Abstract
Dekkera bruxellensis can outcompete Saccharomyces cerevisiae in environments with low sugar concentrations. It is usually regarded as a spoilage yeast but has lately been identified as an alternative ethanol production organism. In this study, global gene expression in the industrial isolate D. bruxellensis CBS 11270 under oxygen and glucose limitation was investigated by whole transcriptome sequencing using the AB SOLiD technology. Among other observations, we noted expression of respiratory complex I NADH-ubiquinone reductase although D. bruxellensis is a Crabtree positive yeast. The observed higher expression of NADH-generating enzymes compared to NAD+-generating enzymes might be the reason for the previously observed NADH imbalance and resulting Custer effect in D. bruxellensis. Low expression of genes involved in glycerol production is probably the molecular basis for high efficiency of D. bruxellensis metabolism under nutrient limitation. No D. bruxellensis homologs to the genes involved in the final reactions of glycerol biosynthesis were detected. A high number of expressed sugar transporter genes is consistent with the hypothesis that the competitiveness of D. bruxellensis is due to a higher affinity for the limiting substrate.
Introduction
The non-conventional yeast Dekkera bruxellensis is frequently associated with high-ethanol technical habitats [1], [2]. It has been documented as one of the main spoilage yeasts during winemaking [3]. However, it also serves as a production organism in certain wine and beer fermentations [4]. Laboratory scale fermentation of lignocellulosic hydrolysate by D. bruxellensis indicated that this organism could potentially be used for the production of second generation biofuels [5]. Thus, D. bruxellensis may not only be regarded as spoilage but also as a very competitive production yeast.
Dekkera bruxellensis hosts a number of metabolic particularities, which are highly interesting both from scientific and technological points of view. D. bruxellensis is a Crabtree positive yeast that was assumed to be capable of cyanide insensitive respiration [6]. In contrast to S. cerevisiae, D. bruxellensis can utilize nitrate as a sole nitrogen source and a correlation between nitrate assimilation and competitiveness has been suggested [2]. On the other hand, outcompetition of S. cerevisiae by D. bruxellensis in glucose limited culture has been demonstrated also in the absence of nitrate [7]. Understanding of its competitive physiology could be improved by studying genome-wide gene expression under conditions where it outcompetes S. cerevisiae. However, for several years only a partial genome sequence was available for this yeast [6], and genome sequences of two wine contaminant strains were published only very recently [8], [9].
Before the advent of whole genome sequencing [10], gene expression analyses were restricted to single genes that had been cloned and sequenced by conventional techniques. With whole genome sequences available, production of microarrays became possible, which enabled analyses of total gene expression. However, microarray analyses are rather expensive for non-conventional organisms and restricted to those genes that are identified by in silico gene prediction models. Recent genome analyses have shown that gene prediction is one of the bottlenecks in genome annotation [11]. New generation sequencing (NGS) techniques enable large-scale sequencing of both genomes and transcriptome to a comparatively reasonable price [12]. Sequencing a whole transcriptome can provide un-biased information about expressed genes and it may even be possible to quantify the gene expression level by counting the number of sequence reads that maps to the specific transcripts. More than 1000 genomes are available but comparatively few trancriptomes have been sequenced [13]. Several techniques that can enable whole transcriptome sequencing are available, including Roche 454 pyrosequencing, Illumina, or AB SOLiD [12]. Each of these techniques has specific advantages and drawbacks, and taking into account rapid development it is hardly possible to determine an ideal approach for transcriptome sequencing.
In this study we intended to develop the technique to study gene expression in D. bruxellensis. This technique was used to investigate gene expression under conditions similar to those in industrial fermentation, i.e. oxygen- and sugar limitation, where it is able to outcompete S. cerevisiae. We chose AB SOLiD because of the low error rate and the ability to produce strand specific sequencing data. Furthermore, specific expertise in this technique was available at the SciLife Genomic platform in Uppsala (Uppsala Genome Center).
Materials and Methods
Strains and culture media
The industrial isolate D. bruxellensis CBS 11270 [14] was used.
Synthetic medium was prepared according to [14] with the following modifications: KH2PO4 9.375 g/l; (NH4)2HPO4 3 g/l; 6.5 g/l YNB, glucose concentration 40 g/l.
Continuous cultivation of D. bruxellensis
Continuous culture of D. bruxellensis was run to simulate conditions of industrial fermentation. The yeast was inoculated to an OD600 of approx. 1.0 in 0.7 l to bioreactors (1.8 l, Belach, Stockholm, Sweden, working volume1.5 l), which before inoculation were flushed with nitrogen until the dissolved oxygen tension (DOT) was 0.0. The cells were grown at pH 3.6 and 36°C, at a stirring speed of 200 rpm. DOT was monitored by a dissolved oxygen (DO) electrode (Broadley James, CA, USA). When the glucose was consumed (monitored by glucose sticks (Biophan G, Kallies Feinchemie AG, Seibritz, Germany)) addition of synthetic medium was initiated at a constant dilution rate of 0.014 h−1. Excess volume was constantly pumped out and samples were taken for HPLC and OD measurements. Within 7 days the culture reached steady state and 12 ml of the culture was collected once every 3 days for RNA extraction. The culture was run for 1 month and 10 samples in total were collected.
