Abstract
Objective
Women are disproportionately affected by musculoskeletal disorders. Parous women appear to be at particularly elevated risk for structural and functional changes in the lower limbs. The combination of increased weight on joints with potentially greater laxity during pregnancy could lead to permanent structural changes in feet. Although arches may become lax during pregnancy, it is unknown whether changes persist. The objective of this study was to determine whether arch height loss persists postpartum.
Design
Forty-nine women completed this longitudinal study. Static and dynamic arch measurements were collected in first-trimester and at 19 weeks postpartum. Linear mixed models were used to determine whether outcome measures significantly changed overall or by parity.
Results
Arch height and rigidity index significantly decreased, with concomitant increases in foot length and arch drop. The first pregnancy accounted for the reduction in arch rigidity and increases in foot length and arch drop. No changes were detected in the center of pressure excursion index.
Conclusions
Pregnancy appears to be associated with a permanent loss of arch height and the first pregnancy may be the most significant. These changes in the feet could contribute to the increased risk for musculoskeletal disorders in women. Further research should assess the efficacy of rehabilitative interventions for prevention of pregnancy-related arch drop.
Keywords: Pregnancy, Arch, Musculoskeletal, Women
Women are disproportionately affected by musculoskeletal disorders, which are a significant cause of functional limitations and disability. Studies have found a higher prevalence of chronic joint pain (1.3x),1 including foot pain (1.3x),2, 3 knee pain (1.3x),4 hip pain (1.4x),4–6 greater trochanteric pain syndrome (3.3x),7 and low back pain (1.2x)8 in women than in men. In addition, women are at higher risk for osteoarthritis,9 especially in the knee (1.8x),10 in comparison with men. Studies have suggested that the increased risk for musculoskeletal problems may, in part, relate to biochemical and biomechanical changes that occur in a woman’s body during pregnancy.
Vullo and colleagues11 have suggested that there may be musculoskeletal changes that persist following pregnancy, as parous women are more likely to develop new lower limb musculoskeletal disorders than are nulliparous women. The increase in body mass12 in combination with a seven to ten-fold increase in the relaxin hormone level during pregnancy13, 14 have the potential to place atypical stresses on the musculoskeletal system. It is possible that acute or chronic pathomechanics, seen as deviant arthrokinematics might contribute to structural changes that may have long-term consequences.
Studies have reported increases in foot length, width, and volume during pregnancy.15, 16 The increased foot width has been attributed to downward movement of the head of the talus in the context of body weight and relaxin effects on the arch, the first metatarsophalangeal joint and the subtalar joint during pregnancy.17 Block and colleagues17 studied the increased hindfoot pronation that occurs during pregnancy and reported that the talus drops approximately 1 cm in association with loss of static arch height and is accompanied by increased subtalar and first metatarsophalangeal joint range of motion. In addition to the anatomic changes in the foot, there are also changes in gait pattern during pregnancy.18–20 Nyska found that, during pregnancy, the center of pressure on the foot shifts posteriorly to compensate for the increased anterior abdominal mass.21
The combination of ligamentous laxity in the arch, increased body mass and the shift in the center of pressure towards the posterior part of the foot during pregnancy may contribute to change in length of the ligaments supporting the arch, leading to loss of arch height. In turn, changes in foot biomechanics that occur with changes in the foot structure can alter the normal control of forces propagating from the foot to more proximal lower limb joints and spine22 and may contribute to pain in the feet, knees, and hips.23 Therefore, disruption of the interaction between skeletal and musculotendinous and ligamentous structures through loss of arch height may predispose to painful musculoskeletal conditions.
Perhaps more important than the foot changes that have been reported during pregnancy is the issue of whether these changes return to baseline or persist postpartum. Although numerous scientific studies have assessed the characteristics of the arch during pregnancy, they have not reported whether the changes persisted long-term. We are aware of only one case report,24 and a myriad of anecdotal reports of changes in arch height and foot width that did not resolve postpartum. However, there is a need to assess the validity of these reports through prospectively studying whether there are permanent changes in foot structure. Therefore, the purpose of this study was to determine whether altered foot structure persists following pregnancy by assessing static arch structure and dynamic arch function during the first trimester of pregnancy and 4–5 months postpartum. We hypothesized that a significant reduction in arch height persists postpartum, evident during static and dynamic conditions.
METHODS
Participants
Sixty-one women between 18 and 40 years of age in their first trimester of pregnancy volunteered for the study. Participants were recruited from clinics affiliated with the University of Iowa Hospitals and Clinics (UIHC), the clinical trials website at the UIHC, and local daycare facilities, schools, and pregnancy exercise classes. Exclusion criteria included: women participating in in-vitro fertilization, prior lower limb joint surgery or spinal surgery, chronic diseases affecting collagen metabolism, and women who were not mobile or had surgeries that may affect their walking. This study was reviewed and approved by the University of Iowa’s Institutional Review Board and all participants participated in an informed consent process, culminating in providing written informed consent prior to enrollment.
Participants’ first visit was during their first trimester (10.3 ± 1.6 weeks) and follow-up visit was approximately 19 weeks postpartum (18.9 ± 4.3 weeks). This duration of follow-up was selected, as changes in the musculoskeletal system have not been reported to persist past six weeks post-partum and the blood levels of hormones known to affect collagen return to normal within 48 hours of delivery. Pregnancy information was collected and a health questionnaire, static (dorsal arch height, foot length, truncated foot length, arch height index, and arch rigidity index) and dynamic (center of pressure excursion index, CPEI) measurements were completed at baseline and follow-up visits. General joint laxity was assessed at baseline.
