Abstract
Autosomal dominant overhydrated cation-leak stomatocytosis in humans has been associated with missense mutations in the erythroid membrane transport genes AE1, RhAG, and GLUT1. Syndromic stomatocytosis has been reported in three dog breeds, but stomatocytosis in Standard Schnauzers is usually asymptomatic, and is accompanied by minimal if any anemia. We have extended the evaluation of a cohort of schnauzers. We found that low-level stomatocytosis was accompanied by increased MCV and increased red cell Na content, and minimal or no reticulocytosis. Red cells from two affected dogs exhibited increased currents in on-cell patches measured in symmetrical NaCl solutions, but Na,K-ATPase and NKCC-mediated cation flux was minimal. Three novel coding polymorphisms found in canine RhAG cDNA and three novel polymorphisms found in canine SLC4A1 cDNA did not cosegregate with MCV or Na content. The GLUT1 cDNA sequence was normal. We conclude that unlike human overhydrated cation-leak stomatocytosis, stomatocytosis in this cohort of Standard Schnauzers is not caused by mutations in the genes encoding RhAG, SLC4A1, or GLUT1.
Keywords: Schnauzer, Stomatocytosis, RhAG, AE1, GLUT1, Erythrocytes
Introduction
Cation-leak stomatocytosis in humans is an autosomal dominant disease of mild-to-moderate hemolytic anemia characterized by elevated red cell Na content associated with diminished K content secondary to increased red cell cation permeability exhibiting a variety of temperature dependence patterns [1–3]. Overhydrated cation stomatocytosis is marked by macrocytosis, and has been associated with missense mutations in the genes encoding the erythroid chloride/bicarbonate exchanger SLC4A1/AE1/Band 3 [4,5], the erythroid ammonia channel RhAG [6,7], and the erythroid glucose transporter SLC2A1/GLUT1 [8,9]. The GLUT1 mutation-associated stomatocytosis syndromes are associated with neuromuscular hyperexcitability.
All stomatocytosis mutations reported to date preserve normal or near-normal polypeptide abundance at the cell surface. The mutant polyeptides have generally exhibited severe loss-of-function phenotypes for transport of physiological solutes. However, all three groups of mutants are associated with increased cation channel activity in the red cell membrane and/or in the surface membrane of Xenopus oocytes overexpressing the heterologous mutant proteins. These phenotypically similar cation channel activities were originally attributed to structurally altered permeation pathways through each of the three, dissimilar stomatocytosis gene products. However, data from Xenopus oocytes [5,7] and from intact patient red cells [10,11] remains consistent with the possibility that all three types of mutants serve to activate one or more additional, as yet undefined cation channel pathway(s) endogenous to red cells and to Xenopus oocytes.
Human red cells express Na,K-ATPase and maintain elevated intracellular K+ content and low intracellular Na+ content throughout erythroid development. Red cells of pig, horse, deer, and rodents exhibit red cell Na and K contents similar to those of humans [12]. In contrast, mature dog red cells of most dog strains exhibit no detectable Na+, K+-ATPase activity, accompanied by elevated intracellular Na+ content and low intracellular K+ content [13]. The ion composition of dog red cells is highly dependent on cell volume. Cell volume perturbations lead to activation of previously quiescent ion transport systems [14], including swelling-activated Na+/Ca2+ exchange and furosemide-sensitive K–Cl cotransport [15] and shrinkage-activated, amiloride-sensitive Na+/H+ exchange [16]. These dog red cell volume-regulatory pathways are highly pH- and Ca2+-sensitive, and appeared to be coupled functionally to membrane receptors [15]. High Na–low K red cells are also found in cats, sheep, and cows, but red cells of camels and goats exhibit intermediate levels of intracellular Na and K [12].
We hypothesized that cation-leak stomatocytosis in high Na cells might arise from mutations known to be associated with the disease in low Na human red cells. Stomatocytosis in dogs has been reported in association with chondrodysplasia in Alaskan Malamutes [17], and in Drentse Patrijshonds associated with growth retardation, hypertrophic gastritis, pelvic limb weakness, diarrhea, polyuria/polydipsia, and central nervous system anomalies [18]. The non-syndromic stomatocytosis in Standard Schnauzers [19–21] is characterized by mild or no anemia, erythroid macrocytosis accompanied by elevations of intracellular [Na+] beyond even the normally high values, increased red cell osmotic fragility, elevated levels of 2,3-diphosphoglycerate, and normal red cell stomatin abundance, without grossly apparent abnormality of the Coomassie Blue protein profile on SDS-PAGE.
We tested AE1, RhAG, and (with lower probability) GLUT1 as candidate genes responsible for stomatocytosis in Standard Schnauzers, and initiated functional characterization of the cation transport abnormality suggested by the elevated intracellular [Na+].
Materials and methods
Clinical subjects
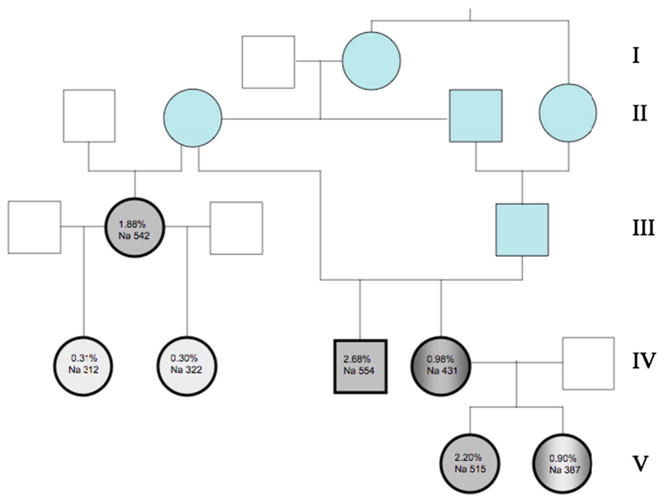
Five Standard Schnauzers between 2 and 13 years of age and from a single breeder were sampled in March, 2011. The same five dogs and two female puppies 45 days of age (born to one of the five adults), were sampled in May, 2011. The pedigree is presented in Fig. 1. Medical histories and complete physical exams indicated each animal to be in good health, without clinical signs of anemia or other disease. Blood samples (~5.0 mL) were collected from the external jugular vein into sterile plastic syringes and immediately transferred into tubes containing EDTA or lithium heparin. In addition, two blood smears were prepared from the tip of the needle.
