Abstract
Using rare intracranial recordings from the posterior interhemispheric region of the human brain, we explored the oscillatory properties of the posteromedial cortex (PMC) during rest. The PMC is a core structure of the default mode network, which is known for its higher activity during the resting state. We found that resting PMC spectral power peaked in the theta band range (4–7 Hz) and was clearly distinguishable from adjacent cortical sites in the occipital lobe displaying peaks in the alpha band range (8–12 Hz). Additionally, the phase of PMC theta oscillations modulated the amplitude of ongoing high gamma (70–180Hz) activity during the resting state. The magnitude of this cross-frequency modulation was shown to fluctuate at time scales comparable to those observed in functional neuroimaging studies of intrinsic functional connectivity networks (~0.1 Hz). The difference of canonical oscillations in the PMC compared to its adjacent cortical sites conforms to functional specialization across anatomical boundaries. Such differences may reflect separate oscillatory preferences between networks that are functionally connected.
Keywords: Electrocorticography, posteromedial cortex, theta oscillations, phase-amplitude coupling, default mode network
1. Introduction
During the resting state, oscillations of differing peak frequencies predominate across the cerebral cortex. The occipital alpha and the rolandic beta rhythms are classical examples of such canonical oscillations (Adrian and Matthews, 1934; Jasper and Penfield, 1949). More recently, there has been growing evidence for the role of canonical oscillations in modulating local cortical activation and distal cortical interaction through phase-amplitude and phase-phase coupling, respectively (Canolty and Knight, 2010).
Similarly, functional magnetic resonance imaging (fMRI) studies have also revealed unique spatio-temporal patterns of resting state activity, in the form of intrinsic functional connectivity (resting state networks), which reflect correlations in hemodynamic signals at slow time scales (< 1 Hz) (Deco et al., 2011). The default mode network (DMN) is a consistently identified resting state network, of which the posteromedial cortex (PMC) forms the central functional-anatomic node (Buckner et al., 2008; Hagmann et al., 2008). However, despite great research interest, little is known about the electrophysiological properties unique to the PMC, in contrast to adjacent sensory regions, during the resting state. Central to this limitation is the difficulty in obtaining electrophysiological interhemispheric recordings from the PMC in conscious human subjects.
Typically the DMN, and the PMC in particular, is described as having higher blood oxygenation level dependent (BOLD) resting activity which deactivates during attentionally demanding cognitive tasks (Fox et al., 2005). Consistent with these fMRI findings, single-unit recordings in non-human primates (Hayden et al., 2009, 2010) and intracranial recordings from human subjects (Dastjerdi et al., 2011; Jerbi et al., 2010; Miller et al., 2009; Ossandon et al., 2011) also show clear support for higher activity in PMC during rest, which is suppressed during tasks of external attention. These data suggest resting activation and task deactivation to be best tracked by power shifts in a broad gamma range at the cortical surface (Dastjerdi et al., 2011; Jerbi et al., 2010; Miller et al., 2009; Ossandon et al., 2011). More generally, electrophysiological investigations outside of the DMN have suggested that modulation in gamma amplitude at slower frequencies, similar to those observed in fMRI data, correlate at spatial scales relevant to the study of resting state brain networks (Leopold et al., 2003; Nir et al., 2008).
In the current study, we go beyond the event related changes of gamma activity in the PMC during cued rest fixation (Dastjerdi et al., 2011). We use intracranial recordings in human subjects to measure the signature of electrophysiological activity within the PMC during spontaneous rest. During this state, we show that theta oscillations predominate in PMC and also modulate ongoing gamma power, which distinguishes this region from other nearby areas such as the visual cortex whose activity is preferentially influenced by canonical alpha, rather than theta, oscillations. Additionally, we show that cross-frequency amplitude modulation for the same frequencies fluctuates at slow time scales akin to those observed for resting state networks with fMRI BOLD signals.
2. Materials and Methods
2.1 Subjects
Direct electrocortical recordings during periods of awake resting state were obtained from subjects implanted with subdural electrodes. Intracranial recordings were performed for clinical reasons related to the surgical treatment of refractory epilepsy. All subjects provided voluntary written consent to participate in research recordings as part of a protocol approved by the Stanford Institutional Review Board office. Results reported here were obtained from 4 subjects (3 female) with electrode coverage over the PMC in addition to other cortical sites (see Fig. 1–4), which is a rare occurrence for this clinical cohort. Electrodes were defined as PMC for each subject based on clear anatomical boundaries as shown in Fig. 1. Anatomical location of electrodes was identified through the alignment of pre-operative magnetic resonance imaging (MRI) and post-operative computed tomography (CT) scans as previously described (Dastjerdi et al., 2011).
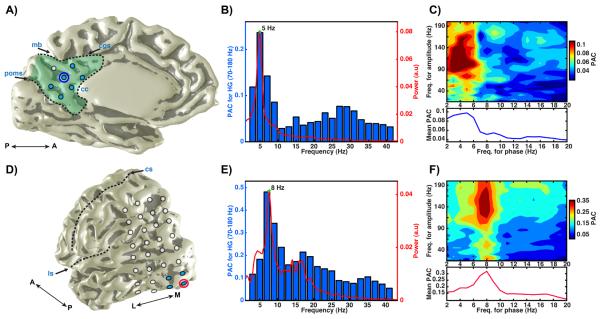
Figure 1. Power spectrum and cross-frequency coupling in PMC.