Measures
Pregnancy Information and Health Questionnaire
Body mass (kg), height (cm), BMI (kg/m2), and pregnancy information (i.e. delivery date and weight at delivery) were retrieved from each participant’s medical record. In addition, participants completed a medical history questionnaire regarding previous ankle, knee or foot surgeries or injuries and shoe size or ach height changes during past pregnancies (if applicable). The questionnaire also included information about the number of previous pregnancies, whether participants had noticed a change in shoe size since age 18, and medical utilization during previous pregnancies. A modified version of this questionnaire was administered at follow-up where participants were asked about perceived changes in shoe size or arch height, arch supporting insole use, lower limb injuries and medical utilization for lower limb concerns during pregnancy.
General Joint Laxity
General joint laxity was recorded during participants’ first visit using the Beighton Hypermobility test, with a score of 4 or greater indicating hypermobility.25, 26
Static Arch Height Index (AHI) and Arch Rigidity Index
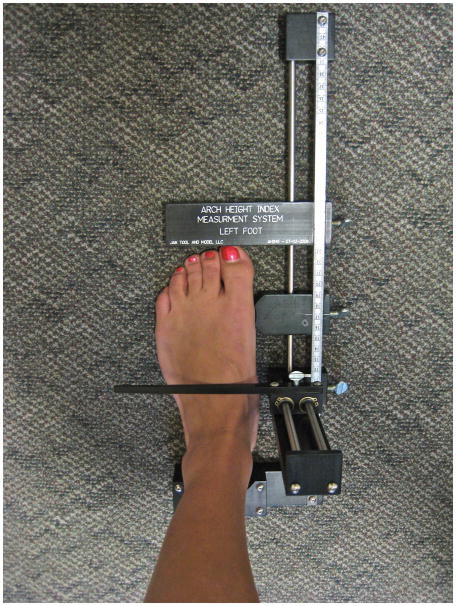
Foot anthropometrics were measured during both standing (weight bearing) and sitting (non-weight bearing) conditions, using the Arch Height Index Measurement System (AHIMS) (Jak Tool and Model, LLC, Matawan, NJ) (Figure 2). The rationale for measurements both standing and sitting is to measure the magnitude of drop in the arch and rigidity of the arch, comparing weight bearing with a relatively non-weight bearing condition. The AHIMS is a reliable and valid method of characterizing arch height based on bony landmarks (intra and inter-rater reliability ICC: 0.96–0.99).27
Figure 2.
Arch Height Index Measurement System (AHIMS) for arch height measurement during weight bearing and non-weight bearing conditions
The relatively non-weight bearing measurement28 was conducted with the participant seated in a chair with hips and knees flexed at 90° and feet resting on the floor. The weight-bearing measurement was conducted with the participant maintaining a natural comfortable stance, with feet shoulder width apart and weight evenly distributed. In each position, once the heel cup was placed firmly against the participant’s heel, a horizontal sliding caliper was slid forward until it gently touched the most prominent toe to obtain the total foot length (ToFL). Another horizontal sliding caliper, with a concave edge, was positioned around the medial aspect of the first metatarsophalangeal joint. The distance from the first metatarsophalangeal joint to the heel cup was measured as the truncated foot length (TrFL). A third caliper was positioned at 50% of the total foot length. An integrated vertical caliper was positioned on the dorsum of the foot to provide a measure of the dorsum height of the foot at 50% of the foot length. The dorsum height was recorded as arch height. Two measurements were taken in each position and averaged unless measurement differences were greater than 2 mm, then a third set of measurements was collected. The arch height index (AHI) was calculated as the dorsum height at 50% of total foot length divided by the ipsilateral truncated foot length (TrFL).
Arch drop was used to determine the amount of flexibility that occurs in the foot when transferring from a seated to a standing position. Arch drop was calculated by subtracting the recorded arch height at standing from the recorded arch height at sitting. The arch rigidity index describes the ability of the foot to maintain the structural arch when placed in a weight-bearing position. The arch rigidity index, a measure of foot flexibility, was determined by dividing the standing AHI by the sitting AHI. A value of 1.0 would indicate a perfectly rigid arch, while smaller values would indicate a more flexible arch.29
Center of Pressure Excursion Index (CPEI)
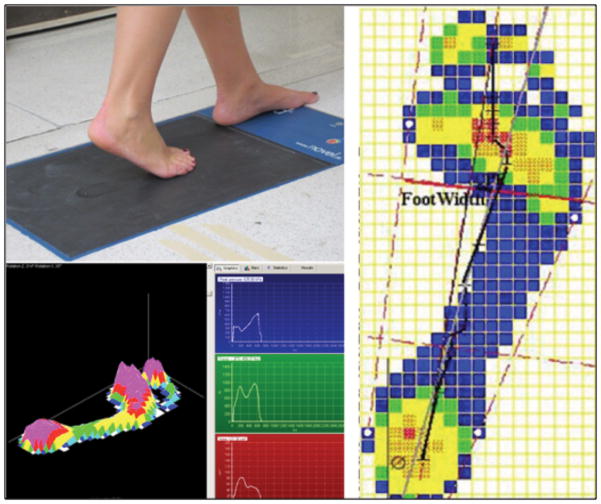
Paired measurements at both baseline and follow-up were collected with participants walking barefoot at their preferred gait speed over either an Emed (N=17) (SF 2016/2, Novel, St. Paul, MN) or a Tekscan (Hugemat 5400, South Boston, MA) (N=34) pressure plate. Five trials per foot were collected and averaged to minimize bias in the measurement. Data were collected at 50 Hz on Emed and 60 Hz on Tekscan with a 15 kPa threshold. Once the distribution of barefoot plantar pressure was collected, a program, custom-written in Matlab (Version 7.8.0 R2009a, Natick, MA, USA), was used to calculate the CPEI: lateral displacement of the center of pressure curve from a reference line drawn from the initial to the final centers of pressure during stance phase of gait, and standardized to the width of the anterior third of the foot. The CPEI is represented by a black line in Figure 3.
