Abstract
Background
Among older women with early stage breast cancer, patients with a short life expectancy (LE) are much less likely to benefit from adjuvant radiation therapy (RT). Little is known about the impact of physicians and regional factors on the use of RT across LE groups.
Objective
To determine the relative contribution of patient, physician and regional factors on the use of RT.
Design
Retrospective cohort.
Subjects
Women age 67–94 diagnosed with stage I breast cancer between 1998–2007 receiving breast-conserving surgery.
Measures
We evaluated patient, physician and regional factors for their association with RT across strata of LE using a three-level hierarchical logistic regression model. Risk-standardized treatment rates (RSTRs) for the receipt of radiation were calculated according to primary surgeon and region.
Results
Approximately 43.6% of the 2,253 women with a short LE received RT, compared to 90.8% of the 11,027 women with a long LE. Among women with a short LE, the probability of receiving RT varied substantially across primary surgeons; RSTRs ranged from 27.7% to 67.3% (mean 43.9%). There was less variability across geographic regions; RSTRs ranged from 42.0–45.2% (mean 43.6%). Short LE patients were more likely to receive RT in areas with high radiation oncologist density (OR 1.59; 95% CI, 1.07–2.36).
Conclusions
While there is wide variation across geographic regions in the use of RT among women with breast cancer and short LE, the regional variation was substantially diminished after accounting for the operating surgeon.
Keywords: breast-conserving surgery, radiation therapy, life expectancy, small-area analysis, SEER program
INTRODUCTION
Standard treatment for women with early stage breast cancer currently includes breast-conserving surgery (BCS) followed by external beam radiation therapy (RT).1, 2 However, recent trial results suggest that women above the age of 70 with early stage breast cancer derive little benefit from adjuvant RT: a study of 636 women found that those receiving RT had lower locoregional tumor recurrence (1%) than those without (4%) but no difference in overall survival.3
Women with a short life expectancy (LE) are particularly unlikely to benefit from RT, given that they are more likely to die from causes other than breast cancer.4 Additionally, side effects are known to accompany adjuvant RT, including long-term mastalgia, aesthetic changes, and rare cardiopulmonary toxicity or secondary tumors.5 RT requires a significant time investment by patients and is expensive. Accordingly, some have suggested that adjuvant RT may be omitted for older women with low risk tumors who are age 70 and older.6–8 Those with a long LE may potentially benefit from RT by reducing the risk of local recurrence and avoiding test anxiety, stress and the need for repeat surgery, although the survival outcome would not be altered.
There are concerns that some women who have weaker indications for treatment continue to receive radiation.9 A commonly used approach to assessing the use of health care services among patients for whom there is questionable benefit (i.e. discretionary use) is to assess geographic variation. While numerous studies have demonstrated substantial regional variation in cancer care, there is little clarity regarding whether variation is a result of regional differences in health system infrastructure, or of underlying variation across physicians.10–13 Some studies have suggested that variation in the use of health care across geographic regions is attributable to the collective attitudes and styles of physicians.14–16 Other studies suggest that variation is more an effect of regional factors such as health care infrastructure, availability of technology, and local legal statutes.17–19 Health reform and quality improvement efforts require a greater understanding of the relative contributions of individual physicians versus regional factors to the use – and overuse – of cancer care.
In addition to identifying the relative contribution of physicians and geographic regions to practice variation, it is important to identify health system factors that affect utilization among patients who are unlikely to benefit.20–22 Factors such as the availability of radiation specialists or facilities may correlate with the likelihood of receiving RT.23, 24 Regional rates of Medicare spending overall and at the end-of-life are markers for local intensity of care. Another factor that might affect use of expensive technologies, including RT, is Certificate of Need (CON) legislation, wherein states require a CON application prior to establishing or expanding a medical facility.25, 26
To address these knowledge gaps, we assessed the use of adjuvant RT among older women with early stage breast cancer across strata of LE. We used hierarchical generalized linear models to assess the impact of the treating physician and geographic region on cancer care across strata of LE. We then determined the impact of clinical and health system factors to variation in treatment among short LE and long LE patients separately.
METHODS
Study Design Overview
We examined the impact of clinical patient factors (age, comorbidities, hormone receptor status), non-clinical patient factors (sociodemographics, access to care), treatment factors (chemotherapy, time from diagnosis to surgery), health system factors (supply of physicians and hospital beds, resource utilization, quality of care), and regional factors (radiation oncologist density, state CON legislation for RT equipment) on the receipt of RT after BCS. We used Hospital Referral Regions (HRRs) as the regional units.27 The Yale Human Investigation Committee determined that this study did not constitute human subjects research.
