Abstract
We review current concepts in CRPS from a neuroimaging perspective and point out topics and potential mechanisms that are suitable to be investigated in the next step towards understanding the pathophysiology of CRPS. We have outlined functional aspects of the syndrome, from initiating lesion via inflammatory mechanisms to CNS change and associated sickness behavior, with current evidence for up-regulation of immunological factors in CRPS, neuroimaging of systemic inflammation, and neuroimaging findings in CRPS. The initiation, maintenances and CNS targets implicated in CRPS and in the neuro-inflammatory reflex are discussed in terms of CRPS symptoms and recent preclinical studies. Potential avenues for investigating CRPS with PET and fMRI are described, along with roles of inflammation, treatment and behavior in CRPS. It is our hope that this outline will provoke discussion and promote further empirical studies on the interactions between central and peripheral inflammatory pathways manifest in CRPS.
Keywords: CRPS, cytokine, fMRI, PET, glia, astrocyte, brain, inflammation
Introduction
Complex Regional Pain Syndrome (CRPS) is a chronic pain syndrome following nerve injury (either defined or subclinical) that may evolve into a number of other manifestations over time that implicate brain changes. These manifestations include hypersensitivity to noxious somatosensory stimuli (hyperalgesia), pain to non-noxious stimuli (allodynia), spreading pain (spontaneous and evoked) (Maleki et al. 2000) that involves undamaged regions in the affected limb to the opposite limb and other parts of the body (e.g., from lower extremities to upper extremities), swelling and skin discoloration, autonomic changes such as coldness, poor circulation, abnormal sweating, hemi-inattention, motor changes (movement disorders including tremor and focal dystonias) as well as changes in emotional and cognitive function. CRPS type 1 occurs without detectable nerve trauma, and minor injuries or a limb fracture often precede the onset. CRPS type 2 CRPS develops following injury of a major peripheral nerve, but the subtype distinction is often not made in the scientific literature (Watts and Kremer 2011). However, even clinically, the differences may be limited because microscopic nerve damage is not possible to detect. The estimated overall incidence rate of CRPS was 26.2 per 100,000 person years with females being affected 3–4 times more than males (de Mos et al. 2007), usually occurring from peripheral nerve injury.
Clinically, CRPS patients are profoundly affected with decreased ability to participate in normal activities of daily living. Following nerve injury a cascade of events is initiated that include peripheral inflammation (Tal 1999) and potentially increased CNS inflammation. The peripheral changes include immune-related genes and members of the complement system (Liang et al. 2012) as well as presence of several pro-inflammatory cytokines (Strong et al. 2012). A number of etiopathophysiological processes have been suggested, including cytokine imbalance and neuroinflammation (Cooper and Clark 2012). Here, we evaluate lines of evidence from animal and human research indicative of focal and disseminated effects of cytokine alterations that may affect brain circuits through changes on glia, astrocytes and neurons.
CRPS The Model: From Peripheral Nerve damage to diffuse CNS Changes
The behavior of sick animals and humans is recognized as part of a motivational system that priorities seeking quiescence to facilitate recovery (Dantzer and Kelley 2007; Hart 1988). Cytokines are signaling proteins secreted by peripheral immune cells, microglia, astrocytes and neurons that signal the detection of pathogens (Galic et al. 2012). An overproduction of cytokines may result in disease (Czura and Tracey 2005; Pavlov and Tracey 2006). The inflammatory reflex is “a physiological pathway in which the autonomic nervous system detects the presence of inflammatory stimuli and modulates cytokine production” (Czura and Tracey 2005). From a functional perspective, the inflammatory processes in traumatic events induce central and peripheral sensitization to increase pain awareness and limit further injury.
Inflammatory cytokines are associated with common symptoms of sickness including loss of appetite, sleepiness, withdrawal, fatigue and pain sensitivity in both rodents (Maier et al. 1993) and humans (Benson et al. 2012). For example, in the common cold, headaches and sinus pain may originate from nasal congestion and pressure changes, but inflammatory mediators within the sinuses may also trigger sensitization of pain or directly stimulate trigeminal nerves (Eccles 2005). Moreover, muscle aches (myalgia) occurs in around 50% of patients with the common cold (Eccles et al. 2003). Of the possible mechanism for this increase in myalgia, the perhaps most studied is that circulating cytokines increase the production of prostaglandin E2 in muscle tissue, thereby stimulating peripheral sensory neurons and inducing hyperalgesia (Baracos et al. 1983). A second route for increased pain responses is via cytokine activation of microglial cells and/or macrophages in the spinal cord and dorsal root ganglion (Yoon et al. 2012). A third route is via direct cytokine effects on brain excitability. In other conditions such as depression it has been postulated that the depressed state may activate peripheral mechanisms resulting in an up-regulation of systemic levels of inflammation and peripheral inflammation may exacerbate depression (Messay et al. 2012). Here, we explore the potential for such a bi-directional process as a model for CRPS.
Evidence for up-regulation of immunological factors in CRPS
There is mixed evidence for pro-inflammatory cytokines profiles in CRPS (Birklein and Schmelz 2008). Van de Beek et al. (2001) found no difference in the production of pro- and anti-inflammatory cytokines when comparing 26 severely affected CRPS patients and 20 healthy controls. However, others have found increased levels of IL-8, IL2, TNF-α receptors and substance P (Maihofner et al. 2005b; Schinkel et al. 2006; Kramer et al. 2011; Uceyler et al. 2007). Case reports of blocking TNF-α with the antibody infliximab have shown success in treatment CRPS (Bernateck et al. 2007), and there is accumulation of TNF-α antibodies in affected hands of patients with early-stage CRPS, but not in clinically unaffected hands or in late-stage CRPS (Bernateck et al. 2010).
Evidence for CNS alterations in CRPS
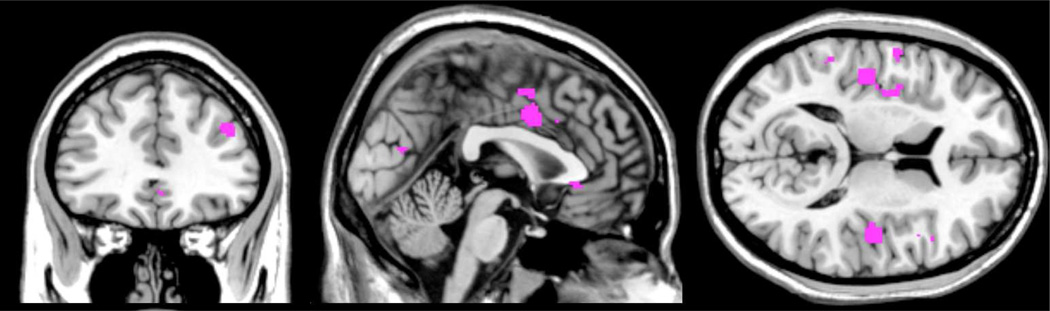
CRPS is thought to involve peripheral and central sensitization of neuronal function (Janig and Baron 2002), and several recent neuroimaging studies have demonstrated changes in brain function. Table 1 provides an overview of the literature, as per July 2012. We performed an activation likelihood estimate (ALE) of the above studies to provide an overview of CNS structures that have been consistently implicated in CRPS. An ALE analysis determines the convergence of activation foci, providing a quantitative meta-analytical estimate of activation probability in the form of a brain map (Eickhoff et al. 2009). As there are still only a handful of studies on alteration in brain activity in CRPS, the resulting ALE maps should be considered preliminary. Results indicate that CRPS patients commonly display alteration in the bilateral parietal lobe, somatosensory cortex, mid insula, mid cingulate and the superior medial frontal gyrus. See Table 2 for details.
Table 1.
Neuroimaging studies of CRPS
| Reference | Subjects | Modality | Paradigm | Key results |
|---|---|---|---|---|
| (Freund et al. 2010) | 10 CRPS, 15 HC | 1.5T fMRI | Electrical pain | increased PCC activation, decreased opercular activation |
| (Freund et al. 2011) | 10 CRPS, 15 HC | 1.5T fMRI | Electrical pain + cogn. suppression |
PAG and cingulate less active during suppression of pain, regardless of stimulation site |
| (Gieteling et al. 2008) | 8 CRPS, 17 HC | 3T fMRI | Motor execution +motor imagining |
imaginary, but not actual movement showed reduced premotor, prefrontal cortex, and anterior insula activation |
| (Gustin et al. 2010) | 20 CRPS | 3T fMRI | Motor execution and morphine+memantine or morph. + placebo |
morphine treatment reduced ACC activation, the addition of memanite further reduced somatosensory activation |
| (Maihofner et al. 2005) | 12 CRPS | 1.5T fMRI | von-Frey hyperalgesia | increased activation of S1 S2, insula, associative- somatosensory, frontal and ACC |
| (Maihofner et al. 2006) | 12 CRPS | 1.5T fMRI | Brush allodynia | increased S1, S2, M1, parietal, frontal cortices, aACC, pACC |
| (Maihofner et al. 2007) | 12 CRPS, 12 HC | 1.5T fMRI | Finger tapping | reorganization of central motor circuits, increased activation of M1 and supplementary motor areas |
| (Lebel et al. 2008) | 8 pediatric CRPS | 3T fMRI | Cold and brush, pre and post recovery |
multiple regions of increased CNS activation, functional abnormalities may persist after pain has resolved |
| (Pleger et al. 2006) | 17 CPRS, 17 HC | 1.5T fMRI | TENS stimulation | SI and SII were significantly reduced parallel impaired tactile discrimination |
| Not included in ALE | ||||
| (Baliki et al. 2011) | 28 CPRS, 46 HC 36 CBP, 20 OA |
3T VBM | Structural changes | decreased gray matter in anterior insula and orbitofrontal cortex |
| (Geha et al. 2008) | 21 CRPS, 21 HC | Gray, white matter | decreased gray matter in VMPFC and accumbmens, decrease in fractional in the left cingulum- callosal bundle, and in insula and basal ganglia connections |
|
| (Mutso et al. 2012) | 30 CRPS 50 HC 38 CBP, 20 OA |
3T structural | Hippocampus volume | Reduced hippocampus volume in CRPS, CBP but not OA |
| (Klega et al. 2010) | 10 CPRS, 10 HC | [18F]-diprenorphine | reduced opioid receptor binding potential in amygdala and parahippocampal gyrus, increased in prefrontal cortex |
|
ACC anterior cingulate cortex, CPRS Complex Regional Pain Syndrome, HC Healthy Controls, CBP Chronic back pain, OA osteoarthritis, PAG periaqueductal gray, PCC posterior cingulate cortex, VMPFC ventromedial prefrontal cortex
Table 2.
Activation likelihood estimated brain regions implicated in inflammatory responses
| Cluster size | Cluster peak MNIxyz | Gray matter regions implicated |
|---|---|---|
| 528 mm3 | −12, −12, −13 | Subthalamic nucleus, Left Parahippocampal Gyrus, Substania Nigra, Globus Pallidus, Uncus |
| 472 mm3 | −44, −5, 9 | Left Insula, Left Precentral Gyrus |
| 360 mm3 | −7, −33, −11 | Left Anterior and Culmen of the Cerebellum, left brainstem, Left Thalamus |
| 320 mm3 | 20, −97, −10 | Right Fusiform Gyrus, Right Lingual Gyrus, Right Inferior Occipital Gyrus |
| 304 mm3 | −38, −59, −12 | Left Temporal Fusiform Gyrus, Left Declive of the Cerebellum |
| 304 mm3 | −29, −60, 35 | Left Middle Temporal Gyrus, Left Precuneus, Left Angular Gyrus |
| 280 mm3 | 48, −42, −9 | Right Temporal Fusiform Gyrus, Right Occipital Fusiform Gyrus |
| 272 mm3 | −5, 17, −13 | Left Anterior Cingulate, Left Caudate Head |
| 200 mm3 | 16, 24, 61 | Right Superior Frontal Gyrus, Right Middle Frontal Gyrus |
Evidence for Inflammation effects on CNS processing
In the last years, several studies have used functional MRI to investigate how systemic inflammation influences brain function. We view this evidence, although not obtained in CRPS patients, as a potential overlapping mechanism:
Typhoid vaccination (Brydon et al. 2008; Harrison et al. 2009b; Harrison et al. 2009a), endotoxin administration (Eisenberger et al. 2010; Eisenberger et al. 2009; Inagaki et al. 2012; Kullmann et al. 2012), and psychological manipulations — social stress (Slavich et al. 2010) and grief (O'Connor et al. 2009) — have been used to induce systemic inflammation and determine brain reactivity. Other studies include the effects of interferon-alpha treatment on brain function in in patients infected with hepatitis C virus (Capuron et al. 2005), and the effects of an antigen challenge TNF alpha production and brain responses in asthma patients (Rosenkranz et al. 2005). Moreover, the glucose metabolism of the cingulate and insula is altered by endotoxin administration (Hannestad et al. 2012b). Also for these studies, we performed an ALE analysis, to identify brain regions that have been consistently linked to systemic inflammation. Due to the relatively few studies, this resulting ALE maps should also be considered preliminary. Nonetheless, the results indicate that the brainstem, amygdala, anterior cingulate, insula and posterior cingulate reliably increase in functional activation as a function of systemic inflammation (see Table 3).