Analytical methods
Ethanol, acetate and glycerol were measured by HPLC as described earlier [15]. Growth was monitored by optical density measurements at a wavelength of 600 nm (OD600) by an Ultrospec 1100 pro spectrophotometer (GE Healthcare Bio-Sciences AB, Sweden).
RNA extraction
Yeast cells were collected by centrifugation at room temperature and 5000 g. RNA isolation was performed immediately after harvesting using the hot acid phenol method [16]. RNA quality was analyzed by BioAnalyzer according to the manufacturer's instructions (Agilent Technologies, Germany). All RNA preparations were pooled together for normalization reasons and 12 µg of total RNA were used for library preparation.
RNA library preparation and sequencing
8 µg total RNA was rRNA depleted using the RiboMinus Eukaryote Kit for RNA-Seq (Invitrogen, USA) according to the manufacturer's instructions.
The sequencing library was prepared using the AB SOLiD Total RNA-Seq Kit according to the manufacturer's instructions: 500 ng rRNA-depleted RNA was fragmented with RNAseIII. AB SOLiD sequencing adapters were ligated to the fragments and the RNA was reverse transcribed. The cDNA was then purified and size selected to a fragment size of 150–250 bp. Templated beads were prepared according to the manufacturer's instruction and sequenced on an AB SOLiD v4 instrument.
Estimation of gene expression levels
Reads were mapped against the recently published genome of D. bruxellensis AWRI1499 [8] using LifeScope Genomic Analysis Software v2.1 (http://www.lifetechnologies.com). The number of reads that align within genomic features was counted with 3 base margins around the annotations. The read count numbers were used to calculate RPKM (Reads Per Kilobase per Million mapped reads) values for each gene - a normalized measure of expression [17].
Protein sequence analysis
BLASTP searches for S. cerevisiae and Komagataella (Pichia) pastoris homologs to D. bruxellensis proteins were carried out at the NCBI BLAST server (http://blast.ncbi.nlm.nih.gov/Blast.cgi) using the RefSeq protein database. Identification of mitochondrial-targeting signals was performed using the SignalP 4.0 Server (http://www.cbs.dtu.dk/services/SignalP/).
Results and Discussion
Continuous cultivation
D. bruxellensis was cultivated under conditions similar to those in industrial fermentation, i.e. oxygen-limitation, pH of 3.6 and 36°C [1], [14]. Steady state glucose concentration was below the detection limit, confirming glucose limitation, the ethanol concentration was 19.0±1.1 g/l, accounting for a yield of 0.475, which agrees well with previously reported data [7]. The OD600 -value was stable at a level of 17.5. Concentrations of glycerol and acetate were below the limit of detection.
Global gene expression levels
Ten samples were taken from steady state of continuous culture and were pooled in order to reduce sample-specific variation and obtain a sufficient amount of RNA for sequencing. Total RNA was isolated and following rRNA depletion, RNA was sequenced on an AB SOLiD instrument. Mapping sequenced reads to the published D. bruxellensis genome [8], we estimated the coverage depth of coding regions. This measure was expressed in number of reads per 1-K base pairs and million mapped reads. 3,221,021 reads mapped to identified genes (8.83% of mapped reads); 33,233,366 (81.17%) of the reads mapped to regions not identified by the gene models used by Curtins et al. 2012 [8]. Although it is possible that single genes were not successfully annotated and that there are some strain specific peculiarities, our results rather indicate a high frequency of transcription events outside the reading frames. Recent studies revealed a complex nature of the yeast transcriptome, 90% of which presented by transcription outside the open reading frames. For example the transcriptome of S. cerevisiae is represented by 10,000 unique transcription units including transcripts of the about 6,000 genes, ORFs with upstream transcription starting site (TSSs), ORFs with internal TSSs, intergenic transcription units, or ORFs with antisense RNAs, snoRNAs and micro-RNAs [18]. Extensive expression of non-coding RNA is a phenomenon generally found in eukaryotes, which has also been demonstrated in the yeasts S. cerevisiae [19]–[21], Schizosaccharomyces pombe [22] and Candida albicans [23]. Our results confirm that expression of non-coding RNA is common among ascomycetous yeasts.
Expression of 3,715 out of 4,861 annotated genes was detected under the tested conditions, which is consistent with number of transcripts typically identified under single condition set in yeasts [19]–[21], [23]. A summary of all detected D. bruxellensis genes' expression levels is presented in Table S1. The 20 transcripts with the highest numbers of mapping reads, i.e. the genes with highest expression levels are presented in Table 1. These highest expressed genes were involved in several physiological functions including translation (AWRI1499_1998, AWRI1499_0355, AWRI1499_3730) and energy metabolism (AWRI1499_4217, AWRI1499_3554, AWRI1499_2550, AWRI1499_1634). High expression of genes involved in protein synthesis is consistent with previously reported data showing that in S. cerevisiae 60% of the transcribed mRNAs are related to the genes involved in the translation process [24]. High expression level of a number of hypothetical proteins (AWRI1499_3102, AWRI1499_2152, AWRI1499_3169) (not shown in Table 1) was also observed. These genes had low or no significant similarity to sequences in other yeast genomes, preventing their functional annotation.
