Abstract
Background
As part of the Family Smoking Prevention and Tobacco Control Act, the United States Food and Drug Administration charged the Tobacco Products Scientific Advisory Committee with developing a report and recommendations regarding the effect of menthol in cigarettes on the public health. The purpose of this study was to examine smoking behaviors, biomarkers of exposure and subjective responses when switching from a novel menthol cigarette to a non-menthol cigarette to isolate the effect of menthol and to approximate the effect a menthol ban might have on smokers.
Methods
Thirty two adult smokers completed this 35-day randomized, open-label, laboratory study. After a 5-day baseline period, participants were randomized to the experimental group (n=22) where they would smoke menthol Camel Crush for 15 days followed by 15 days of non-menthol Camel Crush, or the control group (n=10) where they smoked their own brand cigarette across all periods. Participants attended study visits every five days and completed measures of smoking rate, smoking topography, biomarkers of exposure, and subjective responses.
Results
Although total puff volume tended to increase when the experimental group switched from menthol to non-menthol (p=0.06), there were no corresponding increases in cigarette consumption or biomarkers of exposure (ps>0.1). Subjective ratings related to taste and smell decreased during the non-menthol period (ps<0.01), compared to the menthol.
Conclusions
Results suggest menthol has minimal impact on smoking behaviors, biomarkers of exposure and subjective ratings.
Impact
When controlling for all other cigarette design features, menthol in cigarettes had minimal effect on outcome measures.
Keywords: cigarette, menthol, smoking, nicotine, behavior
The Family Smoking Prevention and Tobacco Control Act (FSPTCA) of 2009 gave the United States Food and Drug Administration (FDA) the authority to regulate tobacco products, and included the power to ban characterizing flavors such as fruit, candy and clove, but not menthol (1). Menthol cigarettes comprise approximately 25% of all cigarettes sold in the United States (2) and therefore regulating menthol would potentially affect a significant number of smokers. However, as part of the FSPTCA (Section 907.e), the FDA charged the Tobacco Products Scientific Advisory Committee (TPSAC) with submitting a report and recommendation to the Secretary of Health and Human Services on the impact of the use of menthol in cigarettes on the public health, including use among children, and ethnic and minority groups (3).
While some previous epidemiological research suggests menthol cigarette smokers are at increased risk for lung cancer (4), other research concludes there is no increased risk to menthol cigarette smokers compared to non-menthol smokers (5,6). This is further complicated by the fact that menthol cigarette smokers tend to be African-American (7), who tend to smoke fewer cigarettes per day, and metabolize nicotine more slowly than Whites (8). Therefore, menthol and non-menthol cigarette smokers differ in ways other than by the presence of menthol in their cigarettes.
Results investigating differences in smoking patterns of menthol cigarette smokers and non-menthol cigarette smokers are equally mixed. While some laboratory studies have reported significantly greater smoking behaviors (daily cigarette consumption or puffing intensity) when smoking menthol cigarettes (9,10), others have reported no difference (11–13), or a decrease in smoking behavior relative to non-menthol cigarette smoking (14,15). These studies varied in research design, such as cross-over or group comparison; duration of surveillance, from one cigarette to several days of smoking; and type of cigarettes used, including preferred own brand, brand switching, commercially available or research cigarette, making it difficult to draw definitive conclusions.
Research into the effect of menthol on biomarkers of exposure and toxin effects is also unclear. Cotinine levels tend to be greater in menthol smokers than non-menthol smokers, but these results did not all reach statistically significant levels (16–19). Recent work by Muscat (20), and Heck (21) reported no difference in nicotine or cotinine levels by menthol status, nor were differences in 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), a metabolite of the carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), reported.
Menthol and non-menthol cigarette smokers may, or may not, differ in their disease risk, how they smoke, and their exposure levels. Further, menthol and non-menthol cigarettes differ in ways other than menthol level, including tar levels, flavorings and filter ventilation levels (22) making it difficult to isolate the effect of menthol. Using a randomized, open label design, the current study was designed to examine smoking behaviors, biomarkers of exposure and subjective responses when switching from a novel menthol cigarette to a non-menthol cigarette to isolate the effect of menthol on these outcome measures and to approximate the effect a menthol ban might have on smokers. Using a cigarette that can be both a menthol and a non-menthol cigarette, we hypothesized there would be minimal differences in smoking behaviors and biomarkers of exposure, and that subjective ratings would be negatively affected when menthol smokers smoked during the non-menthol period.
METHODS
Participants
Participants were recruited via newspaper and internet postings, and initial eligibility was assessed via telephone. Inclusion criteria included: age 21–65; smoking ≥ 10 daily cigarettes, for ≥ 5 years, and smoking menthol flavored cigarettes ≥ 80% of the time; not currently trying to quit smoking, or planning to quit in the next 2 months. Exclusion was based on not meeting any inclusion criteria, as well as: drinking ≥ 25 alcohol-containing drinks per week, using any nicotine replacement or nicotine-containing products other than cigarettes; substance use disorders in the last 5 years; any self-reported psychiatric disorders other than depression; current smoking of marijuana; and females could not be currently pregnant or lactating.
Procedure
Sessions occurred between October 2010 and November 2011. To minimize the effect of diurnal variation on smoking behaviors all sessions commenced between 08:00 and 12:00, and each participant had their sessions scheduled congruent to within one hour. The study design consisted of three periods: Period 1 (own brand), where participants smoked their own brand cigarette to establish baseline measures (Days 1–5, laboratory-smoked cigarettes 1–3); Period 2 (crush, or menthol period), where the experimental group smoked the menthol version of the research cigarette (Days 5–20, laboratory-smoked cigarettes 4–9); and Period 3 (non-crush, or non-menthol period), where the experimental group smoked the non-menthol version of the research cigarette (Days 20–35, laboratory-smoked cigarettes 10–15). Laboratory-smoked cigarette number refers to the order of cigarettes smoked during laboratory sessions during the study (See Table 1). The control group smoked their own brand cigarette across all periods. The study was designed to examine how menthol in cigarettes might affect smoking behaviors, toxin exposure and subjective ratings. However, only a menthol to non-menthol cross-over was considered in the study design. A non-menthol to menthol cross-over has less regulatory policy relevance. The study protocol and informed consent were approved by the university institutional review board and signed informed consent was obtained from all participants at the onset of the first session.
