Abstract
While patients with surgically resected non-invasive mucinous cystic neoplasms (MCNs) of the pancreas are cured, the behavior of surgically resected minimally invasive adenocarcinomas arising in MCN has not been well established. We report 16 surgically resected MCNs with minimal invasion defined as unifocal or multifocal microscopic invasive adenocarcinoma confined to the ovarian stroma of the MCN without capsular or pancreatic parenchymal invasion. Pathological findings were correlated with patient demographics, type of surgery, and long-term follow-up. Our study included 15 females and 1 male ranging in age from 25–66 years. The patients were followed for a mean of 48.6 months (range 12–148 months). The MCNs ranged in size from 3.5–25 cm and were all located in the body/tail of the gland. Lymphovascular invasion was not identified in any of the cases and all lymph nodes were negative for tumor. Ten neoplasms had unifocal invasion, while 6 had multifocal invasion. Twelve of the neoplasms were partially submitted for microscopic examination while 4 were submitted entirely. Only one of the 16 minimally invasive MCNs recurred, and that tumor had been minimally sampled pathologically. Our study demonstrates that the majority of patients with minimally invasive adenocarcinoma arising in MCN are cured by surgery, particularly if the neoplasms are completely examined histologically.
Keywords: mucinous cystic neoplasm, minimally invasive carcinoma, pancreatic cancer
INTRODUCTION
In 1978 Compagno and Oertel separated mucinous cystic neoplasms (MCNs) from serous cystic neoplasms, and defined the histological features of mucinous cystic neoplasms (MCN) as mucin-producing epithelium often supported by “dense, cellular stroma resembling that of the ovary”(1). They emphasized the potential for malignancy in this entity especially when compared to serous cystadenoma (“microcystic adenoma”) which is virtually always benign(1, 2). In the 1990s the diagnostic criteria for MCN were refined to require the presence of ovarian stroma, helping to distinguish MCNs from branch-duct type intraductal papillary mucinous neoplasms (IPMNs)(3, 4).
Among reported series of MCN, 31–72% have low-grade dysplasia, 8.2–25% have intermediate-grade dysplasia, 5.5–15% have high-grade dysplasia, and 3.9–34.4% have an associated invasive carcinoma(5–17). The prognosis for patients with non-invasive MCNs is excellent; essentially all patients with a completely resected MCN without an associated invasive carcinoma are cured (11, 14). The prognosis of patients with an MCN with an associated invasive carcinoma is better than it is for patients with an infiltrating ductal adenocarcinoma not arising in association with an MCN, but the 5-year survival rate is still only 33–56%(6, 11, 14).