Figure 3.
Measurements of arch function (a) pressure platform (top left), (b) foot pressures during walking (bottom left), and (c) center of pressure excursion (black line on right)
Statistical Analysis
Statistical analyses were performed using SAS software version 9.2 (SAS Institute Inc., Cary, NC) with alpha level set at p<0.05. Participants were categorized by parity level as first, second or third or more pregnancies as previous data have suggested a threshold may exist for changes in foot structure and joint laxity26 that occurs in conjunction with a second pregnancy.
A prospective power calculation, based on a mean ± SD arch height of 63 ± 5 mm, with an anticipated change of 3 mm when transitioning from sitting to standing in healthy young women,27 an anticipated clinically significant drop of 4 ± 5 mm within group (i.e. whether pregnancy led to a persistent drop in arch height), and 5 ± 5 mm between groups (i.e. whether there is a threshold for parity at which AHI changes more than at other parity levels), using a one-way ANOVA with Bonferroni’s method of all pair-wise comparisons, demonstrated that a sample size of 16 participants per group (1st pregnancy, 2nd pregnancy, and 3rd pregnancy or greater) would have 85% power to detect a reduction in arch height of at least this magnitude at an alpha level of 0.05. This estimate was conservative, as each subject contributed two observations to the study, enhancing statistical power to detect inter-group differences.
Categorical data were summarized with frequencies and percentages and continuous data were examined with scatter plots and tests for normality prior to summarizing with means and standard deviations. Linear mixed models for repeated measures (2 limbs and 2 time-points per participant) were used to assess for a significant change in standing AHI, comparing early pregnancy with 19 weeks postpartum, while controlling for age, Beighton score, weight gain during pregnancy and difference in body weight between baseline and follow-up (to account for incomplete return to baseline pre-partum weight) in addition to controlling for covariance between limbs within participants. Parity level (1, 2, ≥3) was included in models to test the interaction between change over time and parity. A positive interaction would indicate that there was a differential effect on the degree of change in foot parameters related to pregnancy number. Similar analyses were completed to assess changes in foot length, arch drop, arch rigidity index, and dynamic CPEI.
RESULTS
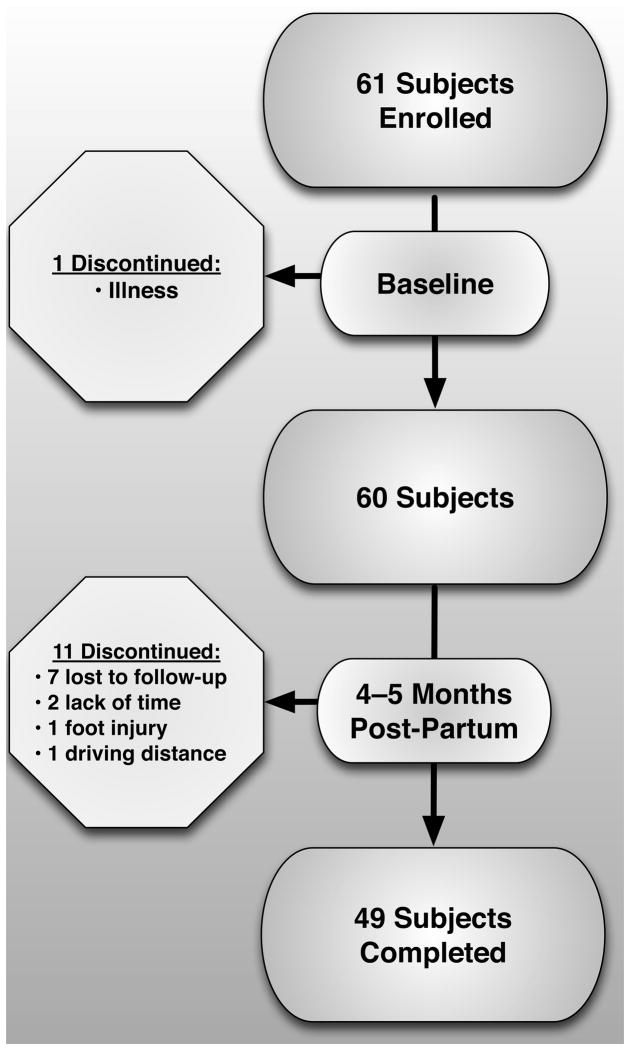
A total of 60 women (mean ± SD age 29.2 ± 4.3 years, and BMI 26.0 ± 5.4 kg/m2) were enrolled in the study (Figure 1). As shown in Table 1, there were 35, 20, and 5 women in the 1st, 2nd, and 3rd or greater parity groups, respectively. Women on their 3rd or greater pregnancy were significantly older (p=0.0008). However, there were no significant differences in BMI, joint laxity score, or gestational age at enrollment, comparing parity groups. As shown in Table 2, there was an average body mass gain during pregnancy of 14.2±4.3 kg. However, body mass at follow-up was only 2.1±3.5 kg greater than at baseline. Eleven women discontinued their participation in the study (7 lost to follow-up, 2 reported lack of time, 1 had a foot injury and 1 due to driving distance). A total of 49 women completed both the baseline and follow-up visits.
