Abstract
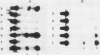
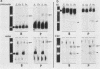
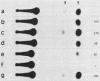
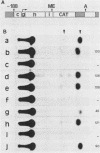
The putative promoter of the variant surface glycoprotein (VSG) gene of Trypanosoma brucei was cloned into a plasmid containing the chloramphenicol acetyltransferase (CAT) gene. After electroporation into trypanosomes, this construct directed the expression of the CAT reporter gene. The essential region for promoter activity was found to reside within 88 bp upstream of the putative transcription start site. Transcription of the CAT construct occurred at approximately the same level in both bloodstream and procyclic forms and was resistant to alpha-amanitin. However, CAT expression appeared to be modulated in the two forms of the parasite. Sequences 3' to the gene seemed to be important in this respect, as CAT activity in bloodstream forms was readily detectable only when the 3' region of a VSG cDNA was placed downstream of the CAT gene. Two separate VSG gene promoter sequences, both cloned from T. brucei AnTat 1.3A, were equally able to direct CAT expression, which suggests that there are a number of potential VSG gene promoters in the genome, although usually only one expression site is fully active at any one time.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aline R. F., Jr, Stuart K. Trypanosoma brucei: conserved sequence organization 3' to telomeric variant surface glycoprotein genes. Exp Parasitol. 1989 Jan;68(1):57–66. doi: 10.1016/0014-4894(89)90008-8. [DOI] [PubMed] [Google Scholar]
- Baltz T., Baltz D., Giroud C., Crockett J. Cultivation in a semi-defined medium of animal infective forms of Trypanosoma brucei, T. equiperdum, T. evansi, T. rhodesiense and T. gambiense. EMBO J. 1985 May;4(5):1273–1277. doi: 10.1002/j.1460-2075.1985.tb03772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellofatto V., Cross G. A. Expression of a bacterial gene in a trypanosomatid protozoan. Science. 1989 Jun 9;244(4909):1167–1169. doi: 10.1126/science.2499047. [DOI] [PubMed] [Google Scholar]
- Ben Amar M. F., Pays A., Tebabi P., Dero B., Seebeck T., Steinert M., Pays E. Structure and transcription of the actin gene of Trypanosoma brucei. Mol Cell Biol. 1988 May;8(5):2166–2176. doi: 10.1128/mcb.8.5.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P. Discontinuous transcription and antigenic variation in trypanosomes. Annu Rev Biochem. 1986;55:701–732. doi: 10.1146/annurev.bi.55.070186.003413. [DOI] [PubMed] [Google Scholar]
- Brun R., Schönenberger Cultivation and in vitro cloning or procyclic culture forms of Trypanosoma brucei in a semi-defined medium. Short communication. Acta Trop. 1979 Sep;36(3):289–292. [PubMed] [Google Scholar]
- Clayton C. E., Fueri J. P., Itzhaki J. E., Bellofatto V., Sherman D. R., Wisdom G. S., Vijayasarathy S., Mowatt M. R. Transcription of the procyclic acidic repetitive protein genes of Trypanosoma brucei. Mol Cell Biol. 1990 Jun;10(6):3036–3047. doi: 10.1128/mcb.10.6.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coquelet H., Tebabi P., Pays A., Steinert M., Pays E. Trypanosoma brucei: enrichment by UV of intergenic transcripts from the variable surface glycoprotein gene expression site. Mol Cell Biol. 1989 Sep;9(9):4022–4025. doi: 10.1128/mcb.9.9.4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cully D. F., Ip H. S., Cross G. A. Coordinate transcription of variant surface glycoprotein genes and an expression site associated gene family in Trypanosoma brucei. Cell. 1985 Aug;42(1):173–182. doi: 10.1016/s0092-8674(85)80113-6. [DOI] [PubMed] [Google Scholar]