Fig. 1.
Pedigree of Standard Schnauzers studied. Generations are listed at right. Individuals in blue, as well as dogs III.2 and IV.3, were previously reported [20]. Individuals studied in the current work are shown in gray with bold outline. Unaffected dogs (IV.1 and IV.2) are in light gray, affected dogs (III.2, IV.3, V.1) are in dark gray, and dogs with intermediate phenotypes (IV.4 and V.2) are in gray gradient. Shown are values for % stomatocytes and red cell Na content (mmol/kg Hb).
Blood samples and hematological indices
Blood was aliquoted, shipped at 4 °C to the laboratories in Milan and Boston, and refrigerated until use. Upon receipt of EDTA-treated blood in Milan, erythrocyte and reticulocyte counts and hematological indices were determined using an automated laser counter (Sysmex XT-2000iV, Kobe, Japan) by a method already validated for dog erythrocytes [22,23]. Heparinized blood was shipped to Boston, and 24–30 h after blood sampling, hematological indices were evaluated with the Advia 120 autoanalyzer, using multispecies software 1.1.07-MS) as calibrated for dog erythrocytes tested within 1–4 h [24] or after up to 48 h storage at 4 °C or 24 °C [25]. Direct comparison of MCV values recorded with the two instruments on 55 fresh canine blood samples in Milan showed no significant difference (p=0.61 by Wilcoxon rank-order test). Values from the two instruments were highly correlated (r2=0.84) without constant or proportional errors (Passing and Bablok regression slope 0.94 with 95% confidence interval 0.84–1.09; intercept 3.51 with confidence interval −6.05–10.43)
Blood smears were stained with May Grünwald-Giemsa in Milan, and microscopically examined for differential leukocyte count and percentage stomatocytes. 20 visual microscopic fields at 400× magnification were photographed (CoolSNAPT-Pro cf, Media Cybernetics, Silver Spring, MD) for image analysis (Image Pro-Plus 6.3, Media Cybernetics). The sum of erythrocytes and of stomatocytes recorded for each dog in the 20 visual fields (8093±91 (s.d.)) was used to calculate the % of stomatocytes.
Intracellular ion measurement
Intracellular ion contents of shipped blood were measured as previously described [26,27]. EDTA-washed blood was passed through cotton to preadsorb leukocytes and pelleted 4 min at 2500 rpm at 4 °C (Sorvall RC28S). Red cell pellets were washed four times with ice-cold Mg2+-free choline wash solution (CWS) containing (in mM) 150 choline Cl, 20 sucrose, and 10 Tris MOPS [3-(N-morpholino)propanesulfonic acid], pH 7.4 (4 °C). Manual spin hematocrits were measured on 50% (v/v) cell suspensions in CWS. Aliquots of these suspensions were diluted with 0.02% Acationox in double-distilled water for measurement of intracellular Na, K, and Mg contents by atomic absorption spectrometry (PerkinElmer 800, Wellesley, MA).
Red cell ion flux measurements
Red cell Na-K pump activity measured in 155 mM choline Cl and 10 mM KCl was estimated as the fraction of Na+ efflux sensitive to 0.1 mM ouabain. Red cell Na+–K+–2Cl− cotransport activity measured in 154 mM choline Cl and 0.1 mM ouabain was estimated as the fraction of Na+ and K+ efflux sensitive to 10 μM bumetanide. Cation leak flux was defined as Na+ and K+ efflux in the presence of 0.1 mM ouabain and 10 μM bumetanide. All determinations were performed in triplicate.
On-cell patch recording on erythrocytes
Washed canine red cells were resuspended in a solution containing (in mM) 140 NaCl, 4 KCl, 1 MgCl2, 1 CaCl2, and 10 HEPES, pH 7.4, then kept at 4 °C until used. Red cells allowed to settle on coverslips were mounted on an inverted microsope in a 200 μl open chamber (WPI, Sarasota, FL) and superfused 15 min at room temperature. Borosilicate pipettes (Corning 8250) pulled with a Sutter P97 puller and fire-polished to resistances of 10–20 MΩ were front-filled and then back-filled. Symmetric bath and pipette solutions contained (in mM) 140 NaCl, 4 KCl, 1 MgCl2, 1 CaCl2, 10 Na HEPES, pH 7.4. On-cell patch currents were recorded at room temperature with the Axopatch 1-D amplifier (Axon Instruments/Molecular Devices, Sunnyvale, CA) as previously described. Holding potential was −Vp=−50 mV (expressed as the negative of the pipette potential (e.g., equivalent to the intracellular potential with respect to the pipette). To determine current–voltage relationships (I-V curves) in Clampex (PCLAMP 10, Axon Instruments), the realtime control window in gap-free mode was used to record current traces of 10–30 s duration at holding potentials ranging from −100 to +100 mV, in 25 mV increments. The bath reference electrode was a silver chlorided wire with a 3 M KCl agar bridge. Data was filtered at 500 Hz, digitized at 2 kHz by Clampex, and analyzed online by Clampfit subroutines of PCLAMP. Holding potentials were expressed as −Vp, the negative of the pipette potential.