Table 1. List of the 20 highest expressed D. bruxellensis genes under oxygen and glucose limitation.
| Gene name | Gene function (according to the annotation by Curtins et al. 2012) | RPKMa |
| AWRI1499_3437 | Nucleolar GTP-binding protein 2 | 66549.06 |
| AWRI1499_1998 | Elongation factor 1-beta | 49138.25 |
| AWRI1499_0355 | 40S ribosomal protein s22 | 45648.69 |
| AWRI1499_0160 | Zinc-regulated transporter 2 | 32387.38 |
| AWRI1499_1299 | G-protein complex beta subunit | 20056.01 |
| AWRI1499_0595 | Covalently linked cell wall glycoprotein, present in the inner layer of the cell wall | 13659.81 |
| AWRI1499_2550 | Glyceraldehyde-3-phosphate dehydrogenase | 8293.73 |
| AWRI1499_1219 | Transcription factor HAC1 | 6184.46 |
| AWRI1499_4217 | Alcohol dehydrogenase | 6038.40 |
| AWRI1499_1850 | Helicase | 5709.36 |
| AWRI1499_3376 | Histone h2b | 5464.61 |
| AWRI1499_3554 | Enolase | 5249.95 |
| AWRI1499_1736 | Glutamine synthetase | 5149.73 |
| AWRI1499_3730 | 60 S ribosomal protein l7 | 4905.53 |
| AWRI1499_4783 | RHO-type GTPase-activating protein | 4697.69 |
| AWRI1499_3397 | Putative COP9 signalosome subunit 7 | 4054.42 |
| AWRI1499_2462 | Calcium calmodulin-dependent protein | 4014.30 |
| AWRI1499_2276 | Oligopeptide transporter | 3838.29 |
| AWRI1499_1956 | Acetohydroxyacid reductoisomerase | 3744.34 |
| AWRI1499_1634 | Triosephosphate isomerase | 3667.94 |
RPKM is a measure of gene expression level expressed as number of reads per 1-K base pairs and million mapped reads.
Expression of D. bruxellensis genes involved in glucose transport
Sugar import has been suggested as one of the key properties of the ability of D. bruxellensis to outcompete S. cerevisiae [6], [7]. The expression levels of several D. bruxellensis genes that have homologs in S. cerevisiae or Komagataella (Pichia) pastoris involved in glucose transport were investigated (Table 2). The D. bruxellensis CBS 11270 homologue to AWRI1499_3136 was expressed at the highest level among the genes with putative glucose transport activity. According to the annotation of the published genome [8], this gene is a low-affinity glucose transporter, however both corresponding homologs of S. cerevisiae [25] and K. pastoris [26] are annotated as high-affinity glucose transporters. The hexose transporter gene AWRI1499_3135 has homologs in S. cerevisiae and K. pastoris that were characterized as high-affinity glucose transporters [25], [26] indicating a similar function of the D. bruxellensis gene. The S. cerevisiae-homologs of AWRI1499_3136 and AWRI1499_3135, HXT6, HXT7 and GAL2 are expressed at glucose limitation [27]. HXT6 has also been described to be up-regulated in oxygen limited chemostate cultures [28]. AWRI1499_4734 has one homolog of S. cerevisiae (HXT2) involved in high-affinity glucose transport and expressed under glucose limitation [25], [27], the affinity of the according K. pastoris gene XP_002489344 was not assessed [26]. HXT2-expression was down-regulated under oxygen limitation in S. cerevisiae chemostate-cultures [28], similarly to the relatively low expression of the D. bruxellensis homolog observed in the present study. The affinities of both S. cerevisiae and K. pastoris homologs of the lowly expressed gene AWRI1499_2919 have not been previously characterized either. The S. cerevisiae-homologs HXT13 and HXT17 were expressed under specific conditions, like growth on non-fermentable carbon source (and thus extremely low glucose concentration, low oxygen or pH 7.7 [29], [28].
Table 2. Putative D. bruxellensis glucose transporter genes expressed under conditions of oxygen and glucose limitation in continuous culture and corresponding S. cerevisiae and K. pastoris homologues.
| D. bruxellensis gene | S. cerevisiae homologs | K. pastoris homologs | Gene function | RPKMa |
| AWRI1499_3136 | HXT6 (4.0×10−146), HXT7 (4.0×10−146) | XP_002490706 (3.0×10−156) | Glucose transporter | 3371.55 |
| AWRI1499_3135 | GAL2 (0), HXT6 (0), HXT7 (0) | XP_002490706 (0) | Hexose transporter | 2234.21 |
| AWRI1499_4734 | HXT2 (6.0×10−38) | XP_002489344 (1.0×10−62) | Hexose transporter | 268.06 |
| AWRI1499_2919 | HXT13 (2.0×10−20), HXT17 (2.0×10−20) | XP_002492232 (7.0×10−72) | High-affinity glucose transporter | 70.72 |
Numbers in parentheses show the Blast E-values of sequence comparison between the D. bruxellensis gene and the respective homologue.