Table 3.
Activation likelihood estimated brain regions implicated in CRPS
| Cluster size | Cluster peak MNIxyz | Gray matter regions implicated |
|---|---|---|
| 1320 mm3 | 0, 12, 52 | Superior Frontal Gyrus, Medial Frontal Gyrus |
| 832 mm3 | −29, −53, 45 | Left Superior Parietal Lobule, Left Inferior Parietal Lobule, Left Angular Gyrus |
| 736 mm3 | 0, 6, 36 | Cingulate Gyrus |
| 696 mm3 | −31, −32, 48 | Left Postcentral Gyrus, Left Inferior Parietal Lobule, Left Precentral Gyrus |
| 648 mm3 | −44, −21, 51 | Left Postcentral Gyrus, Left Inferior Parietal Lobule |
| 616 mm3 | −53, −22, 16 | Left Postcentral Gyrus, Left Insula, Left Transverse Temporal Gyrus |
| 512 mm3 | 30, −52, 43 | Right Superior Parietal Lobule, Right Precuneus, Right Cingulate Gyrus |
| 368 mm3 | 35, 3, 18 | Right Claustrum, Right Insula, Right Precentral Gyrus |
| 224 mm3 | −34, −35, 5 | Left Caudate Tail, Left Transverse Temporal Gyrus |
As illustrated in Figure 1, there is substantial overlap in the brain regions that have been linked to both CRPS and to systemic inflammation independently: notably the middle cingulate, posterior insula, dorsolateral prefrontal cortex and the parietal lobule. Moreover, there may be overlap between inflammatory responses and the structural changes observed in CRPS (Baliki et al. 2011; Geha et al. 2008), i.e. the ventral medial prefrontal cortex and the anterior insula and the nucleus accumbmens.
Fig. 1.
Shared activation in CRPS processing and cytokine related activation, illustrating the potentially shared networks between the conditions. The Activation Likelihood Estimate (ALE) overlap was created as the conjunction of ALE maps from CRPS and cytokine related fMRI studies, not corrected for multiple comparisons.
Another line of (circumstantial) evidence comes from a recent study by Hess and colleagues (Hess et al. 2011) where they demonstrated that in rheumatoid arthritis, the rapid clinical effects of TNF-α neutralization affect central nociceptive activity. Specifically, 24 hours after patients received the TNF-α blocker infliximab (Remicade®) at a dose of 3 mg/kg, fMRI BOLD signal evoked by compressing joints showed diminished spatial extent in somatosensory cortex, the parietal cortex, the posterior cingulate cortex and the medial prefrontal cortex. These effects paralleled pain reduction, but preceded reduction in joint swelling, serum C-reactive protein levels and serum IL-6 levels. These findings provide a potential bridge between the fields of neurology and immunology, in that brain regions associated with immune responses may map onto what has been dubbed an “an immunological homunculus” (Diamond and Tracey 2011; Tracey 2007).
To our knowledge, no study has yet investigated how pain processing is altered by experimental induction of systemic inflammation, and no study has linked pro-inflammatory cytokine levels directly to brain function in CRPS. Such an endeavor would be a highly important contribution to the field, as all the regions implicated above play a key role in sensory and affective components of pain processing, as well as potentially in hemi inattention type manifestations of CRPS. The commonly observed alterations in pain processing observed in CRPS may perhaps, in part, be explained by ongoing systemic inflammation.
Nerve Damage as an initiator of an inflammatory Feedback Loop
Below, we present a basic outline for how CRPS may develop, based on a neuro-inflammatory perspective in terms of contributing events, from initiations in a peripheral injury, via an inflammatory reflex to maintenance, specific CNS targets, and the potential plateauing of the disease.
Initiating Events – Peripheral to CNS Activity
Following injury to a nerve (typically by a sprain, fracture, surgery, or other traumatic injury) a cascade of events take place locally and systemically. These processes have been well characterized and are the subject of a number of reviews (Ren and Dubner 2010; Skaper et al. 2012). The nerve injury is a likely primary driver of neurogenic inflammation in CRPS. The inflammation has best been described at the site (Kim and Moalem-Taylor 2011) and in the spinal cord (Clark et al. 2007; Cao and Zhang 2008). Chemokines (e.g., (CCL2, CCL3, and fractalkine) and cytokines (e.g., IL1, IL6, NF-KB, TNF-alpha) released by neuro-inflammatory immune cells (e.g., leukocytes) that are induced in the injured nerve. Both inflammatory and anti-inflammatory processes are initiated (Austin and Moalem-Taylor 2010). Presumably, a balance between pro- and anti-inflammatory systems, such as IL-10, is lost (Gaba et al. 2012). In some conditions such as CRPS it is assumed that the anti-inflammatory processes may be abnormal or overwhelmed. Recent data show that one-sided inflammation may increase pain systems in the contralateral cord – viz., c-fos activation pattern of spinal Gly/GABA neurons (Hossaini et al. 2011). Such data support a process by which contralateral pain (Schreiber et al. 2008; Hatashita et al. 2008) may progress to other non-injured sites in CRPS (van Rijn et al. 2011). Finally, prior inflammation may contribute to later pain responses in these patients (Hains et al. 2010; Vega-Avelaira et al. 2012), presumably due to sensitization or alteration of pain networks. While inflammatory pain in preclinical models has been shown to activate microglia and astrocytes in the spinal cord, less information is available on central activation of these systems (Wang et al. 2002).
Contributing Events – An Inflammatory Reflex
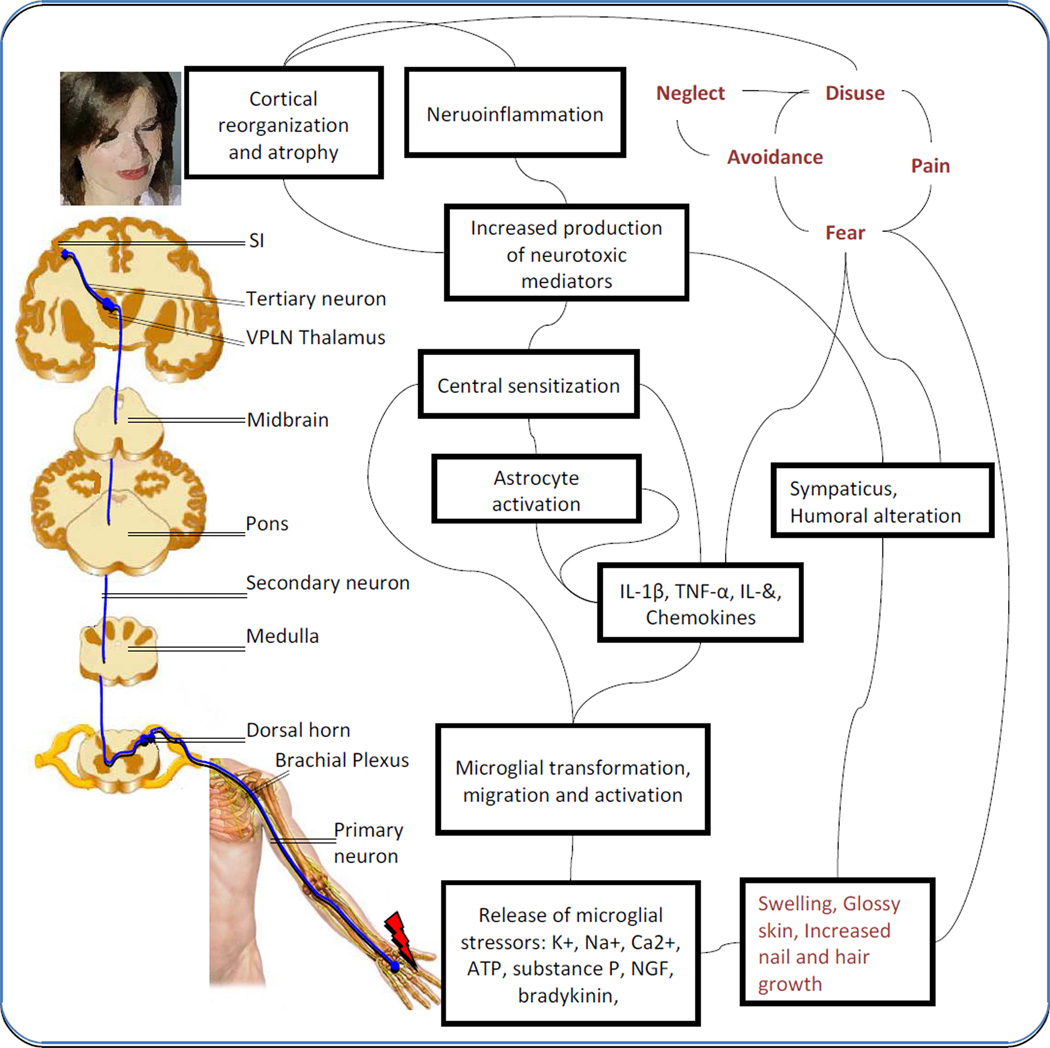
Following initiation of peripheral neuroimmune activation by injury to nerves, a cascade involving peripherally activated signals, may confer changes in neuroimmune systems in the CNS, as illustrated in Figure 2. Microglia are an important source of inflammatory mediators and may have fundamental roles in neuropathic pain (Watkins et al. 2001; Milligan and Watkins 2009) through interactions with mast cells (Skaper et al. 2012). Mechanisms by which this happens is either through direct cytokine passage across the blood-brain barrier (Lossinsky and Shivers 2004) or other processes such as activation of afferent signals by nerves, of which the vagus nerve seems to play a well defined role. This circuit of the inflammatory reflex has also been termed the cholinergic anti-inflammatory reflex because afferent signals in the vagus initiate an efferent output that may inhibit cytokine production. Taken together, the inflammatory reflex constitutes a system that informs the CNS about peripheral stress by then having neuroimmune modulators or chemokines act on neurons in brain regions (Abbadie et al. 2009; Gao and Ji 2010; Thacker et al. 2009) that may produce behavioral manifestations observed in CRPS. The reflex involves immune complexes that drive CNS changes. It is unclear if these drivers are needed to maintain the presumed changes within the brain. An alternative view is that nociceptive drive itself activates a neuroimmune cascade.
Fig. 2.
Cartoon showing CRPS related pathways subsequent to neuronal injury and glial activation. The primary nociceptive pathway is illustrated in blue, biochemical cascades in black, and psychosocial circuits in gray, with potential interactions. Adapted from (Jha et al. 2012; Vlaeyen and Linton 2012; Marinus et al. 2011).
An additional feature relates to the direct effects cytokines may have on desensitization of endogenous opioid receptors (Szabo et al. 2002). In one study, morphine administered to CRPS patients reported no difference in pain reduction vs. placebo (Harke et al. 2001). A PET study on opioid binding receptor potential found that CRPS patients have reduced binding potential (indicative of fewer receptors and/or higher endogenous competition) in the amygdala and parahippocampal gyri contralateral to the effected limb, and increased binding potential in the prefrontal cortex (Klega et al. 2010).