Other serum chemistry values
To check the general health of the dogs, serum was isolated by centrifugation of unheparinized, clotted blood, and spectrophotometric chemical tests (Cobas Mira, Roche Diagnostic) with reagents from Real Time Diagnostic S.r.l. included measurements of serum electrolytes, urea (urease method), cholesterol (CHOD-PAP method), creatinine (Jaffe method), total proteins (biuret method), glucose (GOD-POD method, ALT, AST, and alkaline phosphatase (IFCC method at 37 °C). Serum concentrations of Na+, K+, and Cl− were measured with Ion selective electrodes (ISE) using an automated biochemistry analyzer (ILAB300plus, International Laboratory).
DNA sequencing
Total RNA was isolated from whole blood of affected and unaffected dogs, using the Rneasy Mini Kit (Qiagen). Total RNA from beagle spleen (Zyagen, San Diego, CA) served as a non-Schnauzer control sample. RNA samples were quantitated spectroscopically (Nanodrop 1000, Thermo Scientific), and cDNA was prepared using the Retroscript kit (Ambion). cDNAs encoding AE1/SLC4A1 (33 cycles), RhAG (33 cycles) were PCR-amplified (HotStarTaq, Qiagen) as overlapping fragments, gel-purified, and sequenced employing strategies similar to those previously described [5,7] for the human orthologs. cDNA encoding GLUT1/SLC2A1 was readily amplified from control dog spleen cDNA (33 cycles), but amplification from whole blood cDNA of Schnauzers required 38 cycles of PCR to visualize amplimer on EtBr agarose gel. Amplification for sequencing required a second round of nested or semi-nested PCR (25 cycles) using gel-purified first round product. Oligonucleotides for all cDNAs are listed in Supplemental Tables 1–3. All full length open reading frames were assembled and compared to the NCBI consensus cDNA sequences for AE1 (NM_001048031), for RhAG (N_001110768), and GLUT1 (NM_001159326).
Results
Hematological indices and ion content
The seven dogs studied from the Standard Schnauzer pedigree presented in Fig. 1 are marked by the heavy black outline. As noted previously [20,21], all dogs were grossly normal, without anemia, leukocytosis/leukopenia or thrombocytosis/thrombocytopenia. Stomatocyte counts ranged between 0.9% and 2.68%. All dogs except one had reticulocyte counts within the reference intervals (Table 1). The infrequent borderline reticulocytosis corresponded to the variable, modest reticulocytosis observed previously [20]. Normal dog red cell Na content is high, reflecting the absence of Na,K-ATPase from mature circulating red cells [13,28]. Four of the five dogs with increased stomatocytes had further elevated red cell Na content, and one of the five had an intermediate red cell Na content (Table 2). All five also exhibited elevated red cell K content (Table 2). These five dogs were considered “affected” or of “intermediate” phenotype. Two of the dogs studied had stomatocyte counts of ~0.3% with normal Na content, and were considered “unaffected”. Affected dogs showed elevated values of MCV with reduced CHCM, but little if any elevation of reticulocyte count (Table 1). All dogs showed normal serum values for [Na+], [K+], [Mg2+] (Table 2), [Cl−] urea, creatinine, glucose, total protein, and liver enzyme activities (ALT, AST, Alkaline phosphatase; not shown).
Table 1.
Hematological indices.
| Stomato (%) (n=1) | Hct (%) | MCV (fL) | MCHC CHCM (g/dL) | RDW (%) | HDW (%) | Retics (×109/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresh Smx (n=2) | Ship Advia (n=1) | Fresh Smx (n=2) | Ship Advia (n=2) | Fresh Smx (n=2) | Ship Advia (n=2) | Fresh Smx (n=2) | Ship Advia (n=2) | Ship Advia (n=2) | Fresh Smx (n=2) | Ship Advia (n=1) | ||
| Normal range | 0.0–0.5 | 35–52 | 42–62 | 60–71 | 63–75 | 34–38 | 30–33 | 13–19 | 12–13 | 1.9 + 0.3* | 19.4 – 150.1 | 10.9 – 111 |
| III.2 | 1.88 | 50.4 | 46.5 | 90.6 | 87.9 | 25.2 | 24.9 | 16.7 | 12.0 | 2.64 | 79.8 | 113.7 |
| IV.1 | 0.31 | 46.1 | 46.7 | 67.3 | 66.1 | 35.0 | 33.7 | 16.3 | 14.5 | 3.44 | 54.3 | 74.3 |
| IV.2 | 0.30 | 48.8 | 48.9 | 65.4 | 66.2 | 34.1 | 33.2 | 15.7 | 14.0 | 3.33 | 43.4 | 59.8 |
| IV.3 | 2.68 | 47.2 | 41.1 | 103.3 | 94.8 | 23.9 | 23.6 | 18.9 | 12.4 | 2.95 | 59.7 | 50.1 |
| IV.4 | 0.98 | 44.5 | 39.7 | 94.3 | 92.1 | 25.8 | 24.1 | 16.5 | 12.6 | 2.71 | 62.1 | 80.5 |
| V.1 (n=1) | 2.20 | NA | NA | 86.3 | 87.9 | 26.3 | 24.1 | 14.6 | NA | 3.03 | 91.8 | NA |
| V.2 (n=1) | 0.90 | NA | NA | 72.2 | 76.1 | 31.7 | 29.0 | 14.7 | NA | 3.69 | 115.8 | NA |
Data are from affected (unshaded rows) and unaffected dogs (gray rows). % stomatocytes was calculated from manual counts of 8093±91 cells (mean±s.d) in 20 digitally imaged blood smear visual fields for each individual. Fresh, blood assayed fresh in Milan; Ship, blood assayed after overnight shipment to Boston. Smx: Sysmex XT-2000iV; Advia: Bayer Advia 120. Analyses were performed on blood sampled on one (n=1) or two separate days (n=2). Only single samples from dogs V.1 and V.2 were available for analysis. Reference values for Smx [23] and Advia analysis [24] are for fresh blood, but 24–36 h cold storage yields Advia values within the same range [25].
