Abstract
Aedes aegypti mosquitoes use pharate 1st instar quiescence to cope with fluctuations in water availability hosting a fully developed 1st instar larvae within the chorion. The duration of this quiescence has been shown to affect larval fitness. This study s ought to determine if an extended egg quiescence can elicit a plastic response resulting in an adult phenotype distinct from adults reared from short quiescence eggs. Our findings indicate that extended pharate 1st quiescence affects the performance and reproductive fitness of the adult female mosquito as well as the nutritional status of its progeny via maternal effects in an adaptive manner. This study demonstrates that phenotypic plasticity results as a consequence of the duration of pharate 1st instar quiescence and alternative phenotypes may exist for this mosquito with quiescence serving as a cue possibly signaling the environmental conditions that follow a dry period. These findings have implications for A. aegypti’s success as a vector, geographic distribution, vector capacity and control.
Keywords: Aedes aegypti, egg quiescence, plasticity, nutrition
Introduction
Phenotypic plasticity is the ability of a single genotype to produce more than one alternative form of morphology, physiological state, and/or behavior in response to environmental conditions; it can subsequently result in alternative phenotypes - two or more forms of behavior, physiological response, or structure maintained in the same life stage in a single population and not simultaneously expressed in the same individual (West-Eberhard, 2003). In the case of anticipatory plasticity, phenotypic changes are induced in response to cues which signal future environmental conditions prior to the actual onset of these conditions (Esperk et al., 2012). In tropical and subtropical regions mosquitoes are often exposed to a favorable rainy season with abundant breeding sites and vegetation, and a stressful drier winter (Vinogradova, 2007; Denlinger, 1986; Mori et al., 1981) during which resources may be less plentiful (Gary and Foster, 2001; Foster, 1995; Yaro, et al., 2012). Regardless of the season fluctuations in moisture are however, unpredictable. Aedes aegypti mosquitoes have the ability to undergo aseasonal dormancy as an unhatched 1st instar; this dormant state is referred to as pharate 1st instar quiescence or more simply, egg quiescence (Vinogradova, 2007; Clements, 1992). Aseasonal quiescence is an adaptation to the peculiar conditions of special larval habitats, such as tree and rock holes, or transient ground pools, where water level may be subjected to large and abrupt fluctuations (reviewed in Vinogradova, 2007). As a result of this trait, A. aegypti produce eggs that resist desiccation and can withstand months of dormancy.
A wide variety of studies have shown that the energetic costs of long-term diapause are reflected in lower post-hatching survival and reduced fecundity (reviewed in Hahn and Denlinger, 2007, 2011), however, relatively little work has been conducted examining the fitness costs and consequences of egg quiescence. The duration of extended pharate 1st instar quiescence has been shown to affect larval fitness by reducing nutritional reserves of newly emerged 1st instars, increasing developmental period and decreasing their stress tolerance (Perez and Noriega, 2012). These observations led us to hypothesize that the duration of egg quiescence would result in adaptive adult phenotypes with distinctly different performance and reproductive physiology. To test this hypothesis a laboratory strain of A. aegypti adults reared from extended quiescence eggs were assessed for various parameters of performance and reproductive physiology and compared with adults reared from short quiescence eggs. Our findings indicate that extended egg quiescence produced adult females with a distinct phenotype from those that had undergone short egg quiescence i.e., altered performance and reproductive fitness as well as altered nutritional status of their progeny via maternal effects, perhaps in a manner best suited for the likely suboptimal conditions that follow extended quiescence. These results suggest that alternative phenotypes may exist for this mosquito with implications for its success as a vector, geographic distribution, vector capacity and control.
2. Materials and Methods
2.1 Mosquito rearing and maintenance
A. aegypti (Rockefeller strain) short quiescence pharate 1st instars (eggs less than a week old) were hatched in deoxygenated water. Upon emergence, three replicate groups of 200 of these larvae were reared to adulthood in 23×40×15cm polypropylene plastic pans containing 1L of reconstituted soft water (RSW) (Perez and Noriega, 2012). For the metal stressed treatment, three replicate groups of 200 1st instar larvae from short quiescence eggs were reared to adulthood in one ppm copper (Cu) in RSW. One ppm Cu has been shown to be an ecologically relevant level of metal stress (Sarkar et al., 2004). Larval diet consisted of 750 μl of 10% liver powder suspension (MP Biomedicals Inc., Aurora, OH.) added to the pan every day except for the second day. Pupae were collected every day throughout the pupation period and transferred to an appropriate container for adult emergence. Adult mosquitoes were offered a cotton pad soaked in 3% or 20% sucrose solution ad libitum. These concentrations represented relative sub-optimal and optimal adult environments for comparison purposes. Four-day-old female mosquitoes were fed porcine blood equilibrated to 37 °C, and ATP was added to the blood meal to a final concentration of 1 mM immediately before use. Eggs were oviposited on papers and stored under insectary conditions in Nasco Whirl-Pak bags inside a resealable polypropylene container (adapted from Munstermann, 1985). Insectary conditions were 28 °C and 80% relative humidity under a photoperiod of 16 h light and 8 h dark. Extended quiescence eggs were generated by storing the remaining eggs of the same cohort under insectary conditions for ten weeks. All experiments were then repeated with these ‘old’ eggs. All assays were performed under insectary conditions and started at ~12:00pm.
2.2 Reagents
Reconstituted soft water (RSW) was used in all bioassays. RSW was prepared by adding reagent-grade NaHCO3 (48 mg), CaSO4 2H2O (30 mg), MgSO4 (30 mg) and KCl (2 mg) to 1 liter of deionized water (pH~7.4). All reagents were purchased from Fisher Scientific, Pittsburgh, PA.
2.3 Copper measurement
Reagent grade copper sulfate pentahydrate was dissolved in RSW. Nominal Cu concentrations were confirmed by inductively coupled plasma mass spectrometric analysis (ICP-MS) using an HP 4500 plus IPC-MS instrument (Hewlett-Packard Co., Wilmington, DE), equipped with a Babington-type nebulizer and an ASX-500 autosampler (Cetac Technologies Inc., Omaha, NE).
2.4 Fitness assays
Newly emerged adult females from each treatment were collected, dried at 95° C for 24h and analyzed gravimetrically with a Cahn 29 electrobalance to within one 1/100 of a milligram. For the starvation tolerance assay three replicate groups of 15 newly emerged adult females from each treatment were assayed in one pint mosquito cages and provided deionized water ad libitum. The longevity assay was performed in a similar manner except 3% sucrose was provided. For the filial larvae starvation tolerance assay (maternal effects) three replicate groups of 25 newly emerged 1st instars generated from parents that had been reared from short quiescence and extended quiescence eggs were assayed in 15 cm diameter glass petri dishes containing 100 ml of RSW in the absence of food. The number of larvae found not responsive to mechanical stimulation every 24 h in each assay were counted as “dead” and removed.