Maintenance Events – CNS glia/neuronal/astrocyte interactions
Brain cytokines elevated through peripheral nociceptive drive or through activation of systemic inflammatory responses (e.g., IL-1) following nerve damage. Specifically, following nociceptive induced activation by nerve injury the release of neuroimmune modulators activate glia. CNS neuroinflammation may be exacerbated by the recruitment of microglia and astrocytes creating a feed forward cumulative process that may maintain neuropathic pain (Vallejo et al. 2010). Ongoing CNS inflammation is thus a possible contribution to the manifestations and maintenance of the psychophysical/behavioral responses observed in CRPS. In support of this, expression of some neuroimmune drivers such as TNF-a is increased in a chronic constriction injury (CCI) model of neuropathic pain (Covey et al. 2002). Importantly, the increased expression is observed in discrete brain regions.
Furthermore, neuroinflammation can spread from the primary injury site to second order synapses via remote neuroimmune activation (Banati 2003). Evidence for this comes in the form of elevated translocator protein (TSOP) PET ligand binding in contralateral thalamus in a patient with ongoing phantom limb pain (Banati et al. 2001), likely indicative of microglial activation spread along “neuro-inflammatory tracts” (Cooper and Clark 2012). While phantom limb pain and CRPS are clinically distinct, recent evidence indicates a similar disruption of body schema (Reinersmann et al. 2010) and mirror box therapy has some evidence for both conditions (Lamont et al. 2011). Moreover, microglial activation as well as neuronal loss in has been reported throughout the entire length of the spinal cord, most prominently at the injury level in the posterior horn (Del Valle et al. 2009). However, the link between phantom limb pain and CRPS, and the possibility of anterograde microglia involvement in both disorders, remains speculative.
Compared with the microglial response to nerve injury, astrocyte proliferation begins relatively late and progresses slowly, but is sustained for more than 5 months, a time frame paralleling the development of chronic pain (Zhang et al. 2012). Unlike microglia, astrocytes form networks with themselves and are closely associated with neurons and blood vessels, a close contact that makes it possible for astrocytes to regulate the external chemical environment of neurons during synaptic transmission. Moreover, there is recent evidence that spinal astrocytes but not microglia contribute to the pathogenesis of painful neuropathy (Zhang et al. 2012). As such, preclinical studies are needed to define whether microglia or astrocytes present a better target for novel CRPS treatments. CRPS is reversible in children (Low et al. 2007; Harris et al. 2012), but more chronic in adults following similar initial injuries. This might suggest that the plasticity and maturation/reorganizing capabilities of the CNS are crucial in responding to a persistent inflammatory response.
CNS Targets
Following peripheral nerve injury a number of brain regions are affected by the process. The typical brain regions identified in human neuroimaging studies of pain processing include the anterior cingulate cortex, insular cortex, ventrolateral orbitofrontal area, amygdala, striatum, thalamus, hypothalamus, rostral ventromedial medulla, periaqueductal gray, pons, red nucleus, and medulla oblongata (Apkarian et al. 2005; Geha and Apkarian 2005). As noted by Diamond and Tracey (2011), “The nervous system is hardwired to monitor the presence of cytokines and molecular products of invaders”. In recent reviews of this topic, remote neruroimmune signaling after nerve damage may affect CNS processing by amplifying the gain of the pain-processing pathway (Saab and Hains 2009; Zhuo et al. 2011).
Inflammatory processes have also been postulated to contribute to specific syndromes such as depression (Krishnadas and Cavanagh 2012) and other behavioral manifestations common to this syndrome in CRPS (viz., general malaise, sleep disorders, decreased activity, decreased social interaction). Taken together, these behavioral changes must be driven by altered neural circuits. In CRPS behaviors may be categorized as sensory (pain), emotional (depression or anxiety), cognitive, motor (e.g., movement disorders including dystonia) and other (e.g., hemi-inattention). Thus, a condition expressed following peripheral nerve injury now becomes a disease of the CNS. While immunologic processes are increasingly being described in CRPS (Kramer 2012), little information is available on specific brain targets.
Are some brain regions or circuits simply more susceptible to the inflammatory response in patients who have CRPS? An innate status of “inflammatory disposition” may be present in brain regions in CRPS patients. Although not reported in brains of such patients, preclinical studies have evaluated a number of inflammatory and associated receptors (BDNF, IL-6, IL-1β, IL-18 and NMDA receptors) in different brain regions (thalamus, hippocampus and hypothalamus) of mice with altered reactivity to pain, stress and anxiety related behaviors (Benatti et al. 2011) — indicating that anxiety related behavioral phenotypes constitute intrinsic risk factors for pervasive inflammation. Furthermore, newborn mice exposed to an inflammatory challenge have increased responsiveness to pain and anxiety related behavior (Benatti et al. 2009), further supporting the notion of the peripheral to central inflammatory reflex. Such long-term changes may relate to genetic, epigenetic and environmental processes. Potential interactions with specific brain regions known to confer particular behaviors are summarized below:
CNS Inflammation and Somatosensory Function – Thalamus and Somatosensory Cortex
Simplistically, the somatosensory system involving pain perception includes the thalamus, primary somatosensory cortex and posterior insula (Apkarian et al. 2005; Craig 2003). One of the unusual features of CRPS is the spread of pain from its original location that may envelop the other parts of the limb, other initially uninvolved body regions and extremities. Insights into central sensitization, spreading pain or allodynia have been well documented in other chronic pain conditions including migraine and osteoarthritis albeit not at the same pain intensity (Woolf 2011). Functional imaging studies have implicated anti-inflammatory blockade with TNF-alpha receptor antagonist in diminishing nociceptive CNS activity in the thalamus and somatosensory cortex (Hess et al. 2011). Neuroinflammation as measured by glial cell activation has also been reported in the thalamus in chronic pain (phantom limb) (Banati et al. 2001). Preclinical studies have further implicated glial activation in a model of chronic neuropathic pain where minocycline injected into the somatosensory thalamus (poster lateral nucleus) reverses both microglial activity and hyperalgesia (LeBlanc et al. 2011). Less is known about neuroinflammation in the somatosensory cortex. A recent paper reports on functional and structural changes in the somatosensory cortex following peripheral injury but not related to alterations in neuroimmune modulators (Kim et al. 2012).
CNS Inflammation and Autonomic Function – Hypothalamus
Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis has been observed in multiple chronic pain disorders (Galli et al. 2009) including CRPS (Park and Ahn 2012). One of the commonly observed features in CRPS in autonomic dysfunction manifest as altered temperature regulation, sweating and altered skin color (Marinus et al. 2011), making the hypothalamus a suspect of CNS dysfunction in the disease. In addition, sleep disturbances are present in chronic pain, and thought to be related to hypothalamic (suprachiasmatic) regulation of the sleep-wake cycle, affecting significant (96%) numbers of patients (Sharma et al. 2009). Increased microglial activation is observed in the hypothalamus in rat models of neuropathic pain (Takeda et al. 2009), and increased expression of IL-1 mRNA is observed in the hypothalamus following persistent pain induced formalin injection (Yabuuchi et al. 1996).
Inflammation Cognition and Memory – Hippocampus, Frontal Lobes
Neonatal inflammation increases neurogenesis in the hippocampus. Other studies have implicated abnormal hippocampal function in chronic pain in both animals (Mutso et al. 2012) and humans (Maleki et al. 2012), and patients with CRPS have reduced hippocampal volumes (Mutso et al. 2012). Following peripheral inflammation, alterations in myo-inositol (a presumed marker of glial activation) were observed in the hippocampus that also correlated with increased measures of anxiety-like behavior (Schneider et al. 2012). In a seminal study be Geha and colleagues (2008), gray matter volume and white matter anisotrphy was determined in CRPS patients and healthy subjects. Results indicate gray matter atrophy in the insula, ventromedial prefrontal cortex and nucleus accumbens. The white matter anisotrophy of the cingulumcalosum bundle was decreased. Further, the strength of connectivity between atrophied regions related to anxiety, suggesting that abnormal anatomy of the CRPS brain may be at play in autonomic and cognitive symptoms in CRPS.
CNS Inflammation and Motor Function – Basal Ganglia
In CRPS, putative role for the basal ganglia include motor dysfunction (movement disorders) as well as alterations in mood including reward dysfunction (Geha et al. 2008). Cytokines and glial mediated changes have been observed in the basal ganglia following inflammation or pain in humans (interferon-alpha therapy) (Capuron et al. 2007). In rats, both the chronic constriction injury and the spared nerve injury models of neuropathy, striatum hippocampus and cigulum levels of IL-1β and IL6 are elevated (Al-Amin et al. 2011). Microglial activation with dichlorvos (2,2-dichlorovinyl dimethyl phosphate) produces alterations in the nigrostriatal dopaminergic system in part through increased levels of IL-1β, TNF-α and IL-6 in the midbrain (Binukumar et al. 2011). Interferon-alpha therapy is associated with widespread bilateral increases in glucose metabolism in subcortical regions including the basal ganglia and cerebellum, and decreases in dorsal prefrontal cortex (Capuron et al. 2007) and behavioral symptoms including lassitude, inability to feel, and fatigue.
CNS Inflammation and Inattention – Parietal Lobe
Behavioral observations in CRPS patients indicate that they have hemi-inattention to the affected side or limb(Moseley 2004; Frettloh et al. 2006). Functional imaging studies and neuropsychological testing indicate altered parietal lobe function in CRPS (Lebel et al. 2008; Kolb et al. 2012), a region involved in this type of abnormal processing. While higher neglect scores are reported for CRPS patients there does not seem to be a significant difference between CRPS and other pain conditions (Kolb et al. 2012). Other features of parietal dysfunction include impaired hand size (bigger than it is) estimation (Peltz et al. 2011) and agnosia for object orientation (Robinson et al. 2011), suggesting abnormalities in visiospatial information processing. While no information is available on parietal inflammation in CRPS, direct inaction of rTNF into the parietal lobe in animals induces a significant inflammatory reaction mediated by leukocyte invasion into the perivascular space (Wright and Merchant 1992). In neuropsychiatric systemic lupus erythemetosis (NPSLE) patients, a condition thought to be caused by a number of factors including pro-inflammatory cytokines, fMRI shows increased activation in parietal lobe (Fitzgibbon et al. 2008).
CNS Inflammation and Pain Modulation – Periaqueductal Gray
Endogenous pain modulation is altered in CRPS (Seifert et al. 2009) suggesting abnormalities of pain facilitatory modulation in these patients, possibly through the periaqueductal gray (PAG). Cytokines and other neuromodulators have been implicated in PAG function in a variety of models (Benamar et al. 2008; Heinisch et al. 2011; Hao et al. 2011); for example, elevated cytokines in the PAG result in hyperalgesia (Benamar et al. 2008). Indeed, in CRPS patient the PAG (and cingulate) are significantly less activated during pain suppression, as compared to healthy controls. Notably, this lack of activation was present regardless of stimulating a symptomatic or asymptomatic region, suggestive of a generalized functional change (Freund et al. 2011).
CNS Inflammation and bodily experience – Insula
The insula has been conceptualized as an interoceptive integration region, where ascending sensory pathways conveying proprioceptive and somatosensory information about the body’s internal state, as well as visual and auditory information about the external world, terminate in the posterior insula. This activity is then re-represented in the middle and finally anterior insula, where polysensory integration produces a global meta-representation of the internal feeling state of the individual (Craig 2009). Functional neuroimaging studies indicate the mid-insula region to be involved in pain, interoception, tactile sensation, motion perception and the discrimination between internally and externally generated stimuli (Kurth et al. 2010). Insular lesions may lead to altered pain thresholds and central pain (Garcia-Larrea et al. 2010), and have been associated with a failure to withdraw from and/or absent or inadequate emotional responses to painful stimuli, a syndrome known as pain asymbolia (Berthier et al. 1988). Stroke patients with posterior insula lesions and limb dysfunction may be convinced that their limbs function normally (anosognosia), that their limb does not belong to them (asomatognosia) or attribute their own body parts to other persons (somatoparaphrenia) (Baier and Karnath 2008; Karnath et al. 2005). Such manifestations resemble the estrangement CRPS patients sometimes report to their injured limb. Indeed CRPS patients show alterations in insula structure, connectivity (Geha et al. 2008), and function (Maihofner et al. 2005a; Maihofner et al. 2006). Thus far, direct evidence for a role of the human insula in the inflammatory response has only been demonstrated in asthma patents exposed to antigens (Rosenkranz et al. 2005; Rosenkranz et al. 2012).