A) Medial view of electrode sites covering PMC (green fill) for subject 1 (left hemisphere; A: anterior; P: posterior; excluded electrodes not shown). PMC boundaries (black dashed line) were defined by the marginal branch (mb) of the cingulate sulcus (cgs), the corpus callosum (cc) and the parieto-occipital medial sulcus (poms) as described in Parvizi et al. (2006). B) Power spectrum (red, right y-axis) and phase-amplitude coupling (blue, left y-axis) to HG (70–180 Hz) amplitude for narrow frequency bins (x-axis) for the raw time series in one PMC electrode (circled blue in A). At this PMC site, there is a clear spectral peak in the theta band (5 Hz) of the raw time series at rest, which produces the greatest modulation of resting HG amplitude. In the mean phase-amplitude (x,y) comodulogram (C, upper panel) of 6 PMC electrodes (blue fill in A), the greatest modulation of HG amplitude occurs in the theta range also (C, lower panel shows mean PAC for each phase bin). In contrast to PMC sites, occipital electrodes (D) in the same subject show a strong spectral alpha peak (8 Hz) at rest, which in turn produces the greatest modulation of resting HG amplitude. D) Posterior/lateral view (left hemisphere; L: lateral; M: medial; ls: lateral sulcus; cs: central sulcus) of electrodes covering occipital cortex in the same subject. E) Same as (B), for a single electrode in the primary visual cortex (circled red in D). F) Same as (C), for 4 primary visual electrodes (blue fill in D; F lower panel shows mean PAC for each phase bin). In an event related paradigm, we confirmed the selective response of the chosen PMC and visual electrodes (blue fill in A & D) during cued rest and visual conditions (Dastjerdi et al., 2011).
Figure 4. Slow fluctuation of cross-frequency modulation in PMC.
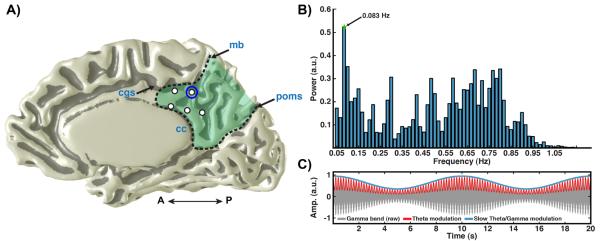
A) Medial view of PMC electrodes (right hemisphere) in subject 4 (labeling as per Fig. 1). B) Power spectrum of theta/HG modulation time series (see methods) for a PMC electrode in A (circle red). Spectrum shows a clear peak at 0.08 Hz (arrow). C) Schematic of the slow modulation of theta/HG cross-frequency coupling (synthetic signal). Ongoing theta modulated (red line; 5 Hz) gamma band oscillations (gray shading; 70–180Hz) fluctuates in magnitude at a slow time scale (blue line; 0.1 Hz).
2.2 Electrophysiological Recording and Analysis
Electrocorticographic (ECoG) recordings from strip and grid electrodes were acquired at 3051.76 Hz and filtered between 0.5–300 Hz using a multichannel research system (Tucker Davis Technologies). Recordings were obtained during continuous periods of spontaneous rest when subjects were awake and resting comfortably in bed with their eyes closed. These resting epochs lasted for 3.5 m, 5 m, 4.5 m and 3.5 m for subjects 1–4, respectively. All data processing was performed off-line using custom routines in MATLAB (MathWorks).
Channels showing epileptiform activity or excessive noise were excluded from analysis. Of the remaining 226 clean electrodes (free of pathological/artifactual activity) from all subjects, 19 were located in PMC (clean/total in PMC = 7/8, 3/3, 4/8 and 5/10 for subjects 1–4 respectively; see Fig. 1–4) and are the focus of data reported here. Preprocessing involved notch filtering the data for line noise (60 Hz plus harmonics) and then referencing to a common average based on all clean electrodes for each subject. Estimates of raw power spectra (Fig. 1b & e) were calculated using a fast Fourier transform (FFT) of 2 s Hamming windows with 50% overlap (Welch's method). This data was used to then calculate band limited power (Fig. 5a) by integrating normalized (to total sum) power for the following frequency ranges: delta (1–3 Hz); theta (4–7 Hz); alpha (8–12 Hz); beta (13–29 Hz); gamma-low (30–79 Hz); gamma-high (80–200 Hz).
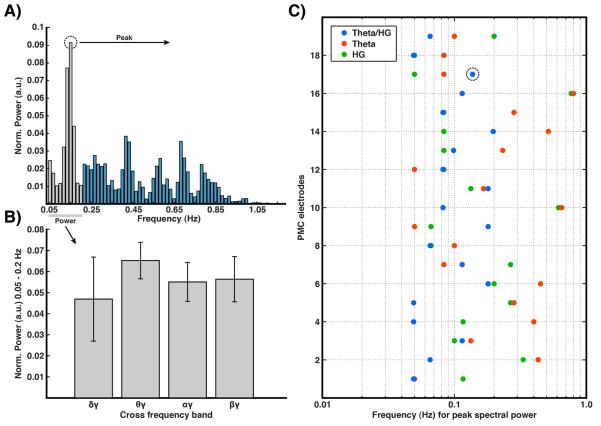
Figure 5. Slow fluctuation across additional frequency bands in PMC.