2.5 Quantification of total neutral lipids
Neutral lipids were quantified using a triglyceride quantification kit (Biovision, Mountain View, CA; cat# K622-100). Three newly emerged adult females from each treatment were assayed in triplicate.
2.6 Quantification of follicles per ovary
A sub-sample of adults was analyzed for follicle quantification. 60-72 h old adult females were anesthetized by chilling for 10 min at 4° C prior to dissection. Ovaries from these mosquitoes were removed, rinsed in Aedes physiological saline (APS) (MgCl2 0.6 mM; KCl 4.0 mM NaHCO3 1.8 mM; NaCl 150.0 mM; HEPES 25.0 mM; CaCl2 1.7 mM) and stained with 0.5% neutral red solution in acetate buffer at pH 5.2 (Sigma–Aldrich, St. Louis, MO) for 5s to more easily visualize follicles (Clifton and Noriega, 2011). The ovaries were rinsed a second time in APS and placed under a coverslip. Photographs were taken of the previtellogenic ovaries using a DM 5500 B Leica fluorescence microscope, a Leica DFC 310 FX mounted camera and Leica LAS imaging software. Ovaries were later scored using Leica LAS imaging software for total follicle count. A mean follicle value was then calculated to represent previtellogenic follicle output for the cohort.
2.7 Quantification of eggs
Mated mosquitoes were offered a blood meal as described above (sec. 2.1). Although blood meal volume was not directly quantified, after feeding each female was visually inspected and judged to have fed to satiety by her fully engorged abdomen. Fifteen fully engorged females were housed in individual containers (1 female/container) and provided 3% sucrose ad libitum. Ninety-six h after blood feeding egg papers were collected, dried under insectary conditions, allowed 5d to complete embryogenesis and photographed with a digital camera. The number of eggs produced per female was later determined by manually counting the number of eggs on each egg paper from those photographs. A mean was then calculated to represent egg output for the cohort.
2.8 Estimation of follicle resorption
The extent to which follicles were resorbed (expressed as a percentage) was determined by subtracting the mean number of oviposited eggs/female from the mean number follicles/female from each treatment combination.
2.9 Quantification of fertility
Fertility (number of viable offspring) was used as an estimate of reproductive fitness. Eggs collected in section 2.7 were hatched as previously described and the number of newly emerged 1st instars counted.
2.10 Data analysis
Graphical representations and statistical analysis were performed with GraphPad Prism Software (San Diego, CA) version 6.00 for Windows. Results were expressed as means and SEM. Survivorship (LT50) was determined by non-linear regression curve fit. Significant differences in survivorship (LT50) were determined with a log rank method. Significant differences for all other results were determined with a one tailed student’s t test or in the case of reproductive output, for graphical clarity, a one way ANOVA with a Tukey test for multiple comparisons was used (P < 0.05).
3. Results
3.1 Extended quiescence altered female adult performance
3.1.1 Extended quiescence reduced female adult body mass
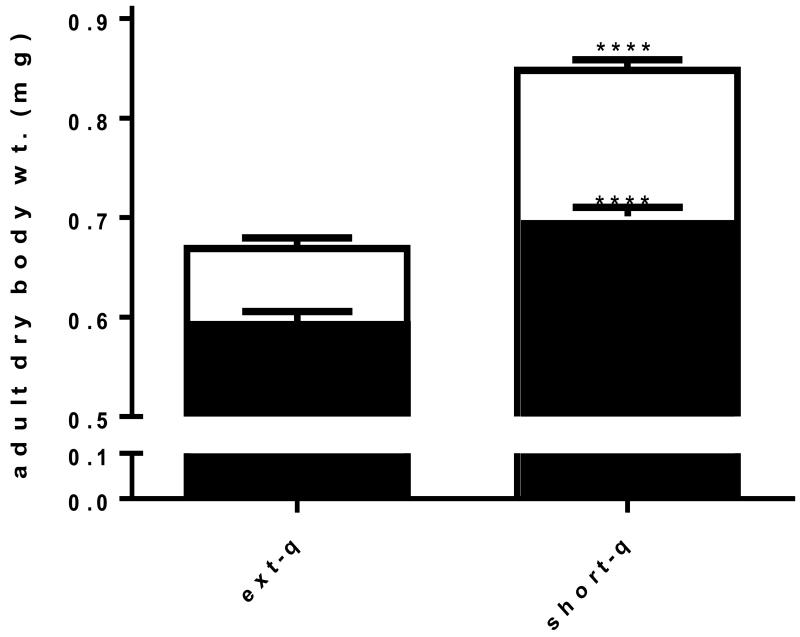
Adult female dry body mass at time of emergence was significantly reduced by 21% when compared with their short quiescence counterparts indicating that an egg quiescence period of ten weeks results in a morphometricly altered adult female. A significant difference in the dry body mass of the larval metal stress treatments suggested a difference in metal physiology response occurred between extended and short quiescence females (Fig. 1).
Fig. 1. Extended quiescence reduced female adult body mass.
Extended quiescence significantly reduced female adult dry body mass at time of emergence by 21% (one tailed t test, p<0.0001, t=11.83 df=95). Larval metal stress reduced adult body size 17.3% in short quiescence females while only 11.4% in extended quiescence females representing a significant difference in metal physiology response between short quiescence and extended quiescence females (one tailed t test, p<0.05, t=7.083 df=117)(Fig. 1). (short-q=short quiescence, ext-q=extended quiescence) (white columns – reared in clean water; black columns-reared with metal stress) (n~50-60).