While extensively invasive carcinomas arising in MCNs clearly are associated with a poor prognosis, the prognosis of patients with a minimally invasive disease (i.e. invasion limited to the ovarian stroma) has not been well studied (11, 14). The purpose of this study was to examine the outcome of a large series of patients with a surgically resected MCN with minimally invasive carcinoma.
MATERIALS AND METHODS
Case Selection and Histological Analysis
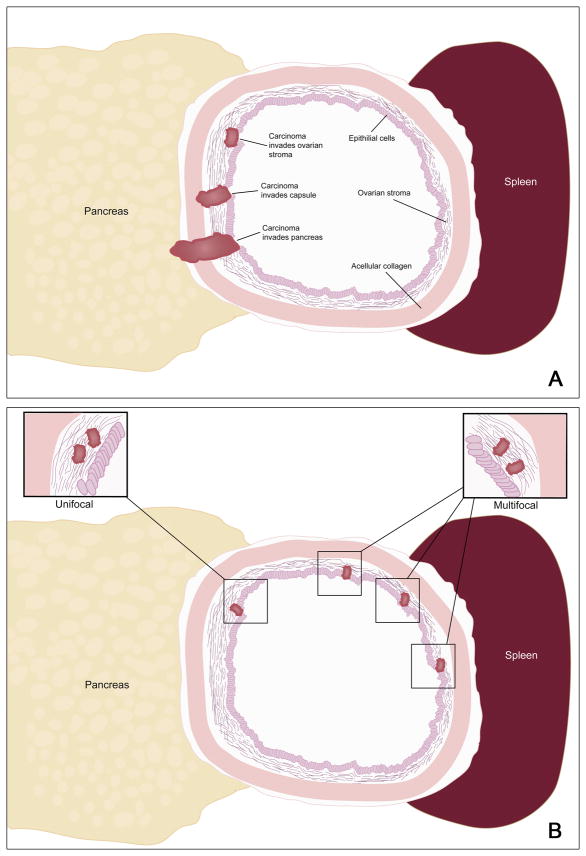
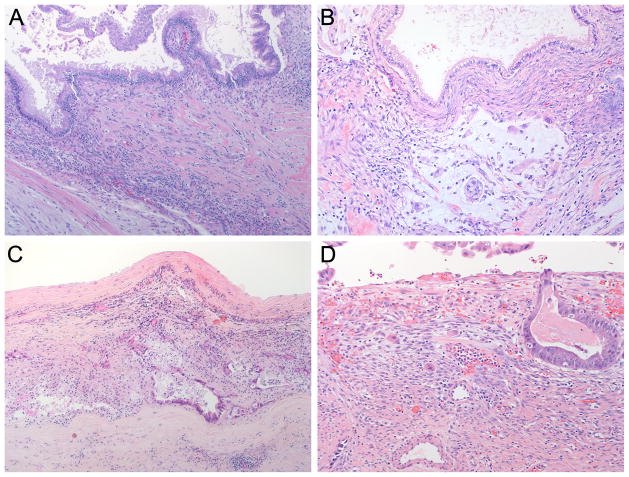
This study was approved by the Institutional Review Board of the Johns Hopkins University School of Medicine, Baltimore, MD. Patients diagnosed with MCN with invasive carcinoma confined to ovarian stroma between March 1984 and September 2010 were identified from the surgical pathology files of the Johns Hopkins Hospital; both in-house and consult cases were included. Cases from the University of Texas MD Anderson Cancer Center and Brigham and Women’s Hospital were identified prospectively. All available hematoxylin and eosin-stained slides were reviewed. Standard criteria were employed for the diagnosis of an MCN, including the requirement of ovarian-type stroma (3, 12). Minimal invasion is defined as invasion histologically confined to the ovarian stroma (Figure 1A). Minimal invasion can be unifocal (confined to one focus) or multifocal (consisting of multiple, discontinuous foci of invasion) (Figure 1B). In every case, foci of invasion characteristically demonstrated haphazard infiltration of the ovarian-type stroma by neoplastic cells (Figures 2 and 3). Stromal desmoplasia was prominent in the areas of infiltration in some of the cases. True invasion is thus distinguished from pseudo-invasion (tangentially sectioned epithelial invaginations), which is characterized by organized glands in stroma lacking desmoplasia.
Figure 1.
Schematic showing the classification of depth of invasion (A) and of multi-focality (B). In this study minimally invasive carcinoma was defined as invasion only into the ovarian stroma.
Figure 2.
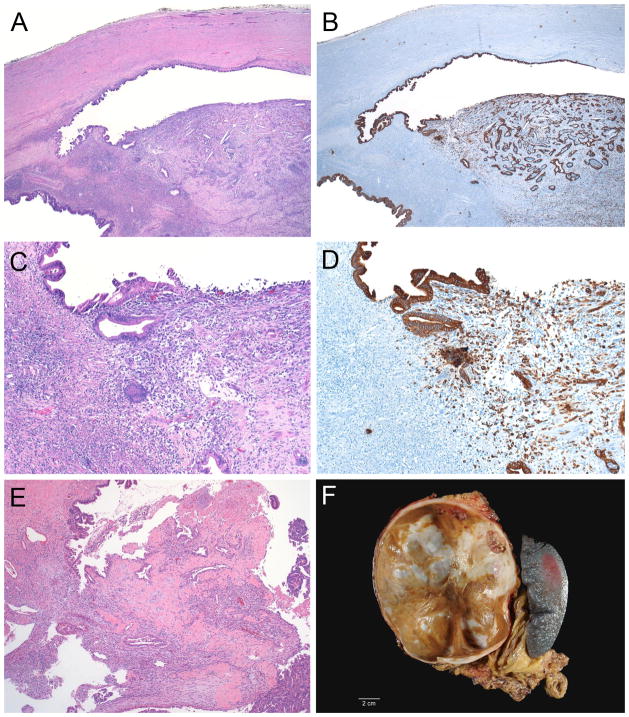
Minimally invasive carcinoma arising in a mucinous cystic neoplasm (case 12, A–D; case 13, E). The carcinoma invades only into the ovarian stroma (A and C). The epithelial nature of the undifferentiated carcinoma in case 12 was confirmed with immunolabeling for cytokeratin (B and D). Note the mural nodule in the gross photograph of case 11 (F).
Figure 3.
Minimally invasive carcinoma arising in a mucinous cystic neoplasm (A–D). Note the individual cells in the ovarian stroma (A and D), and that the overlying non-invasive epithelium does not always harbor high-grade dysplasia (A and B).