A) Example power spectrum of theta/HG modulation time series (as per figure 4b) for a single PMC electrode in subject 1. B) Mean normalized power between 0.05–0.2 Hz, highlighted gray in A, for delta/HG, theta/HG, alpha/HG and beta/HG modulation time series for all PMC electrodes. Given insufficient statistical power, theta/HG produced the greatest power within this range but was not statistically significant. C) Peak frequency of slow modulation spectrum (as shown in A) for theta/HG cross-frequency modulation as well as for theta and HG bands independently across all PMC electrodes. Theta/HG modulation shows consistent spectral peaks distributed around 0.1 Hz (mean 0.10 Hz), with HG and theta bands showing similar, though more varied, peak frequencies. Quantitatively, the standard deviation was clearly greater for the theta and gamma bands alone (0.22 & 0.19, respectively) than for the theta/gamma pair (0.05). A repeated measures analysis of variance showed the variance to be significantly different between these bands (F(2,36) = 6.21, p<.01).
Cross-frequency coupling was explored by focusing on phase-amplitude coupling (PAC) between multiple pairs of differing center frequencies. As the PAC measure employed is sensitive to phase distortion, all band-pass filtering was performed in the time domain with a two-way, non phase-lag, finite impulse response filter from the EEGLAB toolbox (Delorme and Makeig, 2004). Subsequently, the phase and amplitude of each band pass signal was obtained via a Hilbert transform, which gives a complex time series,
| (1) |
where ax[n] and ϕx[n] are the instantaneous amplitudes and phases, respectively. PAC was then estimated using the phase-locking value (PLV) (Lachaux et al., 1999) technique as described by Penny et al. (2008) and recently employed by Voytek et al. (2010) (See Cohen (2008) for a similar approach and Tort et al. (2010) for comparison with other PAC methods). With this approach, PAC is estimated by the PLV between the phase of a narrow band frequency (f1, the phase modulation) and the phase of a differing frequency band (f2, reflecting the amplitude modulation) whose amplitude is first band pass filtered between the phase-modulating frequency range of interest (f1) before its phase is extracted by a secondary Hilbert transform (removing changes in f2 amplitude not related to the specific phase modulating frequency of interest; f1). For example, in the case of theta phase-gamma amplitude PAC, the PLV/PAC is estimated by,
| (2) |
where ϕθ[n] is the theta phase times series and ϕaγθ[n] is the phase time series of the gamma amplitude that has been band pass filtered by the same theta range (for additional details see (Penny et al., 2008; Voytek et al., 2010)). Thus, PLVθγ (i.e. PACθγ) gives the absolute magnitude of the circular mean (vector length) difference between the theta (ϕθ[n]) and theta modulated gamma amplitude (ϕaγθ[n]) phase time series. PAC can therefore range between unity, which reflects perfect PAC, or zero which reflects no PAC.
Before exploring a broad plane of phase-amplitude interactions (comodulogram) (Canolty et al., 2006; Tort et al., 2010), we initially examined the level of PAC for the amplitude of a fixed high gamma band range (HG: 70–180 Hz) relative to the phase of multiple non-overlapping narrow-band frequencies of 2 Hz widths between 2–42 Hz (2–4Hz, 4–6 Hz etc.; Fig. 1), for comparison with the raw power spectrum. To clarify PAC differences between PMC and occipital sites, finer binning of 1 Hz steps between 2–20 Hz was used (Fig. 2). We focused our PAC comparison on occipital electrodes as we sought to make anatomical comparisons within subjects and were limited to the shared electrode locations across PMC subjects, which greatly restricts the possible regions of interest. We also feel the comparison to occipital alpha is a preferable test case in light of its clear empirical history and its anatomical proximity to PMC.
Figure 2. PAC frequencies differ between PMC and occipital cortex.
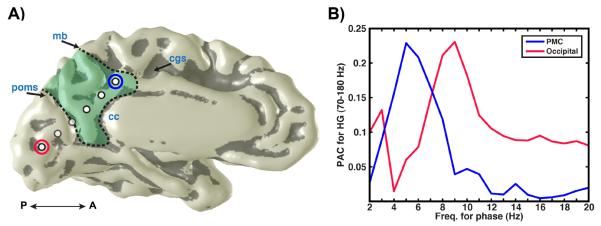
A) Medial view of PMC electrodes (left hemisphere) in subject 2 (labeling as per Fig. 1). B) PAC values (y-axis) for a fixed HG amplitude range across different frequencies of phase modulation (x-axis; 1 Hz bin steps) for example PMC (circled blue in A) and occipital (circled red in A) electrodes. HG amplitude is maximally modulated by theta-phase in PMC, whereas HG modulation is maximal at the alpha range for occipital cortex.
To show that the identified PAC for the HG amplitude range was selective, we created full phase-amplitude comodulograms covering a broad range of possible cross-frequency couplings. Comodulograms (e.g. Fig. 1c & f) were created using the same PAC method described above for amplitude frequencies ranging between 10–205 Hz and phase frequencies ranging between 1–21 Hz. Amplitude bins had a width of 10 Hz with 5 Hz steps (10–20 Hz, 15–25 Hz etc.). Phase bins were 2 Hz in width with 1 Hz steps (1–3 Hz, 2–4 Hz etc.). After identifying strong theta band spectral power and PAC between theta phase and HG amplitude, we explored the phase-angle preference of this modulation by creating theta trough-locked and phase-angle binned averages of HG amplitude in PMC (Fig. 3b & c). For group results theta/HG PAC and phase angle preference were statistically tested against surrogate data. Surrogate data was created using a standard shuffle technique, which splits the phase modulating (e.g. theta phase) time series ϕθ(t) of length n into two segments at a single random time point s (ϕθ(t1…ts)|ϕθ(ts+1…tn)) and reverses their order ϕθ(ts+1…tn)|(ϕθ(t1…ts)) (Canolty et al., 2006; He et al., 2010). This approach preserves the local phase evolution but disrupts the temporal relationship between the two phase series, and is therefore more conservative than phase randomization techniques (Hurtado et al., 2004). Surrogate mean and standard deviation were then calculated for each channel based on the distribution of 300 separate (i.e. single random time point split) shuffle iterations.