Data Sources
We used the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER)-Medicare linked database for years 1998–2007. SEER collects information from tumor registries in 15 states and patient level data are available for sociodemographic and cancer characteristics including sex, age, race, and zip code, as well as tumor stage, grade, size, nodal status, and molecular markers. We used data from the Dartmouth Atlas Project for measures of physician density, percent of physicians in primary care specialties, hospital bed density, resource utilization and quality of care.28 State CON legislation was determined by the American Health Planning Association’s National Directory and the National Conference of State Legislatures.29, 30
Life Expectancy
We used data from individuals without cancer from the 5% random sample of Medicare beneficiaries residing in the SEER regions from 1998–2004 to create life tables according to sex, age and comorbidity. A modification of the Elixhauser method was used to assess for comorbid conditions significantly associated with mortality among cancer patients.31 We validated these life tables in a separate patient population consisting of 18,819 women with stage I or II breast cancer diagnosed during 1998–2004 who received BCS. We used the life tables to assign breast cancer patients to short, medium and long LE categories of <5, 5–10 and ≥10 years and then calculated the actual survival of this cohort. Only International Classification of Diseases, 9th revision (ICD-9) diagnosis codes appearing on an inpatient claim or at least two outpatient claims billed >30 days apart were counted. We validated this stratification schema in a sub-sample of women diagnosed prior to 2002 in order to ensure adequate follow-up, and found that patients predicted to live <5 years had a median survival of 4.73 years with 52.9% dying before 5 years; those predicted to live 5–10 years had a median survival of 9.55 years with 21.6% dying before 5 years; and for those predicted to live 10 years or more the median survival was >10 years with 8.4% dying before 5 years.
Inclusion Criteria
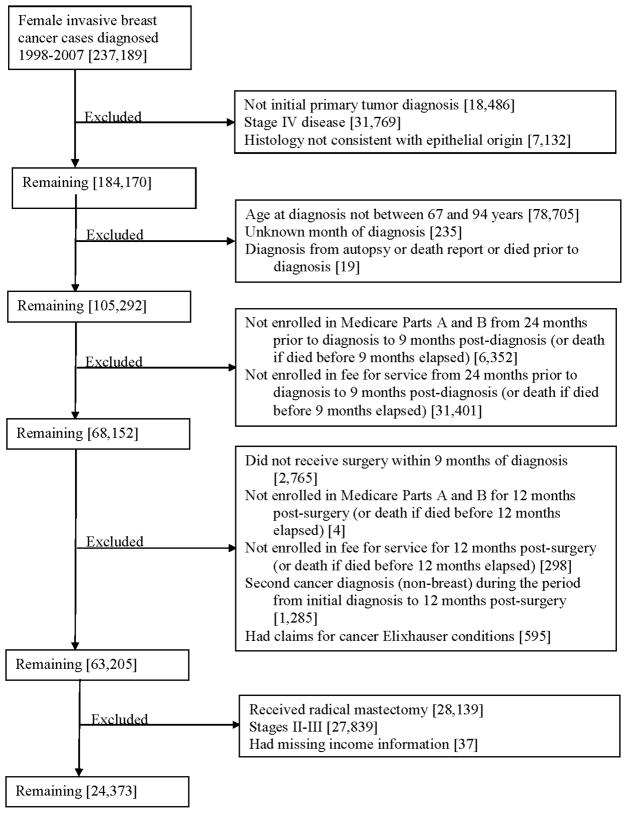
This study included women diagnosed with invasive, stage I breast cancer between 1998–2007, age 67–94 at time of cancer diagnosis, who received primary surgical resection with BCS. The “AJCC stage” variable was used to assign stage during 1998–2003, and the “derived AJCC stage” variable was used during 2004–2007.32 We excluded patients with missing stage, history of a prior or concurrent malignancy, those who did not have Medicare Parts A and B coverage or those enrolled in an HMO during the study period (defined as two years prior to diagnosis through one year after diagnosis), and those with missing income information.