3.1.2 Extended quiescence increased starvation tolerance of female adults
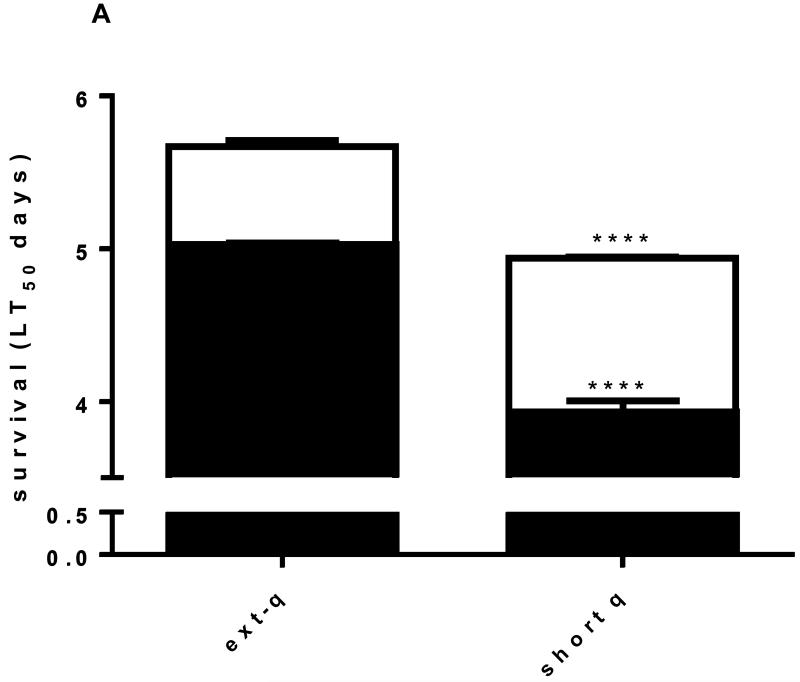
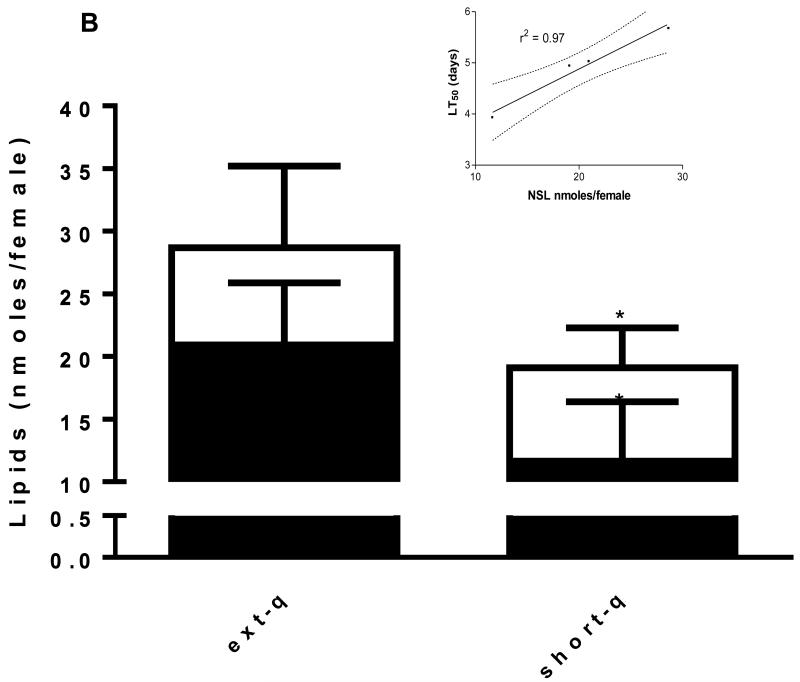
Tolerance to starvation, as estimated by mean survival time (LT50), was 12% greater in newly emerged adult females reared from extended quiescence eggs than adult females reared from short quiescence eggs. Larval metal stress reduced starvation tolerance significantly more in adult females from short quiescence eggs (20% vs. 11.2%) indicating a difference in metal tolerance physiology between the two phenotypes with females from extended quiescence being more tolerant of metal stress (Fig. 2A). Taken together these results indicate that females reared from extended quiescence eggs are more tolerant of short term or immediate stress. Mean neutral storage lipid reserves (NSL, nmoles/female) between extended quiescence and short quiescence treatments also differed significantly in correlation with the starvation tolerance results, i.e. the more NSL, the higher the LT50 value (Fig. 2B and inset).
Fig. 2. Extended quiescence increased starvation tolerance of adult females.
(A) Adult females reared from extended quiescence eggs exhibited 12.9% significantly greater survival time (LT50) on a diet of only water than adult females reared from short quiescence eggs (N=45, log rank, p<0.01). Larval metal stress reduced adult survival times significantly more in the short quiescence females (20%) than in the extended quiescence females (11.4%) when compared to their clean reared counterparts (N=45, log rank, p<0.01). (B) Adult females reared from extended quiescence eggs emerged with 33% significantly more neutral lipid reserves than adult females reared from short quiescence eggs (t test, one tailed, p<0.05, t=2.289, df=4); larval metal stress response reduced lipids significantly more in females from short quiescence than in females from extended quiescence, i.e., 20.0 vs. 11.4% respectively. (t test, one tailed, p<0.05, t=2.350, df=4). Starvation tolerance and neutral lipids reserves are highly correlated (inset). (Legend follows Fig. 1).
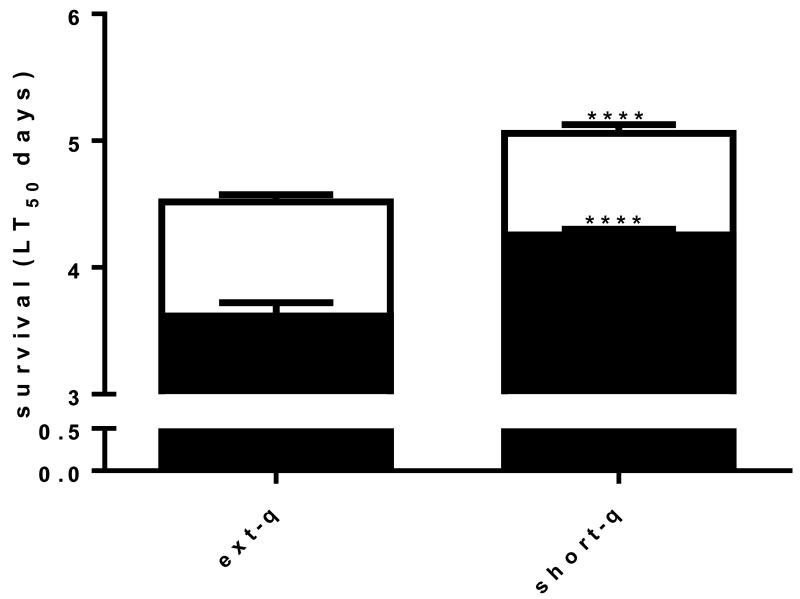
3.1.3 Extended quiescence increased longevity of adult females
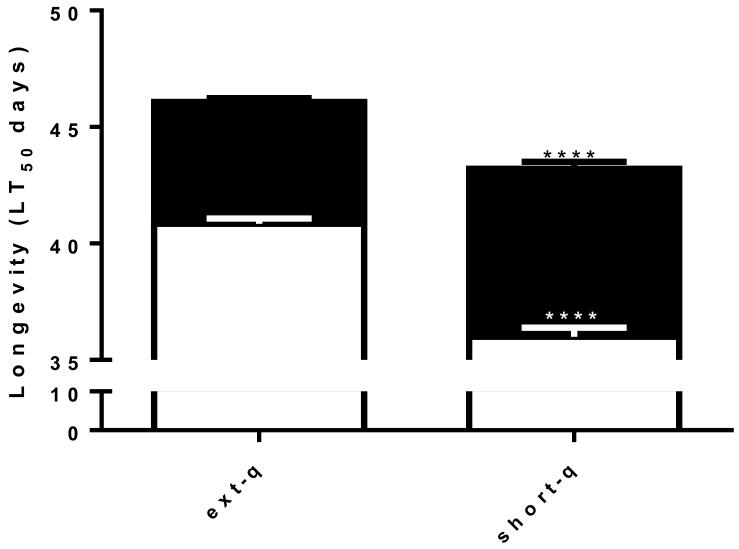
The life span or mean survival time (LT50) of unmated adult females was ~10% greater for females reared from extended quiescence eggs than for females reared from short quiescence eggs suggesting altered physiological states between the two. Larval metal stress had a hormetic effect in both extended and short quiescence treatments further increasing lifespan in each case, however, the magnitude of the effect was significantly greater in females from short quiescence (20 vs. 12%) (Fig. 3).