, HDW reference value is for cold storage. Hct, hematocrit; MCV, mean corpusclar volume; CHCM, corpuscular hemoglobin concentration mean; RDW, red cell distribution width; HDW, hemoglobin distribution width; NA, not available.
Table 2.
Red cell ion content, concentration, and efflux.
| Na (mmol/kg Hb) | K (mmol/kg Hb) | Mg (mmol/kg Hb) | s[Na+] (mM) | s[K+] (mM) | s[Mg2+] (mg/dL) | Ouab.-sens Na efflux | Bumet.-sens Na efflux | |
|---|---|---|---|---|---|---|---|---|
| (mmol/1013cellsxhr) | (mmol/1013cellsxhr) | |||||||
| III.2 | 542 | 24.8 | 10.2 | 144 | 5.0 | 1.9 | 0.11 | 0.00 |
| IV.1 | 312 | 16.9 | 6.8 | 144 | 4.6 | 2.5 | 0.00* | 0.00* |
| IV.2 | 322 | 13.3 | 6.7 | 143 | 4.0 | 2.2 | 0.00* | 0.00* |
| IV.3 | 554 | 23.5 | 9.0 | 141 | 4.5 | 2.3 | 0.00 | 0.14 |
| IV.4 | 431 | 18.9 | 8.0 | 144 | 4.2 | 1.9 | 0.29 | 0.32 |
| V.1 | 515 | 33.3 | 10.2 | 139 | 5.2 | NA | 0.00* | 0.00* |
| V.2 | 387 | 28.3 | 8.7 | 145 | 5.4 | NA | NA | NA |
Cell contents of Na, K, and Mg were determined by atomic absorption on cells shipped overnight to Boston. Serum electrolytes (s [Na+] and s[K+]) were measured by ion selective electrodes in serum prepared from fresh blood (see Methods). Values of cell ion content and serum electrolyte concentrations are means of two triplicate determinations except for V.1 and V.2 (n=1). For Na efflux values without *, n=2 triplicate determinations; with *, n=1 triplicate determination.
NA, not available. Data from unaffected dogs is shaded.
Red cell cation transport
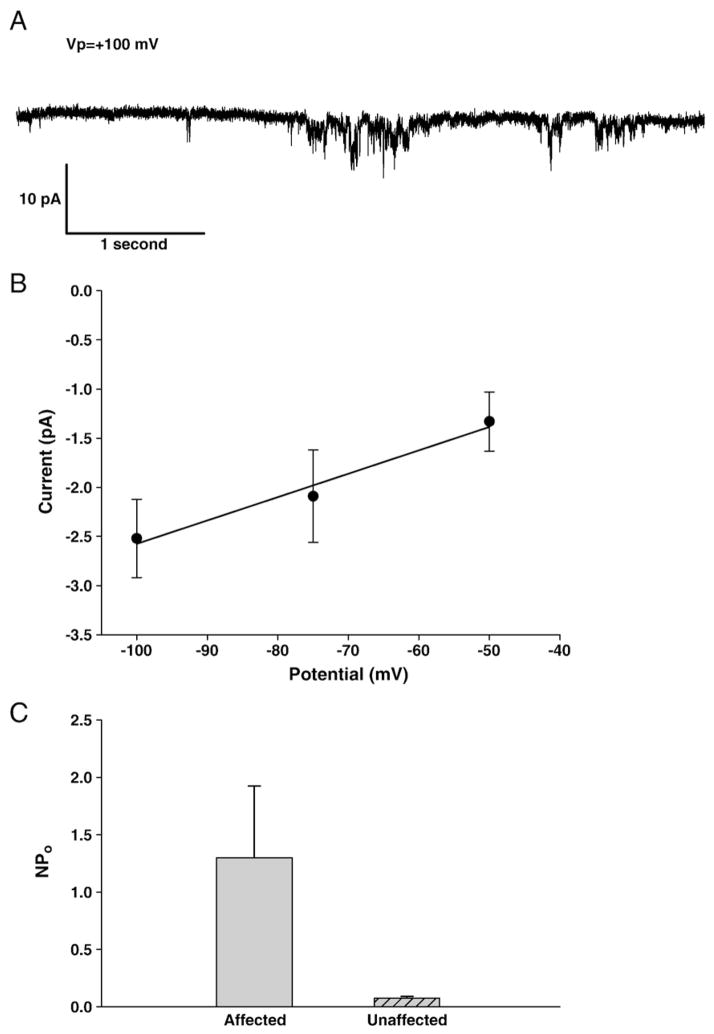
As shown in Fig. 2, on-cell patch records of red cells from affected dogs exhibited increased channel activity. A representative recording shown in Fig. 2A revealed a linear voltage dependence across the limited intracellular-negative voltage range sampled during the lifetime of this gigaseal patch, with an apparent conductance of 24 pS. Mean nPo of this channel activity in red cell membrane patches from two affected dogs was 1.30 (n=6 patches) and 0.08 in one of the unaffected dogs (n=3 patches; p=0.024, Mann–Whitney rank-order test).
Fig. 2.
Ion currents associated with stomatocytosis. A. Representative current trace from on-cell patch of erythrocyte from affected dog III.2, recorded at −Vp=−100 mV. Symmetrical bath and pipet solutions contained (in mM) 145 NaCl, 4 KCl, 1 MgCl2, 1 CaCl2, 10 Na Hepes, pH 7.40. B. Limited current–voltage relationship of cell shown in panel A. C. NPo of currents recorded from red cells of unaffected (IV.1) and affected dogs (III.2 and IV.4).