Patient Outcomes
Patient outcomes were obtained from 3 sources: The Johns Hopkins Hospital Surgical Pathology Database, the medical records of The Johns Hopkins Hospital, and direct follow-up with physicians. Detailed clinical follow-up information was available for all 16 patients.
Statistical Analysis
P-values were determined by Wilcoxon Rank-Sum Test or Log-Rank Test.
RESULTS
As summarized in Table 1, our study included fifteen females and 1 male ranging in age from 25–66 years with mean of 48.4 years (median 51 years). The MCN ranged in size from 3.5–25 cm with mean 11.2 cm (median 10 cm) and were all located in the body/tail of the pancreas. Ten patients had unifocal minimal invasion, while 6 had multifocal minimal invasion (Figure 1B). By definition, all foci of invasion were histologically confined to the ovarian stroma (Figures 1A, 2 and 3). All of the neoplasms contained foci with high-grade dysplasia somewhere in the tumor, but the foci of invasion were not always associated with overlying high-grade dysplasia (Figures 3A and 3B). The six patients with multifocal invasion were older than the ten with unifocal invasion (mean/median 52.2/56 years versus 46.1/47.5 years), however, the difference was not statistically significant (p = 0.1152). The tumors with multifocal invasion were similar in size to those with unifocal invasion (mean/median 11.9/10.5 cm versus 10.8/9.75 cm, p = 0.7445).
Table 1.
Patient Characteristics
| Patient | Age (decade) | Gender | Tumor Size (cm) | Extent of Sampling1 (#sections) | No. sections/1 cm MCN | Lymph Nodes | Chemotherapy | Invasion | Histology of Invasive Carcinoma | Follow-up (months) | Clinical Course2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 30s | F | 10 | RS (47) | 4.7 | 0/6 | No | Unifocal | Tubular ADCA | 148 | AAW |
| 2 | 50s | F | 5 | RS (5) | 1 | NS | No | Multifocal | Tubular ADCA | 42 | DOD |
| 3 | 40s | F | 15 | RS (15) | 1 | NS | No | Unifocal | Tubular ADCA | 76 | AAW |
| 4 | 40s | F | 14 | RS (14) | 1 | 0/2 | No | Unifocal | Tubular ADCA | 66 | AAW |
| 5 | 50s | F | 9.5 | RS (11) | 1.2 | 0/7 | Yes | Unifocal | Tubular ADCA | 65 | AAW |
| 6 | 40s | F | 3.5 | RS (20) | 4.4 | 0/14 | No | Unifocal | Tubular ADCA | 56 | AAW |
| 7 | 60s | F | 4.5 | RS (7) | 1.6 | 0/2 | Yes | Multifocal | Tubular ADCA | 28 | AAW |
| 8 | 50s | F | 8 | RS (8) | 1 | 0/5 | No | Unifocal | Tubular ADCA | 24 | AAW |
| 9 | 50s | M | 5 | ES (76) | 15.2 | 0/12 | No | Unifocal | Tubular ADCA | 19 | AAW |
| 10 | 50s | F | 16 | ES (296) | 18.5 | 0/5 | No | Multifocal | Tubular ADCA | 47 | AAW |
| 11 | 50s | F | 10 | ES (123) | 12.3 | 0/43 | Yes | Multifocal | Tubular ADCA | 70 | AAW |
| 12 | 30s | F | 20.7 | RS (11) | 0.5 | 0/14 | Yes | Unifocal | Undifferentiated carcinoma with spindle cell features | 40 | AAW |
| 13 | 50s | F | 9 | RS (7) | 0.8 | 0/7 | Yes | Unifocal | Tubular ADCA | 12 | AAW |
| 14 | 30s | F | 13 | ES (66) | 5.1 | 0/17 | Yes | Unifocal | Tubular ADCA | 16 | AAW |
| 15 | 50s | F | 11 | RS (40) | 3.6 | 0/5 | Yes | Multifocal | ADCA with squamous features | 46 | AAW |
| 16 | 20s | F | 25 | RS (30) | 1.2 | 0/16 | No | Multifocal | Tubular ADCA | 22 | AAW |
RS: representative sections; ES: entirely sampled.
AAW: alive and well; DOD: died of disease.