Construction of Variables
Patients were assigned to the appropriate HRRs for all analyses according to their zip codes at the time of diagnosis, and the zip code allowed for estimation of median household income based on U.S. Census measures. Using Medicare claims data we determined whether patients had visited a primary care physician in the period of 24 to three months prior to the date of diagnosis, calculated the interval from the date of diagnosis to the date of BCS, and determined whether patients had received RT and/or chemotherapy. Adjuvant RT had to be initiated within nine months of the date of BCS to be included. When patients were evaluated by multiple surgeons, the surgeon performing BCS was considered to be the primary surgeon.
Statistical Analysis
To determine whether regional variation exists in the use of adjuvant RT, we analyzed the percent of women in the total sample and in each LE group who received RT after BCS in each of the 164 HRRs in our sample. We assessed whether the degree of regional variability differed between the LE groups and then identified factors associated with the use of RT among these women. Because we anticipated that use of RT would be dependent on both the surgeon and the HRR, we used a three-level hierarchical logistic regression model with patients clustered by primary surgeon and HRR, with random effects at the surgeon and HRR levels. This model allowed us to estimate the variation within and between surgeons and HRRs, and to estimate the proportion of the variance in the outcome explained by each of these levels.33 Patients with missing surgeon or HRR information were excluded from these models. Treatment rates in each HRR and according to the primary surgeon were calculated and risk adjusted. Number of radiation oncologists per 100,000, metro nature of the HRR, and an indicator for CON regulations for RT equipment were incorporated into this multivariable model. In a secondary analysis we included only women with estrogen receptor-positive status. As a regional characteristic, we defined a metro HRR as one in which 100% of Medicare beneficiaries in that HRR reside in a metro county. Hierarchical models were estimated using the XTMELOGIT routine in Stata statistical software, with quadrature specified as the method of estimation.
To better interpret the variation across surgeons and HRRs, we calculated risk-standardized treatment rates (RSTRs) as the ratio of the predicted probability of RT use to the expected probability of RT use, multiplied by the overall treatment rate for the group.34 The expected probability is the predicted probability for an average facility and surgeon, under the assumption that the random effects are zero. Statistical analyses were performed using SAS version 9.2 and Stata version 12.1.
RESULTS
The study sample consisted of 24,373 women living in 164 HRRs. The short LE patient group had 2,253 women while the medium and long LE groups had 11,093 and 11,027 women, respectively (Table 1). The racial distribution and median household income were similar between groups. The regional characteristics, including measures of physician and hospital density, reimbursement, utilization and quality, were similar in each patient group (Table 1).
Table 1.
Selected sociodemographic, clinical and regional characteristics of patients by life expectancy (LE).
| Total Population | Short LE* | Medium LE* | Long LE* | |
|---|---|---|---|---|
| Patients | 24,373 | 2,253 | 11,093 | 11,027 |
| INDIVIDUAL CHARACTERISTICS, No. (%) | ||||
| Age at diagnosis | ||||
| 67–69 | 4,119 (16.9) | — | 295 (2.7) | 3,824 (34.7) |
| 70–74 | 6,938 (28.5) | — | 1,740 (15.7) | 5,198 (47.1) |