Table 1.
Behavioral, biochemical and subjective measures for the experimental group presented by period, day and cigarette. Values are adjusted means and standard errors.
| OWN BRAND | CRUSH | NON-CRUSH | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Day | 1 | 1 | 5 | 5 | 10 | 10 | 15 | 15 | 20 | 20 | 25 | 25 | 30 | 30 | 35 |
| Cigarette | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
| Biomarkers | |||||||||||||||
| CO session start (ppm) | 26.8 (2.8) | 25.3 (2.2) | 22.3 (1.9) | 23.3 (2.2) | 23.5 (1.9) | 23.5 (2.2) | 22.9 (2.4) | 23.4 (2.4) | |||||||
| CO boost (ppm) | 6.2 (0.8) | 5.9 (0.8) | 5.9 (0.5) | 4.6 (0.5) | 4.0 (0.7) | 3.9 (0.8) | 4.1 (0.5) | 4.3 (0.5) | 3.7 (0.5) | 3.2 (0.6) | 3.7 (0.7) | 3.8 (0.6) | 3.5 (0.5) | 4.6 (0.5) | 3.9 (0.5) |
| Nicotine (ug/ml) | 1.706 (0.30) | 2.68 (0.54) | 2.49 (0.43) | ||||||||||||
| Cotinine (ug/ml) | 4.00 (0.74) | 4.46 (0.69) | 3.74 (0.39) | ||||||||||||
| 8-Oxo-dGuo (ng/ml) | 3.52 (0.46) | 5.10 (1.00) | 4.52 (0.94) | ||||||||||||
| Smoking Topography | |||||||||||||||
| Total Puff Volume (ml) | 612.6 (74.6) | 517.9 (36.8) | 560.7 (42.7) | 600.4 (42.0) | 586.0 (42.1) | 630.7 (42.7) | 601.6 (41.0) | 614.3 (46.3) | 650.3 (47.9) | 664.4 (56.8) | 738.3 (50.4) | 648.7 (48.3) | 623.6 (57.5) | 658.0 (47.4) | 677.9 (57.1) |
| Number of Puffs | 11.2 (0.6) | 11.1 (0.8) | 12.6 (0.7) | 12.2 (0.7) | 11.9 (0.8) | 12.0 (0.6) | 12.4 (0.7) | 12.0 (0.8) | 12.0 (0.9) | 12.4 (1.2) | 13.3 (1.1) | 12.2 (0.9) | 10.9 (1.0) | 12.1 (0.7) | 12.7 (1.0) |
| Puff Duration (s) | 1.7 (0.1) | 1.7 (0.1) | 1.6 (0.1) | 1.7 (0.1) | 1.5 (0.1) | 1.6 (0.1) | 1.6 (0.1) | 1.6 (0.1) | 1.7 (0.2) | 1.8 (0.2) | 1.7 (0.1) | 1.8 (0.2) | 1.9 (0.2) | 1.7 (0.1) | 1.8 (0.1) |
| Interpuff Interval (s) | 30.1 (2.1) | 33.7 (3.2) | 31.4 (3.0) | 30.6 (3.0) | 32.5 (3.9) | 31.5 (2.6) | 29.4 (2.8) | 30.7 (3.2) | 34.4 (2.9) | 36.4 (4.0) | 29.8 (3.5) | 28.2 (2.9) | 31.6 (3.1) | 29.9 (3.1) | 29.4 (3.4) |
| Subjective Ratings | |||||||||||||||
| Strength: (very weak/very strong) | 61.2 (5.6) | 66.4 (5.5) | 65.2 (4.9) | 57.5 (6.3) | 65.9 (5.5) | 66.0 (5.1) | 63.5 (4.8) | 65.8 (5.1) | 63.8 (5.7) | 46.4 (6.5) | 50.8 (4.6) | 55.5 (4.7) | 51.7 (5.2) | 59.1 (4.5) | 53.3 (5.1) |
| Harshness: (very mild/very harsh) | 42.5 (6.0) | 38.3 (5.9) | 43.4 (5.4) | 49.3 (5.3) | 51.6 (6.1) | 50.8 (6.7) | 49.7 (5.8) | 48.1 (5.9) | 46.0 (5.4) | 50.1 (6.7) | 43.5 (5.4) | 50.5 (5.2) | 42.5 (5.9) | 45.9 (5.4) | 45.4 (5.5) |
| Heat: (no heat/very hot) | 21.4 (5.3) | 21.7 (4.5) | 33.5 (6.0) | 38.3 (6.1) | 29.6 (5.5) | 34.5 (6.3) | 37.9 (6.7) | 41.2 (6.4) | 35.7 (6.4) | 31.5 (6.4) | 34.0 (5.7) | 30.0 (4.9) | 29.2 (4.7) | 36.6 (5.8) | 34.5 (5.1) |
| Draw: (easy/difficult) | 25.6 (5.6) | 26.0 (5.4) | 34.5 (5.6) | 32.5 (6.2) | 19.2 (4.1) | 26.8 (4.7) | 25.4 (5.1) | 26.5 (5.0) | 20.2 (4.2) | 33.6 (5.9) | 28.5 (5.7) | 26.8 (5.4) | 27.4 (5.7) | 31.5 (5.8) | 22.6 (4.7) |
| Taste: (very bad/very good) | 56.6 (5.8) | 61.3 (5.3) | 57.6 (4.9) | 49.6 (6.4) | 49.3 (6.0) | 47.3 (6.0) | 51.0 (5.1) | 48.2 (6.2) | 52.3 (5.8) | 29.3 (5.1) | 37.5 (5.6) | 38.9 (5.3) | 40.0 (5.4) | 40.4 (5.9) | 39.0 (5.7) |
| Satisfaction from smoking: (unsatisfying/satisfying) | 55.5 (6.2) | 54.5 (6.7) | 56.0 (6.0) | 51.0 (5.9) | 51.2 (6.3) | 48.8 (6.2) | 49.5 (6.5) | 54.9 (6.3) | 54.5 (6.6) | 35.7 (5.1) | 44.0 (5.6) | 42.2 (5.9) | 46.9 (5.7) | 45.6 (5.9) | 44.8 (5.6) |
| (Burned/Did not burn) too fast in too few puffs | 54.0 (5.7) | 53.1 (5.1) | 60.1 (5.8) | 61.1 (5.7) | 56.1 (6.5) | 64.5 (6.1) | 66.0 (5.9) | 71.0 (5.9) | 65.0 (6.4) | 60.7 (6.4) | 66.5 (5.9) | 65.6 (6.5) | 63.8 (6.5) | 67.0 (6.5) | 69.8 (6.2) |
| Mild taste/Not mild taste | 42.0 (6.1) | 52.3 (6.0) | 45.9 (5.9) | 49.6 (6.6) | 54.9 (7.0) | 53.5 (6.4) | 50.5 (5.9) | 56.2 (5.7) | 47.3 (6.2) | 51.2 (6.6) | 50.7 (5.0) | 45.3 (5.2) | 50.7 (5.1) | 49.9 (5.2) | 46.1 (5.9) |
| It (was/was not) too mild for me | 69.4 (5.1) | 66.7 (6.1) | 68.2 (6.1) | 63.5 (6.8) | 66.9 (5.5) | 61.4 (5.7) | 70.5 (5.0) | 65.5 (5.1) | 67.5 (5.5) | 53.0 (7.1) | 62.8 (5.2) | 58.3 (4.9) | 63.6 (5.1) | 63.8 (6.1) | 62.0 (5.1) |