- Cunningham I. New culture medium for maintenance of tsetse tissues and growth of trypanosomatids. J Protozool. 1977 May;24(2):325–329. doi: 10.1111/j.1550-7408.1977.tb00987.x. [DOI] [PubMed] [Google Scholar]
- De Lange T., Borst P. Genomic environment of the expression-linked extra copies of genes for surface antigens of Trypanosoma brucei resembles the end of a chromosome. Nature. 1982 Sep 30;299(5882):451–453. doi: 10.1038/299451a0. [DOI] [PubMed] [Google Scholar]
- Ehlers B., Czichos J., Overath P. RNA turnover in Trypanosoma brucei. Mol Cell Biol. 1987 Mar;7(3):1242–1249. doi: 10.1128/mcb.7.3.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frebourg T., Brison O. Plasmid vectors with multiple cloning sites and cat-reporter gene for promoter cloning and analysis in animal cells. Gene. 1988 May 30;65(2):315–318. doi: 10.1016/0378-1119(88)90468-4. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Imboden M. A., Laird P. W., Affolter M., Seebeck T. Transcription of the intergenic regions of the tubulin gene cluster of Trypanosoma brucei: evidence for a polycistronic transcription unit in a eukaryote. Nucleic Acids Res. 1987 Sep 25;15(18):7357–7368. doi: 10.1093/nar/15.18.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. J., Standart N. Do the poly(A) tail and 3' untranslated region control mRNA translation? Cell. 1990 Jul 13;62(1):15–24. doi: 10.1016/0092-8674(90)90235-7. [DOI] [PubMed] [Google Scholar]
- Johnson P. J., Kooter J. M., Borst P. Inactivation of transcription by UV irradiation of T. brucei provides evidence for a multicistronic transcription unit including a VSG gene. Cell. 1987 Oct 23;51(2):273–281. doi: 10.1016/0092-8674(87)90154-1. [DOI] [PubMed] [Google Scholar]
- Kooter J. M., Borst P. Alpha-amanitin-insensitive transcription of variant surface glycoprotein genes provides further evidence for discontinuous transcription in trypanosomes. Nucleic Acids Res. 1984 Dec 21;12(24):9457–9472. doi: 10.1093/nar/12.24.9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooter J. M., van der Spek H. J., Wagter R., d'Oliveira C. E., van der Hoeven F., Johnson P. J., Borst P. The anatomy and transcription of a telomeric expression site for variant-specific surface antigens in T. brucei. Cell. 1987 Oct 23;51(2):261–272. doi: 10.1016/0092-8674(87)90153-x. [DOI] [PubMed] [Google Scholar]
- König E., Delius H., Carrington M., Williams R. O., Roditi I. Duplication and transcription of procyclin genes in Trypanosoma brucei. Nucleic Acids Res. 1989 Nov 11;17(21):8727–8739. doi: 10.1093/nar/17.21.8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanham S. M., Godfrey D. G. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol. 1970 Dec;28(3):521–534. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- Muhich M. L., Boothroyd J. C. Polycistronic transcripts in trypanosomes and their accumulation during heat shock: evidence for a precursor role in mRNA synthesis. Mol Cell Biol. 1988 Sep;8(9):3837–3846. doi: 10.1128/mcb.8.9.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy N. B., Pays A., Tebabi P., Coquelet H., Guyaux M., Steinert M., Pays E. Trypanosoma brucei repeated element with unusual structural and transcriptional properties. J Mol Biol. 1987 Jun 20;195(4):855–871. doi: 10.1016/0022-2836(87)90490-6. [DOI] [PubMed] [Google Scholar]
- Murphy W. J., Watkins K. P., Agabian N. Identification of a novel Y branch structure as an intermediate in trypanosome mRNA processing: evidence for trans splicing. Cell. 1986 Nov 21;47(4):517–525. doi: 10.1016/0092-8674(86)90616-1. [DOI] [PubMed] [Google Scholar]