Figure 3. Phase preference of PMC cross-frequency coupling.
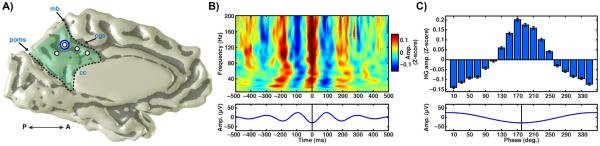
A) Medial view of PMC electrodes (left hemisphere) in subject 3 (labeling as per Fig. 1). B) Time-frequency average of normalized raw time series amplitude between 10–200Hz (Z-score, upper panel) locked to theta wave troughs (lower panel) for a PMC electrode shown in A (circled red). C) Mean HG amplitude (Z-score; upper panel) with standard error, binned to theta phase angle (non-overlapping 20° bins 0–360°, lower panel) for the same electrode. Z-score values were calculated by subtracting the mean from each time point of the gamma-amplitude series and dividing by the standard deviation.
Finally, after characterizing the basic frequency power and in turn cross-frequency coupling (PAC) of PMC electrodes, we then examined a further higher order nesting of the identified cross-frequency interaction in a more exploratory analysis of slow fluctuations. To quantify slow fluctuations in the PMC two frequency ranges were selected around the identified max comodulogram PAC values, F1 being the HG band (as above 70–180 Hz) on the amplitude (y) axis, and F2 being a 3 Hz band centered on the peak PAC value relative to the phase (x) axis (center typically 4–6 Hz). Using these selected bands the analysis then focuses simply on continuous changes in cross-frequency amplitude modulation time series, rather than ongoing changes in the calculated PAC values, which become poorly estimated for short data lengths. Subsequently the raw time series of each electrode was band-pass filtered by the frequency range defined by F1, its amplitude calculated via a Hilbert transform, and then filtered by the range defined by F2. This gives the same amplitude times series (aγθ[n]) whose phase is used for the PAC calculation above (ϕaγθ[n]. However, instead of taking the phase of this theta modulated gamma amplitude (aγθ[n]), the power spectrum was then estimated for the low pass filtered (< 1 Hz) amplitude of this signal (from a second Hilbert transform; aaγθ[n]), using a FFT (60 s Hamming window with 50% overlap). We excluded frequency bins whose period was greater than half the FFT window. This approach is similar to previous studies of slow changes in ECoG gamma power (Nir et al., 2008), but with a focus upon narrow band modulations of gamma power based on identified cross-frequency modulation, and subsequently quantifying the fluctuations of this signal. This basic approach of studying cross-frequency amplitude modulation time series allows a linking between time scales (i.e. to higher order moments), motivated by identified canonical oscillations in the power spectrum and their phase modulation of HG amplitude (PAC). The described slow fluctuations therefore represent shifts in cross-frequency comodulation (i.e. slow changes in the magnitude of theta modulated gamma amplitude).
3. Results
The first finding of our study suggests that the PMC can be distinguished from its nearby cortical regions in terms of its oscillatory properties at rest. As shown in Fig. 1, PMC has a clear spectral peak in the theta range (5 Hz), in contrast to sites in visual cortex of the same subject that show a strong alpha resonance (8 Hz). Furthermore, the PMC shows maximal PAC between theta band phase and HG amplitude while electrodes in the lateral and mesial visual cortex show a clear PAC relationship between alpha band phase and HG amplitude. For the example PMC electrode in Fig. 1a, PAC for HG amplitude is reduced by 42% when comparing theta to alpha phase (θPAC = 0.24; αPAC = 0.14). Conversely, PAC for HG amplitude is reduced by 63% for the example occipital electrode in Fig. 1d when comparing alpha to theta phase (αPAC = 0.48; θPAC = 0.18). In this respect, the dominant local oscillation at a given cortical site can be a strong determinant of cross-frequency coupling, in this case the preferred phase modulating frequency of HG amplitude.
Differences in PAC preference are also observed between PMC and mesial occipital cortex in the same subject where interhemispheric recordings extended across the PMC over the parieto-occipital sulcus into cuneus/occipital lobe as shown in Fig. 2. Using finer frequency steps for phase bins (1 Hz as per comodulograms), a clear separation of PAC value distribution is observed between the occipital alpha phase and the PMC theta phase influence on HG amplitude (Fig. 2b). This distribution of PAC values was significantly different between PMC and occipital cortex (Kolmogorov-Smirnov = 0.63, p<0.001). For the example sites shown in Figure 2, PMC has maximal HG amplitude modulation around 5 Hz theta phase (θPAC = 0.23; αPAC = 0.04), whereas occipital cortex shows peak HG amplitude modulation around a 9 Hz alpha phase (αPAC = 0.23; θPAC = 0.06).
Given the clear modulation of HG amplitude by theta phase in PMC, we explored the phase-angle preference of this modulation. As shown in Fig. 3, HG amplitude is maximal at the trough of the ongoing theta band phase based on the averaged spectrogram amplitude locked to theta wave troughs. To more sensitively assess phase-angle preference, we also studied differences in HG amplitude binned to fixed theta phase angles (Fig. 3b). As shown in Fig. 3c, normalized HG amplitude was maximal around the theta wave trough (~180°) and minimal around the theta wave peak (~0°/360°). For the example PMC electrode in Fig. 3a, PAC for HG amplitude is maximal for theta phase and reduced by 53% when compared to alpha phase (θPAC = 0.32; αPAC = 0.15).