| 75–79 | 6,607 (27.1) | 158 (7.0) | 4,444 (40.1) | 2,005 (18.2) |
| 80–84 | 4,307 (17.7) | 728 (32.3) | 3,579 (32.3) | — |
| 85–94 | 2,402 (9.9) | 1,367 (60.7) | 1,035 (9.3) | — |
| Race | ||||
| White | 22,481 (92.2) | 2,101 (93.3) | 10,187 (91.8) | 10,193 (92.4) |
| Black | 996 (4.1) | 96 (4.3) | 501 (4.5) | 399 (3.6) |
| Other | 896 (3.7) | 56 (2.5) | 405 (3.7) | 435 (3.9) |
| Median household income | ||||
| < $33,000 | 3,678 (15.1) | 384 (17.0) | 1,761 (15.9) | 1,533 (13.9) |
| $33–40,000 | 3,189 (13.1) | 281 (12.5) | 1,479 (13.3) | 1,429 (13.0) |
| $40–50,000 | 5,101 (20.9) | 530 (23.5) | 2,331 (21.0) | 2,240 (20.3) |
| $50–63,000 | 5,250 (21.5) | 486 (21.6) | 2,458 (22.2) | 2,306 (20.9) |
| > $63,000 | 7,155 (29.4) | 572 (25.4) | 3,064 (27.6) | 3,519 (31.9) |
| Patient residence | ||||
| Metro area | 21,358 (87.6) | 2,006 (89.0) | 9,798 (88.3) | 9,544 (86.6) |
| Non-metro area | 3,014 (12.4) | 247 (11.0) | 1,295 (11.7) | 1,473 (13.4) |
| Comorbidity index (Elixhauser) | ||||
| 0 | 11,769 (48.3) | 30 (1.3) | 3,696 (33.3) | 8,043 (72.9) |
| 1–2 | 9,493 (38.9) | 797 (35.4) | 5,712 (51.5) | 2,984 (27.1) |
| 3 or more | 3,111 (12.8) | 1,426 (63.3) | 1,685 (15.2) | — |
| Mean LE ± SD | 9.0 (3.5) | 3.7 (0.4) | 6.9 (1.2) | 12.3 (1.9) |
| Received chemotherapy | ||||
| Yes | 1,817 (7.5) | 67 (3.0) | 593 (5.3) | 1,157 (10.5) |
| No | 22,556 (92.5) | 2,186 (97.0) | 10,500 (94.7) | 9,870 (89.5) |
| Time to BCS | ||||
| ≤ 45 days | 18,444 (75.7) | 1,671 (74.1) | 8,323 (75.0) | 8,450 (76.6) |
| > 45 days | 5,929 (24.3) | 582 (25.9) | 2,770 (25.0) | 2,577 (23.4) |
| Hormone Receptor status | ||||
| Positive | 19,685 (80.8) | 1,770 (78.6) | 8,975 (80.9) | 8,940 (81.1) |
| Negative | 2,110 (8.7) | 186 (8.2) | 909 (8.2) | 1,015 (9.2) |
| Unknown | 2,578 (10.6) | 297 (13.2) | 1,209 (10.9) | 1,072 (9.7) |
| Recent visit to a PCP** | ||||
| Yes | 23,637 (97.0) | 2,212 (98.2) | 10,891 (98.2) | 10,534 (95.5) |
| No | 736 (3.0) | 41 (1.8) | 202 (1.8) | 493 (4.5) |
| REGIONAL CHARACTERISTICS, Mean (SD)*** | ||||
| MDs per 100,000 people | 209 (29.8) | 210 (29.7) | 210 (29.9) | 208 (29.8) |
| Radiation Oncologists per 100,000 people | 1 (0.3) | 1 (0.3) | 1 (0.3) | 1 (0.3) |
| Percent of MDs in primary care | 36 (2.9) | 35 (2.9) | 36 (2.9) | 36 (2.8) |
| Hospital beds per 1,000 people | 2 (0.5) | 2 (0.5) | 2 (0.5) | 2 (0.5) |
| Total Medicare reimbursements per enrollee (Part A and B) | 8,993 (1,484) | 9,181 (1,454) | 9,052 (1,465) | 8,895 (1,503) |
| Surgical discharges per 1,000 Medicare enrollees | 97 (13.5) | 98 (12.8) | 97 (13.4) | 97 (13.7) |
| Non-HMO Mortality: ASR-adjusted % of deaths among Medicare enrollees without HMO coverage | 5 (0.4) | 5 (0.4) | 5 (0.4) | 5 (0.5) |
| CMS Hospital Compare technical process quality measures composite quality score (AMI, CHF, Pneumonia)**** | 94 (1.8) | 94 (1.9) | 94 (1.8) | 94 (1.8) |
| State CON status, No. (%) | ||||
| Yes | 8,778 (36.0) | 793 (35.2) | 4,000 (36.1) | 3,985 (36.1) |
| No | 15,595 (64.0) | 1,460 (64.8) | 7,093 (63.9) | 7,042 (63.9) |
AMI indicates Acute Myocardial Infarction; ASR indicates Age, Sex, and Race; BCS indicates breast-conserving surgery; CHF indicates Congestive Heart Failure; CMS indicates Centers for Medicare & Medicaid Services; CON indicates Certificate of Need; HMO indicates Health Maintenance Organization.
Short LE median survival = 4.73 years, 52.9% dying before 5 years; medium LE median survival = 9.55 years, 21.6% dying before 5 years; long LE median survival >10 years, 8.4% dying before 5 years.
Indicates an office visit with a primary care physician in the 24 through 3 months prior to diagnosis.
Data from 2006.
Data from 2007.