Fig. 3. Extended quiescence increased longevity of adult females.
Adult female mosquitoes reared from extended quiescence eggs exhibited 10.2% significantly longer life span than adult female mosquitoes reared from short quiescence eggs (40.1 vs. 36.0days) (N=45, log rank, p<0.01). The hormetic effect of metal stress differed significantly between extended and short quiescence females, an increase of 12.8 vs. 20% respectively over their clean reared counterparts (46.1 vs. 43.2 days) (log rank test p<0.01). (Legend follows Fig. 1)
3.2 Extended quiescence altered reproductive strategy
3.2.1 Effect of extended quiescence on reproduction
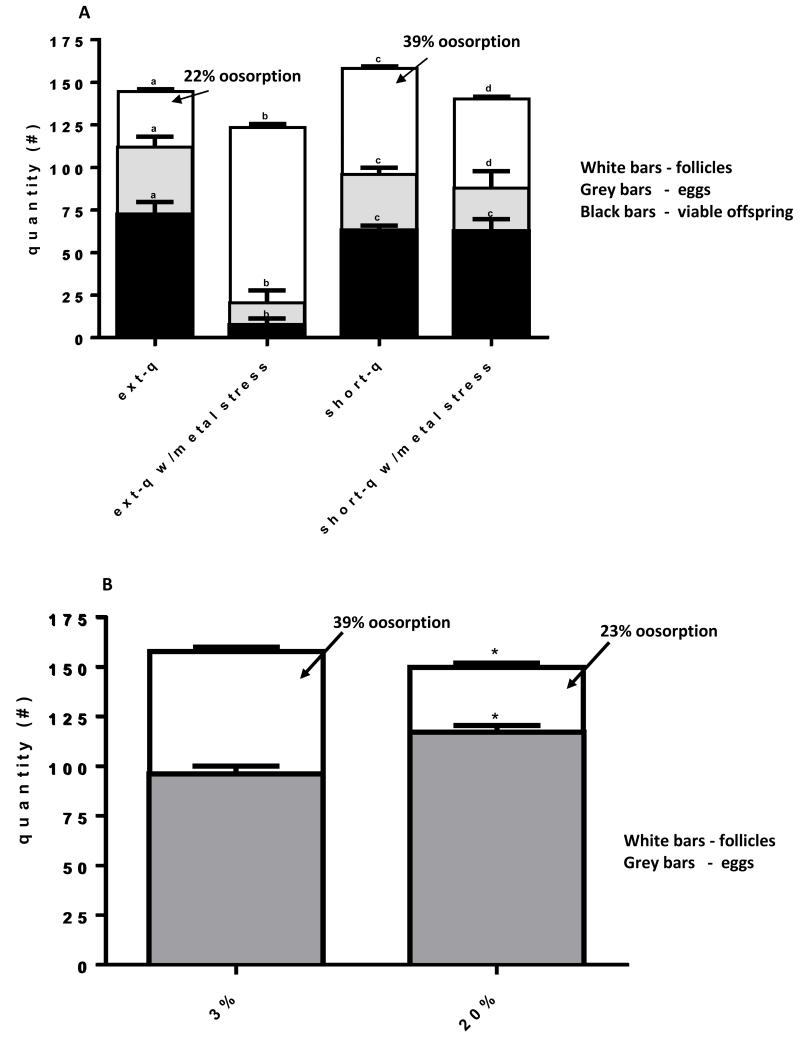
Adult females reared from extended quiescence eggs developed significantly less resting stage follicles than females reared from short quiescence eggs, yet laid more eggs and yielded ~14% more viable offspring under the sub optimal 3% sucrose regime (Fig. 4A). Altered follicular resorption is responsible for this difference; under the sub optimal 3% sucrose regime females reared from extended quiescence eggs resorbed 22% of the resting stage follicles while females reared from short quiescence eggs resorbed 39%. However, when adult females reared from short quiescence eggs were given the enriched 20% sucrose diet they resorbed only 23% of their follicles and the subsequent number of eggs developed was similar to those of females reared from quiescent eggs (Fig. 4B).
Fig. 4. Extended quiescence altered reproductive strategy.
(A) Adult females reared from extended quiescence eggs developed significantly fewer follicles (n=30 pairs of ovaries from 15 females) (white bars) than females reared from short quiescence eggs (3% sucrose) but matured more eggs (gray bars) that resulted in more viable offspring (black bars) than their short quiescence counterparts. Although the reproductive profile among all four treatment combinations was significantly different, the metal stress response in extended quiescence females was dramatically altered (one way ANOVA p<0.05, with Tukey test of multiple comparisons, df=58)(n=15 females). (B) Adults reared from short quiescence eggs on a diet of 20% sucrose resulted in less oosorption and more eggs being matured. Consequently, the number of eggs matured approximates the number of eggs developed by adults reared from extended quiescence eggs under the 3% sucrose diet (n=15) (t test, one tailed, p<0.05).
3.2.2 Response to larval metal stress
Larval metal stress reduced the number of resting stage follicles similarly in females reared from both extended and short quiescence eggs (14.5% vs. 11.7%), however, the mean number of eggs matured and viable offspring were dramatically reduced (>5× fold) only in the extended quiescence phenotype (Fig. 4A). Reproduction in extended quiescence females was particularly sensitive to larval metal stress whereas the opposite held true for the other forms of stress analyzed.