In light of this clear cross-frequency coupling between theta and HG bands in the PMC, the raw time series for each electrode was then examined for evidence of slow amplitude fluctuations within the filtered range of theta modulated gamma amplitude (aγθ[n]). Whereby slow fluctuations were identified as peaks below 1 Hz in the power spectrum of the amplitude for this modulation time series (i.e. FFT of aaγθ[n]). In this analysis, we used frequency ranges identified with maximal PAC values (max phase frequency within the theta band) in order to better identify signals that are likely to be influencing local spontaneous cortical activity, and as a link to the specific pre-identified PAC for each channel. As can be seen in Fig. 4, the magnitude of theta/HG amplitude modulation fluctuates at slow time scales, which for the example electrode shown is maximally peaked at 0.083 Hz, a value that is consistent with previous ECoG and fMRI data (Baria et al., 2011; Cordes et al., 2001; Nir et al., 2008). However, additional peaks in spectral power are also observed and may equally reflect signals of interest as discussed below.
To more broadly understand the nature of these slow fluctuations, we compared theta/HG modulation with other canonical oscillations (i.e. delta, alpha & beta modulation of HG; Fig. 5). Taking the mean power between 0.5–0.2 Hz (highlighted gray in Fig. 5a) for all PMC electrodes, theta/HG produced the largest power level, but was not significantly greater than the same value for delta, alpha or beta HG modulation (Fig. 5b). When comparing across all PMC electrodes the mean maximal spectral peak (as per Fig. 4) was 0.1 Hz (Fig. 5c). To compare the contribution of independent changes in theta and HG power, we computed the same power spectrum peak values as was done for theta/HG for these bands. It is of note that when comparing the values from theta/HG modulation to HG or theta bands independently, greater variation of the peak frequency is observed for the theta and HG bands. The standard deviation, across all PMC electrodes, of the peak spectral power frequency for theta and HG bands alone was 0.22 and 0.19, respectively. While the standard deviation of theta/HG peak frequency across all PMC electrodes was 0.05, and therefore a significant difference in variance of peak frequency across the bands was observed (F(2,36) = 6.21, p<0.01).
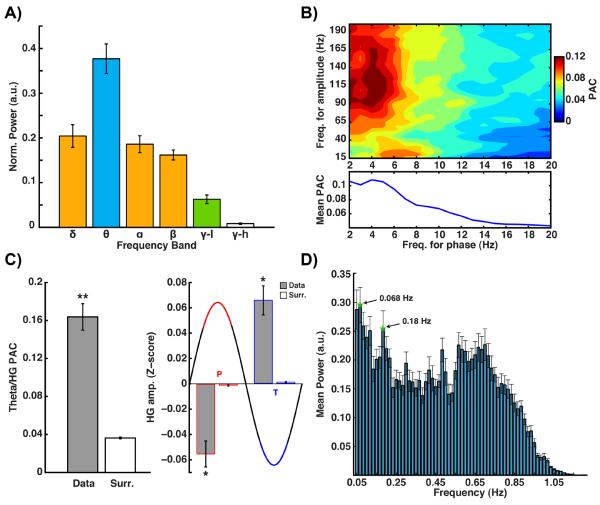
Findings from all four subjects were verified in the analysis of pooled data from all PMC sites recorded (n=19). As shown in Fig. 6, group data show that, across different subjects, the PMC is particularly dominated by theta band oscillations (significantly more power than all other bands, p<0.001, Bonferroni corrected), which in turn produces the chief modulation of HG amplitude at rest (Fig. 6a & b). Comparison of PAC values with shuffled surrogate data showed the theta phase modulation of HG amplitude to be statistically significant (p<0.00001,Wilcoxon rank sum), and therefore the PMC values for PAC reflected genuine temporal cross-frequency coupling (Fig. 6c). The nature of this modulation is for HG amplitude to be maximal at theta phase trough (180°). When compared with surrogate data, HG amplitude was significantly greater for theta wave troughs (t(18) = 5.03, p<0.001, one sided) and significantly lower for theta wave peaks (t(18) = −4.57, p<0.001, one sided) (Fig. 6c). Consequently, HG amplitude was significantly different for theta wave troughs vs. peaks (t(18) = 4.89, p<.001, one sided). Across subjects, the magnitude of the theta modulated gamma amplitude signal itself also showed slower time scale fluctuations maximally at 0.068 Hz (peak also at 0.18 Hz) across all PMC sites (Fig 6d).
Figure 6. Power spectrum, cross-frequency coupling and slow fluctuations in PMC.
A) Normalized mean band power (with standard error) for all PMC electrodes (n = 19, all subjects). Bands with differing fill color are significantly different at p<0.001 (corrected). B) Mean comodulogram (upper panel) for all PMC electrodes (lower panel shows mean PAC for each phase bin). C) Mean theta/HG PAC value for real and surrogate data (left plot, ** = p<0.00001); mean normalized HG amplitude (Z-score) for real and surrogate data (right plot) at theta wave peak (P, red) or trough (T, blue) (* = p<0.001). Peak reflects 90° angle centered at 0°, troughs reflect 90° angle centered at 180°. D) Mean power spectrum, with standard error, of slow theta/HG fluctuations.