In the short LE group (greater than 50% 5-year mortality in the validation sample), 43.6% of women received RT. More women in the medium LE group received RT, 76.4% of the 11,093 patients. The use of RT among women in the long LE group (<10% 5-year mortality) was higher, with 90.8% receiving RT overall (Table 2). Chemotherapy use was more common in the long LE group where 10.5% received chemotherapy compared to 5.3% in the medium LE group and 3.0% in the short LE group, suggesting more aggressive care. The time from diagnosis to BCS was similar in all groups with 25.9% of patients in the short LE group waiting over 45 days for surgery compared to 23.4% in the long LE group.
Table 2.
Receipt of radiation therapy (RT) according to patient, physician and health system factors among the total population and among patients with differing life expectancy (LE).
| Percentage of Women Receiving RT | |||||
|---|---|---|---|---|---|
| Total Population | Short LE* | Medium LE* | Long LE* | P value for difference in RT use (Long vs. Short LE) | |
| Received RT | |||||
| Yes | 79.9 | 43.6 | 76.4 | 90.8 | |
| INDIVIDUAL CHARACTERISTICS | |||||
| Race | .02 | ||||
| White | 80.0 | 43.4 | 76.5 | 91.0 | |
| Black | 75.6 | 44.8 | 72.5 | 87.0 | |
| Other | 83.3 | 50.0 | 79.3 | 91.3 | |
| Median household income | < .001 | ||||
| < $33,000 | 76.2 | 37.0 | 74.2 | 88.3 | |
| $33–40,000 | 80.3 | 39.9 | 76.5 | 92.2 | |
| $40–50,000 | 78.7 | 43.8 | 74.0 | 91.8 | |
| $50–63,000 | 81.3 | 49.6 | 78.9 | 90.5 | |
| > $63,000 | 81.6 | 44.6 | 77.6 | 91.0 | |
| Recent visit to a PCP** | < .001 | ||||
| Yes | 80.4 | >43.9 | <76.9 | 91.6 | |
| No | 65.8 | <27.0 | >51.0 | 75.0 | |
| Time to BCS | .01 | ||||
| ≤ 45 days | 80.6 | 43.8 | 76.9 | 91.6 | |
| > 45 days | 77.6 | 43.0 | 74.9 | 88.4 | |
| Received chemotherapy | < .001 | ||||
| Yes | 82.2 | 56.7 | 84.7 | 91.8 | |
| No | 79.2 | 43.2 | 76.0 | 90.7 | |
| REGIONAL CHARACTERISTICS | |||||
| State CON status | .07 | ||||
| Yes | 79.8 | 41.4 | 75.9 | 91.4 | |
| No | 80.0 | 44.8 | 76.7 | 90.5 | |
| Patient residence | .002 | ||||
| Metro area | 80.1 | 45.0 | 76.8 | 90.9 | |
| Non-metro area | 78.3 | 32.4 | 73.5 | 90.2 | |
BCS indicates breast-conserving surgery; CON indicates Certificate of Need.
Short LE median survival = 4.73 years, 52.9% dying before 5 years; medium LE median survival = 9.55 years, 21.6% dying before 5 years; long LE median survival >10 years, 8.4% dying before 5 years.
Indicates an office visit with a primary care physician in the 24 through 3 months prior to diagnosis; the frequency in the short LE group was changed to a range due to SEER-Medicare requirements in order to ensure that no cell sizes <10 can be observed or derived from the data
There was a statistically significant effect of race on the receipt of RT in bivariate analysis, but this was not observed in the adjusted model (Table 3). There was no consistent trend according to median household income. Women receiving chemotherapy in the short LE group were nearly twice as likely to receive RT after BCS as those not receiving chemo, with an odds ratio of 1.91 (P = .04; 95% CI, 1.04–3.54). Although comorbidity burden was not associated with RT in the short LE group, patients with 1-2 comorbidities in the long LE group were less likely than those without comorbidities to undergo treatment (OR 0.81, P = .01; 95% CI, 0.68–0.96). Also in the long LE group, women who had BCS more than 45 days after diagnosis were less likely to receive adjuvant RT (OR 0.73, P < .001; 95% CI, 0.62–0.86). Patients who had visited a primary care physician in the 24 through 3 months prior to diagnosis in both the short, medium and long LE groups were more likely to receive RT than those without recent primary care contact. There was no significant effect based on residence in a state with or without CON legislation (Table 4).
Table 3.