3.3 Extended quiescence produced maternal effects
3.3.1 Extended quiescence reduced tolerance to starvation of offspring
The maternal effects of extended quiescence were observed in the reduced starvation tolerance of newly emerged 1st instar progeny of adults reared from eggs having under gone quiescence (11.9%). The effect of larval metal stress on maternal effects for these two treatments differed only slightly, although significantly (20.0 vs. 16.0%) suggesting that P1 generation may also be phenotypically different (Fig. 5).
Fig. 5. Extended Quiescence reduced tolerance to starvation of progeny 1st instar larvae.
Extended quiescence resulted in maternal effects i.e., an 11.9% reduction in starvation tolerance of newly emerged 1st instar progeny (4.5 vs. 5.1 d). The metal stress response differed significantly also; it was greater in the extended quiescence condition (20 vs. 16%). (N=75, log rank test, p<0.01). (Legend follows Fig. 1)
4. Discussion
Rainfall pattern is the most conspicuous aspect of tropical seasons. The wet/dry cycle is responsible for the seasonal growth of vegetation and small puddles that provide suitable habitats for container breeding mosquitoes. The subsequent increase in plant growth provides carbohydrate rich food resources for many phytophagous insects that during the drier season are less abundant. Egg quiescence occurs in mosquitoes of the genera Ochlerotatus, Aedes, and Psorophora; these tropical and sub-tropical container inhabiting species face the challenge of unpredictable alternating dry and wet periods utilizing egg quiescence to survive dry conditions (Vinogradova, 2007). Our results suggest that A. aegypti egg quiescence may serve as a cue signaling the sub-optimal adult environmental conditions that follow a dry period. The resulting adult phenotype is subsequently better fit for these conditions. This unique characteristic may have contributed to its wide geographic distribution and success as a vector.
4.1 Quiescence and female adult performance
Our observation that A. aegypti adult female mosquitoes reared from extended quiescence eggs have smaller body mass at emergence, yet more lipid reserves and greater fecundity than their short quiescence counterparts might seem to contradict the current literature stating that female body size, lipid reserves and fecundity are all positively correlated (Caroci, et al., 2004; Blackmore and Lord, 2000; Briegel, 1990). However insects, being capable of a high degree of phenotypic plasticity, including seasonal polyphenism, are able to adjust the relative proportion of teneral reserves so as to develop phenotypes best suited to environmental changes (Brakefield, et al., 2007, Moran, 1992; West-Eberhardt, 2003). It has also been demonstrated that altered insulin signaling in Drosophila melanogaster can produce a phenotype with smaller somatic body size, hypertrophied fat bodies with high lipid stores that are long lived (Tatar et al., 2001). Altered metabolic rate may also be responsible. It has been shown in a Lepidopteron model that insect body size is proportional with metabolic rate (Brakefield, et al., 2007; Pijge, et al., 2007) and a high metabolic rate would entail greater maintenance requirements resulting in lower adult quality (Gotthard et al., 1994).
Our results also demonstrated that unmated adult female mosquitoes reared from extended quiescence eggs exhibited greater starvation tolerance and longer lifespan than females reared from short quiescence eggs. Many organisms encounter periods of starvation in their life and most organisms have evolved some form of adaptive physiology to cope with them. It has often been suggested that the mechanisms that enhance survival under starvation also underlie the regulation of longevity. In a range of well-studied animal species, long-lived individuals appear to be more resistant to multiple stresses, often including starvation (Pijpe et al., 2007). Mosquitoes emerging at a dry/wet period interface with greater adult lipid reserves, starvation tolerance and longevity would be at a physiological advantage at a time when environmental conditions may be more challenging than during the wet season. Our previous work has already demonstrated that larvae hatched from extended quiescence eggs exhibit a longer larval developmental period and presumably a slower growth rate than larvae hatched from eggs having undergone a brief quiescence. Therefore, it may be possible that smaller mosquitoes with a lower metabolic rate can emerge with greater neutral lipid reserves, altered metabolism and increased adult survivorship. These individuals emerging at the dry/wet season interface might have been physiologically reprogrammed by the extended quiescence period for improved performance and fitness during the weeks that follow. Alternatively, extended quiescence may increase adult longevity by acting as a period of larval dietary restriction. Periods of famine often trigger what appear to be metabolic switches that paradoxically extend the normal life span, without sacrificing subsequent reproduction and survival when favorable conditions return (Austad, 1989; Kirkwood and Austad, 2000). For example, dietary restriction in A. aegypti larvae has been shown to extend adult lifespan (Joy, et al., 2010). Dietary restriction is thought to increase lifespan though lowered metabolic rate and decreased TOR signaling as an active, highly conserved stress response that evolved early in life’s history to increase an organism’s chance of surviving adversity (Britton, et al., 2002; Powers, et al., 2006; Sinclair, 2005).
A. aegypti larval breeding sites are heterogeneous and dynamic environments with metals and other physiological stressors (Barrera et al., 2006, 2008; Huber et al., 2008). Larval environmental conditions can alter the growth and development of mosquitoes and subsequently their adult phenotypes, reproduction and progeny (Blackmore and Lord, 2000; Caroci, et al., 2004; reviewed in Merrit et al., 1992). We, therefore, examined the response to larval metal stress on these two adult phenotypes. Larval environmental metal stress resulted in reduced body mass and lipid reserves that were most likely responsible for reduced starvation tolerance in adults reared from both extended quiescence and short quiescence eggs, however the response was greater in the short quiescence condition. This overall reduction in performance in both phenotypes is most likely a result of the caloric cost of metal detoxification, sequestration and elimination (Mireji et al., 2010; Pook, et al., 2009; Servia, et al., 2006; Perez and Noriega, unpublished), but why the response was greater in short quiescence adults is unclear. We also witnessed a hormetic effect which was greater in adults reared from short quiescence. Hormesis is defined as a favorable biological response to low exposures of a stressor (reviewed in Sinclair, 2005). Longevity has been shown to be increased by moderate metal or oxidative stress in several studies with D. melanogaster and C. elegans (Baldal et al., 2006; Barsyte et al., 2001; Broughton et al., 2005).