Figure 1.
Summary of participant involvement
Table 1.
Baseline Participant Characteristics by Parity Group
| Parity Group | 1st (mean±SD) | 2nd (mean±SD) | ≥3rd (mean±SD) | All | p-value comparing parity groups |
|---|---|---|---|---|---|
| Variable | |||||
| N | 35 | 20 | 5 | 60 | N/A |
| Age (years) | 27.7±3.7 | 30.7±4.3 | 34.0±2.9 | 29.2±4.3 | 0.0008 |
| BMI (kg/m2) | 25.6±5.4 | 26.2±6.0 | 27.7±1.6 | 26.0±5.4 | 0.7056 |
| Beighton Score | 2.1±2.2 | 2.9±2.3 | 2.2±3.9 | 2.4±2.4 | 0.5246 |
| Week of Pregnancy at Initial Visit | 10.5±1.6 | 10.1±1.5 | 9.6±1.5 | 10.3±1.6 | 0.4207 |
Table 2.
Follow-up Participant Characteristics by Parity Group
| Parity Group | 1st (mean±SD) | 2nd (mean±SD) | ≥3rd (mean±SD) | All | p-value comparing parity groups |
|---|---|---|---|---|---|
| Variable | |||||
| N | 29 | 17 | 3 | 49 | N/A |
| BMI (kg/m2) | 26.4±3.8 | 26.7±5.7 | 27.9±0.7 | 26.6±4.4 | 0.8411 |
| Weight change from baseline (kg) | 3.3±3.1 | 0.2±3.4 | 1.9±4.3 | 2.1±3.5 | 0.0140 |
| Weight gain during pregnancy (kg) | 15.1±3.7 | 12.3±4.9 | 16.2±4.8 | 14.2±4.3 | 0.0840 |
| Postpartum week | 19.0±5.1 | 18.6±3.2 | 19.3±2.3 | 18.9±4.3 | 0.9436 |
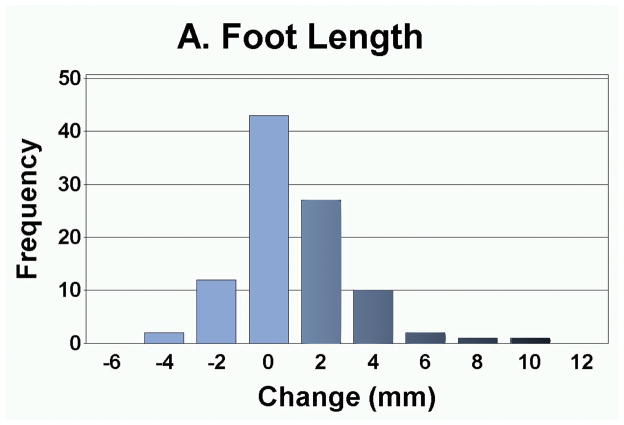
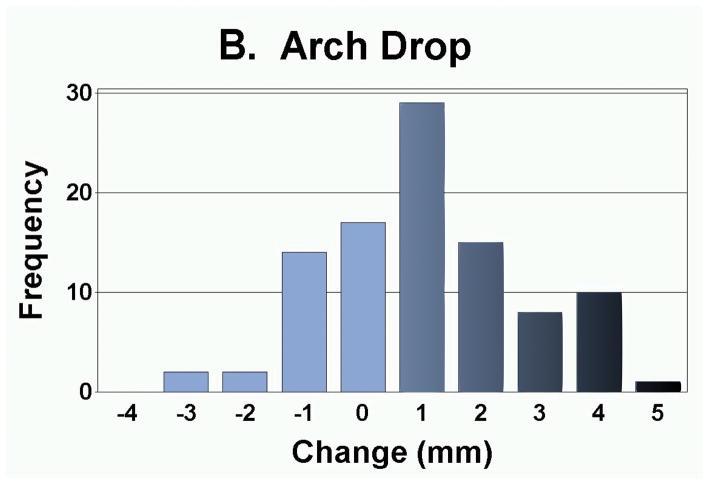
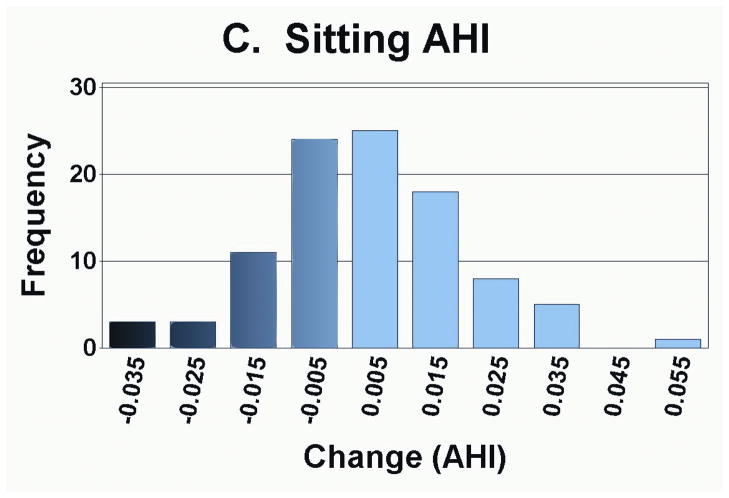
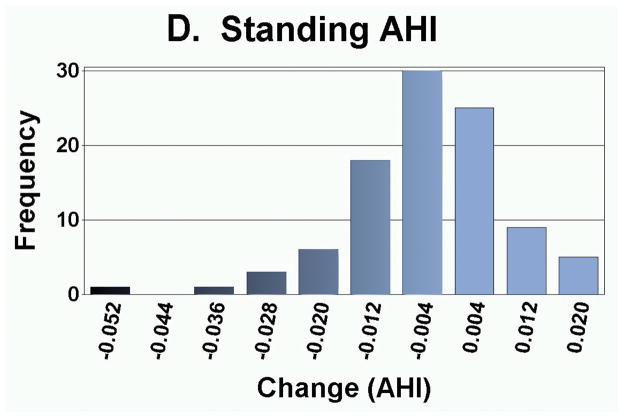
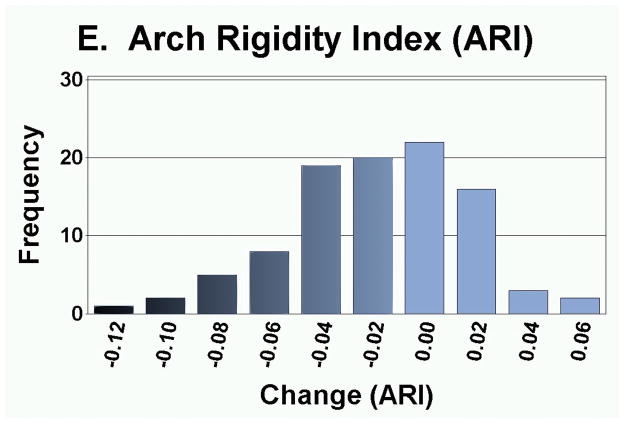
As presented in Table 3, the measurement of foot anthropometrics showed that on average, there was a significant decrease in arch height and arch rigidity index at follow-up, with concurrent increases in foot length and arch drop. However, there were no statistically significant changes in the CPEI between baseline and follow-up (1st pregnancy, 18.4±0.9% to 18.7±0.9%; 2nd pregnancy, 18.3±1.1% to 18.3±1.1%; and 3rd or greater pregnancy, 18.9±2.6% to 20.5±2.7%). In addition to finding significant differences in the mean change in each foot parameter, the range of magnitudes and frequencies are depicted in Figures 4A–4E. Specifically, 30 of 49 women had an increase in foot length (most of them on the order of 2–10 mm), and 35 of 49 participants had an arch drop (many in the 1–5 mm range), with similar numbers for reduction in arch height index and arch rigidity.