Results of hierarchical logistic regression models for receipt of radiation therapy (RT) according to demographic, clinical and health system characteristics for patients with differing life expectancy (LE).
| Short LE* | Medium LE* | Long LE* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | Wald P | OR (95% CI) | P | Wald P | OR (95% CI) | P | Wald P | |
| Intercept | 0.25 [0.52,1.21] | .09 | 7.34 [4.09,13.19] | < .001 | 3.40 [2.27,5.10] | < .001 | |||
|
| |||||||||
|
INDIVIDUAL
CHARACTERISTICS
| |||||||||
| Age at diagnosis | < .001 | < .001 | < .001 | ||||||
| 67–69 | omitted | ref | ref | ||||||
| 70–74 | omitted | 0.94 [0.63,1.41] | 0.78 | 0.88 [0.75,1.04] | .13 | ||||
| 75–79 | ref | 0.53 [0.35,0.78] | 0.001 | 0.62 [0.50,0.76] | < .001 | ||||
| 80–84 | 0.64 [0.42,0.97] | .04 | 0.23 [0.15,0.34] | < .001 | omitted | ||||
| 85–94 | 0.17 [0.11,0.26] | < .001 | 0.06 [0.04,0.10] | < .001 | omitted | ||||
|
| |||||||||
| Race | .26 | .07 | .41 | ||||||
| White | ref | ref | ref | ||||||
| Black | 1.27 [0.75,2.16] | .38 | 0.74 [0.57,0.96] | 0.02 | 0.89 [0.62,1.27] | .52 | |||
| Other | 1.60 [0.84,3.03] | .15 | 0.97 [0.71,1.32] | 0.84 | 1.27 [0.84,1.91] | .25 | |||
|
| |||||||||
| Median household income | .03 | 0.01 | .002 | ||||||
| < $33,000 | ref | ref | Ref | ||||||
| $33–40,000 | 1.09 [0.75,1.60] | .64 | 1.17 [0.96,1.42] | 0.13 | 1.62 [1.23,2.13] | .001 | |||
| $40–50,000 | 1.28 [0.92,1.78] | .14 | 0.98 [0.82,1.17] | 0.82 | 1.54 [1.21,1.97] | .001 | |||
| $50–63,000 | 1.71 [1.22,2.39] | .002 | 1.28 [1.06,1.54] | 0.01 | 1.28 [1.00,1.63] | .046 | |||
| >$63,000 | 1.29 [0.92,1.81] | .14 | 1.26 [1.05,1.52] | 0.01 | 1.42 [1.12,1.80] | .004 | |||
|
| |||||||||
| Hormone Receptor status | .002 | < .001 | < .001 | ||||||
| Negative | ref | ref | ref | ||||||
| Positive | 0.74 [0.52,1.08] | .12 | 0.63 [0.51,0.79] | < .001 | 1.12 [0.86,1.45] | .40 | |||
| Unknown | 0.47 [0.30,0.74] | .001 | 0.39 [0.30,0.51] | < .001 | 0.70 [0.51,0.96] | .03 | |||
|
| |||||||||
| Chemotherapy | |||||||||
| No | ref | ref | ref | ||||||
| Yes | 1.91 [1.04,3.54] | .04 | 1.31 [1.00,1.72] | 0.05 | 1.10 [0.86,1.42] | .45 | |||
|
| |||||||||
|
INDIVIDUAL
CHARACTERISTICS
| |||||||||
| Comorbidity index (Elixhauser) | .39 | < .001 | .01 | ||||||
| 0 | ref | ref | ref | ||||||
| 1–2 | 1.46 [0.58,3.66] | .42 | 0.88 [0.77,0.99] | 0.04 | 0.81 [0.68,0.96] | .01 | |||
| 3 or more | 1.24 [0.49,3.14] | .65 | omitted | omitted | |||||
|
| |||||||||
| Time to BCS | |||||||||
| ≤45 days | ref | ref | ref | ||||||
| >45 days | 0.85 [0.67,1.07] | .16 | 0.90 [0.79,1.01] | 0.08 | 0.73 [0.62,0.86] | < .001 | |||
|
| |||||||||
| Recent visit to a PCP** | |||||||||
| No | ref | ref | ref | ||||||
| Yes | 8.16 [2.58,25.82] | < .001 | 2.66 [1.86,3.79] | < .001 | 3.86 [2.98,5.00] | < .001 | |||
|
| |||||||||
|
REGIONAL
CHARACTERISTICS
| |||||||||
| Radiation Oncologists per 100,000 people | 1.59 [1.07,2.36] | .02 | 1.56 [1.20,2.01] | .001 | 1.32 [0.96,1.81] | .09 | |||
|
| |||||||||
| State CON status | 0.84 [0.65,1.09] | .20 | 0.91 [0.76,1.09] | .29 | 0.95 [0.76,1.18] | .62 | |||
|
| |||||||||
| Metro residence | 0.96 [0.74,1.25] | .77 | 0.88 [0.73,1.05] | .16 | 0.76 [0.61,0.95] | .02 | |||
|
| |||||||||
| N | 2166 | 10816 | 10775 | ||||||
| C-statistic | 0.826 | 0.846 | 0.839 | ||||||
|
| |||||||||
| Var | 95% CI | Var % | Var | 95% CI | Var % | Var | 95% CI | Var % | |