Replica measurements of net cation efflux from dog red cells confirmed minimal Na-K pump activity (0–0.6 mmol/1013 cells×h), defined as 0.1 mM ouabain-sensitive Na efflux, in affected (n=4) and unaffected dogs (n=2). Na+–K+–2Cl− cotransport activity, measured in red cells from the same dogs as Na and K effluxes sensitive to 10 μM bumetanide, was similarly low (0–0.6 mmol Na/1013 cells×h and 0–0.1 mmol K/1013 cells×h). Basal Na efflux measured in the absence of inhibitors was 3.0–6.2 mmol/1013 cells×h, whereas basal K efflux was 0.1–1.1 mmol/1013 cells×h in red cells of the same 6 dogs. Thus, even in the setting of red cell Na content higher than the normally elevated levels of dog red cells, stomatocytic Standard Schnauzer red cells fail to attenuate or delay the proteolytic degradation of Na, K-ATPase activity at the reticulocyte stage observed in normal dog red cells [29].
DNA sequence analysis of candidate genes for hereditary stomatocytosis
Based on hereditary stomatocytosis in humans, three genes were evaluated as candidate genes for hereditary stomatocytosis in Standard Schauzers: AE1/SLC4A1, RhAG, and GLUT1. In two affected dogs (III.2 and V.1) the cDNA sequence for AE1 was indistinguishable from NCBI RefSeq NM_001048031. In one unaffected dog (V.2), three novel heterozygous single nucleotide polymorphisms were present. One was heterozygous coding polymorphism a77g (D5G), which lies within the 7 N-terminal amino acids of dog AE1 that are not present in the AE1 polypeptide of human or mouse. Two silent heterozygous polymorphisms were also present: a1107g (V348V) and g1161a (P386).
The cDNA sequence of Schnauzer RhAG revealed three coding polymorphisms compared to NCBI RefSeq XM_539554.2: M220I (dogs IV.3, IV.4, V.1, and V.2), Y255F (all dogs except IV.2 and V.1), and R312Q, (all dogs except IV.2 and V.1). These polymorphisms are shown in Fig. 3 in the context of the twelve transmembrane span topographical model of RhAG based on hydropathy predictions and on the crystal structures of bacterial Amt [30] and Rh50 polypeptides [31]. Polymorphism M220I, but not Y255F or R312Q, was also found in control beagle spleen cDNA. Isoleucine in the position analogous to dog RhAG 220 is present also in RhaG of mouse and cow. Polymorphism Y255F is reflected by phenylalanine at the analogous position in primates and in chicken. Canine RhAG polymorphism R312Q is notable in that glutamine has not been observed at the analogous position in any other reported RhAG polypeptide, and arginine at that position has been noted only in chicken RhAG. The analogous residue in human RhAG is lysine (see alignments in [32]). However, as shown in Table 3, none of these coding polymorphisms cosegregated with the phenotypes of stomatocytosis, elevated MCV, or elevated red cell Na (Tables 1 and 2).
Fig. 3.
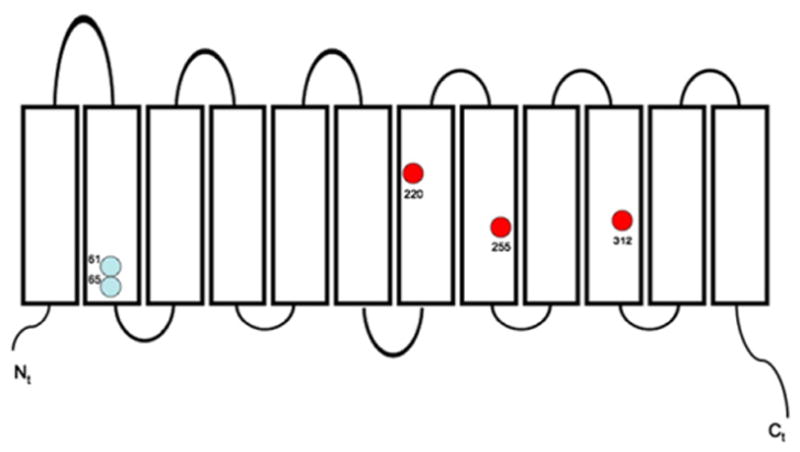
Schematic diagram of the RhAG polypeptide, showing approximate locations of the two known human stomatocytosis mutations (light blue) and the three novel polymorphisms of dog RhAG found in the Standard Schnauzer cohort with stomatocytosis.
Table 3.
RhAG polymorphisms.
| REFSEQ | M220 | Y255 | R312 |
|---|---|---|---|
| III.1 affected | M/M | Y/F | R/Q |
| IV.1 unaffected | M/M | Y/F | R/Q |
| IV.2 unaffected | M/M | Y/Y | R/R |
| IV.3 affected | M/I | Y/F | R/Q |
| IV.4 intermediate | M/I | Y/F | R/Q |
| V.1 affected | M/I | Y/Y | R/R |
| V.2 intermediate | M/I | Y/F | R/Q |
| Beagle spleen RNA (Zyagen DR-701) | M/I | Y/Y | R/R |
In addition, we detected the following silent polymorphisms in dog RhAG cDNAs: T63T/C (dogs IV.4, V.1, V.2, and beagle), C306A (all dogs except V.1, in which C306C/A was found), C654C/T (dogs IV.3, IV.4, V.1, V.2, and beagle), and T800T/C (dog IV.2 and beagle). Full-length RhAG cDNAs from dog V.2 and from beagle were PCR-amplified with High Fidelity PCR System (Roche) and cloned into T-cloning vector pCRII (Invitrogen) for allele analysis of polymorphisms. In beagle allele 1 was 63C-306A-654T-696A(220Ile)-800A(255Tyr)-971G(312Arg), and allele 2 was 63T-306A-654C-696G(220Met)-800A(255Tyr)-971G(312Arg). In dog V.II allele 1 was the same as beagle allele 1, and allele 2 was 63T-306A-654C-696G(220Met)-800T(255Phe)-971A(312Gln). Therefore, we have shown, at least for Schnauzer V.II, that RhAG allele 1 carries 220I-255Y-312R, and allele 2 has 220M-255F-312Q.