4. Discussion
4.1 Significant findings
Using direct intracranial recordings from the human PMC, we made the following observations: 1) within the PMC there is a predominance of theta oscillations, peaking in the 4–5 Hz range, during the resting state; 2) This differentiates the PMC from its neighboring visual cortices that oscillate in the alpha range during the resting state; 3) the amplitude of ongoing resting gamma oscillations within the PMC is modulated to be maximal at the theta wave trough; 4) the power of theta-modulated high gamma power as well as theta and gamma bands alone were subject to slow fluctuations at time scales similar to those observed for resting state networks with fMRI and ECoG (~0.1 Hz) (Baria et al., 2011; Cordes et al., 2001; Nir et al., 2008).
4.2 Canonical oscillations within the hub of the default mode network (DMN)
As a functional and structural hub of the DMN, the PMC has been implicated in a wide variety of cognitive processes and clinical conditions (Buckner et al., 2008). We sought to quantify the key oscillatory properties of PMC at rest that could potentially provide some basic building blocks for understanding the cognitive electrophysiology of this region. Similar to other canonical rhythms across the cortical surface, our data suggests that the signature of canonical oscillations in the PMC is in the range of theta band frequency, which modulates HG power at rest. More importantly, the oscillatory preference in the PMC was easily distinguishable from adjacent cortical sites, which displayed their own spectral preference (e.g. occipital 8–10 Hz alpha).
4.3 Cross-frequency coupling of theta and high gamma bands during resting state
Canolty et al. (2006) provided clear evidence for the modulation of HG power by theta band phase in human cortex across a large sampling of cortical sites on the lateral surface. More recently, Voytek et al. (2010) reported a shift from theta-gamma to alpha-gamma PAC particularly at posterior sites during visual task conditions. Our findings support these anatomical differences in PAC, but do so for data acquired during passive resting states. Using a novel technique to explore multiple modes of phase modulation, Miller et al. (2010) recently reported clear differences in cross-frequency coupling at the gyral scale in visual cortex during task conditions. Together such data suggests that phase-amplitude modulation can be identified at rest; that it differs between putative functional and structural brain areas; and that it can be selectively modified by task conditions. In addition to local couplings (single channel), PAC in human ECoG can also be observed between distal (inter-electrode) sites and show a variety of phase and amplitude frequency preferences (Maris et al., 2011). Given the evidence that canonical cortical oscillations influence local excitability (Canolty and Knight, 2010; Jacobs et al., 2007; Lakatos et al., 2005), it will be of future interest to explore if these rhythms also provide a platform for selective communication between brain regions that share similar oscillatory preferences (Canolty et al., 2010), either intrinsically at rest or transiently through task modulation. In light of this suggestion, we hypothesize that the functional significance of theta oscillations in PMC is related to a shared oscillatory dynamic with the medial temporal lobe (MTL) and other association cortices. The role of theta oscillations in coordinating ensemble activity in the hippocampus is well characterized (Buzsaki, 2002), and given the strong anatomical connections between PMC and MTL (Parvizi et al., 2006), and the clear involvement of PMC in episodic memory processing (Cabeza and St Jacques, 2007; Hassabis and Maguire, 2009; Schacter et al., 2007; Sestieri et al., 2011; Spreng and Grady, 2010; Vannini et al., 2011; Wagner et al., 2005), this interaction shows some promise but requires further investigation. Of particular interest is the possibility of heterogeneity in PAC across PMC, which may accord with observed functional subdivisions (Dastjerdi et al., 2011). Theta predominance may be but one of several oscillatory modes within the PMC when exploring across a wider range of cognitive states, which is more pronounced at sites/subregions directly interacting with the MTL such as the retrosplenial cortex (Andrews-Hanna et al., 2010).
4.4 Slow Fluctuations
Our analysis also revealed magnitude shifts of the theta modulated HG signal in PMC at slow time scales. These changes in magnitude showed a more consistent spectral peak when considering the cross-frequency theta modulation of HG amplitude, than for theta or HG amplitude individually. Given that HG activity likely reflects local activation, and that at rest most cortical regions show dominant low frequency “canonical” oscillations, our findings may suggest a novel way of looking for slow changes of excitability by studying shifts of power in the cross-frequency interaction between the local HG and canonical oscillatory activity (e.g., magnitude changes in the theta modulated HG amplitude, as we have reported here). It should be noted that modulations in theta or HG power by themselves are not independent from the combination of the two. Rather, what we have shown is that in terms of slow modulation, their combination is less variable across electrodes and possibly a more interpretable process given the a priori identification of their significant cross-frequency interaction. Indeed, while theta phase can account for some of the variance in the HG amplitude, additional factors on multiple time scales will be influencing this process also (e.g. other cross-frequency influences Fig. 5). These slower fluctuations may reflect an additional nesting (Lakatos et al., 2005) linked to increased magnitude of the ongoing canonical oscillation (e.g., theta or alpha), which is known to enhance the strength of HG amplitude phase modulation (Canolty et al., 2006), highlighting the close relationship between the slow modulations of theta or HG bands independently with their mutual modulation.
Additionally, it should be noted that the spectrum of slow fluctuations shows power peaks at additional frequencies >0.1 Hz which may also be of interest, but are typically not observed with hemodynamic signals due to low pass filtering and poor signal to noise at frequencies which overlap with known artifact signals (e.g. cardiovascular/ballistic processes). Therefore the importance of these other frequencies should not be excluded, along with the role of other canonical oscillations in modulating gamma amplitude. Elucidating these contributions and their anatomical variation is a clear direction for future investigations.