Progressive Nature of CRPS Central Changes – Brain Cascades
Evolution of CRPS may be defined into “three possible CRPS subtypes: (1) a relatively limited syndrome with vasomotor signs predominating, (2) a relatively limited syndrome with neuropathic pain/sensory abnormalities predominating, and (3) a florid CRPS syndrome similar to "classic RSD" descriptions” (Bruehl et al. 2002). These findings have been supported by earlier reports noting that (1) 75% of patients reported initial symptoms of pain, swelling, coldness, and color changes; (2) 71% had weakness and inability to move the extremity as initial symptoms; (3) Sleep disturbance was reported by 80%. One potential explanation for the subtypes includes progressive involvement of different brain circuits: hypothalamic; somatosensory; and more florid and integrated circuit involvement – hypothalamic, somatosensory; basal ganglia; hippocampal; frontal and parietal cortices. Many variables worsen over the course of the illness (Schwartzman et al. 2009a) but, in contrast to more devastating neuro-inflammatory disorders such as MS, the disease seems to plateau. How may this occur?
If there were “nodal” regions that are initially involved or activated in the neuro-immune reflex, these would potentially be the thalamus and hypothalamus because of their involvement in nociceptive transmission and the stress response. Changes in other areas may relate to processes that involve consecutive damaging/neuroimmune changes in other brain regions mediated through connections (axonal pathology) or secondary to the neuroimmune onslaught. Such changes may also be secondary to primary sites affected – thus remote changes may occur due to neuronal alterations. Following focal brain lesions or damage there is an associate “spread of death” (Viscomi et al. 2009), that may set up secondary immune responses in diffuse brain regions. In addition, as shown in a model of contralateral spread in mono-arthritic models, antidromic activity of this nature is known to result in the peripheral release of pro-inflammatory and vasoactive neuropeptides (Kelly et al. 2007). Although no large studies have been conducted evaluating the cumulative aggregation of symptoms (and thus putative brain regions) to support such a notion, the evolution of some basic processes would seem to be consistent with this. Thus, for example, the spread of pain from the original site, the evolution of motor changes, the unfolding of depression and other emotional changes would seem consistent with an underlying process that temporally captures involvement of these different regions.
Imaging Astrocytes, Microglia, Peptides and the Inflammatory Process in CRPS
Human in vivo imaging of glial function is becoming more viable. In response to injury, microglia migrate to the site of injury, and express multiple cell surface proteins, including the translocator protein (18kDa) (TSPO, formerly known as the peripheral benzodiazepine receptor.) This conditional expression makes TSPO a prime target for PET imaging. Microglia responses have been document in a variety of pain and nerve injury models (Milligan and Watkins 2009). In human studies, increased TSPO expression has been reported in the thalamus after peripheral nerve injuries (Banati et al. 2001) and in widespread cortical regions after traumatic brain injury (Folkersma et al. 2011). There are multiple candidate PET tracers for microglial activity, with PK11195 being most commonly used (Ching et al. 2012). However, several higher affinity TSPO radioligand suitable for imaging of microglial activation are emerging, such as PRB28 (Kreisl et al. 2010). In a very recent study on baboons, lipopolysaccharide administration led to a significant microglial response, most prominently in the accumbmens, insula and frontal cortex (Hannestad et al. 2012a). However, there are several caveats to TSPO imaging, including a common genetic polymorphism that alters ligand binding properties (Kreisl et al. 2012), and recent evidence that also reactive astrocytes can contribute to the signal in addition to reactive microglia (Lavisse et al. 2012).
Astrocytes are the most abundant brain cell type in terms of their number and volume, and they constitute 40% to 50% of all glial cells. Astrocyte reaction has been demonstrated in peripheral nerve injury and in tissue inflammation models. For example, peripheral chronic nerve lesion is associated with breakdown of the blood spinal cord barrier permeability and activation of astrocytes (Gordh et al. 2006). Although most studies have focused on the role of astrocyte activation at the spinal cord dorsal horn level, alterations can occur at supraspinal areas, such as the rostral ventromedial medulla and in the forebrain (Raghavendra et al. 2004). The enzyme MAO-B exists on the outer mitochondrial membrane, occurring predominantly in astrocytes (Fowler et al. 2005). When astrocytes become activated [as customarily defined by their greatly enhanced glial fibrillary acidic protein (GFAP) binding] they express high levels of MAO-B (Ekblom et al. 1993), thereby providing an indirect target for PET imaging. L-deprenyl (Selegeline) is a selective irreversible MAO-B inhibitor that has been carbon-11 labeled, allowing for PET imaging of a proxy to astrocyte activity (Fowler et al. 1987). A deuterium substitution on the L-deprenyl molecule causes a significant reduction in the rate of trapping, thereby further enhancing the tracer’s sensitivity to subtle changes in MAO-B concentration (Fowler et al. 1995). Thus far, studies using this deuterium substituted deprenyl (DED) tracer have been performed to asses MAO-B function and astrocytosis in epilepsy (Bergstrom et al. 1998), amyotrophic lateral sclerosis (Johansson et al. 2007), Creutzfeldt–Jakob disease (Engler et al. 2012) and Alzheimer’s (Santillo et al. 2011; Carter et al. 2012).
Substance P is a neuropeptide that modulates pain both peripherally and centrally, primarily through the neurokinin-1 (NK1) receptor. Substance P and its primary receptor, NK1, are widely distributed throughout the brain with high density in the striatum, the amygdala and the dorsolateral prefrontal cortex. We have recently demonstrated significant alterations of NK1 receptors using GR205171 PET in chronic neck pain after a whiplash trauma (Linnman et al. 2010) , in epilepsy (Danfors et al. 2011) and between the sexes (Engman et al. 2012). In CRPS, there are multiple lines of evidence implicating the substance P system, including elevated serum levels (Schinkel et al. 2009) and an animal model of CRPS where continuous substance P application caused a significant and long-lasting decrease in paw withdrawal thresholds upon mechanical stimulation, edema and enhanced leukocyte-endothelial interaction (Gradl et al. 2007).
Given the white matter changes associated with CRPS (Geha et al. 2008), a possible avenue of inquiry would be the evaluation of CRPS patients with respect to astocytosis, microglial activity and neurkinin-1 receptors, ideally in a longitudinal study with parallel (PET-MR) assessment of brain structure, function and measurements of immunological components. Substance P is released from the terminals of specific sensory nerves and NK1 receptors are expressed on both neurons and astrocytes, but about 9 times less on microglia. Further, there is a tantalizing possibility that glial cells can regulate neuronal substance P release. MAO-B expression occurs primarily in astrocytes, while TSPO expression occurs in activated microglia and to a lesser degree in active astrocytes. Thus, the three systems are somewhat orthogonal, and site specific PET probes may be used indicate different pathological mechanisms.
Gender - Hormones and Immunosensitization
One of the major issues in CRPS is the predominance (3–4 times) of women who have the condition compared with men. Estrogens may play a role in the sex difference observed in neurological diseases with inflammatory components (reviewed in Czlonkowska et al. 2006). However, in a population based case control study, there was no association between CRPS and estrogen exposure (de Mos et al. 2009). fMRI studies on sex differences in pain reactivity in healthy subjects indicate that men have activation of the somatosensory and insular cortex, and that women have higher medial prefrontal activation (Derbyshire et al. 2002; Moulton et al. 2006; Paulson et al. 1998; Straube et al. 2009; Kong et al. 2010). In addition to task related fMRI, resting state functional connectivity studies also indicate sex differences (Biswal et al. 2010; Kilpatrick et al. 2006). Recently, we demonstrated significant sex differences in pain induced functional connectivity of the PAG. In men, higher pain led to an increased functional connectivity between the PAG and the amygdala and also between the PAG and the putamen (Linnman et al. 2012a). A potential link to inflammatory aspects was demonstrated by Eisenberger et al. (2009) in an fMRI study on endotoxin administration and depression: Women, but not men, exposed to endotoxin, showed increases in IL-6 associated with increases in dorsal anterior cingulate cortex and anterior insula activation, mediating the relationship between IL-6 increases and depressed mood. If such differences are also present in clinical pain conditions, and the relation to inflammatory responses, remains to be determined.
Immunomodulatory effects of Medications and other Treatments
Immunomodulators have been proposed as useful pharmacotherapies for CRPS and include glucocorticoids, tumor necrosis factor-α antagonists, thalidomide, bisphosphonates, and immunoglobulins (Dirckx et al. 2012). More commonly used medications that may be useful in CRPS include gabapentin (van de Vusse et al. 2004), ketamine (Azari et al. 2012), and lidocaine (Schwartzman et al. 2009b). All are reported to have anti-inflammatory responses. Gabapentin modulates immunoreactivity in neuropathic mice (Schwartzman et al. 2009b). Ketamine is reported to have anti-inflammatory effects through interactions with inflammatory cells recruitment, cytokine production, and inflammatory mediators regulation (Loix et al. 2011). Exercise may also enhance anti-inflammatory function (Walsh et al. 2011). Taken together, the data suggests that anti-inflammatory and neuroimmune modulating treatments adjusted to the temporal nature of the CRPS process (duration, intensity, age). With respect to physical exercise (including physical therapy), data is suggestive that patients improve (Smith 2005) through mechanisms yet not defined but may relate to exercise induced neuromodulation of the exacerbated inflammatory process in these patients.
Behavioral aspects
CRPS is often viewed as a biopsychosocial disorder, for which successful treatment must target concurrently the biological, psychological, and social components (Bruehl and Chung 2006). A recent review of the evidence for such treatments found that “…randomized controlled studies of psychological interventions for CRPS, alone or in the multidisciplinary context, are almost entirely absent from the literature. The clinical studies available, however, do suggest that psychological interventions are likely to be a useful part of a comprehensive multidisciplinary treatment package” (Bruehl and Chung 2006). Pain related fear might however be a consequence, rather than a cause of CRPS: In early CRPS, pain severity but not fear of movement was related to functional limitations. In chronic patients, however, functional limitations beyond and above the contribution of pain severity were predicted by patients perceived harmfulness of activities (de Jong et al. 2011). Pain exposure therapies show initial evidence (van de Meent et al. 2011; de Jong et al. 2005). Fear of pain can shape pro-inflammatory immune system responses to noxious stimulation: Experimental pain induced levels of IL-6 were significantly correlated to pain catastrophizing in healthy volunteers (Edwards et al. 2008). In chronic pain patients, particularly in women, focusing on the negative aspects of their pain condition lead to elevated levels of IL-6, indicating that women display an increased and delayed inflammatory responses following negative emotional expression (Darnall et al. 2010).
Specificity and Sensitivity
A major obstacle for the neuroimaging community is defining specificity in results. This is evident in recent reviews indicating that a wide rage of behaviors are processed in similar brain regions, for example the anterior cingulate (Shackman et al. 2011) and the periaqueductal gray (Linnman et al. 2012b). The effective spatial and temporal resolution of fMRI is increasing, 7 Tesla fMRI with sub-millimeter resolution is in proccess (Polimeni et al. 2010; Linnman et al. 2012b). Simultaneous PET-fMRI (Judenhofer et al. 2008) and ultra high resolution diffusion weighted imaging (Miller et al. 2011) are other emerging technologies. In the case of CRPS, studying more subjects, preferably longitudinally, to define subtypes, cytokine levels and behavioral correlates to CNS function may be a fast route to success. Furthermore, recent studies have begun contrasting CRPS with other pain states (Baliki et al. 2011; Mutso et al. 2012) to define common and specific structural alterations in distinct disease states. With regard to the neuro-inflammation hypothesis presented here, direct comparisons between CPRS patients and other disorders with inflammatory components such as depression, multiple sclerosis and rheumatoid arthritis are on the agenda.