| Smoke (seemed/did not seem) harsh | 67.5 (6.0) | 55.0 (6.7) | 55.8 (7.3) | 61.1 (7.0) | 61.4 (6.9) | 53.8 (7.3) | 55.0 (6.6) | 56.1 (5.9) | 58.2 (6.6) | 45.2 (7.1) | 51.5 (6.9) | 53.7 (6.8) | 54.8 (6.9) | 51.3 (5.7) | 55.5 (7.0) |
| (Did not leave/Left) a good aftertaste in my mouth | 46.6 (5.8) | 50.3 (6.8) | 50.2 (6.2) | 48.5 (6.8) | 52.2 (6.5) | 46.7 (6.5) | 46.4 (6.5) | 44.7 (5.9) | 50.2 (6.7) | 21.9 (4.7) | 32.3 (4.9) | 32.0 (5.4) | 34.0 (5.1) | 32.2 (5.4) | 32.0 (4.8) |
| Somehow it (seemed/did not seem) stale | 78.5 (5.7) | 72.5 (7.1) | 76.6 (6.2) | 76.9 (5.8) | 82.2 (5.1) | 79.3 (5.3) | 78.3 (5.7) | 78.1 (5.7) | 78.1 (5.9) | 53.5 (8.1) | 71.8 (6.4) | 68.6 (6.9) | 73.3 (6.6) | 72.0 (6.4) | 72.8 (6.9) |
| Smoke seemed (very weak/very strong) | 58.5 (5.4) | 62.5 (4.8) | 59.8 (5.5) | 49.7 (6.3) | 65.0 (5.5) | 64.9 (5.1) | 68.2 (4.2) | 64.4 (5.1) | 61.9 (5.6) | 47.8 (6.7) | 49.6 (5.2) | 56.3 (5.3) | 58.3 (5.5) | 57.5 (4.3) | 55.0 (5.2) |
| Smoke smell: (unpleasant/pleasant) | 49.7 (5.2) | 49.5 (6.5) | 51.3 (5.3) | 55.5 (5.8) | 51.5 (5.5) | 49.5 (6.0) | 51.9 (5.9) | 49.7 (5.6) | 53.9 (5.9) | 37.8 (5.0) | 40.6 (5.2) | 38.0 (5.8) | 45.5 (6.0) | 40.9 (6.1) | 39.4 (5.9) |
On Day 1, participants brought a pack of their cigarettes to verify preferred brand, completed demographic and smoking history questions; nicotine dependence was assessed using the well-validated, reliable Fagerstrom Test of Nicotine Dependence (23,24). All participants smoked their own preferred brand cigarette between Day 1 and 5. On Day 5, participants were randomized to either the experimental or control condition in a 3:1 ratio. Participants were instructed on how to crush the capsule to release the menthol from the capsule and compliance was assessed by slicing open the used filters to inspect capsule integrity. Participants in both conditions had cigarettes provided to them from Day 5 through Day 35. Participants were provided with 20% more cigarettes than reported smoking during Day 1 so that if a session required to be re-scheduled, or the participant chose to smoke more cigarettes, they would not extinguish their supply. Participants were explicitly told that they were not required to smoke all of the cigarettes provided to them, and were incentivized to return all of their used and unused cigarettes to the research staff at the onset of each laboratory session.
At the beginning of each session, a baseline breath carbon monoxide (CO) sample was taken, a calendar which logged cigarette consumption was reviewed, and used cigarette filters were counted to verify daily cigarette consumption. At each session two cigarettes were smoked interspersed by 45 minutes, using a smoking topography device. CO boost and subjective ratings were assessed for each cigarette smoked. On Days 5, 20, and 35 a urine sample was obtained for biomarker analysis.
Cigarettes
Cigarettes used in the experimental condition were Camel Crush cigarettes (R.J. Reynolds, Winston-Salem, NC). These cigarettes contain a small, menthol-filled capsule located in the filter that breaks open upon squeezing the capsule and releases menthol into the cigarette. Independent evaluation of Camel Crush verifies there is no menthol present prior to crushing, minimal between cigarette variation in menthol presence after crushing, and equivalent levels of nicotine, cotinine, and total particulate matter analytes, including NNK, before and after crushing (25), making this cigarette ideally suited to isolate menthol’s effects.
Measures
Smoking Behaviors
Smoking behaviors included daily cigarette count, and total puff volume, as measured through a smoking topography device. The smoking topography device is often used in smoking behavior research (26,27), and is a reliable measure of smoking behaviors (28,29). Total puff volume, the sum of the volumes of all puffs taken while consuming the cigarette was selected as the primary measure (other measures reported in Table 1) because it provides a metric to quantify smoke exposure that also allows participants to compensate in different ways, such as taking more puffs or greater volume per puff (27,30).