Table 3.
Changes in Foot Anthropometrics for Cohort (least sqare means ± SE and ranges)
| Variable | Baseline (mean±SD) | Follow-up (mean±SD) | Difference (mean±SD) (range) | p-value |
|---|---|---|---|---|
| Standing Foot Length (mm) | 244.0±1.4 | 244.7±1.4 | 0.7±0.2 | 0.0008* |
| Sitting AHI | 0.368±0.003 | 0.372±0.003 | 0.004±0.002 | 0.0799 |
| Standing AHI | 0.336±0.003 | 0.332±0.003 | −0.003±0.001 | 0.0067* |
| Arch Drop (mm) | 4.7±0.2 | 5.6±0.2 | 0.9±0.2 | <0.0001* |
| Arch Rigidity | 0.904±0.006 | 0.885±0.005 | −0.019±0.005 | <0.0001* |
| CPEI | 18.4±0.7% | 18.7±0.7% | 0.3±0.7% | 0.5095 |
Figure 4.
Distribution of changes in (a) foot length, (b) arch drop, (c) sitting Arch Height Index (AHI), (d) standing AHI, and (e) Arch Rigidity Index (ARI) (Darker shaded bars indicate the direction of structural loss)
Analyses of the interaction between parity and change in foot parameters revealed that the 1st pregnancy group primarily drove the overall outcomes reported in Table 3, with smaller or no significant effects detected in the higher parity groups. In the 1st pregnancy group (least square mean ± SE), foot length increased by 1.4 ± 0.3 mm (p<0.0001), arch drop increased by 1.0 ± 0.2 mm (p<0.0001) and arch rigidity decreased by 0.019 ± 0.004 (p<0.0001), without significant change in the other variables. In the 2nd pregnancy group, arch drop increased by 0.7 ± 0.3 (p=0.0131) and arch rigidity decreased by 0.015 ± 0.006 (p=0.0064), without significant change in the other variables. With these exceptions, there were not statistically significant changes in the static measures of arch height or dynamic measures of arch function by parity group, comparing baseline and follow-up visits.