RPKM is a measure of gene expression level expressed as number of reads per 1-K base pairs and million mapped reads.
In addition, high expression of a putative low glucose sensor, AWRI1499_1677, possibly involved in the induction of hexose transporters was observed. The corresponding homologue of S. cerevisiae, SNF3 [30] was identified to have high sequence similarity (Blast E-value 3.0×10−177).
Expression of genes involved in the central carbon metabolism
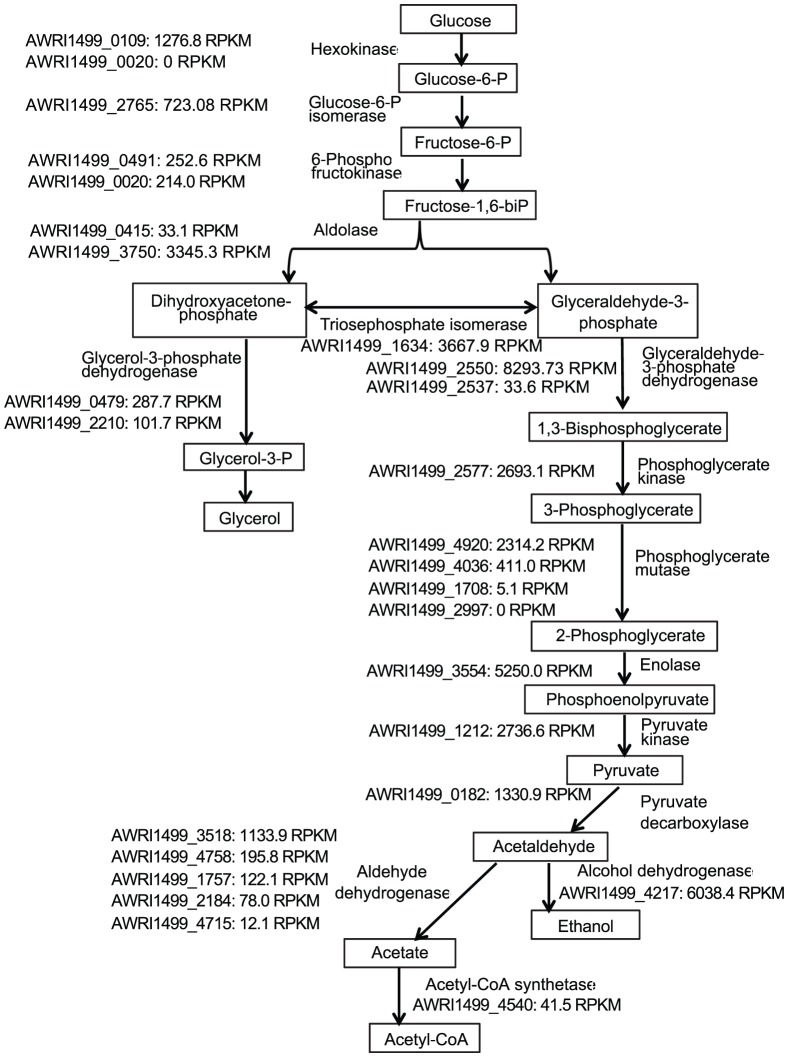
Expression of genes involved in glycolysis, alcoholic fermentation and acetate formation is summarized in Figure 1. Almost all genes involved in glycolysis were expressed at high levels with RPKM higher than 1,000. For several steps in glycolysis isoenzyme genes were identified. Among them, isoenzymes of hexokinase (AWRI1499_0020) and phosphoglycerate mutase (AWRI1499_2997) were not transcribed. The two isoforms of 6-phospho- fructokinase, catalyzing an energy consuming reaction (AWRI1499_0491, AWRI1499_0020) were expressed at the lowest level. As mentioned above, two glycolysis genes were also detected among the 20 genes with the highest expression level (glyceraldehyde-3-phosphate dehydrogenase, AWRI1499_2550, and enolase, AWRI1499_3554). The genes involved in the initial ATP-consuming phase of glycolysis were expressed at levels lower than 1,300 RPKM, while the genes of energy-generating C3 -phase were mainly expressed at levels higher than 2,500 RPKM.
Figure 1. Expression of genes involved in the central carbon metabolism.
RPKM is a measure of gene expression level expressed as a number of reads per 1-K base pairs and million mapped reads.
Low expression of glucose-6-phosphate dehydrogenase (AWRI1499_0062) with RPKM 98.69 compared to expression of hexokinase (AWRI1499_0020) with RPKM 1,276.84 may indicate that glycolysis presents the main carbon flow compared to the pentose phosphate pathway. This is in accordance with results of metabolic flux analysis in S. cerevisiae [31] and in respiratory yeasts under oxygen limited conditions [15]. On the other hand, a relatively high activity of glucose-6-phosphate dehydrogenase has been observed in oxygen limited D. bruxellensis batch cultures, which was about 40% of that observed for hexokinase [32]. This result may indicate physiological differences between cells grown under glucose excess or -limitation or transcription-independent levels of enzyme regulation.