4.2 Extended quiescence alters reproductive success
We have previously shown that an extended quiescence can be metabolically expensive for A. aegypti larvae and in that regard quiescence is similar to diapause. Unlike diapause which results in lower post diapause fecundity, our results suggest that the extended quiescence period of 1st instars adjusts the reproductive strategy of adults in a manner that might help reestablish local mosquito populations at the end of a dry period, an unfavorable time which requires maximum fertility, i.e., maximum viable offspring. This altered reproductive performance, however, comes at a cost, i.e., maternal effects; 1st instar larvae from females reared from quiescent eggs are less fit.
Reproductive trade-offs in adult mosquitoes occur primarily through the process of oosorption (Clifton and Noriega, 2011 and 2012). By resorbing excess reproductive tissues, insects can alter previous reproductive decisions by redirecting resources away from reproduction in favor of competing physiological activities. Y-model allocation theory predicts that a limited resource is divided between survival and reproduction (e.g. starvation resistance and longevity vs. fecundity). The costs of reproduction involving trade-offs between egg production and mortality are well documented in insects and other animals with trade-offs imposing constraints among survival, growth, resource acquisition and reproduction; nutrient limitation being the central mechanism underlying these trade-offs (Boggs, 2009; Chippindale, et al., 1993). Seasonal variation in the reproductive parameters of Anopheles gambiae have also been demonstrated (Yaro, et al., 2012).
Adult A. aegypti females derived from extended quiescence and short quiescence eggs and fed the sub-optimal 3% diet resorbed dramatically different proportions of their follicles (22% vs. 39% respectively). However, when females reared from short quiescence eggs where fed the enriched 20% diet they resorbed only 23% of their follicles. From these data we conclude that adult females reared from short quiescence eggs and fed the sub-optimal 3% sugar diet failed to predict their adult environment and had to resorb excess reproductive material. In a natural setting, females derived from short quiescence eggs would have expected an optimal environment that the wet season would provide or that the 20% sucrose diet represents.
The reduction in the number of resting stage follicles developed under both quiescence conditions produced by larval metal stress is most likely due to a reallocation of resources due to the above mentioned ‘cost’ of metal stress. The number of ovarioles a female will produce is determined during the 2nd - 3rd instar stage (Ronquillo and Horsefall, 1969). It is reasonable to hypothesize that newly emerged larvae sensing a stressful environment will allocate resources to somatic rather than reproductive purposes (reviewed in Boggs, 2009; Postma et al., 1995; Servia, 2006). Most interestingly, larval metal stress reduced fecundity and fertility dramatically only after extended quiescence. Although adult females reared from extended quiescence eggs are apparently better fit than females reared from short quiescence eggs in the absence of a stressor; in the presence of a stressor the opposite holds true. The reasons for this are unclear; however, this is strong evidence that adult females reared from extended quiescent eggs are physiologically distinct from females reared from short quiescence eggs.
4.3 Extended quiescence and maternal effects
Extended quiescence reduced tolerance to starvation of offspring 1st instar larvae under both clean and metal stressed conditions. The fertilized mosquito egg is a closed system totally dependent on maternally-derived lipid reserves to complete embryogenesis and maintain its quiescent metabolism (Clements, 1992; Vinogradova, 2007). As a consequence, a nutrient limited adult has fewer resources to devote to reproduction and therefore less maternally derived nutrients are passed on to filial larvae. Maternal effects, therefore, reflect the nutritional status of the parents, i.e., filial pharate 1st instar larvae derived from extended quiescence and containing less nutrient reserves will perform accordingly. Adult females reared from extended quiescence eggs exhibited greater fertility than adult females reared from short quiescence eggs at the cost of the next generation when presumably environmental conditions might improve. Then, if mesic conditions follow, females will produce eggs with higher lipid content enabling those eggs to endure a prolonged quiescence to survive any subsequent dry period.
4.4 Implications for Vector Ecology
Aseasonal pharate 1st instar quiescence is an adaptation to the large and abrupt fluctuations in water availability of larval container habitats. It results in an asynchronous hatching of eggs. Usually the first flooding induces hatching of the majority of the eggs, whereas the remainder of the eggs may hatch much later after a subsequent flooding episode. Asynchronous hatching is based on intrapopulation variation in sensitivity of individuals to environmental cues. Such arrest of hatching is a very useful adaptation favoring conservation of populations with unpredictable water sources (Vinogradova, 2007 and references therein,). Such an adaptation may have inadvertently contributed to A. aegypti’s wide geographic distribution and success as an anthropophilic vector. Furthermore, egg quiescence that results in adult plasticity would give this species of mosquito a great advantage in colonizing and maintaining population abundances in new geographic areas by producing adults best suited to novel environments. Although A. aegypti is considered a tropical mosquito and its distribution appears to be influenced by climate in some temperate regions of the world, contemporary and historical records document persistence outside these regions (Christophers, 1960). The peridomestic behavior of A. aegypti and its desiccation-resistant eggs afford this species an alternative mode of long-distance dispersal via human-mediated transportation within and between continents. Over the last 25 years, there has been a global increase in both the distribution of A. aegypti and epidemic dengue virus activity (Jansen and Beebe, 2010). While climate influences the geographical distribution of this mosquito species, the close association of A. aegypti with humans and the domestic environment allows this species to persist in regions that may otherwise be unsuitable based on climatic factors alone. Quiescence dependent adult plasticity would greatly enhance this dispersal process by producing adults with an adaptive advantage in challenging and novel environments and allowing for sustained populations. For example a newly emerged female resulting from an extended quiescence egg would have greater lipid reserves available for longer host and oviposition seeking flights. Future work needs to determine if the plasticity described here is simply a norm of reaction (continuous range of phenotypes) or a case of alternative phenisim (discrete phenotypes).
Environmental conditions experienced during the mosquito’s immature stages can subsequently affect adult phenotypes in a manner that may be important determinants of vector capacity (Merrit et al., 1992). We propose that A. aegypti mosquitoes hatched from eggs that have undergone an extended quiescence in an urban environment are likely to exhibit greater vector capacity than mosquitoes hatched from short quiescence eggs. Laboratory studies have indicated that vector competence (i.e. the internal physiological factors that govern the infection of human pathogens in a mosquito) correlates with the quality of the larval environment and adversely affects larval survival, developmental rates and produces smaller adults with compromised fitness (Alto et al., 2008; Alto et al., 2012; Muturi, 2011; Telang, 2012). As a consequence, eggs that have undergone an extended quiescence in an urban environment could (a) contribute to increased vector capacity via increased dengue viral load of adult females and (b) have implications for vector control strategies. Following quiescence a mosquito population is in its most vulnerable state. A. aegypti adult mosquitoes reared from quiescent eggs and living in stressed urban environments produce far less viable offspring resulting in a seasonal population bottleneck, a phenomenon that mosquito control boards could use to their advantage.