Summary and Conclusions
Neuro-inflammation following nerve injury is a potential process or mechanism by which many of the CRPS clinical manifestations may be produced. Changes from pain in the periphery, to spreading pain and more complex processes such as neglect-like symptoms, autonomic changes, and dystonias could all result from the initiation and maintenance of neuroimmune and cytokine induced changes. The evidence reviewed here supports a potential role for these processes that affect brain systems. That said, there is still a paucity of direct evidence for a neuro-inflammatory mechanism in CRPS. We have attempted to provide an overview of the many potential interactions that are ripe for direct tests, and of the multiple avenues of further inquiry. As such, continued translational efforts and the use of neuroimaging techniques such as functional-, morphological- and diffusion-MRI, PET imaging of neuro-receptor and glial systems, can provide further insights into neuro-inflammation at the onset and during the course of CRPS.
Acknowledgments
Supported by grants to David Borsook from the Mayday Foundation, New York, National Institute of Neurological Disorders and Stroke (NINDS) R01NS065051 and NS064050; and to Clas Linnman from the International Association for the Study of Pain (IASP) early career award and IASP Research Grant, funded by the Scan|Design Foundation BY INGER & JENS BRUUN.
Footnotes
The authors declare that they have no conflict of interest.
References
- Abbadie C, Bhangoo S, De Koninck Y, Malcangio M, Melik-Parsadaniantz S, White FA. Chemokines and pain mechanisms. Brain Res Rev. 2009;60(1):125–134. doi: 10.1016/j.brainresrev.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Amin H, Sarkis R, Atweh S, Jabbur S, Saade N. Chronic dizocilpine or apomorphine and development of neuropathy in two animal models II: effects on brain cytokines and neurotrophins. Experimental neurology. 2011;228(1):30–40. doi: 10.1016/j.expneurol.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9(4):463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Austin PJ, Moalem-Taylor G. The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. J Neuroimmunol. 2010;229(1-2):26–50. doi: 10.1016/j.jneuroim.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Azari P, Lindsay DR, Briones D, Clarke C, Buchheit T, Pyati S. Efficacy and safety of ketamine in patients with complex regional pain syndrome: a systematic review. CNS Drugs. 2012;26(3):215–228. doi: 10.2165/11595200-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Baier B, Karnath HO. Tight link between our sense of limb ownership and self-awareness of actions. Stroke. 2008;39(2):486–488. doi: 10.1161/STROKEAHA.107.495606. [DOI] [PubMed] [Google Scholar]
- Baliki MN, Schnitzer TJ, Bauer WR, Apkarian AV. Brain morphological signatures for chronic pain. PloS one. 2011;6(10):e26010. doi: 10.1371/journal.pone.0026010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banati RB. Neuropathological imaging: in vivo detection of glial activation as a measure of disease and adaptive change in the brain. Br Med Bull. 2003;65:121–131. doi: 10.1093/bmb/65.1.121. [DOI] [PubMed] [Google Scholar]
- Banati RB, Cagnin A, Brooks DJ, Gunn RN, Myers R, Jones T, Birch R, Anand P. Long-term trans-synaptic glial responses in the human thalamus after peripheral nerve injury. Neuroreport. 2001;12(16):3439–3442. doi: 10.1097/00001756-200111160-00012. [DOI] [PubMed] [Google Scholar]
- Baracos V, Rodemann HP, Dinarello CA, Goldberg AL. Stimulation of muscle protein degradation and prostaglandin E2 release by leukocytic pyrogen (interleukin-1). A mechanism for the increased degradation of muscle proteins during fever. The New England journal of medicine. 1983;308(10):553–558. doi: 10.1056/NEJM198303103081002. [DOI] [PubMed] [Google Scholar]
- Benamar K, Geller EB, Adler MW. Elevated level of the proinflammatory chemokine, RANTES/CCL5, in the periaqueductal grey causes hyperalgesia in rats. European journal of pharmacology. 2008;592(1-3):93–95. doi: 10.1016/j.ejphar.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Benatti C, Alboni S, Capone G, Corsini D, Caggia F, Brunello N, Tascedda F, Blom JM. Early neonatal inflammation affects adult pain reactivity and anxiety related traits in mice: genetic background counts. Int J Dev Neurosci. 2009;27(7):661–668. doi: 10.1016/j.ijdevneu.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Benatti C, Alboni S, Montanari C, Caggia F, Tascedda F, Brunello N, Blom JM. Central effects of a local inflammation in three commonly used mouse strains with a different anxious phenotype. Behavioural brain research. 2011;224(1):23–34. doi: 10.1016/j.bbr.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Benson S, Kattoor J, Wegner A, Hammes F, Reidick D, Grigoleit JS, Engler H, Oberbeck R, Schedlowski M, Elsenbruch S. Acute experimental endotoxemia induces visceral hypersensitivity and altered pain evaluation in healthy humans. Pain. 2012;153(4):794–799. doi: 10.1016/j.pain.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Bergstrom M, Kumlien E, Lilja A, Tyrefors N, Westerberg G, Langstrom B. Temporal lobe epilepsy visualized with PET with 11C-L-deuterium-deprenyl-analysis of kinetic data. Acta Neurologica Scandinavica. 1998;98(4):224–231. doi: 10.1111/j.1600-0404.1998.tb07300.x. [DOI] [PubMed] [Google Scholar]
- Bernateck M, Karst M, Gratz KF, Meyer GJ, Fischer MJ, Knapp WH, Koppert W, Brunkhorst T. The first scintigraphic detection of tumor necrosis factor-alpha in patients with complex regional pain syndrome type 1. Anesthesia and analgesia. 2010;110(1):211–215. doi: 10.1213/ANE.0b013e3181c4bab7. [DOI] [PubMed] [Google Scholar]
- Bernateck M, Rolke R, Birklein F, Treede RD, Fink M, Karst M. Successful intravenous regional block with low-dose tumor necrosis factor-alpha antibody infliximab for treatment of complex regional pain syndrome 1. Anesthesia and analgesia. 2007;105(4):1148–1151. doi: 10.1213/01.ane.0000278867.24601.a0. table of contents. [DOI] [PubMed] [Google Scholar]
- Berthier M, Starkstein S, Leiguarda R. Asymbolia for pain: a sensory-limbic disconnection syndrome. Ann Neurol. 1988;24(1):41–49. doi: 10.1002/ana.410240109. [DOI] [PubMed] [Google Scholar]
- Binukumar BK, Bal A, Gill KD. Chronic dichlorvos exposure: microglial activation, proinflammatory cytokines and damage to nigrostriatal dopaminergic system. Neuromolecular Med. 2011;13(4):251–265. doi: 10.1007/s12017-011-8156-8. [DOI] [PubMed] [Google Scholar]
- Birklein F, Schmelz M. Neuropeptides, neurogenic inflammation and complex regional pain syndrome (CRPS) Neuroscience letters. 2008;437(3):199–202. doi: 10.1016/j.neulet.2008.03.081. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kotter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107(10):4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruehl S, Chung OY. Psychological and behavioral aspects of complex regional pain syndrome management. Clin J Pain. 2006;22(5):430–437. doi: 10.1097/01.ajp.0000194282.82002.79. [DOI] [PubMed] [Google Scholar]
- Bruehl S, Harden RN, Galer BS, Saltz S, Backonja M, Stanton-Hicks M. Complex regional pain syndrome: are there distinct subtypes and sequential stages of the syndrome? Pain. 2002;95(1-2):119–124. doi: 10.1016/s0304-3959(01)00387-6. [DOI] [PubMed] [Google Scholar]
- Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biological Psychiatry. 2008;63(11):1022–1029. doi: 10.1016/j.biopsych.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Zhang YQ. Spinal glial activation contributes to pathological pain states. Neuroscience and biobehavioral reviews. 2008;32(5):972–983. doi: 10.1016/j.neubiorev.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili M, Woolwine BJ, Nemeroff CB, Berns GS, Miller AH. Anterior cingulate activation and error processing during interferon-alpha treatment. Biological Psychiatry. 2005;58(3):190–196. doi: 10.1016/j.biopsych.2005.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili MF, Lawson DH, Fornwalt FB, Woolwine B, Berns GS, Nemeroff CB, Miller AH. Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2007;32(11):2384–2392. doi: 10.1038/sj.npp.1301362. [DOI] [PubMed] [Google Scholar]
- Carter SF, Scholl M, Almkvist O, Wall A, Engler H, Langstrom B, Nordberg A. Evidence for astrocytosis in prodromal Alzheimer disease provided by 11Cdeuterium-L-deprenyl: a multitracer PET paradigm combining 11CPittsburgh compound B and 18F-FDG. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2012;53(1):37–46. doi: 10.2967/jnumed.110.087031. [DOI] [PubMed] [Google Scholar]
- Ching AS, Kuhnast B, Damont A, Roeda D, Tavitian B, Dolle F. Current paradigm of the 18-kDa translocator protein (TSPO) as a molecular target for PET imaging in neuroinflammation and neurodegenerative diseases. Insights Imaging. 2012;3(1):111–119. doi: 10.1007/s13244-011-0128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AK, Gentry C, Bradbury EJ, McMahon SB, Malcangio M. Role of spinal microglia in rat models of peripheral nerve injury and inflammation. European journal of pain. 2007;11(2):223–230. doi: 10.1016/j.ejpain.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Cooper MS, Clark VP. Neuroinflammation, Neuroautoimmunity, and the Co-Morbidities of Complex Regional Pain Syndrome. J Neuroimmune Pharmacol. 2012 doi: 10.1007/s11481-012-9392-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey WC, Ignatowski TA, Renauld AE, Knight PR, Nader ND, Spengler RN. Expression of neuron-associated tumor necrosis factor alpha in the brain is increased during persistent pain. Reg Anesth Pain Med. 2002;27(4):357–366. doi: 10.1053/rapm.2002.31930. [DOI] [PubMed] [Google Scholar]
- Craig AD. Pain mechanisms: labeled lines versus convergence in central processing. Annu Rev Neurosci. 2003;26:1–30. doi: 10.1146/annurev.neuro.26.041002.131022. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Czlonkowska A, Ciesielska A, Gromadzka G, Kurkowska-Jastrzebska I. Gender differences in neurological disease: role of estrogens and cytokines. Endocrine. 2006;29(2):243–256. doi: 10.1385/ENDO:29:2:243. [DOI] [PubMed] [Google Scholar]
- Czura CJ, Tracey KJ. Autonomic neural regulation of immunity. Journal of internal medicine. 2005;257(2):156–166. doi: 10.1111/j.1365-2796.2004.01442.x. [DOI] [PubMed] [Google Scholar]
- Danfors T, Ahs F, Appel L, Linnman C, Fredrikson M, Furmark T, Kumlien E. Increased neurokinin-1 receptor availability in temporal lobe epilepsy: a positron emission tomography study using [(11)C]GR205171. Epilepsy Res. 2011;97(1-2):183–189. doi: 10.1016/j.eplepsyres.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain, Behavior, and Immunity. 2007;21(2):153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnall BD, Aickin M, Zwickey H. Pilot study of inflammatory responses following a negative imaginal focus in persons with chronic pain: analysis by sex/gender. Gender medicine. 2010;7(3):247–260. doi: 10.1016/j.genm.2010.06.003. [DOI] [PubMed] [Google Scholar]
- de Jong JR, Vlaeyen JW, de Gelder JM, Patijn J. Pain-related fear, perceived harmfulness of activities, and functional limitations in complex regional pain syndrome type I. The journal of pain : official journal of the American Pain Society. 2011;12(12):1209–1218. doi: 10.1016/j.jpain.2011.06.010. [DOI] [PubMed] [Google Scholar]
- de Jong JR, Vlaeyen JW, Onghena P, Cuypers C, den Hollander M, Ruijgrok J. Reduction of pain-related fear in complex regional pain syndrome type I: the application of graded exposure in vivo. Pain. 2005;116(3):264–275. doi: 10.1016/j.pain.2005.04.019. [DOI] [PubMed] [Google Scholar]