Expression of five aldehyde dehydrogenases (except AWRI1499_2185) among six annotated in the D. bruxellensis genome was detected. At least three (AWRI1499_3518, AWRI1499_1757, AWRI1499_4715) aldehyde dehydrogenase genes are predicted to be located in mitochondria. Two out of five aldehyde dehydrogenases of S. cerevisiae (ALD5 and ALD4) were reported to be mitochondrial proteins [33]–[35].
Two aldehyde dehydrogenase genes AWRI1499_4758 and AWRI1499_2184 were identified to have high similarity to ALD2 of S. cerevisiae with Blast E-values of 2.0×10−145 and 9.0×10−43, respectively. The S. cerevisiae ALD2 gene was previously reported to be induced in response to ethanol, and involved in ethanol oxidation to acetate [33]. Products of these two genes did not contain any detectable mitochondrial-targeting signal, which suggests they are more likely to be located in the cytoplasm.
High similarities of AWRI1499_3518, AWRI1499_1757 and AWRI1499_4715 to ALD4 of S. cerevisiae, the major isoform catalyzing aldehyde conversion to acetate might indicate a similar function of the corresponding D. bruxellensis genes. Remarkably, AWRI1499_3518 representing a gene of the mitochondrial isoform had highest RPKM among all putative aldehyde dehydrogenase genes, indicating that this gene can potentially be regarded as the major aldehyde dehydrogenase in D. bruxellensis.
Acetyl-CoA synthetase 2 (AWRI1499_4540) was expressed at low level with RPKM 41.15, which is consistent with the expression of ACS2 of S. cerevisiae under anaerobic conditions [36]. Expression of acetyl-CoA synthetase 1 (AWRI1499_3236) was in principle not detectable (1.14 RPKM, data not shown). Expression of ACS1 of S. cerevisiae is mainly associated with aerobic conditions. Low expression of acetyl-CoA synthetase, blocking the acetaldehyde oxidizing pathway was previously speculated to be involved in the high acetate producing capacity of D. bruxellensis [3], and activities of this enzyme were not detectable both in aerobic and oxygen limited batch culture [32]. Thus the observed gene expression pattern would promote secretion of acetate. However, since no acetate was detected in the medium under these conditions, other regulation mechanisms than transcription must exist, preventing acetate formation. The alcohol dehydrogenase (AWRI1499_4217), homologous to ADH1 of S. cerevisiae (Blast E-value 4.0×10−180) was highly expressed. Aldehyde dehydrogenase reactions are generating reduced redoxfactors, which can only to a little extend be re-oxidized under low oxygen conditions. Thus, acetaldehyde will be preferentially channeled towards ethanol on the cost of acetate formation. This is consistent with the observed high ethanol yield. The physiological function of the remarkably high number of expressed aldehyde dehydrogenases is not clear. However, increased activities of aldehyde dehydrogenase under oxygen limitation have been described earlier in the respiratory yeasts Scheffersomyces stipitis and Wickerhamomyces anomalus [15], [37]. Another alcohol dehydrogenase (AWRI1499_3822), which has a high degree of identity to the sequence of the ADH7 gene in S. cerevisiae (Blast E-value 3.0×10−24) was expressed with RPKM of 80.38. Glycerol-3-phosphate dehydrogenase (AWRI1499_0479, AWRI1499_2210) was expressed at low level with 287.7 and 101.7 RPKM, respectively, which is consistent with the low glycerol producing capacity of D. bruxellensis. No specific genes involved in dephosphorylation of glycerol-3-phosphate, the final step of glycerol biosynthesis were identified.
Aldehyde dehydrogenase and glyceraldehyde-3-phosphate dehydrogenase genes, both involved in NADH synthesis were expressed at high levels. In contrast to this, one of the enzymes involved in the NADH reoxidation under oxygen limited conditions, glycerol-3-phosphate dehydrogenase (AWRI1499_0479, AWRI1499_2210) was expressed at least 4 times lower than the genes of the mentioned NADH-generated enzymes. This anyway explains the observed low capacity of D. bruxellensis to form glycerol under oxygen limited conditions. Overall expression of genes coding for NADH-generating proteins, involved in the central carbon metabolism were apparently higher than those coding for NADH-reoxidizing enzymes, including even the highly expressed ADH-gene. This is consistent with previously reported data speculating on an NADH imbalance as metabolic basis of the Custer effect in D. bruxellensis [38], [39].
Expression of D. bruxellensis genes that do not have homologs in S. cerevisiae
Out of the 4,969 genes annotated by Curtin et al., there were no S. cervisiae homologs for 2,242 genes in D. bruxellensis [8]. Expression of some of these genes was observed under the chosen culture conditions (Table 3).