Fluctuations in gamma band power at slow time scales during non-task and task conditions was reported by Nir et al. (2008) to be maximally correlated across interhemispheric sites (e.g. auditory cortex) for a range below 0.1 Hz (cf. 0.1–1 Hz or <1 Hz). The slow shifts in theta modulated gamma power reported here fall into a similar range (max power ~0.07 Hz, Fig. 6). The frequency of slow fluctuation in the magnitude of theta-modulated HG power, as we report here, is familiar to those studying slow oscillations in fMRI data as a means to identify resting state networks (Cordes et al., 2001). Recent evidence also suggests that similar to resting canonical oscillations these slow fluctuations may differ across functional-anatomic boundaries (Baria et al., 2011). Our finding of a third-order nesting of slow fluctuations of electrocortical signals is intriguing but exploratory at this stage and in need of future examination. For example, consistent with the notion of hierarchically nested oscillations, future investigations should seek to relate the slow cross-frequency modulation reported here with infra-slow (0.01–0.1 Hz) fluctuations of raw potentials recorded at the scalp or cortical surface, and their behavioral correlates (Monto et al., 2008). More generally it is important to emphasize that the maximal peaks of the slow spectra reported here may not be the most important nor sole frequency of interest to the study resting state networks. As electrophysiological data is more amenable to the analysis of a wider frequency range, a broader domain of slow fluctuation may be relevant to understanding long range resting state or task correlations.
4.5 Conclusion
Functional brain networks show both intrinsic resting and task related correlations across multiple spatial scales (Fox et al., 2005; Leopold et al., 2003; Tsodyks et al., 1999). Also this functional connectivity reflects temporal correlation between local and distant brain regions at fast and slow temporal scales. As the core node of the DMN, the PMC is functionally connected to a host of cortical sites in the temporal, parietal and frontal lobes (Parvizi et al 2006). Although a large neuroimaging literature exists for the PMC and its interactions with these other cortical networks, little is known about the electrophysiology of this region and its functional interactions. We recently reported an increase of broadband gamma activity within the PMC during short and extended episodes of rest (Dastjerdi et al., 2011) that likely reflects local increases in population spiking activity (Miller, 2010). In this study, we now report the effect of theta band oscillations in coordinating local PMC activity at fast and slow time scales, which may be an important signature for functional communication between the PMC and other nodes of the DMN.
Highlights
The predominant resting oscillation in the human posteromedial cortex (PMC) is in the theta band (3–7Hz) range
The resting PMC theta oscillations distinguish it from the adjacent occipital cortex where resting alpha oscillations predominate
The phase of theta oscillations modulates the amplitude of high gamma activity in the human PMC
Theta/Gamma cross-frequency modulation shows magnitude fluctuations at very slow frequencies (~0.1 Hz)
PMC theta oscillations may be linked to its functional interactions with the medial temporal lobes and other associative cortices.
Acknowledgements
We thank Bradley Voytek for open code sharing; Kai Miller, Dora Hermes, Mohammad Dastjerdi and Christopher Honey for helpful comments. This study was funded by a seed grant from Stanford University Institute of Medicine and the Stanford Institute for NeuroInnovation and Translational Neurosciences (SINTN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adrian ED, Matthews A. The Berger rhythm: potential changes from the occipital lobes in man. Brain. 1934;57:355–385. doi: 10.1093/brain/awp324. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baria AT, Baliki MN, Parrish T, Apkarian AV. Anatomical and Functional Assemblies of Brain BOLD Oscillations. J Neurosci. 2011;31:7910–7919. doi: 10.1523/JNEUROSCI.1296-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Cabeza R, St Jacques P. Functional neuroimaging of autobiographical memory. Trends Cogn Sci. 2007;11:219–227. doi: 10.1016/j.tics.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM, Knight RT. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313:1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty RT, Ganguly K, Kennerley SW, Cadieu CF, Koepsell K, Wallis JD, Carmena JM. Oscillatory phase coupling coordinates anatomically dispersed functional cell assemblies. Proc Natl Acad Sci U S A. 2010;107:17356–17361. doi: 10.1073/pnas.1008306107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends Cogn Sci. 2010;14:506–515. doi: 10.1016/j.tics.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX. Assessing transient cross-frequency coupling in EEG data. J Neurosci Methods. 2008;168:494–499. doi: 10.1016/j.jneumeth.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Dastjerdi M, Foster BL, Nasrullah S, Rauschecker AM, Dougherty RF, Townsend JD, Chang C, Greicius MD, Menon V, Kennedy DP, Parvizi J. Differential electrophysiological response during rest, self-referential, and non-self-referential tasks in human posteromedial cortex. Proc Natl Acad Sci U S A. 2011;108:3023–3028. doi: 10.1073/pnas.1017098108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Jirsa VK, McIntosh AR. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat Rev Neurosci. 2011;12:43–56. doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Maguire EA. The construction system of the brain. Philos Trans R Soc Lond B Biol Sci. 2009;364:1263–1271. doi: 10.1098/rstb.2008.