Biomarkers of Exposure
CO samples at the onset of each session (CO baseline), then prior to and 4 minutes after smoking each cigarette (the difference defined as CO boost) were collected (26,31). Participants provided urine samples on the final day of each period, from which nicotine, cotinine, and 7,8-dihydro-8-oxo-2´-deoxyguanosine (8-oxo-dGuo) assays were performed. Nicotine and cotinine, the proximate metabolite of nicotine, were assessed because of the addictive properties of nicotine.
8-oxo-dGuo is a useful marker of oxidative stress (32,33) that has been proposed as a potential biomarker of cancer risk and carcinogenesis in vivo (34–36), and has shown sensitivity to smoking status (33). All markers were derived from urine samples and underwent analysis using LC-MS techniques described elsewhere (33,37).
Subjective Ratings
To assess subjective responses, participants completed a rating scale of cigarette features that uses a 100 mm visual analog scale with descriptive anchors, and participants place a vertical line to indicate their rating. All subjective rating items, their respective descriptor anchors, and results for the experimental group are presented in Table 1. The subjective items have been used by the tobacco industry and elsewhere (26). Menthol is primarily marketed as a flavoring and therefore subjective ratings when switching menthol presence is important to characterize.
Statistical Analysis
Descriptive statistics were used to characterize the study sample, and unpaired t-tests and chi-square used to examine differences between control (n=15) and experimental groups (n=45), and to test for differences between study completers (n=32) and non-completers (i.e., randomized at Day 5 but did not complete all subsequent sessions; n=28), regardless of randomization condition.
Random effects maximum likelihood regressions (Stata-xt-reg; Stata Corporation, College Station, TX) were used to assess the effects of period on smoking behavior and subjective ratings. Each model included terms for condition (experimental versus control), period (baseline, crush, no crush), and day, and the interaction term. All 35 days were included in the model for cigarettes per day. For smoking topography, CO boost, and subjective ratings, an additional term was included to control for the cigarette smoked during the session (first or second). Biomarker measures derived from the urine samples were assessed using repeated measures ANOVA. Sex, race and nicotine dependence score were included as covariates in all models.
RESULTS
Subjects
Seventy-eight participants met eligibility criteria and attended the first session; 60 returned for the second session at Day 5 and are the basis for analyses. Equal numbers of White (n=29) and African-American (n=29) participated, one participant reported more than one race, and one was Asian; two reported Hispanic ethnicity irrespective of race. Participants had a mean age of 38.4 (SD=11.7). Sixty percent of the study sample was female (n=36), most had completed high school (92%), many had some college education (68%). Participants reported smoking on average 18.0 daily cigarettes (SD=8.0), had been smoking daily for 21.3 years (SD=11.3), and had a nicotine dependence score of 5.8 (SD=1.8). Those not returning for Day 5 (n=18) did not descriptively differ from those completing Day 5 (n=60).
There were no significant differences between experimental and control groups for baseline cigarettes per day, CO, nicotine dependence, age, sex, race or other demographic or smoking characteristics (years smoking, type of cigarette) all ps>0.18. Among study completers, there were no descriptive differences between the control group (n=10) and the experimental group (n=22). Groups did not significantly differ by descriptive measures (p’s >.2). There were no significant group differences at baseline in total puff volume, puff duration, interpuff interval or number of puffs (p’s >.3), subjective ratings (p’s>.15), or biomarkers (p’s>.4) Compliance with crushing (98.9%) and not crushing (99.5%) the menthol capsule as appropriate per period was excellent.
Smoking topography
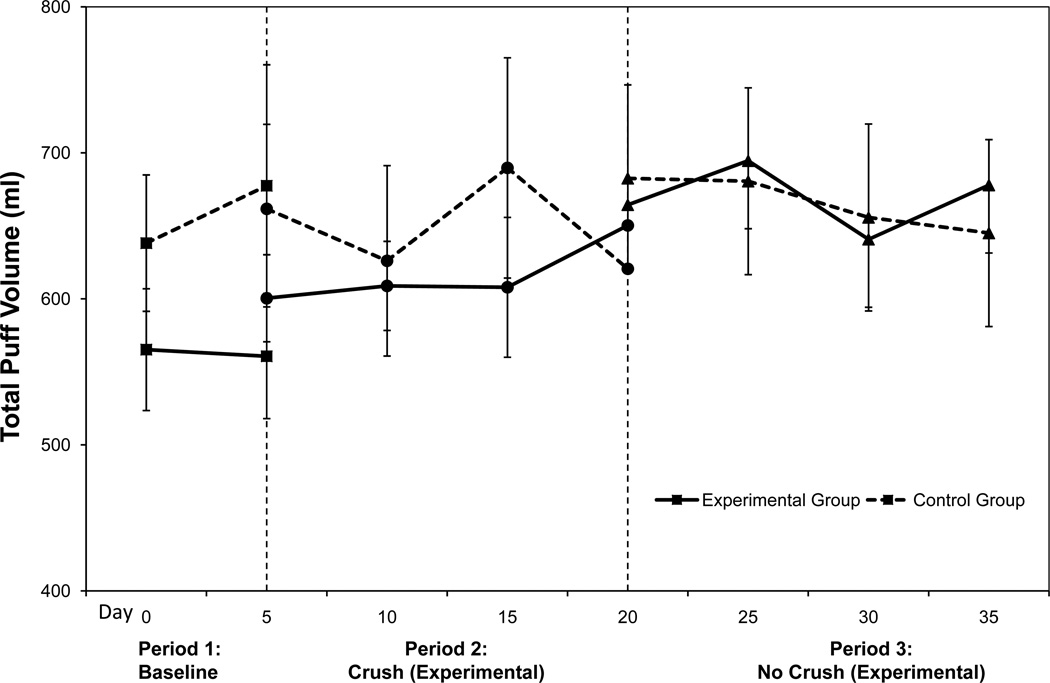
There were significant condition×period interactions for total puff volume (β=91.7, z=2.19, p=0.028) and puff duration (β=0.17, z=2.26, p=0.024). As shown in Figure 1, the experimental group exhibited a marginal increase in total puff volume from period 1 (own brand) to period 2 (menthol; p=0.06) and from period 2 (menthol) to period 3 (non-menthol; p=0.06) and a significant increase between periods 1 and 3 (p=0.02). The control group showed no significant changes in total puff volume (all ps>0.4). For puff duration, there was a significant increase from period 2 (menthol) to period 3 (non-menthol) for the experimental group (p=0.03) and a marginal increase from period 1 to period 2 in the control group (p=0.07; see Table 1). There were no significant condition or period differences for interpuff interval or number of puffs (all ps>0.3).
