb06019.x. [DOI] [PubMed] [Google Scholar]
- [42].Nijs J, Paul L, Wallman K. Chronic fatigue syndrome: an approach combining self-management with graded exercise to avoid exacerbations. Journal of Rehabilitation Medicine. 2008;40:241–247. doi: 10.2340/16501977-0185. [DOI] [PubMed] [Google Scholar]
- [43].Davenport TE, Stevens SR, Van Ness MJ, Snell CR, Little T. Conceptual model for physical therapist management of Chronic Fatigue Syndrome/Myalgic Encephalomyelitis. Physical Therapy. 2010;90:1–13. doi: 10.2522/ptj.20090047. [DOI] [PubMed] [Google Scholar]
- [44].Campbell B. The Patient’s Guide to Chronic Fatigue Syndrome and Fibromyalgia. 2011 Available at: http://www.cfidsselfhelp.org/library/the-patients-guide-chronic-fatigue-syndrome-fibromyalgia. [Google Scholar]
- [45].Cox D, Ludlam S, Mason L, Wagner S, Sharpe M. Adaptive pacing therapy (APT) for CFS/ME. Manual for therapists. [Internet] 2004 Available from: http://www.pacetrial.org/docs/apt-therapist-manual.pdf.
- [46].Van Cauwenbergh D, De Kooning M, Ickmans K, Nijs J. How to exercise people with chronic fatigue syndrome: evidence-based practice guidelines. European Journal of Clinical Investigation. 2012 doi: 10.1111/j.1365-2362.2012.02701.x. Forthcoming. [DOI] [PubMed] [Google Scholar]
- [47].Jason LA, Sorenson M, Porter N, Belkairous N. An etiological model for Myalgic Encephalomyelitis/chronic fatigue syndrome. Neuroscience & Medicine. 2011;2:14–27. doi: 10.4236/nm.2011.21003. Available on the Internet: http://www.scirp.org/journal/nm/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gupta A. Unconscious amygdalar fear conditioning in a subset of chronic fatigue syndrome patients. Medical Hypotheses. 2002;59:727–735. doi: 10.1016/s0306-9877(02)00321-3. [DOI] [PubMed] [Google Scholar]
- [49].Arnsten A FT. Stress signaling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Baram TZ, Hatalski C G. Neuropeptide-mediated excitability: A key triggering mechanism for seizure generation in the developing brain. Trends in Neuroscience. 1998;21:471–476. doi: 10.1016/s0166-2236(98)01275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Cook DB, O’Connor PJ, Lange G, Steffener J. Functional neuroimaging correlates of mental fatigue induced by cognition among chronic fatigue syndrome patients and controls. NeuroImage. 2007;36(1):108–122. doi: 10.1016/j.neuroimage.2007.02.033. [DOI] [PubMed] [Google Scholar]
- [52].Jason LA, Sorenson M, Evans M, Brown A, Flores S, Sunnquist M, Schafer C. The implications of sensitization and kindling for chronic fatigue syndrome. In: Gotsiridze-Columbus N, editor. Encephalopathies: Symptoms, causes and potential complications. Nova Science; New York: Forthcoming. [Google Scholar]
- [53].Kaufman MP, Hayes SG. The exercise pressor reflex. Clinical Autonomic Research. 2002;12:429–439. doi: 10.1007/s10286-002-0059-1. [DOI] [PubMed] [Google Scholar]