The cDNA sequence of SLC2A1/GLUT1 amplified from commercial beagle spleen RNA was identical to NCBI Refseq NM_001159326. Complete GLUT1 cDNA sequences of two affected dogs, III.2, IV.4, and of dog V.2 with an intermediate phenotype, were also identical to the Refseq sequence. The GLUT1 cDNA sequence of affected dog V.1 had a gap in coverage between nt 240–780 (RefSeq numbering), but was otherwise identical to the Refseq sequence.
The abundance of GLUT1 mRNA was much lower than those of AE1 and RhAG mRNAs, consistent with the very low glucose transport activity [33] and GLUT1 polypeptide abundance [34] in dog erythrocytes, as compared to human red cells.
Discussion
Mutations in three genes encoding solute transport proteins of the red cell plasma membrane have been implicated in autosomal dominant overhydrated stomatocytosis with cation leak: AE1/SLC4A1, RhAG, and SLC2A1/GLUT1. In this study we have ruled out each of these genes as candidates for the causative gene of hereditary cation-leak stomatocytosis in Standard Schnauzers.
In the course of this work, we have confirmed the presence of macrocytosis and stomatocytes without anemia and with little or no reticulocytosis in this Standard Schnauzer cohort. We have directly demonstrated for the first time substantially elevated red cell Na content, elevated red cell contents of K and Mg, and elevated Na leak fluxes in stomatocytic dogs. These were accompanied by greatly increased ion channel activity in on-cell patches of red cells of affected dogs, characterized by a unitary conductance of ~24 pS, and consistent with nonspecific cation channel activity.
Unlike human red cells, dog red cells are devoid of Na+, K+-ATPase activity. Thus dog cells have elevated intracelllar [Na+] and low intra-celllar [K+].
Nonetheless, stomatocytosis in Schnauzer red cells was associated with substantially higher Na content than measured in red cells from unaffected dogs. In contrast to red cells from humans with cation-leak stomatocytosis, red cells from stomatocytic dogs showed no elevation of Na+, K+-ATPase activity (ouabain-sensitive Na efflux). Na+–K+–2Cl− cotransport activity (10 μM bumetanide-sensitive Na efflux) was similarly unstimulated. The elevated Na+ leak flux in red cells from affected dogs likely reflected at least a contribution from the increased single channel activity detected in those cells. The apparent single channel conductance of this channel activity was similar in magnitude to those observed in red cells from human patients with stomatocytosis associated with missense mutations RhAG F65S [7] and SLC4A1/AE1 R730C [5]. Increased whole cell conductance with some apparent cation selectivity was observed in red cells from syndromic stomatocytosis patients with missense mutations in SLC2A1/GLUT1 [8], but unitary conductance values were not reported.
The molecular identity or identities of the ion channel(s) activated in human stomatocytosis remain unknown. Initial hypotheses suggested that stomatocytosis missense mutations in SLC4A1/AE1 [4], RhAG [6], and GLUT1 [8] converted each of these transporters into novel and distinct cation leak pathways accompanied by loss or reduction of physiological transport function. However, additional studies in Xenopus oocytes [5,7,35] and proband red cells [5,7,10] were also consistent with the hypothesis that each of the above mutant polypeptides, expressed at normal or near-normal abundance in the red cell membrane, perturbed membrane structure or cell signaling pathways to alter the threshold for activation of one or more endogenous cation transporter(s) or channel(s).
In the current work we have presented evidence for increased ion channel activity in red cells from Standard Schnauzers with stomatocytosis, with apparent single channel conductance resembling those detected in human red cells with mutant AE1/SLC4A1 and RhAG polypeptides. As for the human red cell cation pathway activated in cation-leak stomatocytosis, the molecular identity of this channel(s) in red cells of stomatocytic dogs is not known. A dog red cell candidate protein that might, either as mutant disease gene or as a secondarily activated wildtype protein, mediate the increased conductance of red cells from stomatocytic Standard Schnauzers is the sodium-calcium exchanger (likely NCX1), which in normal dog red cells contributes to volume homeostasis [36]. Even a heterozygous loss of the normal Na+ extrusion function of Na+/Ca2+ might suffice to explain increased red cell Na content in stomatocytosis of Standard Schnauzers. However, the attendant increased red cell ion conductance would likely require activation of an endogenous conductance by, or de novo intrinsic conductance of, the mutant polypeptide.
The molecular bases of the stomatocytic shape change and its often low penetrance, and the relationship of shape change to increased red cell Na content and ion conductance, remain unclear. Further pharmacological, physiological, and genetic studies on red cells from stomatocytosis patients and from affected dogs are needed to resolve these questions. Such studies will provide more data to address the hypothesis that orthologous permeability pathways are activated in stomatocytic anemias associated with mutations of the three defined human stomatocytosis genes, and the still undefined stomatocytosis genes in Standard Schnauzers and in syndromic stomatocytoses of other canine breeds.
Supplementary materials related to this article can be found online at doi:10.1016/j.bcmd.2012.02.003.
Supplementary Material
Acknowledgments
The authors thank Dr. Pierangelo Moretti for serum biochemical measurements.
This work was supported by NIH grants HL090632 (AR) and HL077655 (SLA).
Role of the funding source
The funders were not involved in the design, conduct, analysis, or interpretation of the study.
Abbreviations
- OHSt
overhydrated stomatocytosis
- DHSt
dehydrated stomatocytosis
- SLC4A1
solute carrier 4A1
- AE1
anion exchanger 1
- RhAG
Rhesus antigen ammonia carrier
- MCV
mean corpuscular volume
- CHCM
corpuscular hemoglobin concentration, mean
- RDW
red cell distribution width
- HDW
hemoglobin distribution width
Footnotes
Contributions
SLA, BES, AR, and SP designed the study. BES, DHV, JA, UB, and SP performed experiments and collected data. BES, AR, DHV, SP and SLA analyzed and interpreted data. The manuscript was drafted by SLA, and reviewed, edited, and approved by the other authors.