Figure 1.
Total puff volume across all visits for each condition. Values are adjusted means and standard errors. Vertical dashed lines represent the switch from baseline to crush and from crush to no crush periods.
Cigarettes per day
Across both conditions, there was a main effect of period (β=2.8, z=3.45, p=0.001) on cigarettes per day. There was a significant increase in cigarettes smoked from period 1 (baseline) (mean=13.2, SE=0.81) to period 2 (menthol; mean=14.6, SE=0.69, p=0.0001), but no change from period 2 to period 3 (non-menthol; mean 14.5, SE=0.71, p=0.89) for the experimental group. The control group had a non-significant trend toward an increase in cigarettes per day from period 1 to period 3 compared to the experimental group (condition×period interaction, β=−1.07, z=−1.65, p=0.10). Whites smoked more daily cigarettes than non-Whites (17.0, SE=0.21 vs. 12.6 SE=.17, p=0.028), and significantly increased their smoking throughout the study (β=3.98, p=0.001) regardless of condition.
Carbon monoxide measures
For baseline CO, there were no significant main effects of condition (p=0.21), period (ps>0.31), or interaction (p=0.51).
Across all periods, the experimental group had significantly lower CO boost compared to the control group (β=−3.37, z=−2.99, p=0.003). There was a significant decrease in CO boost from period 1 (baseline) to period 2 (menthol; mean difference=1.6ppm, SE=0.47, p=0.033), but there was no difference between period 2 and period 3 (non-menthol; p=0.32) for the experimental group, nor was there a significant interaction (p=0.4).
Nicotine and cotinine
Mean values for nicotine were 1.706µg/ml (SE=0.30), 2.68µg/ml (SE=0.54), and 2.49µg/ml (SE=0.43) for the own brand, menthol, and non-menthol periods for the experimental group, and 2.17µg/ml (SE=0.82), 3.41µg/ml (SE=1.02), and 2.80µg/ml (SE=1.13) for the corresponding time points in the control group. There was no significant condition effect or interaction effect, but there was a trend in time (p=.09), such that nicotine levels increased slightly between Day 5 and Day 20. Cotinine values had a similar pattern as the nicotine values; there were no significant main effects or interaction effect. Whites had significantly higher cotinine levels than non-white participants (p=.004), but no differences in nicotine levels. There were no significant interaction effects with race.
8-oxo-dGuo
Mean values for 8-oxo-dGuo were 3.52ng/ml (SE=0.46), 5.10ng/ml (SE=1.00), and 4.52ng/ml (SE=.94) for the own brand, menthol, and non-menthol periods for the experimental condition, and 2.91ng/ml (SE=0.61), 3.57ng/ml (SE=0.53), and 2.78ng/ml (SE=0.81) for the corresponding time points in the control condition. There were no significant main effects or interaction effect.
Subjective ratings
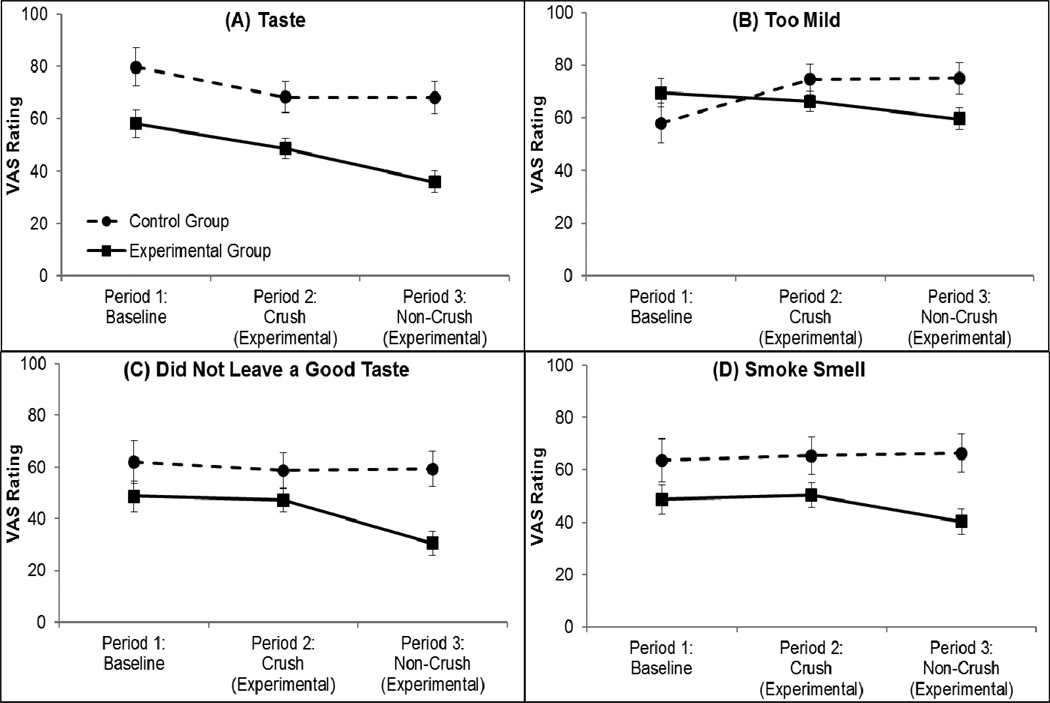
Descriptive statistics for all subjective ratings for the experimental group are presented in Table 1, and only subjective items with a significant effect are reported here. There were significant condition×period interactions for Taste (β=−10.7, z=−2.03, p=0.043), Too Mild (β=−19.9, z=−3.3, p=0.001), Did Not Leave a Good Taste (β=−15.6, z=−2.5, p=0.011), and Smoke Smell (β=−11.2, z=−2.36, p=0.018); see Figure 2.
Figure 2.