Table 3. Expression of D. bruxellensis genes that do not have homologs in S. cerevisiae.
| Gene name | Gene function | RPKMa |
| AWRI1499_4733 | Beta-glucosidase | 1825.03 |
| AWRI1499_4881 | Nitrate reductase | 469.55 |
| AWRI1499_4189 | NADH-ubiquinone oxidoreductase subunit | 352.67 |
| AWRI1499_4883 | Nitrate transporter | 287.59 |
| AWRI1499_3467 | Dihydroorotate dehydrogenase | 196.93 |
| AWRI1499_4880 | Beta-galactosidase | 121.43 |
| AWRI1499_4315 | Sucrose transporter | 72.39 |
| AWRI1499_1979 | Alternative oxidase mitochondrial precursor | 49.67 |
| AWRI1499_4882 | Nitrite reductase | 2.15 |
RPKM is a measure of gene expression level expressed as number of reads per 1-K base pairs and million mapped reads.
A low level of expression of NADH-ubiquinone oxidoreductase (complex I of the respiratory chain) was observed. In a previous study it was speculated that complex I might be active in D. bruxellensis, which is Crabtree-positive, while the genes for this complex are missing in other Crabtree-positive yeasts [6], [40] and our results indeed show that this complex is present in D. bruxellensis.
Dekkera bruxellensis expressed a dihydroorotate dehydrogenase gene encoding for a mitochondrial isoform of this enzyme typical for respiratory yeasts. This apparently reflects a difference in the pyrimidine metabolism between D. bruxellensis and S. cerevisiae. S. cerevisiae has lost the gene of the mitochondrial isoform (URA9) and instead expresses another gene (URA1), encoding for cytoplasmic isoform of this enzyme. The cytoplasmatic isoform is most probably the product of horizontal gene transfer between yeasts and a relative of Lactococcus lactis. It has been suggested that expression of a dihydroorotate dehydrogenase independent on the respiratory chain is essential for anaerobic synthesis of uracil [41]. However, we recently demonstrated that anaerobic growth of D. bruxellensis only requires addition of amino acids, uracil addition was not essential [7].
Other differences were the expression of beta-galactosidase and beta-glucosidase, which supports the notation that D. bruxellensis has broader spectrum of consumable sugars, compared to S. cerevisiae. These results are consistent with previously reported data on cellobiose-fermenting [14], [42] and lactose-assimilating capacity of D. bruxellensis [43]. Expression of a sucrose transporter in D. bruxellensis indicates a different mechanism of sucrose consumption compared to S. cerevisiae, which is probably a key for the high competitiveness of this yeast in sucrose-based fermentations [2]. Most genes of the nitrate assimilation pathway were also moderately expressed, confirming earlier results by de Barros Pita et al. that the nitrate assimilation pathway is not completely repressed in the presence of ammonium [2].
D. bruxellensis' competitiveness towards S. cerevisiae under nutrient limited conditions was previously hypothesized to be due to the higher efficiency of its energy metabolism [14]. This hypothesis is consistent with the gene expression data presented in this study. Low expression of the genes involved in glycerol biosynthesis may contribute to directing of the C3-compounds towards energy-providing reactions of the glycolysis and thus to ATP conservation within the cell. Glycerol production as such is energy consuming, since the cell does not get back the ATP used for glucose phosphorylation. Expression of components of the respiratory chain including complex I under low oxygen condition indicates that the yeast is using even trace amounts of oxygen for NADH re-oxidation, providing another resource for ATP generation. However, there is also a significant expression of a variety of sugar transporters, several of them most probably with high affinity to glucose. A higher affinity to glucose may explain why D. bruxellensis consumed almost 100% of the sugar in a glucose limited co-culture with S. cerevisiae [7]. However, this hypothesis needs to be proven by directly measuring glucose affinities. The combination of high energy efficiency and high affinity glucose transport may also explain wine spoilage by this yeast, since in the final stage of wine making conditions are similar to the one tested in this study, i.e. low sugar, low oxygen and acid pH.
Supporting Information
Expression levels of D. bruxellensis genes under oxygen and glucose limitation. Count designates the amount of reads mapped to the reference gene, RPKM is a measure of gene expression level expressed as number of reads per 1-K base pairs and million mapped reads.
(DOCX)
Acknowledgments
The authors would like to acknowledge support of Uppsala Genome Center and UPPMAX for providing assistance in massive parallel sequencing and computational infrastructure.