References
- 1.Stewart GW. Hemolytic disease due to membrane ion channel disorders. Curr Opin Hematol. 2004;11:244–250. doi: 10.1097/01.moh.0000132240.20671.33. [DOI] [PubMed] [Google Scholar]
- 2.Delaunay J. The hereditary stomatocytoses: genetic disorders of the red cell membrane permeability to monovalent cations. Semin Hematol. 2004;41:165–172. doi: 10.1053/j.seminhematol.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Bruce LJ. Hereditary stomatocytosis and cation-leaky red cells — recent developments. Blood Cells Mol Dis. 2009;42:216–222. doi: 10.1016/j.bcmd.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Bruce LJ, Robinson HC, Guizouarn H, Borgese F, Harrison P, King MJ, Goede JS, Coles SE, Gore DM, Lutz HU, Ficarella R, Layton DM, Iolascon A, Ellory JC, Stewart GW. Monovalent cation leaks in human red cells caused by single amino-acid substitutions in the transport domain of the band 3 chloride–bicarbonate exchanger, AE1. Nat Genet. 2005;37:1258–1263. doi: 10.1038/ng1656. [DOI] [PubMed] [Google Scholar]
- 5.Stewart AK, Kedar PS, Shmukler BE, Vandorpe DH, Hsu A, Glader B, Rivera A, Brugnara C, Alper SL. Functional characterization and modified rescue of novel AE1 mutation R730C associated with overhydrated cation leak stomatocytosis. Am J Physiol Cell Physiol. 2010;300:C1034–C1046. doi: 10.1152/ajpcell.00447.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruce LJ, Guizouarn H, Burton NM, Gabillat N, Poole J, Flatt JF, Brady RL, Borgese F, Delaunay J, Stewart GW. The monovalent cation leak in overhydrated stomatocytic red blood cells results from amino acid substitutions in the Rh-associated glycoprotein. Blood. 2009;113:1350–1357. doi: 10.1182/blood-2008-07-171140. [DOI] [PubMed] [Google Scholar]
- 7.Stewart AK, Shmukler BE, Vandorpe DH, Rivera A, Heneghan JF, Li X, Hsu A, Karpatkin M, O’Neill AF, Bauer DE, Heeney MM, John K, Kuypers FA, Gallagher PG, Lux SE, Brugnara C, Westhof CM, Alper SL. Loss-of-function and gain-of-function phenotypes of stomatocytosis mutant RhAG F65S. Am J Physiol Cell Physiol. 2011;301:C1325–C1343. doi: 10.1152/ajpcell.00054.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber YG, Storch A, Wuttke TV, Brockmann K, Kempfle J, Maljevic S, Margari L, Kamm C, Schneider SA, Huber SM, Pekrun A, Roebling R, Seebohm G, Koka S, Lang C, Kraft E, Blazevic D, Salvo-Vargas A, Fauler M, Mottaghy FM, Munchau A, Edwards MJ, Presicci A, Margari F, Gasser T, Lang F, Bhatia KP, Lehmann-Horn F, Lerche H. GLUT1 mutations are a cause of paroxysmal exertion-induced dyskinesias and induce hemolytic anemia by a cation leak. J Clin Invest. 2008;118:2157–2168. doi: 10.1172/JCI34438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flatt JF, Guizouarn H, Burton NM, Borgese F, Tomlinson RJ, Forsyth RJ, Baldwin SA, Levinson BE, Quittet P, Aguilar-Martinez P, Delaunay J, Stewart GW, Bruce LJ. Stomatin-deficient cryohydrocytosis results from mutations in SLC2A1: a novel form of GLUT1 deficiency syndrome. Blood. 2011;118:5267–5277. doi: 10.1182/blood-2010-12-326645. [DOI] [PubMed] [Google Scholar]
- 10.Bogdanova A, Goede JS, Weiss E, Bogdanov N, Bennekou P, Bernhardt I, Lutz HU. Cryohydrocytosis: increased activity of cation carriers in red cells from a patient with a band 3 mutation. Haematologica. 2010;95:189–198. doi: 10.3324/haematol.2009.010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Genetet S, Ripoche P, Picot J, Bigot S, Delaunay J, Armari-Alla C, Colin Y, Mouro-Chanteloup I. The human RhAG ammonia channel is impaired by the Phe65Ser mutation in overhydrated stomatocytic red cells. Am J Physiol Cell Physiol. 2012;302:C419–C428. doi: 10.1152/ajpcell.00092.2011. [DOI] [PubMed] [Google Scholar]
- 12.Bogner P, Sipos K, Ludany A, Somogyi B, Miseta A. Steady-state volumes and metabolism-independent osmotic adaptation in mammalian erythrocytes. Eur Biophys J. 2002;31:145–152. doi: 10.1007/s00249-001-0198-7. [DOI] [PubMed] [Google Scholar]
- 13.Parker JC. Dog red blood cells. Adjustment of salt and water content in vitro. J Gen Physiol. 1973;62:147–156. doi: 10.1085/jgp.62.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parker JC, McManus TJ, Starke LC, Gitelman HJ. Coordinated regulation of Na/H exchange and [K–Cl] cotransport in dog red cells. J Gen Physiol. 1990;96:1141–1152. doi: 10.1085/jgp.96.6.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parker JC. Volume-activated transport systems in dog red blood cells. Comp Biochem Physiol A Comp Physiol. 1988;90:539–542. doi: 10.1016/0300-9629(88)90664-0. [DOI] [PubMed] [Google Scholar]
- 16.Parker JC, Castranova V. Volume-responsive sodium and proton movements in dog red blood cells. J Gen Physiol. 1984;84:379–401. doi: 10.1085/jgp.84.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinkerton PH, Fletch SM, Brueckner PJ, Miller DR. Hereditary stomatocytosis with hemolytic anemia in the dog. Blood. 1974;44:557–567. [PubMed] [Google Scholar]