Regarding awareness of foot changes during pregnancy, 11 participants (1st pregnancy N=9) noticed a change in shoe size and 5 participants (1st pregnancy N=3) noticed a change in arch height. In addition, 7 participants reported using shoes with arch supports “sometimes” and 9 participants reported using shoes with arch supports “most of the time” during pregnancy. Two participants reported discussing concerns about their feet with a physician during or after their pregnancy. However, there were no statistically significant associations between self-report of shoe size change, arch height change, or use of arch supports with the change in arch height index, arch rigidity, foot length or arch drop.
DISCUSSION
The purpose of this study was to determine whether there are changes in foot structure that persist following pregnancy, through assessing static (dorsal arch height, foot length, AHI and arch rigidity index) and dynamic (CPEI) measurements during the first trimester of pregnancy and 19 weeks postpartum. We hypothesized that a significant reduction in arch height persists postpartum, evident during static and dynamic conditions. The results of this study showed a significant decrease in arch height and arch rigidity index, with concomitant increases in foot length and arch drop. In the “1st pregnancy” group, there was a significant increase in foot length and arch drop with a reduction in arch rigidity. These findings suggest that pregnancy is associated with a permanent loss of arch height and rigidity and that the first pregnancy may be the most significant with the effect being attenuated with later pregnancies.
The results of the present study are consistent with previous studies assessing structural changes in the feet during pregnancy15, 16 and postpartum.24 Bohemen and Gendi reported significant loss of medial arch and increased foot size in two pregnant women with no joint hypermobility,24 postulating that “The problem may often be so minor as to not cause concern and this may explain why there have not been any cases reported in medical journals.” Alvarez et al,16 after serial measurements of the volume, length, and width of the feet of 17 pregnant women, found significant changes in volume between the 13th and 35th week of gestation and between the 13th week and eight weeks postpartum. The 0.8±0.5 mm increase in foot length in that smaller study was not statistically significant, but is consistent with the findings of the current study. In a recent study, Wetz et al found a significant increase in foot length, width and volume and a slight decrease in foot height in 40 women who were seen three different times during pregnancy.15 The foot length increase of 1.8 mm was greater in magnitude than that detected in our study, likely because the prior study compared 3rd with 1st trimester of pregnancy, whereas the current study assessed for the residual change that persists following completion of pregnancy.
Although the mean magnitudes of change were small, the high frequency of arch height loss was consistent with our prior work, which revealed that pregnancy may lead to a permanent change in shoe size with a dose-effect for each additional pregnancy detected in that larger sample.30 The frequencies of increase in foot length and arch drop as well as loss of arch height and rigidity suggest that there may be sub-groups of women that sustain these lasting changes with pregnancy and preventive strategies may be best focused on these women. Interestingly, Beighton laxity score, weight gain during pregnancy, and residual increased weight following pregnancy were not associated with the outcomes.
The discovery of measurable residual changes in the foot size and structure following pregnancy is consistent with the findings of a prior self-report study24 and could potentially suggest a mechanism for increased risk of lower limb musculoskeletal disease in postpartum women. Loss of arch height has been correlated to calcaneal eversion/inversion (r=0.8),31 and may lead to excessive pronation of the foot. A pronated foot posture causes increased rotation of the tibia32 that can be communicated across the knee to the femur. The presence of rotational torques, in turn, can cause shear stress on the medial tibiofemoral and lateral patellofemoral compartments of the knee. In a cross-sectional study of 1,903 older adults, limbs with a lower arch height had 1.31 times the odds of ipsilateral knee pain and 1.43 times the odds of medial tibiofemoral cartilage damage in comparison with limbs with a higher arch height.33 As pronation of the foot with arch collapse has been shown to be coupled with internal rotation of the hip,34 the persistent loss of arch height with pregnancy could plausibly lead to alterations in articular contact stress at the knee and hip.
A possible mechanism for the changes in arch height and rigidity observed with pregnancy may relate to the combination of increased magnitude and anterior displacement of body mass in the context of a hormonal milieu known to increase collagen extensibility during pregnancy. For example, relaxin is a peptide hormone, produced by the placenta and chorion during pregnancy, which enhances collagen breakdown. Although the role of relaxin in widening the pubic symphysis and softening the cervix has been better described, it is believed that this systemic hormone may also contribute to laxity of ligaments in peripheral joints as well.26, 35 It is therefore conceivable that cumulative weight bearing on feet with more lax ligaments could lead to permanent changes in the length and competence of those ligaments.
While most static measurements (foot length, arch height index, arch drop and arch rigidity) significantly changed, indicating a more flexible/flatter arch following pregnancy, we did not detect a change in the dynamic measure of foot function, CPEI. It may be that the small static structural changes were insufficient to alter dynamic loading of the foot. Another possible explanation relates to the fact that the baseline CPEI values of approximately 18% were already in a range that could be considered to be over-pronated (closer to pesplanus feet), so there may have been a “floor effect” in that there may have been insufficient room to further over-pronate. Alternatively, the larger variability in the CPEI measurement (intra-subject variability in CPEI of 15–20% over 5 trials on both the Emed and the Tekscan devices) could have contributed to insufficient sensitivity to detect small dynamic changes, whereas the static measurements of arch structure were inherently less variable. A larger sample size or a more sensitive measure, such as 3D motion analysis may be useful in better clarifying the biomechanical significance of the changes in foot function that persist following pregnancy.