Funding Statement
This study has been supported by the Swedish Energy Authority (Statens Energimyndigheten, STEM), project number 34134-1 and the Research program MicroDrivE at the Faculty for Natural Resources and Agriculture at the Swedish University of Agricultural Sciences. Work performed at Uppsala Genome Center has been funded by RFI/VR “SNISS” (Swedish National Infrastructure for large Scale Sequencing and Science for Life Laboratory) Uppsala. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Passoth V, Blomqvist J, Schnürer J (2007) Dekkera bruxellensis and Lactobacillus vini form a stable ethanol-producing consortium in a commercial alcohol production process. Appl Environ Microbiol 73: 4354–4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Barros Pita W, Leite FC, de Souza Liberal AT, Simoes DA, de Morais MA Jr (2011) The ability to use nitrate confers advantage to Dekkera bruxellensis over S. cerevisiae and can explain its adaptation to industrial fermentation processes. Antonie van Leeuwenhoek 100: 99–107. [DOI] [PubMed] [Google Scholar]
- 3. Silva P, Cardoso H, Geros H (2004) Studies on the wine spoilage capacity of Brettanomyces/Dekkera spp . Am J Enol Viticult 55: 65–72. [Google Scholar]
- 4. Martens H, Iserentant D, Verachtert H (1997) Microbiological aspects of a mixed yeast-bacterial fermentation in the production of a special Belgian acidic ale. J Inst Brewing 103: 85–91. [Google Scholar]
- 5. Blomqvist J, South E, Tiukova I, Momeni MH, Hansson H, et al. (2011) Fermentation of lignocellulosic hydrolysate by the alternative industrial ethanol yeast Dekkera bruxellensis . Lett Appl Microbiol 53: 73–78. [DOI] [PubMed] [Google Scholar]
- 6. Woolfit M, Rozpedowska E, Piskur J, Wolfe KH (2007) Genome survey sequencing of the wine spoilage yeast Dekkera (Brettanomyces) bruxellensis . Eukaryot Cell 6: 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blomqvist J, Nogue VS, Gorwa-Grauslund M, Passoth V (2012) Physiological requirements for growth and competitiveness of Dekkera bruxellensis under oxygen-limited or anaerobic conditions. Yeast 29: 265–274. [DOI] [PubMed] [Google Scholar]
- 8. Curtin CD, Borneman AR, Chambers PJ, Pretorius IS (2012) De-novo assembly and analysis of the heterozygous triploid genome of the wine spoilage yeast Dekkera bruxellensis AWRI1499. PloS One 7: e33840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Piskur J, Ling Z, Marcet-Houben M, Ishchuk OP, Aerts A, et al. (2012) The genome of wine yeast Dekkera bruxellensis provides a tool to explore its food-related properties. Int J Food Microbiol 157: 202–209. [DOI] [PubMed] [Google Scholar]
- 10. Goffeau A, Barrell BG, Bussey H, Davis RW, Dujon B, et al. (1996) Life with 6000 genes. Science 274: 546–567. [DOI] [PubMed] [Google Scholar]
- 11. Shah T, de Villiers E, Nene V, Hass B, Taracha E, et al. (2006) Using the transcriptome to annotate the genome revisited: application of massively parallel signature sequencing (MPSS). Gene 366: 104–108. [DOI] [PubMed] [Google Scholar]
- 12. Wall PK, Leebens-Mack J, Chanderbali AS, Barakat A, Wolcott E, et al. (2009) Comparison of next generation sequencing technologies for transcriptome characterization. BMC Genomics 10: 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yassour M, Kapian T, Fraser HB, Levin JZ, Pfiffner J, et al. (2009) Ab initio construction of a eukaryotic transcriptome by massively parallel mRNA sequencing. Proc Natl Acad Sci USA 106: 3264–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blomqvist J, Eberhard T, Schnürer J, Passoth V (2010) Fermentation characteristics of Dekkera bruxellensis strains. Appl Microbiol Biot 87: 1487–1497. [DOI] [PubMed] [Google Scholar]
- 15. Fredlund E, Blank LM, Schnürer J, Sauer U, Passoth V (2004) Oxygen- and glucose-dependent regulation of central carbon metabolism in Pichia anomala . Appl Environ Microbiol 70: 5905–5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xue YT, Haas SA, Brino L, Gusnanto A, Reimers M, et al. (2004) A DNA microarray for fission yeast: minimal changes in global gene expression after temperature shift. Yeast 21: 25–39. [DOI] [PubMed] [Google Scholar]
- 17. Mortazavi A, Williams BA, Mccue K, Schaeffer L, Wold B (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5: 621–628. [DOI] [PubMed] [Google Scholar]
- 18. Ito T, Miura F, Onda M (2008) Unexpected complexity of the budding yeast transcriptome. Iubmb Life 60: 775–781. [DOI] [PubMed] [Google Scholar]