- 18.Slappendel RJ, van der Gaag I, van Nes JJ, van den Ingh TS, Happe RP. Familial stomatocytosis–hypertrophic gastritis (FSHG), a newly recognised disease in the dog (Drentse Patrijshond) Vet Q. 1991;13:30–40. doi: 10.1080/01652176.1991.9694282. [DOI] [PubMed] [Google Scholar]
- 19.Brown DE, Weiser MG, Thrall MA, Giger U, Just CA. Erythrocyte indices and volume distribution in a dog with stomatocytosis. Vet Pathol. 1994;31:247–250. doi: 10.1177/030098589403100213. [DOI] [PubMed] [Google Scholar]
- 20.Bonfanti U, Comazzi S, Paltrinieri S, Bertazzolo W. Stomatocytosis in 7 related Standard Schnauzers. Vet Clin Pathol. 2004;33:234–239. doi: 10.1111/j.1939-165x.2004.tb00379.x. [DOI] [PubMed] [Google Scholar]
- 21.Paltrinieri S, Comazzi S, Ceciliani F, Prohaska R, Bonfanti U. Stomatocytosis of Standard Schnauzers is not associated with stomatin deficiency. Vet J. 2007;173:200–203. doi: 10.1016/j.tvjl.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Lilliehook I, Tvedten H. Validation of the Sysmex XT-2000iV hematology system for dogs, cats, and horses. I. Erythrocytes, platelets, and total leukocyte counts. Vet Clin Pathol. 2009;38:163–174. doi: 10.1111/j.1939-165X.2009.00125.x. [DOI] [PubMed] [Google Scholar]
- 23.Bourges-Abella N, Geffre A, Concordet D, Braun JP, Trumel C. Canine reference intervals for the Sysmex XT-2000iV hematology analyzer. Vet Clin Pathol. 2011;40:303–315. doi: 10.1111/j.1939-165X.2011.00333.x. [DOI] [PubMed] [Google Scholar]
- 24.Moritz A, Fickenscher Y, Meyer K, Failing K, Weiss DJ. Canine and feline hematology reference values for the ADVIA 120 hematology system. Vet Clin Pathol. 2004;33:32–38. doi: 10.1111/j.1939-165x.2004.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 25.Furlanello T, Tasca S, Caldin M, Carli E, Patron C, Tranquillo M, Lubas G, Solano-Gallego L. Artifactual changes in canine blood following storage, detected using the ADVIA 120 hematology analyzer. Vet Clin Pathol. 2006;35:42–46. doi: 10.1111/j.1939-165x.2006.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 26.Rivera A, Jarolim P, Brugnara C. Modulation of Gardos channel activity by cytokines in sickle erythrocytes. Blood. 2002;99:357–603. doi: 10.1182/blood.v99.1.357. [DOI] [PubMed] [Google Scholar]
- 27.Rivera A, Rotter MA, Brugnara C. Endothelins activate Ca(2+)-gated K(+) channels via endothelin B receptors in CD-1 mouse erythrocytes. Am J Physiol. 1999;277:C746–C754. doi: 10.1152/ajpcell.1999.277.4.C746. [DOI] [PubMed] [Google Scholar]
- 28.Maede Y, Inaba M. (Na, K)-ATPase and Ouabain binding in reticulocytes from dogs with high K and low K erythrocytes and their changes during maturation. J Biol Chem. 1985;260:3337–3343. [PubMed] [Google Scholar]
- 29.Inaba M, Maede Y. Na,K-ATPase in dog red cells. Immunological identification and maturation-associated degradation by the proteolytic system. J Biol Chem. 1986;261:16099–16105. [PubMed] [Google Scholar]
- 30.Khademi S, O’Connell J, III, Remis J, Robles-Colmenares Y, Miercke LJ, Stroud RM. Mechanism of ammonia transport by Amt/MEP/Rh: structure of AmtB at 1.35 A. Science. 2004;305:1587–1594. doi: 10.1126/science.1101952. [DOI] [PubMed] [Google Scholar]
- 31.Lupo D, Li XD, Durand A, Tomizaki T, Cherif-Zahar B, Matassi G, Merrick M, Winkler FK. The 1.3-A resolution structure of Nitrosomonas europaea Rh50 and mechanistic implications for NH3 transport by Rhesus family proteins. Proc Natl Acad Sci U S A. 2007;104:19303–19308. doi: 10.1073/pnas.0706563104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang CH, Peng J. Evolutionary conservation and diversification of Rh family genes and proteins. Proc Natl Acad Sci U S A. 2005;102:15512–15517. doi: 10.1073/pnas.0507886102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner R, Zimmer G, Lacko L. An interspecies approach to the investigation of the red cell membrane glucose transporter. Biochim Biophys Acta. 1984;771:99–102. doi: 10.1016/0005-2736(84)90115-9. [DOI] [PubMed] [Google Scholar]
- 34.Montel-Hagen A, Kinet S, Manel N, Mongellaz C, Prohaska R, Battini JL, Delaunay J, Sitbon M, Taylor N. Erythrocyte Glut1 triggers dehydroascorbic acid uptake in mammals unable to synthesize vitamin C. Cell. 2008;132:1039–1048. doi: 10.1016/j.cell.2008.01.042. [DOI] [PubMed] [Google Scholar]
- 35.Stewart AK, Vandorpe DH, Heneghan JF, Chebib F, Stolpe K, Akhavein A, Edelman EJ, Maksimova Y, Gallager PG, Alper SL. The GPA-dependent, spherostomatocytosis mutant AE1 E758K induces GPA-independent, endogenous cation transport in amphibian oocytes. Am J Physiol Cell Physiol. 2010:283–297. doi: 10.1152/ajpcell.00444.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parker JC. Volume-activated cation transport in dog red cells: detection and transduction of the volume stimulus. Comp Biochem Physiol. 1992:615–618. doi: 10.1016/0300-9629(92)90713-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.