The MCN was entirely submitted for histological examination in 4 cases with a range of 5.1–18.5 sections, and a mean/median of 12.8/13.75 sections per 1 cm of the greatest linear dimension of the MCN. Of the twelve cases where the MCN was partially submitted for histological examination, the mean number of sections submitted per 1 cm of the greatest dimension of the MCN was 1.8 (range 0.5–4.7 sections, median 1.1 sections). Although the mean number of sections submitted per 1 cm of the greatest dimension of the MCN was higher in the cases where multifocal invasion was identified (mean of 6.4 sections per 1 cm MCN) compared to those where only unifocal invasion was identified (mean of 3.5 sections per 1 cm MCN), the difference was not statistically significant (p = 0.2742). Of the cases with multifocal invasion, the MCN was entirely submitted in 2 cases, was extensively sampled (mean of 2.1 sections per 1 cm MCN) in 3 cases, and was lightly sampled in 1 case (1 section per 1 cm MCN).
The histological type of invasive carcinoma was conventional ductal adenocarcinoma in 14 cases (Figures 2E, 3B and 3C), undifferentiated carcinoma with spindle cell features (sarcomatoid carcinoma) in 1 case (Figure 2A–D), and adenocarcinoma with squamous features in 1 case. Lymphovascular invasion was absent in all cases. The pancreatic parenchymal margin was negative in all cases. Lymph nodes were submitted for histological examination in 14 of 16 cases (range 2–43 lymph nodes, mean/median 11.1/7 lymph nodes) and were all negative for metastatic carcinoma.
The patients were followed for 12–148 months (mean/median 48.6/44 months). Follow-up for patients with unifocal (mean of 52.2/48 months) and multifocal (mean of 42.5/44 months) invasive carcinoma were comparable (p = 0.2636). Seven of the patients received adjuvant chemotherapy: 3 with multifocal invasion, 4 with unifocal invasion.
Only one of the 16 minimally invasive MCNs recurred, and that tumor had been lightly sampled (5 sections for the 5 cm tumor). No lymph nodes were submitted for histological examination. The single patient whose carcinoma recurred was found to have multifocal invasive conventional adenocarcinoma and had received no chemotherapy. Her tumor recurred in the pancreatic bed 36 months after initial resection. The recurrence was identified by CT scan and was surgically unresectable. The patient died of disease 42 months after the initial resection.
All other patients are alive and well. The patient with sarcomatoid carcinoma presented with unifocal invasion and received chemotherapy. The tumor was lightly sampled (0.5 section per 1 cm MCN). The patient with adenocarcinoma with squamous features presented with multifocal invasion and received adjuvant chemotherapy. The tumor was more extensively sampled (3.6 sections per 1 cm MCN). The results are summarized in Table 1.
DISCUSSION
We describe 16 minimally invasive carcinomas arising from MCNs. Invasive carcinoma was intracapsular, confined within the ovarian stroma of the MCN in all cases (Figure 1A, Figure 2, and Figure 3). After a mean of 48 months of follow-up, only one of the 16 carcinomas recurred, and this patient’s tumor had only been lightly sampled (5 sections for 5 cm tumor) for histological examination leaving open the possibility that more extensive invasion was present elsewhere in the unsampled lesion. This observation highlights the importance of extensive, if not complete, histologic sampling of mucinous cystic neoplasms(3, 5).
Our finding that minimally invasive carcinomas arising from MCN have an excellent prognosis is supported by previous reports in the literature (11, 14). Crippa et al reported a large series of MCNs, of which 14 were intracapsular invasive carcinomas, defined as invasion that “did not go beyond the outer layer of the wall (14).” Only 3 of these 14 carcinomas with intracapsular invasion, all three with multifocal invasion, recurred (14). Similarly, Zamboni et al reported three patients with minimal invasion confined to the ovarian stroma and five additional cases with invasion beyond the ovarian stroma but still confined to the tumor capsule (Figure 1A), and all three patients with invasion confined to the ovarian stroma were alive and well after a mean follow-up of 22 months (11). Two of 5 patients with deeper capsular invasion died of disease (11). Thus, even using the definitions employed in these other series, only 16% (6 of 38) of the patients in the combination of our series, and those reported by Crippa and Zamboni recurred.
We conclude that invasive carcinoma confined to the ovarian stroma of MCN has an excellent prognosis. It is important to sample MCNs well, and the single recurrence in our series, that of a patient whose MCN was incompletely sampled, suggests that more extensive invasion may have remained in the unsampled tissue (5). The majority of patients with minimally invasive adenocarcinoma arising in MCN are cured, particularly if the neoplasms are completely examined histologically.