Subjective ratings related to taste and flavor across all periods for each condition. Panel (A) Taste, panel (B) Too Mild, panel (C) Did Not Leave a Good Taste, and panel (D) Smoke Smell. Values are adjusted means and standard errors.
For Taste, the experimental group reported significantly worse taste from period 1 (baseline) to period 2 (menthol; p=0.007) and from period 2 to period 3 (non-menthol; p=0.0004). Although the control group reported worse Taste from period 1 to period 2 (p=0.02) despite remaining on their own cigarettes, there was no change between periods 2 and 3 (p=0.97). For Too Mild, there was no change from period 1 (baseline) to period 2 (menthol; p=0.41) and a marginal decrease from period 2 to period 3 (non-menthol; p=0.10) in the experimental group, indicating that period 3 (non-menthol) cigarettes were less mild than all previous cigarettes; and, a decrease in Too Mild ratings from period 1 to period 2 (p=0.002), but no change from period 2 to period 3 (p=0.93) among the control group. For Did Not Leave a Good Taste, the experimental group reported a worse after taste during period 3 (non-menthol) compared to period 1 (baseline; p=0.005) and compared to period 2 (menthol; p=.001), whereas the control group reported no changes between periods (p=0.90). For Smoke Smell, the experimental group reported marginally less pleasant smell from period 1 (baseline) compared to period 2 (menthol; p=0.09), and significantly less pleasant from period 2 to period 3, (menthol to non-menthol periods; p=0.002). The control group showed no changes for smoke smell (ps>0.6). Additionally, for Satisfying, there were main effects of condition (β=−22.5, z=−2.7, p=0.008) and period (β=−6.4, z=−2.5, p=0.012) such that across all periods, the experimental group rated their cigarettes as less satisfying than the control group, and across both conditions, cigarettes were rated as less satisfying from period 1 to period 2 and from period 1 to period 3 (mean=51.3, SE=3.9, p=0.02).
DISCUSSION
This study is the first to examine the effect removing menthol might have on smokers through an extensive panel of behavioral, exposure and subjective rating measures, and for an extended duration. By using a new cigarette that allows all other cigarette design features and constituents to remain constant, results can be interpreted as attributable to menthol presence. Previous brand switching studies, where smokers switched from a menthol cigarette to a non-menthol cigarette, were inadequate because the cigarettes differed in other ways. For the same reason, comparisons between groups of menthol and non-menthol smokers had shortcomings, as well as the inherent differences in the composition of the groups who self-select toward the different cigarette types.
Although there was a slight increase in daily cigarette consumption once cigarettes were supplied to both groups (post-Day 5), there was no meaningful change in daily cigarette consumption in the experimental group when the menthol flavor was removed from their cigarettes. Total puff volume significantly increased when the experimental group switched from menthol to non-menthol smoking, but only by an average of 65ml (11% increase). These puffing differences did not correspond to increased biomarker levels. The lack of behavioral and exposure differences in this study are important to note because they add to the data that support the nominal impact of menthol on smoking and exposure levels that have been recently reported (20,38).
Under the auspices of the FSPTCA, the TPSAC was charged with reviewing the scientific evidence related to the health risks of menthol cigarette smoking (3). Their conclusions regarding menthol’s effects on smoking behaviors noted many limitations in previous research, including cigarette design differences beyond menthol presence, the difference in racial composition between menthol and non-menthol groups, and the lack of laboratory studies on the topic. This current laboratory study adds to the empirical evidence by using a product that allows for isolating the effect of menthol in cigarettes, and makes a new contribution to better understanding how menthol affects smoking behavior and exposure. Further, by including an own brand period, smokers serve as their own control or comparison, thus accounting for individual differences including race, metabolic differences, and smoking behaviors, in the model.
Subjective ratings directly associated with taste and flavor, specifically Taste, Mildness, After taste, and Smoke smell were all significantly negatively impacted during the non-menthol period. Interestingly, characteristics associated with nicotine level (strength and harshness) or physical characteristics (e.g., filter ventilation, such as draw, heat and burn time) (26,39), were not affected by the switch to non-menthol. One interpretation of this pattern of results might be that menthol is primarily affecting taste and sensory responses, which has been discussed by Carpenter and colleagues (40), and less associated with nicotine delivery or facilitating of easier and bigger puffing.
What this study did not investigate is the effect menthol might have on recruiting and retaining new adolescent smokers, a topic investigated by others (41,42) that suggests young smokers often start smoking menthol cigarettes then switch to non-menthol as their smoking progresses. Menthol as a means to attract and retain new smokers is an important topic that clearly extends beyond the scope of this project. While some have posed the question of what menthol smokers would do if menthol were banned (43), few modeling estimates exist to propose how a menthol ban might prevent smoking initiation (44). Also, the study recruited all menthol smokers over the age of 21 and therefore cannot address minority and adolescent smoking of menthol cigarettes.
There are approximately 19.2 million menthol cigarette smokers in the U.S. (45), and to assign significant efforts to achieve a ban on menthol is not a small effort and will impact many people. Recent conclusions by the TPSAC state that it is inconclusive whether menthol affects smoking behavior and smoke exposure, but also note several shortcomings in the studies reviewed. This study adds to the scientific literature, and bridges previously identified gaps in the knowledge, and our results suggest minimal impact on smoking behaviors and exposures due to menthol presence when using a cigarette that can control for all other aspects of cigarette design. Menthol may have less effect on smoking behaviors in established smokers than on adolescents and smoking initiation.
Acknowledgments
GRANT SUPPORT
This work was supported by the National Institutes of Health (R01-120594, R01-120594-S1 to A.A.S.; R01-130961 to A.A.S. and I.A.B; and P30-ES013508 T.M.P; and P50-CA143187; C.E.L.).
Footnotes
CONFLICTS OF INTEREST: There are no actual, potential or perceived conflicts of interest with this manuscript.
REFERENCES
- 1. [Accessed July 30, 2012];H.R. 1256-111th Congress: Family Smoking Prevention and Tobacco Control Act. 2009. from http://www.govtrack.us/congress/bills/111/hr1256.