- de Mos M, de Bruijn AG, Huygen FJ, Dieleman JP, Stricker BH, Sturkenboom MC. The incidence of complex regional pain syndrome: a populationbased study. Pain. 2007;129(1-2):12–20. doi: 10.1016/j.pain.2006.09.008. [DOI] [PubMed] [Google Scholar]
- de Mos M, Huygen FJ, Stricker BH, Dieleman JP, Sturkenboom MC. Estrogens and the risk of complex regional pain syndrome (CRPS) Pharmacoepidemiol Drug Saf. 2009;18(1):44–52. doi: 10.1002/pds.1683. [DOI] [PubMed] [Google Scholar]
- Del Valle L, Schwartzman RJ, Alexander G. Spinal cord histopathological alterations in a patient with longstanding complex regional pain syndrome. Brain, Behavior, and Immunity. 2009;23(1):85–91. doi: 10.1016/j.bbi.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Derbyshire SW, Nichols TE, Firestone L, Townsend DW, Jones AK. Gender differences in patterns of cerebral activation during equal experience of painful laser stimulation. J Pain. 2002;3(5):401–411. doi: 10.1054/jpai.2002.126788. [DOI] [PubMed] [Google Scholar]
- Diamond B, Tracey KJ. Mapping the immunological homunculus. Proc Natl Acad Sci U S A. 2011;108(9):3461–3462. doi: 10.1073/pnas.1100329108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirckx M, Stronks DL, Groeneweg G, Huygen FJ. Effect of immunomodulating medications in complex regional pain syndrome: a systematic review. Clin J Pain. 2012;28(4):355–363. doi: 10.1097/AJP.0b013e31822efe30. [DOI] [PubMed] [Google Scholar]
- Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718–725. doi: 10.1016/S1473-3099(05)70270-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles R, Loose I, Jawad M, Nyman L. Effects of acetylsalicylic acid on sore throat pain and other pain symptoms associated with acute upper respiratory tract infection. Pain Med. 2003;4(2):118–124. doi: 10.1046/j.1526-4637.2003.03019.x. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Kronfli T, Haythornthwaite JA, Smith MT, McGuire L, Page GG. Association of catastrophizing with interleukin-6 responses to acute pain. Pain. 2008;140(1):135–144. doi: 10.1016/j.pain.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping. 2009;30(9):2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biological Psychiatry. 2010;68(8):748–754. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. An fMRI study of cytokine-induced depressed mood and social pain: the role of sex differences. NeuroImage. 2009;47(3):881–890. doi: 10.1016/j.neuroimage.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom J, Jossan SS, Bergstrom M, Oreland L, Walum E, Aquilonius SM. Monoamine oxidase-B in astrocytes. Glia. 1993;8(2):122–132. doi: 10.1002/glia.440080208. [DOI] [PubMed] [Google Scholar]
- Engler H, Nennesmo I, Kumlien E, Gambini JP, Lundberg P, Savitcheva I, Langstrom B. Imaging astrocytosis with PET in Creutzfeldt-Jakob disease: case report with histopathological findings. Int J Clin Exp Med. 2012;5(2):201–207. [PMC free article] [PubMed] [Google Scholar]
- Engman J, Ahs F, Furmark T, Linnman C, Pissiota A, Appel L, Frans O, Langstrom B, Fredrikson M. Age, sex and NK1 receptors in the human brain - A positron emission tomography study with [(11)C]GR205171. Eur Neuropsychopharmacol. 2012 doi: 10.1016/j.euroneuro.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Fitzgibbon BM, Fairhall SL, Kirk IJ, Kalev-Zylinska M, Pui K, Dalbeth N, Keelan S, Robinson E, During M, McQueen FM. Functional MRI in NPSLE patients reveals increased parietal and frontal brain activation during a working memory task compared with controls. Rheumatology (Oxford) 2008;47(1):50–53. doi: 10.1093/rheumatology/kem287. [DOI] [PubMed] [Google Scholar]
- Folkersma H, Boellaard R, Yaqub M, Kloet RW, Windhorst AD, Lammertsma AA, Vandertop WP, van Berckel BN. Widespread and prolonged increase in (R)-(11)C-PK11195 binding after traumatic brain injury. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2011;52(8):1235–1239. doi: 10.2967/jnumed.110.084061. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Logan J, Volkow ND, Wang GJ. Translational neuroimaging: positron emission tomography studies of monoamine oxidase. Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging. 2005;7(6):377–387. doi: 10.1007/s11307-005-0016-1. [DOI] [PubMed] [Google Scholar]
- Fowler JS, MacGregor RR, Wolf AP, Arnett CD, Dewey SL, Schlyer D, Christman D, Logan J, Smith M, Sachs H, et al. Mapping human brain monoamine oxidase A and B with 11C-labeled suicide inactivators and PET. Science. 1987;235(4787):481–485. doi: 10.1126/science.3099392. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Wang GJ, Logan J, Xie S, Volkow ND, MacGregor RR, Schlyer DJ, Pappas N, Alexoff DL, Patlak C, et al. Selective reduction of radiotracer trapping by deuterium substitution: comparison of carbon-11-L-deprenyl and carbon-11-deprenyl-D2 for MAO B mapping. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1995;36(7):1255–1262. [PubMed] [Google Scholar]
- Frettloh J, Huppe M, Maier C. Severity and specificity of neglect-like symptoms in patients with complex regional pain syndrome (CRPS) compared to chronic limb pain of other origins. Pain. 2006;124(1-2):184–189. doi: 10.1016/j.pain.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Freund W, Wunderlich AP, Stuber G, Mayer F, Steffen P, Mentzel M, Schmitz B, Weber F. The role of periaqueductal gray and cingulate cortex during suppression of pain in complex regional pain syndrome. Clin J Pain. 2011;27(9):796–804. doi: 10.1097/AJP.0b013e31821d9063. [DOI] [PubMed] [Google Scholar]
- Gaba A, Grivennikov SI, Do MV, Stumpo DJ, Blackshear PJ, Karin M. Cutting Edge: IL-10-Mediated Tristetraprolin Induction Is Part of a Feedback Loop That Controls Macrophage STAT3 Activation and Cytokine Production. J Immunol. 2012 doi: 10.4049/jimmunol.1201126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galic MA, Riazi K, Pittman QJ. Cytokines and brain excitability. Frontiers in neuroendocrinology. 2012;33(1):116–125. doi: 10.1016/j.yfrne.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli U, Gaab J, Ettlin DA, Ruggia F, Ehlert U, Palla S. Enhanced negative feedback sensitivity of the hypothalamus-pituitary-adrenal axis in chronic myogenous facial pain. European journal of pain. 2009;13(6):600–605. doi: 10.1016/j.ejpain.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther. 2010;126(1):56–68. doi: 10.1016/j.pharmthera.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Larrea L, Perchet C, Creac'h C, Convers P, Peyron R, Laurent B, Mauguiere F, Magnin M. Operculo-insular pain (parasylvian pain): a distinct central pain syndrome. Brain : a journal of neurology. 2010;133(9):2528–2539. doi: 10.1093/brain/awq220. [DOI] [PubMed] [Google Scholar]
- Geha PY, Apkarian AV. Brain imaging findings in neuropathic pain. Curr Pain Headache Rep. 2005;9(3):184–188. doi: 10.1007/s11916-005-0060-1. [DOI] [PubMed] [Google Scholar]
- Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. 2008;60(4):570–581. doi: 10.1016/j.neuron.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordh T, Chu H, Sharma HS. Spinal nerve lesion alters blood-spinal cord barrier function and activates astrocytes in the rat. Pain. 2006;124(1-2):211–221. doi: 10.1016/j.pain.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Gradl G, Finke B, Schattner S, Gierer P, Mittlmeier T, Vollmar B. Continuous intra-arterial application of substance P induces signs and symptoms of experimental complex regional pain syndrome (CRPS) such as edema, inflammation and mechanical pain but no thermal pain. Neuroscience. 2007;148(3):757–765. doi: 10.1016/j.neuroscience.2007.06.024. [DOI] [PubMed] [Google Scholar]
- Hains LE, Loram LC, Weiseler JL, Frank MG, Bloss EB, Sholar P, Taylor FR, Harrison JA, Martin TJ, Eisenach JC, Maier SF, Watkins LR. Pain intensity and duration can be enhanced by prior challenge: initial evidence suggestive of a role of microglial priming. The journal of pain : official journal of the American Pain Society. 2010;11(10):1004–1014. doi: 10.1016/j.jpain.2010.01.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannestad J, Gallezot JD, Schafbauer T, Lim K, Kloczynski T, Morris ED, Carson RE, Ding YS, Cosgrove KP. Endotoxin-induced systemic inflammation activates microglia: [(11)C]PBR28 positron emission tomography in nonhuman primates. NeuroImage. 2012a;63(1):232–239. doi: 10.1016/j.neuroimage.2012.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannestad J, Subramanyam K, Dellagioia N, Planeta-Wilson B, Weinzimmer D, Pittman B, Carson RE. Glucose metabolism in the insula and cingulate is affected by systemic inflammation in humans. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2012b;53(4):601–607. doi: 10.2967/jnumed.111.097014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S, Liu S, Zheng X, Zheng W, Ouyang H, Mata M, Fink DJ. The role of TNFalpha in the periaqueductal gray during naloxone-precipitated morphine withdrawal in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36(3):664–676. doi: 10.1038/npp.2010.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harke H, Gretenkort P, Ladleif HU, Rahman S, Harke O. The response of neuropathic pain and pain in complex regional pain syndrome I to carbamazepine and sustained-release morphine in patients pretreated with spinal cord stimulation: a double-blinded randomized study. Anesthesia and analgesia. 2001;92(2):488–495. doi: 10.1097/00000539-200102000-00039. [DOI] [PubMed] [Google Scholar]
- Harris EJ, Schimka KE, Carlson RM. Complex regional pain syndrome of the pediatric lower extremity: a retrospective review. J Am Podiatr Med Assoc. 2012;102(2):99–104. doi: 10.7547/1020099. [DOI] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biological Psychiatry. 2009a;66(5):407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Dolan RJ, Critchley HD. Neural origins of human sickness in interoceptive responses to inflammation. Biological Psychiatry. 2009b;66(5):415–422. doi: 10.1016/j.biopsych.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neuroscience and biobehavioral reviews. 1988;12(2):123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Hatashita S, Sekiguchi M, Kobayashi H, Konno S, Kikuchi S. Contralateral neuropathic pain and neuropathology in dorsal root ganglion and spinal cord following hemilateral nerve injury in rats. Spine. 2008;33(12):1344–1351. doi: 10.1097/BRS.0b013e3181733188. [DOI] [PubMed] [Google Scholar]
- Heinisch S, Palma J, Kirby LG. Interactions between chemokine and muopioid receptors: anatomical findings and electrophysiological studies in the rat periaqueductal grey. Brain, Behavior, and Immunity. 2011;25(2):360–372. doi: 10.1016/j.bbi.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess A, Axmann R, Rech J, Finzel S, Heindl C, Kreitz S, Sergeeva M, Saake M, Garcia M, Kollias G, Straub RH, Sporns O, Doerfler A, Brune K, Schett G. Blockade of TNF-alpha rapidly inhibits pain responses in the central nervous system. Proc Natl Acad Sci U S A. 2011;108(9):3731–3736. doi: 10.1073/pnas.1011774108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossaini M, Sarac C, Jongen JL, Holstege JC. Spinal glycinergic and GABAergic neurons expressing C-fos after capsaicin stimulation are increased in rats with contralateral neuropathic pain. Neuroscience. 2011;196:265–275. doi: 10.1016/j.neuroscience.2011.08.050. [DOI] [PubMed] [Google Scholar]