Acknowledgments
Sources of Support: Supported by NIH P50CA062924, The Sol Goldman Pancreatic Cancer Research Center, and The Michael Rolfe Foundation for Pancreatic Cancer Research
References
- 1.Compagno J, Oertel JE. Mucinous cystic neoplasms of the pancreas with overt and latent malignancy (cystadenocarcinoma and cystadenoma). A clinicopathologic study of 41 cases. American Journal of Clinical Pathology. 1978;69:573–580. doi: 10.1093/ajcp/69.6.573. [DOI] [PubMed] [Google Scholar]
- 2.Compagno J, Oertel JE. Microcystic adenomas of the pancreas (glycogen-rich cystadenomas): a clinicopathologic study of 34 cases. American Journal of Clinical Pathology. 1978;69:289–298. doi: 10.1093/ajcp/69.1.289. [DOI] [PubMed] [Google Scholar]
- 3.Hruban RH, Pitman MB, Klimstra DS. Tumors of the pancreas. Atlas of tumor pathology. Washington, DC: American Registry of Pathology and Armed Forces Institute of Pathology; 2007. [Google Scholar]
- 4.Tanaka M, Fernandez-Del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–197. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Wilentz RE, Albores-Saavedra J, Zahurak M, et al. Pathologic examination accurately predicts prognosis in mucinous cystic neoplasms of the pancreas. Am J Surg Pathol. 1999;23:1320–1327. doi: 10.1097/00000478-199911000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Wilentz RE, Hruban RH, Albores-Saavedra J. Prognosis of invasive mucinous cystadenocarcinomas of the pancreas: a study of over 29,000 patients from the SEER database. Modern Pathology. 1999;12:169A. [Google Scholar]
- 7.Sarr MG, Carpenter HA, Prabhakar LP, et al. Clinical and pathologic correlation of 84 mucinous cystic neoplasms of the pancreas: can one reliably differentiate benign from malignant (or premalignant) neoplasms? Annals of Surgery. 2000;231:205–212. doi: 10.1097/00000658-200002000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Borgne J, de Calan L, Partensky C. Cystadenomas and cystadenocarcinomas of the pancreas: a multiinstitutional retrospective study of 398 cases. French Surgical Association. Annals of Surgery. 1999;230:152–161. doi: 10.1097/00000658-199908000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki Y, Atomi Y, Sugiyama M, et al. Cystic neoplasm of the pancreas: a Japanese multiinstitutional study of intraductal papillary mucinous tumor and mucinous cystic tumor. Pancreas. 2004;28:241–246. doi: 10.1097/00006676-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Klöppel G, Kosmahl M. Cystic lesions and neoplasms of the pancreas. The features are becoming clearer. Pancreatology. 2001;1:648–655. doi: 10.1159/000055876. [DOI] [PubMed] [Google Scholar]
- 11.Zamboni G, Scarpa A, Bogina G, et al. Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol. 1999;23:410–422. doi: 10.1097/00000478-199904000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Zamboni G, Klöppel G, Hruban RH, et al. Mucinous cystic neoplasms of the pancreas. In: Hamilton SR, Aaltonen LA, editors. World Health Organization Classification of Tumours Pathology and Genetics of Tumours of the Digestive System. Lyon: IARCPress; 2000. pp. 234–236. [Google Scholar]
- 13.Zamboni G, Castelli P, Pea M, et al. Mucinous cystic tumor of the pancreas recurring after 11 years as cystadenocarcinoma with foci of choriocarcinoma and osteoclast-like giant cell tumor. Surgical Pathology. 1994;5:253–262. [Google Scholar]
- 14.Crippa S, Salvia R, Warshaw AL, et al. Mucinous cystic neoplasm of the pancreas is not an aggressive entity: lessons from 163 resected patients. AnnSurg. 2008;247:571–579. doi: 10.1097/SLA.0b013e31811f4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kosmahl M, Pauser U, Peters K, et al. Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: a review of 418 cases and a classification proposal. Virchows Arch. 2004;445:168–178. doi: 10.1007/s00428-004-1043-z. [DOI] [PubMed] [Google Scholar]
- 16.Wilentz RE, Albores-Saavedra J, Hruban RH. Mucinous cystic neoplasms of the pancreas. Semin Diagn Pathol. 2000;17:31–42. [PubMed] [Google Scholar]
- 17.Yamao K, Yanagisawa A, Takahashi K, et al. Clinicopathological Features and Prognosis of Mucinous Cystic Neoplasm With Ovarian-Type Stroma: A Multi-Institutional Study of the Japan Pancreas Society. Pancreas. 2010;40:67–71. doi: 10.1097/MPA.0b013e3181f749d3. [DOI] [PubMed] [Google Scholar]