Interestingly, differing results by parity level were found primarily for the 1st pregnancy group and to a lesser extent in the 2nd pregnancy group. This may indicate that the greatest effects occur with the 1st pregnancy or that the relatively low number of participants in the 3rd or greater pregnancy group may have affected the sensitivity to detect a parity threshold. Alternatively, there may not be a parity threshold, but rather a cumulative effect of multiple pregnancies.
In summary, there is an abundance of evidence that women suffer disproportionate risk for numerous musculoskeletal problems that cause suffering in the post-reproductive years. The results of the present study suggest that pregnancy appears to be associated with a permanent loss of arch height and rigidity that could potentially lead to abnormal arthrokinematics in the lower limb and ultimately place atypical stresses on the musculoskeletal system in postpartum women. The results of this study also suggest the need to assess whether the use of inexpensive, well-tolerated and widely available arch supporting orthoses during pregnancy could potentially protect the long-term musculoskeletal health of women.
CONCLUSIONS
Pregnancy appears to be associated with a persistent loss of arch height and rigidity as well as greater arch drop and foot lengthening, and the first pregnancy may be the most significant. These changes in the feet could potentially contribute to the increased risk for subsequent musculoskeletal disorders in women.
Acknowledgments
We appreciate the dedication of the participants who committed their time to making this study possible. This study also benefited from the recruitment efforts of Ms. Ranae Molkenthin.
Footnotes
Disclosures:
Financial disclosure statements have been obtained, and no conflicts of interest have been reported by the authors or by any individuals in control of the content of this article. Funding was provided by an American Geriatrics Society 2006 Dennis W. Jahnigen Career Development Scholars Award and a National Institutes on Aging Paul B. Beeson Career Development Award in Aging Research (1K23AG030945). A portion of this work was presented at Women’s Health 2012: The 20th Annual Congress (Washington, DC).
References
- 1.Pleis JR, Lethbridge-Cejku M. Summary health statistics for U.S. adults: National Health Interview Survey, 2006. Vital Health Stat. 2007;10(235):1–153. [PubMed] [Google Scholar]
- 2.Garrow AP, Silman AJ, Macfarlane GJ. The Cheshire Foot Pain and Disability Survey: a population survey assessing prevalence and associations. Pain. 2004;110(1–2):378–84. doi: 10.1016/j.pain.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 3.Benvenuti F, Ferrucci L, Guralnik JM, Gangemi S, Baroni A. Foot pain and disability in older persons: an epidemiologic survey. J Am Geriatr Soc. 1995;43(5):479–84. doi: 10.1111/j.1532-5415.1995.tb06092.x. [DOI] [PubMed] [Google Scholar]
- 4.Andersen RE, Crespo CJ, Bartlett SJ, Bathon JM, Fontaine KR. Relationship between body weight gain and significant knee, hip, and back pain in older Americans. Obes Res. 2003;11(10):1159–62. doi: 10.1038/oby.2003.159. [DOI] [PubMed] [Google Scholar]
- 5.Tuchsen F, Hannerz H, Burr H, Lund T, Krause N. Risk factors predicting hip pain in a 5-year prospective cohort study. Scand J Work Environ Health. 2003;29(1):35–9. doi: 10.5271/sjweh.702. [DOI] [PubMed] [Google Scholar]
- 6.Christmas C, Crespo CJ, Franckowiak SC, Bathon JM, Bartlett SJ, Andersen RE. How common is hip pain among older adults? Results from the Third National Health and Nutrition Examination Survey. J Fam Pract. 2002;51(4):345–8. [PubMed] [Google Scholar]
- 7.Segal NA, Felson DT, Torner JC, Zhu Y, Curtis JR, Niu J, et al. Greater trochanteric pain syndrome: epidemiology and associated factors. Arch Phys Med Rehabil. 2007;88(8):988–92. doi: 10.1016/j.apmr.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354(9178):581–5. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- 9.Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13(9):769–81. doi: 10.1016/j.joca.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Felson DT, Zhang Y, Hannan MT, Naimark A, Weissman B, Aliabadi P, et al. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum. 1997;40(4):728–33. doi: 10.1002/art.1780400420. [DOI] [PubMed] [Google Scholar]
- 11.Foley S, Ding C, Cicuttini F, Jones G. Physical activity and knee structural change: a longitudinal study using MRI. Medicine and science in sports and exercise. 2007;39(3):426–34. doi: 10.1249/mss.0b013e31802d97c6. [DOI] [PubMed] [Google Scholar]
- 12.Chesley LC. Weight changes and Water Balance in Noramal and Toxic pregnancy. Am J Obste Gyn. 1944;48:565–93. [Google Scholar]
- 13.Marnach ML, Ramin KD, Ramsey PS, Song SW, Stensland JJ, An KN. Characterization of the relationship between joint laxity and maternal hormones in pregnancy. Obstet Gynecol. 2003;101(2):331–5. doi: 10.1016/s0029-7844(02)02447-x. [DOI] [PubMed] [Google Scholar]