Highlights.
-
➢
Extended quiescence serves as an environmental cue predicting adult environment
-
➢
Pharate 1st instar extended quiescence modifies adult phenotype
-
➢
Adult response to larval stress differs between phenotypes
-
➢
Costs of pharate 1st instar extended quiescence are reflected in maternal effects
Acknowledgements
We would like to thank Dr. Marcela Nouzova, Dr. Crisalejandra Rivera and especially Dr. Mark Clifton for the critical reading and feedback of the manuscript. This work was supported by NIH/NIGMS R25 GM061347 and the FIU MBRS RISE Biomedical Research Initiative to MHP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alto BW, Lounibos LP, Mores CN, Reiskind MH. Larval competition alters susceptibility of adult Aedes mosquitoes to dengue infection. Proceedings of the Royal Society B: Biological Sciences. 2008;275:463–471. doi: 10.1098/rspb.2007.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto BW, Muturi EJ, Lampman RL. Effects of nutrition and density in Culex pipiens. Medical and Veterinary Entomology. 2012:396–406. doi: 10.1111/j.1365-2915.2012.01010.x. [DOI] [PubMed] [Google Scholar]
- Austad SN. Life extension by dietary restriction in the bowl and doily spider, Frontinella pyramitela. Experimental Gerontology. 1989;24:83–92. doi: 10.1016/0531-5565(89)90037-5. [DOI] [PubMed] [Google Scholar]
- Amador BR, Clark G. Ecological Factors Influencing Aedes aegypti (Diptera: Culicidae) Productivity in Artificial Containers in Salinas, Puerto Rico. Journal of Medical Entomology. 2006;43:484–492. doi: 10.1603/0022-2585(2006)43[484:efiaad]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Baldal EA, Brakefield PM, Zwaan BJ. Multitrait evolution in lines of Drosophila melanogaster selected for increased starvation resistance: The role of metabolic rate and implications for the evolution of longevity. Evolution. 2006;60:1435–1444. [PubMed] [Google Scholar]
- Barrera R, Amador M, Clark GG. Ecological Factors Influencing Aedes aegypti (Diptera: Culicidae) Productivity in Artificial Containers in Salinas, Puerto Rico. Journal of Medical Entomology. 2006;43:484–492. doi: 10.1603/0022-2585(2006)43[484:efiaad]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Barrera R, Amador M, Diaz A, Smith J, Munoz-Jordan JL, Rosario Y. Unusual productivity of Aedes aegypti in septic tanks and its implications for dengue control. Medical and Veterinary Entomology. 2008:62–69. doi: 10.1111/j.1365-2915.2008.00720.x. [DOI] [PubMed] [Google Scholar]
- Barsyte D, Lovejoy DA, Lithgow GJ. Longevity and heavy metal resistance in daf-2 and age-1 long-lived mutants of Caenorhabditis elegans. Journal of the Federation of American Societies for Experimental Biology. 2001;15:627–634. doi: 10.1096/fj.99-0966com. [DOI] [PubMed] [Google Scholar]
- Blackmore MS, Lord CC. The relationship between size and fecundity in Aedes albopictus. Journal of Vector Ecology. 2000;25:212–217. [PubMed] [Google Scholar]
- Boggs CL. Understanding insect life histories and senescence through a resource allocation lens. Functional Ecology. 2009;23:27–37. [Google Scholar]
- Brakefield P, Pijpe J, Zwaan B. Developmental plasticity and acclimation both contribute to adaptive responses to alternating seasons of plenty and of stress in Bicyclus butterflies. Journal of Biosciences. 2007;32:465–475. doi: 10.1007/s12038-007-0046-8. [DOI] [PubMed] [Google Scholar]
- Briegel H. Metabolic relationship between female body size, reserves, and fecundity of Aedes aegypti. Journal of Insect Physiology. 1990;36:165–172. [Google Scholar]
- Britton JS, Lockwood WK, Li L, Cohen SM, Edgar BA. Drosophila’s Insulin/PI3-Kinase Pathway Coordinates Cellular Metabolism with Nutritional Conditions. Developmental Cell. 2002;2:239–249. doi: 10.1016/s1534-5807(02)00117-x. [DOI] [PubMed] [Google Scholar]
- Broughton SJ, Piper MDW, Ikeya T, Bass TM, Jacobson J, Driege Y, Martinez P, Hafen E, Withers DJ, Leevers SJ, Partridge L. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proceedings of the National Academy of Sciences. 2005;102:3105–3110. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroci AS, Li Y, Noriega FG. Reduced juvenile hormone synthesis in mosquitoes with low teneral reserves reduces ovarian previtellogenic development in Aedes aegypti. Journal of Insect Physiology. 2004;207:2685–2690. doi: 10.1242/jeb.01093. [DOI] [PubMed] [Google Scholar]
- Chippindale AK, Leroi AM, Kim SB, Rose MR. Phenotypic plasticity and selection in Drosophila life-history evolution. I. Nutrition and the cost of reproduction. Journal of Evolutionary Biology. 1993;6:171–193. [Google Scholar]
- Christophers R. Aëdes aegypti (L.) the Yellow Fever Mosquito: its Life History, Bionomics and Structure. Cambridge University Press; 1960. [Google Scholar]
- Clements AN. The Biology of Mosquitoes. 1st ed. Chapman and Hall; London: 1992. [Google Scholar]
- Clifton ME, Noriega FG. Nutrient limitation results in juvenile hormone-mediated resorption of previtellogenic ovarian follicles in mosquitoes. Journal of Insect Physiology. 2011;57:1274–1281. doi: 10.1016/j.jinsphys.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton ME, Noriega FG. The fate of follicles after a blood meal is dependent on previtellogenic nutrition and juvenile hormone in Aedes aegypti. Journal of Insect Physiology. 2012;58:1007–1019. doi: 10.1016/j.jinsphys.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denlinger DL. Dormancy in Tropical Insects. Annual Review of Entomology. 1986;31:239–264. doi: 10.1146/annurev.en.31.010186.001323. [DOI] [PubMed] [Google Scholar]
- Esperk T, Stefanescu C, Teder T, Wiklund C, Kaasik A, Tammaru T. Distinguishing between anticipatory and responsive plasticity in a seasonally polyphenic butterfly. Evolutionary Ecology. 2012:1–18. [Google Scholar]
- Foster WA. Mosquito sugar feeding and reproductive energetics. Annual Review of Entomology. 1995;40:443–474. doi: 10.1146/annurev.en.40.010195.002303. [DOI] [PubMed] [Google Scholar]