- 2.Lawrence D, Rose A, Fagan P, Moolchan ET, Gibson JT, Backinger CL. National patterns and correlates of mentholated cigarette use in the United States. Addiction. 2010;105:13–31. doi: 10.1111/j.1360-0443.2010.03203.x. [DOI] [PubMed] [Google Scholar]
- 3.Tobacco Products Scientific Advisory Committee. (TPSAC) [Accessed July 30, 2012];Menthol Cigarettes and Public Health: Review of the Scientific Evidence and Recommendations. 2011 http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/TobaccoProductsScientificAdvisoryCommittee/UCM269697.pdf.
- 4.Sidney S, Tekawa IS, Friedman GD, Sadler MC, Tashkin DP. Mentholated cigarette use and lung cancer. Arch Intern Med. 1995;155(7):727–732. [PubMed] [Google Scholar]
- 5.Murray RP, Connett JE, Skeans MA, Tashkin DP. Menthol cigarettes and health risks in Lung Health Study data. Nicotine Tob Res. 2007;9(1):101–107. doi: 10.1080/14622200601078418. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter CL, Jarvik ME, Morgenstern H, McCarthy WJ, London SJ. Mentholated cigarette smoking and lung-cancer risk. Ann Epidemiol. 1999;9(2):114–120. doi: 10.1016/s1047-2797(98)00042-8. [DOI] [PubMed] [Google Scholar]
- 7.US Department of Health and Human Services. (USDHHS) Tobacco Use Among U.S. Racial/Ethnic Minority Groups-African Americans, American Indians and Alaska Natives, Asian Americans and Pacific Islanders, and Hispanics: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 1998. [Google Scholar]
- 8.Perez-Stable EJ, Fuentes-Afflick E. Role of clinicians in cigarette smoking prevention. West J Med. 1998;169(1):23–29. [PMC free article] [PubMed] [Google Scholar]
- 9.McCarthy WJ, Caskey NH, Jarvik ME, Gross TM, Rosenblatt MR, Carpenter C. Menthol vs nonmenthol cigarettes: effects on smoking behavior. Am J Public Health. 1995;85(1):67–72. doi: 10.2105/ajph.85.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahijevych K, Parsley LA. Smoke constituent exposure and stage of change in black and white women cigarette smokers. Addict Behav. 1999;24(1):115–120. doi: 10.1016/s0306-4603(98)00031-8. [DOI] [PubMed] [Google Scholar]
- 11.Pickworth WB, Moolchan ET, Berlin I, Murty R. Sensory and physiologic effects of menthol and non-menthol cigarettes with differing nicotine delivery. Pharmacol Biochem Behav. 2002;71(1–2):55–61. doi: 10.1016/s0091-3057(01)00623-2. [DOI] [PubMed] [Google Scholar]
- 12.Caskey NH, Jarvik ME, McCarthy WJ, Rosenblatt MR, Gross TM, Carpenter CL. Rapid smoking of menthol and nonmenthol cigarettes by black and white smokers. Pharmacol Biochem Behav. 1993;46(2):259–263. doi: 10.1016/0091-3057(93)90350-3. [DOI] [PubMed] [Google Scholar]
- 13.Miller GE, Jarvik ME, Caskey NH, Segerstrom SC, Rosenblatt MR, McCarthy WJ. Cigarette mentholation increases smokers' exhaled carbon monoxide levels. Experimental and Clinical Psychopharmacology. 1994;2(2):154–160. [Google Scholar]
- 14.Jarvik ME, Tashkin DP, Caskey NH, McCarthy WJ, Rosenblatt MR. Mentholated cigarettes decrease puff volume of smoke and increase carbon monoxide absorption. Physiol Behav. 1994;56(3):563–570. doi: 10.1016/0031-9384(94)90302-6. [DOI] [PubMed] [Google Scholar]
- 15.Nil R, Battig K. Separate effects of cigarette smoke yield and smoke taste on smoking behavior. Psychopharmacology (Berl) 1989;99(1):54–59. doi: 10.1007/BF00634452. [DOI] [PubMed] [Google Scholar]
- 16.Williams JM, Gandhi KK, Steinberg ML, Foulds J, Ziedonis DM, Benowitz NL. Higher nicotine and carbon monoxide levels in menthol cigarette smokers with and without schizophrenia. Nicotine Tob Res. 2007;9(8):873–881. doi: 10.1080/14622200701484995. [DOI] [PubMed] [Google Scholar]
- 17.Mustonen TK, Spencer SM, Hoskinson RA, Sachs DP, Garvey AJ. The influence of gender, race, and menthol content on tobacco exposure measures. Nicotine Tob Res. 2005;7(4):581–590. doi: 10.1080/14622200500185199. [DOI] [PubMed] [Google Scholar]
- 18.Clark PI, Gautam S, Gerson LW. Effect of menthol cigarettes on biochemical markers of smoke exposure among black and white smokers. Chest. 1996;110(5):1194–1198. doi: 10.1378/chest.110.5.1194. [DOI] [PubMed] [Google Scholar]
- 19.Ahijevych K, Gillespie J, Demirci M, Jagadeesh J. Menthol and nonmenthol cigarettes and smoke exposure in black and white women. Pharmacol Biochem Behav. 1996;53(2):355–360. doi: 10.1016/0091-3057(95)02034-9. [DOI] [PubMed] [Google Scholar]
- 20.Muscat JE, Stellman SD, Caraballo RS, Richie JP. Time to First Cigarette after Waking Predicts Cotinine Levels. Cancer Epidemiol Biomarkers Prev. 2009;18(12):3415–3420. doi: 10.1158/1055-9965.EPI-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heck JD. Smokers of menthol and nonmenthol cigarettes exhibit similar levels of biomarkers of smoke exposure. Cancer Epidemiol Biomarkers Prev. 2009;18(2):622–629. doi: 10.1158/1055-9965.EPI-08-0550. [DOI] [PubMed] [Google Scholar]
- 22.Borgerding M, Bodnar J, Wingate D. [Accessed August 28, 2012];The 1999 Massachusetts Benchmark Study: Final Report. 2000 Jul; Available at http://legacy-dcucsfedu/documentStore/e/p/m/epm46a00/Sepm46a00pdf.