- Inagaki TK, Muscatell KA, Irwin MR, Cole SW, Eisenberger NI. Inflammation selectively enhances amygdala activity to socially threatening images. NeuroImage. 2012;59(4):3222–3226. doi: 10.1016/j.neuroimage.2011.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, Jeon S, Suk K. Glia as a Link between Neuroinflammation and Neuropathic Pain. Immune Netw. 2012;12(2):41–47. doi: 10.4110/in.2012.12.2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson A, Engler H, Blomquist G, Scott B, Wall A, Aquilonius SM, Langstrom B, Askmark H. Evidence for astrocytosis in ALS demonstrated by [11C](L)-deprenyl-D2 PET. J Neurol Sci. 2007;255(1-2):17–22. doi: 10.1016/j.jns.2007.01.057. [DOI] [PubMed] [Google Scholar]
- Judenhofer MS, Wehrl HF, Newport DF, Catana C, Siegel SB, Becker M, Thielscher A, Kneilling M, Lichy MP, Eichner M, Klingel K, Reischl G, Widmaier S, Rocken M, Nutt RE, Machulla HJ, Uludag K, Cherry SR, Claussen CD, Pichler BJ. Simultaneous PET-MRI: a new approach for functional and morphological imaging. Nature medicine. 2008;14(4):459–465. doi: 10.1038/nm1700. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Baier B, Nagele T. Awareness of the functioning of one's own limbs mediated by the insular cortex? The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25(31):7134–7138. doi: 10.1523/JNEUROSCI.1590-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S, Dunham JP, Donaldson LF. Sensory nerves have altered function contralateral to a monoarthritis and may contribute to the symmetrical spread of inflammation. Eur J Neurosci. 2007;26(4):935–942. doi: 10.1111/j.1460-9568.2007.05737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick LA, Zald DH, Pardo JV, Cahill LF. Sex-related differences in amygdala functional connectivity during resting conditions. Neuroimage. 2006;30(2):452–461. doi: 10.1016/j.neuroimage.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Kim CF, Moalem-Taylor G. Detailed characterization of neuro-immune responses following neuropathic injury in mice. Brain research. 2011;1405:95–108. doi: 10.1016/j.brainres.2011.06.022. [DOI] [PubMed] [Google Scholar]
- Kim SK, Eto K, Nabekura J. Synaptic structure and function in the mouse somatosensory cortex during chronic pain: in vivo two-photon imaging. Neural plasticity. 2012;2012:640259. doi: 10.1155/2012/640259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klega A, Eberle T, Buchholz HG, Maus S, Maihofner C, Schreckenberger M, Birklein F. Central opioidergic neurotransmission in complex regional pain syndrome. Neurology. 2010;75(2):129–136. doi: 10.1212/WNL.0b013e3181e7ca2e. [DOI] [PubMed] [Google Scholar]
- Kolb L, Lang C, Seifert F, Maihofner C. Cognitive correlates of "neglect-like syndrome" in patients with complex regional pain syndrome. Pain. 2012;153(5):1063–1073. doi: 10.1016/j.pain.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Kong J, Loggia ML, Zyloney C, Tu P, Laviolette P, Gollub RL. Exploring the brain in pain: activations, deactivations and their relation. Pain. 2010;148(2):257–267. doi: 10.1016/j.pain.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer HH. Immunological Aspects of the Complex Regional Pain Syndrome (CRPS) Curr Pharm Des. 2012 doi: 10.2174/138161212802502206. [DOI] [PubMed] [Google Scholar]
- Kramer HH, Eberle T, Uceyler N, Wagner I, Klonschinsky T, Muller LP, Sommer C, Birklein F. TNF-alpha in CRPS and 'normal' trauma--significant differences between tissue and serum. Pain. 2011;152(2):285–290. doi: 10.1016/j.pain.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Kreisl WC, Fujita M, Fujimura Y, Kimura N, Jenko KJ, Kannan P, Hong J, Morse CL, Zoghbi SS, Gladding RL, Jacobson S, Oh U, Pike VW, Innis RB. Comparison of [(11)C]-(R)-PK 11195 and [(11)C]PBR28, two radioligands for translocator protein (18 kDa) in human and monkey: Implications for positron emission tomographic imaging of this inflammation biomarker. NeuroImage. 2010;49(4):2924–2932. doi: 10.1016/j.neuroimage.2009.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisl WC, Jenko KJ, Hines CS, Hyoung Lyoo C, Corona W, Morse CL, Zoghbi SS, Hyde T, Kleinman JE, Pike VW, McMahon FJ, Innis RB. A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012 doi: 10.1038/jcbfm.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnadas R, Cavanagh J. Depression: an inflammatory illness? Journal of neurology, neurosurgery, and psychiatry. 2012;83(5):495–502. doi: 10.1136/jnnp-2011-301779. [DOI] [PubMed] [Google Scholar]
- Kullmann JS, Grigoleit JS, Lichte P, Kobbe P, Rosenberger C, Banner C, Wolf OT, Engler H, Oberbeck R, Elsenbruch S, Bingel U, Forsting M, Gizewski ER, Schedlowski M. Neural response to emotional stimuli during experimental human endotoxemia. Human Brain Mapping. 2012 doi: 10.1002/hbm.22063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214(5-6):519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont K, Chin M, Kogan M. Mirror box therapy: seeing is believing. Explore (NY) 2011;7(6):369–372. doi: 10.1016/j.explore.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Lavisse S, Guillermier M, Herard AS, Petit F, Delahaye M, Van Camp N, Ben Haim L, Lebon V, Remy P, Dolle F, Delzescaux T, Bonvento G, Hantraye P, Escartin C. Reactive astrocytes overexpress TSPO and are detected by TSPO positron emission tomography imaging. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(32):10809–10818. doi: 10.1523/JNEUROSCI.1487-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel A, Becerra L, Wallin D, Moulton EA, Morris S, Pendse G, Jasciewicz J, Stein M, Aiello-Lammens M, Grant E, Berde C, Borsook D. fMRI reveals distinct CNS processing during symptomatic and recovered complex regional pain syndrome in children. Brain : a journal of neurology. 2008;131(Pt 7):1854–1879. doi: 10.1093/brain/awn123. [DOI] [PubMed] [Google Scholar]
- LeBlanc BW, Zerah ML, Kadasi LM, Chai N, Saab CY. Minocycline injection in the ventral posterolateral thalamus reverses microglial reactivity and thermal hyperalgesia secondary to sciatic neuropathy. Neuroscience letters. 2011;498(2):138–142. doi: 10.1016/j.neulet.2011.04.077. [DOI] [PubMed] [Google Scholar]
- Liang DY, Li X, Shi X, Sun Y, Sahbaie P, Li WW, Clark JD. The complement component C5a receptor mediates pain and inflammation in a postsurgical pain model. Pain. 2012;153(2):366–372. doi: 10.1016/j.pain.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnman C, Appel L, Furmark T, Soderlund A, Gordh T, Langstrom B, Fredrikson M. Ventromedial prefrontal neurokinin 1 receptor availability is reduced in chronic pain. Pain. 2010;149(1):64–70. doi: 10.1016/j.pain.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Linnman C, Beucke JC, Jensen KB, Gollub RL, Kong J. Sex similarities and differences in pain-related periaqueductal gray connectivity. Pain. 2012a;153(2):444–454. doi: 10.1016/j.pain.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnman C, Moulton EA, Barmettler G, Becerra L, Borsook D. Neuroimaging of the periaqueductal gray: state of the field. NeuroImage. 2012b;60(1):505–522. doi: 10.1016/j.neuroimage.2011.11.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loix S, De Kock M, Henin P. The anti-inflammatory effects of ketamine: state of the art. Acta Anaesthesiol Belg. 2011;62(1):47–58. [PubMed] [Google Scholar]
- Lossinsky AS, Shivers RR. Structural pathways for macromolecular and cellular transport across the blood-brain barrier during inflammatory conditions. Review. Histol Histopathol. 2004;19(2):535–564. doi: 10.14670/HH-19.535. [DOI] [PubMed] [Google Scholar]
- Low AK, Ward K, Wines AP. Pediatric complex regional pain syndrome. J Pediatr Orthop. 2007;27(5):567–572. doi: 10.1097/BPO.0b013e318070cc4d. [DOI] [PubMed] [Google Scholar]
- Maier SF, Wiertelak EP, Martin D, Watkins LR. Interleukin-1 mediates the behavioral hyperalgesia produced by lithium chloride and endotoxin. Brain research. 1993;623(2):321–324. doi: 10.1016/0006-8993(93)91446-y. [DOI] [PubMed] [Google Scholar]
- Maihofner C, Forster C, Birklein F, Neundorfer B, Handwerker HO. Brain processing during mechanical hyperalgesia in complex regional pain syndrome: a functional MRI study. Pain. 2005a;114(1-2):93–103. doi: 10.1016/j.pain.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Maihofner C, Handwerker HO, Birklein F. Functional imaging of allodynia in complex regional pain syndrome. Neurology. 2006;66(5):711–717. doi: 10.1212/01.wnl.0000200961.49114.39. [DOI] [PubMed] [Google Scholar]
- Maihofner C, Handwerker HO, Neundorfer B, Birklein F. Mechanical hyperalgesia in complex regional pain syndrome: a role for TNF-alpha? Neurology. 2005b;65(2):311–313. doi: 10.1212/01.wnl.0000168866.62086.8f. [DOI] [PubMed] [Google Scholar]
- Maleki J, LeBel AA, Bennett GJ, Schwartzman RJ. Patterns of spread in complex regional pain syndrome, type I (reflex sympathetic dystrophy) Pain. 2000;88(3):259–266. doi: 10.1016/S0304-3959(00)00332-8. [DOI] [PubMed] [Google Scholar]
- Maleki N, Becerra L, Brawn J, McEwen B, Burstein R, Borsook D. Common hippocampal structural and functional changes in migraine. Brain Struct Funct. 2012 doi: 10.1007/s00429-012-0437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinus J, Moseley GL, Birklein F, Baron R, Maihofner C, Kingery WS, van Hilten JJ. Clinical features and pathophysiology of complex regional pain syndrome. Lancet Neurol. 2011;10(7):637–648. doi: 10.1016/S1474-4422(11)70106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messay B, Lim A, Marsland AL. Current understanding of the bi-directional relationship of major depression with inflammation. Biology of Mood & Anxiety Disorders. 2012;2(1):4. doi: 10.1186/2045-5380-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KL, Stagg CJ, Douaud G, Jbabdi S, Smith SM, Behrens TE, Jenkinson M, Chance SA, Esiri MM, Voets NL, Jenkinson N, Aziz TZ, Turner MR, Johansen-Berg H, McNab JA. Diffusion imaging of whole, post-mortem human brains on a clinical MRI scanner. NeuroImage. 2011;57(1):167–181. doi: 10.1016/j.neuroimage.2011.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nature reviews Neuroscience. 2009;10(1):23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley GL. Why do people with complex regional pain syndrome take longer to recognize their affected hand? Neurology. 2004;62(12):2182–2186. doi: 10.1212/01.wnl.0000130156.05828.43. [DOI] [PubMed] [Google Scholar]
- Moulton EA, Keaser ML, Gullapalli RP, Maitra R, Greenspan JD. Sex differences in the cerebral BOLD signal response to painful heat stimuli. American journal of physiology. 2006;291(2):R257–R267. doi: 10.1152/ajpregu.00084.2006. [DOI] [PubMed] [Google Scholar]
- Mutso AA, Radzicki D, Baliki MN, Huang L, Banisadr G, Centeno MV, Radulovic J, Martina M, Miller RJ, Apkarian AV. Abnormalities in hippocampal functioning with persistent pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(17):5747–5756. doi: 10.1523/JNEUROSCI.0587-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor MF, Irwin MR, Wellisch DK. When grief heats up: pro-inflammatory cytokines predict regional brain activation. NeuroImage. 2009;47(3):891–896. doi: 10.1016/j.neuroimage.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Ahn RS. Hypothalamic-pituitary-adrenal axis function in patients with complex regional pain syndrome type 1. Psychoneuroendocrinology. 2012;37(9):1557–1568. doi: 10.1016/j.psyneuen.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Minoshima S, Morrow TJ, Casey KL. Gender differences in pain perception and patterns of cerebral activation during noxious heat stimulation in humans. Pain. 1998;76(1-2):223–229. doi: 10.1016/s0304-3959(98)00048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA, Tracey KJ. Controlling inflammation: the cholinergic antiinflammatory pathway. Biochem Soc Trans. 2006;34(Pt 6):1037–1040. doi: 10.1042/BST0341037. [DOI] [PubMed] [Google Scholar]