- 14.Schauberger CW, Rooney BL, Goldsmith L, Shenton D, Silva PD, Schaper A. Peripheral joint laxity increases in pregnancy but does not correlate with serum relaxin levels. Am J Obstet Gynecol. 1996;174(2):667–71. doi: 10.1016/s0002-9378(96)70447-7. [DOI] [PubMed] [Google Scholar]
- 15.Wetz HH, Hentschel J, Drerup B, Kiesel L, Osada N, Veltmann U. Changes in shape and size of the foot during pregnancy. Orthopade. 2006;35(11):1124, 6–30. doi: 10.1007/s00132-006-1011-1. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez R, Stokes IA, Asprinio DE, Trevino S, Braun T. Dimensional changes of the feet in pregnancy. J Bone Joint Surg Am. 1988;70(2):271–4. [PubMed] [Google Scholar]
- 17.Block RA, Hess LA, Timpano EV, Serlo C. Physiologic changes in the foot during pregnancy. J Am Podiatr Med Assoc. 1985;75(6):297–9. doi: 10.7547/87507315-75-6-297. [DOI] [PubMed] [Google Scholar]
- 18.Lymbery JK, Gilleard W. The stance phase of walking during late pregnancy: temporospatial and ground reaction force variables. J Am Podiatr Med Assoc. 2005;95(3):247–53. doi: 10.7547/0950247. [DOI] [PubMed] [Google Scholar]
- 19.Jelen K, Tetkova Z, Halounova L, Pavelka K, Koudelka T, Ruzicka P. Shape characteristics of the foot arch: dynamics in the pregnancy period. Neuro Endocrinol Lett. 2005;26(6):752–6. [PubMed] [Google Scholar]
- 20.Foti T, Davids JR, Bagley A. A biomechanical analysis of gait during pregnancy. J Bone Joint Surg Am. 2000;82(5):625–32. [PubMed] [Google Scholar]
- 21.Nyska M, Sofer D, Porat A, Howard CB, Levi A, Meizner I. Planter foot pressures in pregnant women. Isr J Med Sci. 1997;33(2):139–46. [PubMed] [Google Scholar]
- 22.Erhart JC, Mundermann A, Mundermann L, Andriacchi TP. Predicting changes in knee adduction moment due to load-altering interventions from pressure distribution at the foot in healthy subjects. J Biomech. 2008;41(14):2989–94. doi: 10.1016/j.jbiomech.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 23.Dahle LK, Mueller MJ, Delitto A, Diamond JE. Visual assessment of foot type and relationship of foot type to lower extremity injury. J Orthop Sports Phys Ther. 1991;14(2):70–4. doi: 10.2519/jospt.1991.14.2.70. [DOI] [PubMed] [Google Scholar]
- 24.Bohemen EK. Flatfeet in Pregrancy. British Journal of Rheumatology. 1996;35(4):396–7. doi: 10.1093/rheumatology/35.4.396-a. [DOI] [PubMed] [Google Scholar]
- 25.Grahame R, Bird HA, Child A. The revised (Brighton 1998) criteria for the diagnosis of benign joint hypermobility syndrome (BJHS) J Rheumatol. 2000;27(7):1777–9. [PubMed] [Google Scholar]
- 26.Calguneri M, Bird HA, Wright V. Changes in joint laxity occurring during pregnancy. Ann Rheum Dis. 1982;41(2):126–8. doi: 10.1136/ard.41.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butler RJ, Hillstrom H, Song J, Richards CJ, Davis IS. Arch height index measurement system: establishment of reliability and normative values. J Am Podiatr Med Assoc. 2008;98(2):102–6. doi: 10.7547/0980102. [DOI] [PubMed] [Google Scholar]
- 28.Zifchock RA, Davis I, Hillstrom H, Song J. The effect of gender, age, and lateral dominance on arch height and arch stiffness. Foot Ankle Int. 2006;27(5):367–72. doi: 10.1177/107110070602700509. [DOI] [PubMed] [Google Scholar]
- 29.Richards CJ, Card K, Song J, Hillstrom H, Butler RJ, Davis IM. A novel arch height index measurement system (AHIMS): intra- and inter-rater reliability. Proceedings of the Proceedings of the American Society of Biomechanics 26th Annual Meeting; 2003. [Google Scholar]
- 30.Segal N, Eagles M. The effects of pregnancy on shoe size and knee laxity. Am J Phys Med Rehabil. 2010;89(4):S41. [Google Scholar]
- 31.Pietrosimone BG, Saliba SA, Hart JM, Hertel J, Kerrigan DC, Ingersoll CD. Effects of disinhibitory transcutaneous electrical nerve stimulation and therapeutic exercise on sagittal plane peak knee kinematics and kinetics in people with knee osteoarthritis during gait: a randomized controlled trial. Clinical Rehabilitation. 2010;24(12):1091–101. doi: 10.1177/0269215510375903. [DOI] [PubMed] [Google Scholar]
- 32.Coplan JA. Rotational motion of the knee: a comparison of normal and pronating subjects. J Orthop Sports Phys Ther. 1989;10(9):366–9. doi: 10.2519/jospt.1989.10.9.366. [DOI] [PubMed] [Google Scholar]
- 33.Gross KD, Felson DT, Niu J, Hunter DJ, Guermazi A, Roemer FW, et al. Association of flat feet with knee pain and cartilage damage in older adults. Arthritis care & research. 2011;63(7):937–44. doi: 10.1002/acr.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Souza TR, Pinto RZ, Trede RG, Kirkwood RN, Fonseca ST. Temporal couplings between rearfoot-shank complex and hip joint during walking. Clinical biomechanics. 2010;25(7):745–8. doi: 10.1016/j.clinbiomech.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 35.Unemori EN, Amento EP. Relaxin modulates synthesis and secretion of procollagenase and collagen by human dermal fibroblasts. J Biol Chem. 1990;265(18):10681–5. [PubMed] [Google Scholar]