- 19. David L, Huber W, Granovskaia M, Toedling J, Palm CJ, et al. (2006) A high-resolution map of transcription in the yeast genome. Proc Natl Acad Sci USA 103: 5320–5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miura F, Kawaguchi N, Sese J, Toyoda A, Hattori M, et al. (2006) A large-scale full-length cDNA analysis to explore the budding yeast transcriptome. Proc Natl Acad Sci USA 103: 17846–17851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu ZY, Wei W, Gagneur J, Perocchi F, Clauder-Munster S, et al. (2009) Bidirectional promoters generate pervasive transcription in yeast. Nature 457: 1033–U1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ni T, Tu K, Wang Z, Song S, Wu H, et al. (2010) The prevalence and regulation of antisense transcripts in Schizosaccharomyces pombe . PloS One 5: e15271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sellam A, Hogues H, Askew C, Tebbji F, van het Hoog M, et al. (2010) Experimental annotation of the human pathogen Candida albicans coding and noncoding transcribed regions using high-resolution tiling arrays. Genome Biol 11: R71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Warner JR (1999) The economics of ribosome biosynthesis in yeast. Trends Biochem Sci 24: 437–440. [DOI] [PubMed] [Google Scholar]
- 25. Reifenberger E, Freidel K, Ciriacy M (1995) Identification of novel HXT genes in Saccharomyces cerevisiae reveals the impact of individual hexose transporters on glycolytic flux. Mol Microbiol 16: 157–167. [DOI] [PubMed] [Google Scholar]
- 26. De Schutter K, Lin YC, Tiels P, Van Hecke A, Glinka S, et al. (2009) Genome sequence of the recombinant protein production host Pichia pastoris . Nat Biotechnol 27: 561–566. [DOI] [PubMed] [Google Scholar]
- 27. Diderich JA, Schepper M, van Hoek P, Luttik MA, van Dijken JP, et al. (1999) Glucose uptake kinetics and transcription of HXT genes in chemostat cultures of Saccharomyces cerevisiae . J Biol Chem 274: 15350–15359. [DOI] [PubMed] [Google Scholar]
- 28. Rintala E, Wiebe MG, Tamminen A, Ruohonen L, Penttilä M (2008) Transcription of hexose transporters of Saccharomyces cerevisiae is affected by change in oxygen provision. BMC Microbiol 8: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Greatrix BW, van Vuuren HJ (2006) Expression of the HXT13, HXT15 and HXT17 genes in Saccharomyces cerevisiae and stabilization of the HXT1 gene transcript by sugar-induced osmotic stress. Curr Genet 49: 205–217. [DOI] [PubMed] [Google Scholar]
- 30. Neigeborn L, Carlson M (1984) Genes affecting the regulation of SUC2 gene expression by glucose repression in Saccharomyces cerevisiae . Genetics 108: 845–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fiaux J, Cakar ZP, Sonderegger M, Wuthrich K, Szyperski T, et al. (2003) Metabolic-flux profiling of the yeasts Saccharomyces cerevisiae and Pichia stipitis . Eukaryot Cell 2: 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Galafassi S, Merico A, Pizza F, Hellborg L, Molinari F, et al. (2011) Dekkera/Brettanomyces yeasts for ethanol production from renewable sources under oxygen-limited and low-pH conditions. J Ind Microbiol Biotechnol 38: 1079–1088. [DOI] [PubMed] [Google Scholar]
- 34. Tessier WD, Meaden PG, Dickinson FM, Midgley M (1998) Identification and disruption of the gene encoding the K(+)-activated acetaldehyde dehydrogenase of Saccharomyces cerevisiae . FEMS Microbiol Lett 164: 29–34. [DOI] [PubMed] [Google Scholar]
- 35. Wang X, Bai Y, Ni L, Weiner H (1997) Saccharomyces cerevisiae aldehyde dehydrogenases. Identification and expression. Advances in experimental medicine and biology 414: 277–280. [DOI] [PubMed] [Google Scholar]
- 36. van den Berg MA, de Jong-Gubbels P, Kortland CJ, van Dijken JP, Pronk JT, et al. (1996) The two acetyl-coenzyme A synthetases of Saccharomyces cerevisiae differ with respect to kinetic properties and transcriptional regulation. J Biol Chem 271: 28953–28959. [DOI] [PubMed] [Google Scholar]
- 37. Passoth V, Zimmermann M, Klinner U (1996) Peculiarities of the regulation of fermentation and respiration in the Crabtree-negative, xylose-fermenting yeast Pichia stipitis . Appl Biochem Biotech 57–58: 201–212. [DOI] [PubMed] [Google Scholar]
- 38. Scheffers WA (1966) Stimulation of fermentation in yeasts by acetoin and oxygen. Nature 210: 533–534. [DOI] [PubMed] [Google Scholar]
- 39. van Dijken JP, Scheffers A (1986) Redox balances in the metabolism of sugars by yeasts. FEMS Microbiol Rev 32: 199–224. [Google Scholar]
- 40. Prochazka E, Polakova S, Piskur J, Sulo P (2010) Mitochondrial genome from the facultative anaerobe and petite-positive yeast Dekkera bruxellensis contains the NADH dehydrogenase subunit genes. FEMS Yeast Res 10: 545–557. [DOI] [PubMed] [Google Scholar]
- 41. Gojkovic Z, Knecht W, Zameitat E, Warneboldt J, Coutelis JB, et al. (2004) Horizontal gene transfer promoted evolution of the ability to propagate under anaerobic conditions in yeasts. Mol Genet Genomics 271: 387–393. [DOI] [PubMed] [Google Scholar]
- 42. Smith MT, Yamazaki M, Poot GA (1990) Dekkera, Brettanomyces and Eeniella - electrophoretic comparison of enzymes and DNA-DNA homology. Yeast 6: 299–310. [DOI] [PubMed] [Google Scholar]
- 43. Conterno L, Joseph CML, Arvik TJ, Henick-Kling T, Bisson LF (2006) Genetic and physiological characterization of Brettanomyces bruxellensis strains isolated from wines. Am J Enol Viticult 57: 139–147. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expression levels of D. bruxellensis genes under oxygen and glucose limitation. Count designates the amount of reads mapped to the reference gene, RPKM is a measure of gene expression level expressed as number of reads per 1-K base pairs and million mapped reads.
(DOCX)