- Peltz E, Seifert F, Lanz S, Muller R, Maihofner C. Impaired hand size estimation in CRPS. The journal of pain : official journal of the American Pain Society. 2011;12(10):1095–1101. doi: 10.1016/j.jpain.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Polimeni JR, Fischl B, Greve DN, Wald LL. Laminar analysis of 7T BOLD using an imposed spatial activation pattern in human V1. NeuroImage. 2010;52(4):1334–1346. doi: 10.1016/j.neuroimage.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci. 2004;20(2):467–473. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- Reinersmann A, Haarmeyer GS, Blankenburg M, Frettloh J, Krumova EK, Ocklenburg S, Maier C. Left is where the L is right. Significantly delayed reaction time in limb laterality recognition in both CRPS and phantom limb pain patients. Neuroscience letters. 2010;486(3):240–245. doi: 10.1016/j.neulet.2010.09.062. [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nature medicine. 2010;16(11):1267–1276. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson G, Cohen H, Goebel A. A case of complex regional pain syndrome with agnosia for object orientation. Pain. 2011;152(7):1674–1681. doi: 10.1016/j.pain.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Rosenkranz MA, Busse WW, Johnstone T, Swenson CA, Crisafi GM, Jackson MM, Bosch JA, Sheridan JF, Davidson RJ. Neural circuitry underlying the interaction between emotion and asthma symptom exacerbation. Proc Natl Acad Sci U S A. 2005;102(37):13319–13324. doi: 10.1073/pnas.0504365102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz MA, Busse WW, Sheridan JF, Crisafi GM, Davidson RJ. Are There Neurophenotypes for Asthma? Functional Brain Imaging of the Interaction between Emotion and Inflammation in Asthma. PloS one. 2012;7(8):e40921. doi: 10.1371/journal.pone.0040921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab CY, Hains BC. Remote neuroimmune signaling: a long-range mechanism of nociceptive network plasticity. Trends in neurosciences. 2009;32(2):110–117. doi: 10.1016/j.tins.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Santillo AF, Gambini JP, Lannfelt L, Langstrom B, Ulla-Marja L, Kilander L, Engler H. In vivo imaging of astrocytosis in Alzheimer's disease: an (1)(1)C-L-deuteriodeprenyl and PIB PET study. European journal of nuclear medicine and molecular imaging. 2011;38(12):2202–2208. doi: 10.1007/s00259-011-1895-9. [DOI] [PubMed] [Google Scholar]
- Schinkel C, Gaertner A, Zaspel J, Zedler S, Faist E, Schuermann M. Inflammatory mediators are altered in the acute phase of posttraumatic complex regional pain syndrome. Clin J Pain. 2006;22(3):235–239. doi: 10.1097/01.ajp.0000169669.70523.f0. [DOI] [PubMed] [Google Scholar]
- Schinkel C, Scherens A, Koller M, Roellecke G, Muhr G, Maier C. Systemic inflammatory mediators in post-traumatic complex regional pain syndrome (CRPS I) - longitudinal investigations and differences to control groups. Eur J Med Res. 2009;14(3):130–135. doi: 10.1186/2047-783X-14-3-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P, Weber-Fahr W, Schweinfurth N, Ho YJ, Sartorius A, Spanagel R, Pawlak CR. Central metabolite changes and activation of microglia after peripheral interleukin-2 challenge. Brain, Behavior, and Immunity. 2012;26(2):277–283. doi: 10.1016/j.bbi.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Schreiber KL, Beitz AJ, Wilcox GL. Activation of spinal microglia in a murine model of peripheral inflammation-induced, long-lasting contralateral allodynia. Neuroscience letters. 2008;440(1):63–67. doi: 10.1016/j.neulet.2008.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzman RJ, Erwin KL, Alexander GM. The natural history of complex regional pain syndrome. Clin J Pain. 2009a;25(4):273–280. doi: 10.1097/AJP.0b013e31818ecea5. [DOI] [PubMed] [Google Scholar]
- Schwartzman RJ, Patel M, Grothusen JR, Alexander GM. Efficacy of 5-day continuous lidocaine infusion for the treatment of refractory complex regional pain syndrome. Pain Med. 2009b;10(2):401–412. doi: 10.1111/j.1526-4637.2009.00573.x. [DOI] [PubMed] [Google Scholar]
- Seifert F, Kiefer G, DeCol R, Schmelz M, Maihofner C. Differential endogenous pain modulation in complex-regional pain syndrome. Brain : a journal of neurology. 2009;132(Pt 3):788–800. doi: 10.1093/brain/awn346. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12(3):154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Agarwal S, Broatch J, Raja SN. A web-based cross-sectional epidemiological survey of complex regional pain syndrome. Reg Anesth Pain Med. 2009;34(2):110–115. doi: 10.1097/AAP.0b013e3181958f90. [DOI] [PubMed] [Google Scholar]
- Skaper SD, Giusti P, Facci L. Microglia and mast cells: two tracks on the road to neuroinflammation. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26(8):3103–3117. doi: 10.1096/fj.11-197194. [DOI] [PubMed] [Google Scholar]
- Slavich GM, Way BM, Eisenberger NI, Taylor SE. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. Proc Natl Acad Sci U S A. 2010;107(33):14817–14822. doi: 10.1073/pnas.1009164107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TO. How effective is physiotherapy in the treatment of complex regional pain syndrome type I? A review of the literature. Musculoskeletal Care. 2005;3(4):181–200. doi: 10.1002/msc.9. [DOI] [PubMed] [Google Scholar]
- Straube T, Schmidt S, Weiss T, Mentzel HJ, Miltner WH. Sex differences in brain activation to anticipated and experienced pain in the medial prefrontal cortex. Hum Brain Mapp. 2009;30(2):689–698. doi: 10.1002/hbm.20536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong JA, Xie W, Coyle DE, Zhang JM. Microarray analysis of rat sensory Ganglia after local inflammation implicates novel cytokines in pain. PloS one. 2012;7(7):e40779. doi: 10.1371/journal.pone.0040779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo I, Chen XH, Xin L, Adler MW, Howard OM, Oppenheim JJ, Rogers TJ. Heterologous desensitization of opioid receptors by chemokines inhibits chemotaxis and enhances the perception of pain. Proc Natl Acad Sci U S A. 2002;99(16):10276–10281. doi: 10.1073/pnas.102327699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Muramatsu M, Chikuma T, Kato T. Effect of memantine on the levels of neuropeptides and microglial cells in the brain regions of rats with neuropathic pain. J Mol Neurosci. 2009;39(3):380–390. doi: 10.1007/s12031-009-9224-5. [DOI] [PubMed] [Google Scholar]
- Tal M. A Role for Inflammation in Chronic Pain. Curr Rev Pain. 1999;3(6):440–446. doi: 10.1007/s11916-999-0071-4. [DOI] [PubMed] [Google Scholar]
- Thacker MA, Clark AK, Bishop T, Grist J, Yip PK, Moon LD, Thompson SW, Marchand F, McMahon SB. CCL2 is a key mediator of microglia activation in neuropathic pain states. European journal of pain. 2009;13(3):263–272. doi: 10.1016/j.ejpain.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117(2):289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uceyler N, Eberle T, Rolke R, Birklein F, Sommer C. Differential expression patterns of cytokines in complex regional pain syndrome. Pain. 2007;132(1-2):195–205. doi: 10.1016/j.pain.2007.07.031. [DOI] [PubMed] [Google Scholar]
- Vallejo R, Tilley DM, Vogel L, Benyamin R. The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain practice : the official journal of World Institute of Pain. 2010;10(3):167–184. doi: 10.1111/j.1533-2500.2010.00367.x. [DOI] [PubMed] [Google Scholar]
- van de Beek WJ, Remarque EJ, Westendorp RG, van Hilten JJ. Innate cytokine profile in patients with complex regional pain syndrome is normal. Pain. 2001;91(3):259–261. doi: 10.1016/S0304-3959(00)00443-7. [DOI] [PubMed] [Google Scholar]
- van de Meent H, Oerlemans M, Bruggeman A, Klomp F, van Dongen R, Oostendorp R, Frolke JP. Safety of "pain exposure" physical therapy in patients with complex regional pain syndrome type 1. Pain. 2011;152(6):1431–1438. doi: 10.1016/j.pain.2011.02.032. [DOI] [PubMed] [Google Scholar]
- van de Vusse AC, Stomp-van den Berg SG, Kessels AH, Weber WE. Randomised controlled trial of gabapentin in Complex Regional Pain Syndrome type 1 [ISRCTN84121379] BMC Neurol. 2004;4:13. doi: 10.1186/1471-2377-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn MA, Marinus J, Putter H, Bosselaar SR, Moseley GL, van Hilten JJ. Spreading of complex regional pain syndrome: not a random process. Journal of neural transmission. 2011;118(9):1301–1309. doi: 10.1007/s00702-011-0601-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Avelaira D, McKelvey R, Hathway G, Fitzgerald M. The emergence of adolescent onset pain hypersensitivity following neonatal nerve injury. Molecular pain. 2012;8(1):30. doi: 10.1186/1744-8069-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscomi MT, Florenzano F, Latini L, Molinari M. Remote cell death in the cerebellar system. Cerebellum (London, England) 2009;8(3):184–191. doi: 10.1007/s12311-009-0107-7. [DOI] [PubMed] [Google Scholar]
- Vlaeyen JW, Linton SJ. Fear-avoidance model of chronic musculoskeletal pain: 12 years on. Pain. 2012;153(6):1144–1147. doi: 10.1016/j.pain.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Walsh NP, Gleeson M, Shephard RJ, Woods JA, Bishop NC, Fleshner M, Green C, Pedersen BK, Hoffman-Goetz L, Rogers CJ, Northoff H, Abbasi A, Simon P. Position statement. Part one: Immune function and exercise. Exerc Immunol Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- Wang H, Sun H, Della Penna K, Benz RJ, Xu J, Gerhold DL, Holder DJ, Koblan KS. Chronic neuropathic pain is accompanied by global changes in gene expression and shares pathobiology with neurodegenerative diseases. Neuroscience. 2002;114(3):529–546. doi: 10.1016/s0306-4522(02)00341-x. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends in neurosciences. 2001;24(8):450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- Watts D, Kremer MJ. Complex regional pain syndrome: a review of diagnostics, pathophysiologic mechanisms, and treatment implications for certified registered nurse anesthetists. Aana J. 2011;79(6):505–510. [PubMed] [Google Scholar]
- Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3) Suppl:S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JL, Merchant RE. Histopathological effects of intracerebral injections of human recombinant tumor necrosis factor-alpha in the rat. Acta Neuropathol. 1992;85(1):93–100. doi: 10.1007/BF00304638. [DOI] [PubMed] [Google Scholar]
- Yabuuchi K, Maruta E, Minami M, Satoh M. Induction of interleukin-1 beta mRNA in the hypothalamus following subcutaneous injections of formalin into the rat hind paws. Neuroscience letters. 1996;207(2):109–112. doi: 10.1016/0304-3940(96)12505-2. [DOI] [PubMed] [Google Scholar]
- Yoon SY, Patel D, Dougherty PM. Minocycline blocks lipopolysaccharide induced hyperalgesia by suppression of microglia but not astrocytes. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yoon SY, Dougherty PM. Evidence That Spinal Astrocytes but Not Microglia Contribute to the Pathogenesis of Paclitaxel-Induced Painful Neuropathy. The journal of pain : official journal of the American Pain Society. 2012 doi: 10.1016/j.jpain.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo M, Wu G, Wu LJ. Neuronal and microglial mechanisms of neuropathic pain. Mol Brain. 2011;4:31. doi: 10.1186/1756-6606-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]