- Pays E., Coquelet H., Pays A., Tebabi P., Steinert M. Trypanosoma brucei: posttranscriptional control of the variable surface glycoprotein gene expression site. Mol Cell Biol. 1989 Sep;9(9):4018–4021. doi: 10.1128/mcb.9.9.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pays E., Coquelet H., Tebabi P., Pays A., Jefferies D., Steinert M., Koenig E., Williams R. O., Roditi I. Trypanosoma brucei: constitutive activity of the VSG and procyclin gene promoters. EMBO J. 1990 Oct;9(10):3145–3151. doi: 10.1002/j.1460-2075.1990.tb07512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pays E. Pseudogenes, chimaeric genes and the timing of antigen variation in African trypanosomes. Trends Genet. 1989 Dec;5(12):389–391. doi: 10.1016/0168-9525(89)90181-9. [DOI] [PubMed] [Google Scholar]
- Pays E., Steinert M. Control of antigen gene expression in African trypanosomes. Annu Rev Genet. 1988;22:107–126. doi: 10.1146/annurev.ge.22.120188.000543. [DOI] [PubMed] [Google Scholar]
- Pays E., Tebabi P., Pays A., Coquelet H., Revelard P., Salmon D., Steinert M. The genes and transcripts of an antigen gene expression site from T. brucei. Cell. 1989 Jun 2;57(5):835–845. doi: 10.1016/0092-8674(89)90798-8. [DOI] [PubMed] [Google Scholar]
- Pays E., Van Assel S., Laurent M., Darville M., Vervoort T., Van Meirvenne N., Steinert M. Gene conversion as a mechanism for antigenic variation in trypanosomes. Cell. 1983 Sep;34(2):371–381. doi: 10.1016/0092-8674(83)90371-9. [DOI] [PubMed] [Google Scholar]
- Roditi I., Carrington M., Turner M. Expression of a polypeptide containing a dipeptide repeat is confined to the insect stage of Trypanosoma brucei. Nature. 1987 Jan 15;325(6101):272–274. doi: 10.1038/325272a0. [DOI] [PubMed] [Google Scholar]
- Roditi I., Schwarz H., Pearson T. W., Beecroft R. P., Liu M. K., Richardson J. P., Bühring H. J., Pleiss J., Bülow R., Williams R. O. Procyclin gene expression and loss of the variant surface glycoprotein during differentiation of Trypanosoma brucei. J Cell Biol. 1989 Feb;108(2):737–746. doi: 10.1083/jcb.108.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenko G., Le Blancq S., Smith J., Lee M. G., Rattray A., Van der Ploeg L. H. Procyclic acidic repetitive protein (PARP) genes located in an unusually small alpha-amanitin-resistant transcription unit: PARP promoter activity assayed by transient DNA transfection of Trypanosoma brucei. Mol Cell Biol. 1990 Jul;10(7):3492–3504. doi: 10.1128/mcb.10.7.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed B., Sheen J. Y. A simple phase-extraction assay for chloramphenicol acyltransferase activity. Gene. 1988 Jul 30;67(2):271–277. doi: 10.1016/0378-1119(88)90403-9. [DOI] [PubMed] [Google Scholar]
- Shapiro D. J., Blume J. E., Nielsen D. A. Regulation of messenger RNA stability in eukaryotic cells. Bioessays. 1987 May;6(5):221–226. doi: 10.1002/bies.950060507. [DOI] [PubMed] [Google Scholar]
- Sutton R. E., Boothroyd J. C. Evidence for trans splicing in trypanosomes. Cell. 1986 Nov 21;47(4):527–535. doi: 10.1016/0092-8674(86)90617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschudi C., Ullu E. Polygene transcripts are precursors to calmodulin mRNAs in trypanosomes. EMBO J. 1988 Feb;7(2):455–463. doi: 10.1002/j.1460-2075.1988.tb02833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Valerio D., De Lange T., Bernards A., Borst P., Grosveld F. G. An analysis of cosmid clones of nuclear DNA from Trypanosoma brucei shows that the genes for variant surface glycoproteins are clustered in the genome. Nucleic Acids Res. 1982 Oct 11;10(19):5905–5923. doi: 10.1093/nar/10.19.5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T. C., Rudenko G., Borst P. Three small RNAs within the 10 kb trypanosome rRNA transcription unit are analogous to domain VII of other eukaryotic 28S rRNAs. Nucleic Acids Res. 1986 Dec 9;14(23):9471–9489. doi: 10.1093/nar/14.23.9471. [DOI] [PMC free article] [PubMed] [Google Scholar]