- 23.Pomerleau CS, Carton SM, Lutzke ML, Flessland KA, Pomerleau OF. Reliability of the fagerstrom tolerance questionnaire and the fagerstrom test for nicotine dependence. Addict Behav. 1994;19(1):33–39. doi: 10.1016/0306-4603(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 24.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: A revision of the Fagerstrom tolerance questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 25.Gordon SM, Brinkman MC, Meng RQ, et al. Effect of cigarette menthol content on mainstream smoke emissions. Chem Res Toxicol. 2011;24(10):1744–1753. doi: 10.1021/tx200285s. [DOI] [PubMed] [Google Scholar]
- 26.Strasser AA, Ashare RL, Kozlowski LT, Pickworth WB. The effect of filter vent blocking and smoking topography on carbon monoxide levels in smokers. Pharmacol Biochem Behav. 2005;82(2):320–329. doi: 10.1016/j.pbb.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Strasser AA, Benowitz NL, Pinto AG, et al. Nicotine metabolite ratio predicts smoking topography and carcinogen biomarker level. Cancer Epidemiol Biomarkers Prev. 2011;20(2):234–238. doi: 10.1158/1055-9965.EPI-10-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strasser AA, Tang KZ, Sanborn PM, Zhou JY, Kozlowski LT. Behavioral filter vent blocking on the first cigarette of the day predicts which smokers of light cigarettes will increase smoke exposure from blocked vents. Experimental and Clinical Psychopharmacology. 2009;17(6):405–412. doi: 10.1037/a0017649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee EM, Malson JL, Waters AJ, Moolchan ET, Pickworth WB. Smoking topography: reliability and validity in dependent smokers. Nicotine Tob Res. 2003;5(5):673–679. doi: 10.1080/1462220031000158645. [DOI] [PubMed] [Google Scholar]
- 30.Benowitz NL, Jacob P, 3rd, Bernert JT, et al. Carcinogen exposure during short-term switching from regular to "light" cigarettes. Cancer Epidemiol Biomarkers Prev. 2005;14(6):1376–1383. doi: 10.1158/1055-9965.EPI-04-0667. [DOI] [PubMed] [Google Scholar]
- 31.Zacny JP, Stitzer ML, Brown FJ, Yingling JE, Griffiths RR. Human cigarette smoking: effects of puff and inhalation parameters on smoke exposure. Journal of Pharmacology Experimental Therapeutics. 1987;240(2):554–564. [PubMed] [Google Scholar]
- 32.Hu CW, Wang CJ, Chang LW, Chao MR. Clinical-scale high-throughput analysis of urinary 8-oxo-7,8-dihydro-2'-deoxyguanosine by isotope-dilution liquid chromatographytandem mass spectrometry with on-line solid-phase extraction. Clin Chem. 2006;52(7):1381–1388. doi: 10.1373/clinchem.2005.063735. [DOI] [PubMed] [Google Scholar]
- 33.Mesaros C, Arora JS, Wholer A, Vachani A, Blair IA. 8-Oxo-2'-deoxyguanosine as a biomarker of tobacco-smoking-induced oxidative stress. Free Radic Biol Med. 2012;53(3):610–617. doi: 10.1016/j.freeradbiomed.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu LL, Chiou CC, Chang PY, Wu JT. Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin Chim Acta. 2004;339(1–2):1–9. doi: 10.1016/j.cccn.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Kasai H. Analysis of a form of oxidative DNA damage, 8-hydroxy-2'-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat Res. 1997;387(3):147–163. doi: 10.1016/s1383-5742(97)00035-5. [DOI] [PubMed] [Google Scholar]
- 36.Cooke MS, Lunec J, Evans MD. Progress in the analysis of urinary oxidative DNA damage. Free Radic Biol Med. 2002;33(12):1601–1614. doi: 10.1016/s0891-5849(02)01146-2. [DOI] [PubMed] [Google Scholar]
- 37.Rangiah K, Hwang WT, Mesaros C, Vachani A, Blair IA. Nicotine exposure and metabolizer phenotypes from analysis of urinary nicotine and its 15 metabolites by LCMS. Bioanalysis. 2011;3(7):745–761. doi: 10.4155/bio.11.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benowitz NL, Samet JM. The threat of menthol cigarettes to U.S.public health. N Engl J Med. 2011;364(23):2179–2181. doi: 10.1056/NEJMp1103610. [DOI] [PubMed] [Google Scholar]
- 39.Strasser AA, Lerman C, Sanborn PM, Pickworth WB, Feldman EA. New lower nicotine cigarettes can produce compensatory smoking and increased carbon monoxide exposure. Drug Alcohol Depend. 2007;86(2–3):294–300. doi: 10.1016/j.drugalcdep.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 40.Carpenter CM, Wayne GF, Connolly GN. The role of sensory perception in the development and targeting of tobacco products. Addiction. 2007;102(1):136–147. doi: 10.1111/j.1360-0443.2006.01649.x. [DOI] [PubMed] [Google Scholar]
- 41.Hersey JC, Ng SW, Nonnemaker JM, et al. Are menthol cigarettes a starter product for youth? Nicotine Tob Res. 2006;8(3):403–413. doi: 10.1080/14622200600670389. [DOI] [PubMed] [Google Scholar]
- 42.Villanti AC, Giovino GA, Barker DC, Mowery PD, Sevilimedu V, Abrams DB. Menthol Brand Switching Among Adolescents and Young Adults in the National Youth Smoking Cessation Survey. Am J Public Health. 2012 Jul;102(7):1310–1312. doi: 10.2105/AJPH.2011.300632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Connor RJ, Bansal-Travers M, Carter LP, Cummings KM. What would menthol smokers do if menthol in cigarettes were banned? Behavioral intentions and simulated demand. Addiction. 2012 Jul;107(7):1330–1338. doi: 10.1111/j.1360-0443.2012.03822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levy DT, Pearson JL, Villanti AC, et al. Modeling the future effects of a menthol ban on smoking prevalence and smoking-attributable deaths in the United States. Am J Public Health. 2011 Jul;101(7):1236–1240. doi: 10.2105/AJPH.2011.300179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caraballo R. Menthol and Demographics. Washington, D.C.: Food and Drug Administration Tobacco Products Scientific Advisory Committee; 2010. Mar 30, [Google Scholar]