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY, Smith DV, Platt ML. Electrophysiological correlates of default-mode processing in macaque posterior cingulate cortex. Proc Natl Acad Sci U S A. 2009;106:5948–5953. doi: 10.1073/pnas.0812035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY, Smith DV, Platt ML. Cognitive control signals in posterior cingulate cortex. Front Hum Neurosci. 2010;4:223. doi: 10.3389/fnhum.2010.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He BJ, Zempel JM, Snyder AZ, Raichle ME. The temporal structures and functional significance of scale-free brain activity. Neuron. 2010;66:353–369. doi: 10.1016/j.neuron.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado JM, Rubchinsky LL, Sigvardt KA. Statistical method for detection of phase-locking episodes in neural oscillations. J Neurophysiol. 2004;91:1883–1898. doi: 10.1152/jn.00853.2003. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Kahana MJ, Ekstrom AD, Fried I. Brain oscillations control timing of single-neuron activity in humans. J Neurosci. 2007;27:3839–3844. doi: 10.1523/JNEUROSCI.4636-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper H, Penfield W. Electrocorticograms in man: effect of voluntary movement upon the electrical activity of the precentral gyrus. Archiv Psychiatrie Zeitschrift Neurol. 1949;183:163–174. [Google Scholar]
- Jerbi K, Vidal JR, Ossandon T, Dalal SS, Jung J, Hoffmann D, Minotti L, Bertrand O, Kahane P, Lachaux JP. Exploring the electrophysiological correlates of the default-mode network with intracerebral EEG. Front Syst Neurosci. 2010;4:27. doi: 10.3389/fnsys.2010.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Hum Brain Mapp. 1999;8:194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos P, Shah AS, Knuth KH, Ulbert I, Karmos G, Schroeder CE. An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J Neurophysiol. 2005;94:1904–1911. doi: 10.1152/jn.00263.2005. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Murayama Y, Logothetis NK. Very slow activity fluctuations in monkey visual cortex: implications for functional brain imaging. Cereb Cortex. 2003;13:422–433. doi: 10.1093/cercor/13.4.422. [DOI] [PubMed] [Google Scholar]
- Maris E, van Vugt M, Kahana M. Spatially distributed patterns of oscillatory coupling between high-frequency amplitudes and low-frequency phases in human iEEG. NeuroImage. 2011;54:836–850. doi: 10.1016/j.neuroimage.2010.09.029. [DOI] [PubMed] [Google Scholar]
- Miller KJ. Broadband spectral change: evidence for a macroscale correlate of population firing rate? J Neurosci. 2010;30:6477–6479. doi: 10.1523/JNEUROSCI.6401-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KJ, Hermes D, Honey CJ, Sharma M, Rao RP, den Nijs M, Fetz EE, Sejnowski TJ, Hebb AO, Ojemann JG, Makeig S, Leuthardt EC. Dynamic modulation of local population activity by rhythm phase in human occipital cortex during a visual search task. Front Hum Neurosci. 2010;4:197. doi: 10.3389/fnhum.2010.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KJ, Weaver KE, Ojemann JG. Direct electrophysiological measurement of human default network areas. Proc Natl Acad Sci U S A. 2009;106:12174–12177. doi: 10.1073/pnas.0902071106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monto S, Palva S, Voipio J, Palva JM. Very slow EEG fluctuations predict the dynamics of stimulus detection and oscillation amplitudes in humans. J Neurosci. 2008;28:8268–8272. doi: 10.1523/JNEUROSCI.1910-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir Y, Mukamel R, Dinstein I, Privman E, Harel M, Fisch L, Gelbard-Sagiv H, Kipervasser S, Andelman F, Neufeld MY, Kramer U, Arieli A, Fried I, Malach R. Interhemispheric correlations of slow spontaneous neuronal fluctuations revealed in human sensory cortex. Nat Neurosci. 2008;11:1100–1108. doi: 10.1038/nn.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossandon T, Jerbi K, Vidal JR, Bayle DJ, Henaff MA, Jung J, Minotti L, Bertrand O, Kahane P, Lachaux JP. Transient suppression of broadband gamma power in the default-mode network is correlated with task complexity and subject performance. J Neurosci. 2011;31:14521–14530. doi: 10.1523/JNEUROSCI.2483-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvizi J, Van Hoesen GW, Buckwalter J, Damasio A. Neural connections of the posteromedial cortex in the macaque. Proc Natl Acad Sci USA. 2006;103:1563–1568. doi: 10.1073/pnas.0507729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny WD, Duzel E, Miller KJ, Ojemann JG. Testing for nested oscillation. J Neurosci Methods. 2008;174:50–61. doi: 10.1016/j.jneumeth.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nat Rev Neurosci. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Sestieri C, Corbetta M, Romani GL, Shulman GL. Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. J Neurosci. 2011;31:4407–4420. doi: 10.1523/JNEUROSCI.3335-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Grady CL. Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. J Cogn Neurosci. 2010;22:1112–1123. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- Tort AB, Komorowski R, Eichenbaum H, Kopell N. Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J Neurophysiol. 2010;104:1195–1210. doi: 10.1152/jn.00106.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsodyks M, Kenet T, Grinvald A, Arieli A. Linking spontaneous activity of single cortical neurons and the underlying functional architecture. Science. 1999;286:1943–1946. doi: 10.1126/science.286.5446.1943. [DOI] [PubMed] [Google Scholar]
- Vannini P, O'Brien J, O'Keefe K, Pihlajamaki M, Laviolette P, Sperling RA. What goes down must come up: role of the posteromedial cortices in encoding and retrieval. Cereb Cortex. 2011;21:22–34. doi: 10.1093/cercor/bhq051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B, Canolty RT, Shestyuk A, Crone NE, Parvizi J, Knight RT. Shifts in gamma phase-amplitude coupling frequency from theta to alpha over posterior cortex during visual tasks. Front Hum Neurosci. 2010;4:191. doi: 10.3389/fnhum.2010.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]