- Gary RE, Foster WA. Effects of Available Sugar on the Reproductive Fitness and Vectorial Capacity of the Malaria Vector Anopheles gambiae (Diptera: Culicidae) Journal of Medical Entomology. 2001;38:22–28. doi: 10.1603/0022-2585-38.1.22. [DOI] [PubMed] [Google Scholar]
- Gotthard K, Nylin S, Wiklund C. Adaptive variation in growth rate: life history costs and consequences in the speckled wood butterfly, Pararge aegeria. Oecologia. 1994;99:281–289. doi: 10.1007/BF00627740. [DOI] [PubMed] [Google Scholar]
- Hahn DA, Denlinger DL. Meeting the energetic demands of insect diapause: Nutrient storage and utilization. Journa l of Insect Physiology. 2007;53:760–773. doi: 10.1016/j.jinsphys.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Hahn DA, Denlinger DL. Energetics of Insect Diapause. Annual Reviews of Entomology. 2011;56:103–121. doi: 10.1146/annurev-ento-112408-085436. [DOI] [PubMed] [Google Scholar]
- Huber K, Ba Y, Dia I, Mathiot C, Sall AA, Diallo M. Aedes aegypti in Senegal: Genetic Diversity and Genetic Structure of Domestic and Sylvatic Populations. Am J Trop Med Hyg. 2008:218–229. [PubMed] [Google Scholar]
- Jansen CC, Beebe NW. The dengue vector Aedes aegypti: what comes next? Microbes and Infection. 2010;12:272–279. doi: 10.1016/j.micinf.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Joy TK, Arik AJ, Corby-Harris V, Johnson AA, Riehle MA. The impact of larval and adult dietary restriction on lifespan, reproduction and growth in the mosquito Aedes aegypti. Experimental Gerontology. 2010;45:685–690. doi: 10.1016/j.exger.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood TBL, Austad SN. Why do we age? Nature. 2000;408:233–238. doi: 10.1038/35041682. [DOI] [PubMed] [Google Scholar]
- Merritt RW, Dadd RH, Walker ED. Feeding Behavior, Natural Food, and Nutritional Relationships of Larval Mosquitoes. Entomology. 1992:349–374. doi: 10.1146/annurev.en.37.010192.002025. [DOI] [PubMed] [Google Scholar]
- Mireji PO, Keating J, Hassanali A, Mbogo CM, Muturi MN, Githure JI, Beier JC. Biological cost of tolerance to heavy metals in the mosquito Anopheles gambiae. Medical and Veterinary Entomology. 2010;24 doi: 10.1111/j.1365-2915.2010.00863.x. 2 109-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran NA. The Evolutionary Maintenance of Alternative Phenotypes. The American Naturalist. 1992;139:971–989. [Google Scholar]
- Mori A, Oda T, Wada Y. Studies on the egg diapause and overwintering of Aedes albopictus in Nagasaki. Tropical Medicine. 1981;23:79–90. [Google Scholar]
- Munstermann LE, Wasmuth LM. In: Handbook of Insect Rearing. Singh P, Moore RF, editors. Elsevier Amsterdam; 1985. [Google Scholar]
- Muturi EJ, Kim C-H, Alto BW, Berenbaum MR, Schuler MA. Larval environmental stress alters Aedes aegypti competence for Sindbis virus. Tropical Medicine & International Health. 2011;16:955–964. doi: 10.1111/j.1365-3156.2011.02796.x. [DOI] [PubMed] [Google Scholar]
- Perez MH, Noriega FG. Aedes aegypti pharate 1st instar quiescence affects larval fitness and metal tolerance. Journal of Insect Physiology. 2012;58:824–829. doi: 10.1016/j.jinsphys.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijpe J, Brakefield P, Zwaan B. Phenotypic plasticity of starvation resistance in the butterfly Bicyclus anynana. Evolutionary Ecology. 2007;21:589–600. [Google Scholar]
- Pook C, Lewis C, Galloway T. The metabolic and fitness costs associated with metal resistance in Nereis diversicolor. Marine Pollution Bulletin. 2009;58:1063–1071. doi: 10.1016/j.marpolbul.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Postma JF, Kleunen A, Admiraal W. Alterations in life-history traits of Chironomus riparius (diptera) obtained from metal contaminated rivers. Archives of Environmental Contamination and Toxicology. 1995;29:469–475. [Google Scholar]
- Powers RW, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes and Development. 2006;20:174–184. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquillo MC, Horsfall WR. Genesis of the reproductive system of mosquitoes. I. Female of Aedes stimulans (Walker) Journal of Morphology. 1969;129:249–280. [Google Scholar]
- Sarkar S, Duttagupta AK, Mal TK. Effects of heavy metals on population growth and metallothionein gene expression in the mosquito Culex quinquefasciatus, from Calcutta, India. Environmental Pollution. 2004;127:183–193. doi: 10.1016/j.envpol.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Servia M, Péry A, Heydorff M, Garric J, Lagadic L. Effects of copper on energy metabolism and larval development in the midge, Chironomus riparius. Ecotoxicology. 2006;15:229–240. doi: 10.1007/s10646-005-0054-0. [DOI] [PubMed] [Google Scholar]
- Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mechanisms of Ageing and Development. 2005;126:987–1002. doi: 10.1016/j.mad.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. A Mutant Drosophila Insulin Receptor Homolog That Extends Life-Span and Impairs Neuroendocrine Function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- Telang A, Qayum AA, Parker A, Sacchetta BR, Byrnes GR. Larval nutritional stress affects vector immune traits in adult yellow fever mosquito Aedes aegypti (Stegomyia aegypti) Medical and Veterinary Entomology. 2012;26:271–281. doi: 10.1111/j.1365-2915.2011.00993.x. [DOI] [PubMed] [Google Scholar]
- Vinogradova E, Alekseev V, Stasio B, Gilbert J. In: Diapause in Aquatic Insects, with Emphasis on Mosquitoes: in Diapause in Aquatic Invertebrates Theory and Human Use. Dumont HJ, Werger MJA, editors. Springer Netherlands; 2007. pp. 83–113. [Google Scholar]
- West-Eberhard M. Developmental Plasticity and Evolution. Oxford University Press; USA: 2003. [Google Scholar]
- Yaro AS, Traor AI, Huestis DL, Adamou A, Timbin S, Kassogu Y, Diallo M, Dao A, Traor SF, Lehmann T. Dry season reproductive depression of Anopheles gambiae in the Sahel. Journal of Insect Physiology. 2012;58:1050–1059. doi: 10.1016/j.jinsphys.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]