|
| |||||||||
| % | % | % | |||||||
| Explained variance† | 0.60 | 13.6 | 0.98 | 19.3 | 0.18 | 4.6 | |||
| Surgeon variance‡ | 0.52 | [0.31,0.74] | 11.8 | 0.80 | [0.71,0.89] | 15.7 | 0.54 | [0.43,0.65] | 13.3 |
| HRR variance†† | 0.01 | [0.00,0.05] | 0.23 | 0.03 | [0.01,0.05] | 0.5 | 0.04 | [0.01,0.06] | 0.9 |
| Residual variance‡‡ | 3.29 | 74.3 | 3.29 | 64.5 | 3.29 | 81.2 | |||
|
| |||||||||
| Total | 4.43 | 100.0 | 5.10 | 100.0 | 4.05 | 100.0 | |||
Note that odds ratios presented here are adjusted for all terms in the model. Two age category variables were omitted from each model because there were no patients in the respective categories. BCS indicates breast-conserving surgery; CON indicates Certificate of Need.
Short LE median survival = 4.73 years, 52.9% dying before 5 years; medium LE median survival = 9.55 years, 21.6% dying before 5 years; long LE median survival >10 years, 8.4% dying before 5 years.
Indicates an office visit with a primary care physician in the 24 through 3 months prior to diagnosis.
Variance in values predicted by the model.
Variance of the estimated random surgeon effects.
Variance of the estimated random HRR effects; negative estimated values truncated at zero.
Residual variance of the logit model, fixed at π2/3.
Table 4.
Current status of Certificate of Need (CON) legislation for radiation therapy (RT) among SEER regions. Bold indicates that state does have CON legislation, but it does not cover RT equipment.
| Yes CON for Radiation | No CON for Radiation |
|---|---|
|
| |
| Connecticut | San Francisco-Oakland, San Jose-Monterey, |
| Detroit (Michigan) | Los Angeles, Greater California |
| Atlanta, Greater Georgia, Rural Georgia | Louisiana |
| Hawaii | New Jersey |
| Iowa | New Mexico |
| Kentucky | Seattle-Puget Sound (Washington) |
| Utah | |
There was variation at the HRR level in the use of RT after BCS, but it did not differ with respect to LE. Among short LE patients the proportion of the variance in the model explained by HRR was 0.23%, although statistically this was not significantly different from zero, compared to 0.9% for the long LE patients (Table 3). Although the patient’s geographic region contributed to the eventual receipt of RT, we found that the patient’s primary surgeon had an impact on the model that was approximately 15 to 51 times greater than that of HRR in both LE groups. The surgeon accounted for 11.8% of variance in the short LE patients and 13.3% in the long LE patients. Among medium LE patients, the proportion of variance explained by HRR was 0.5% and that by surgeons was 15.7%, indicating a 31x larger impact of the surgeons on the model.
The risk-standardized treatment rates (RSTRs) demonstrated much greater variability among the surgeons than among HRRs (Figure 1). Among women with a short LE, the RSTRs according to the primary surgeon ranged from 27.7% to 67.3% (mean 43.9% ± 7.8%, IQR 38.6–52.0%). There was less variability across geographic regions, with regional RSTRs ranging from 42.0–45.2% (mean 43.6% ± 0.4%, IQR 43.4–43.8%). Among women with a long LE, the variation according to the primary surgeon ranged from 76.3% to 96.5% (mean 90.3% ± 3.3%, IQR 89.7–92.2%), while RSTRs for the HRRs ranged from only 88.8% to 91.9% (mean 90.6% ± 0.5%, IQR 90.6–90.9%). Surgeons in this sample saw a median of 1 (IQR 0–3) patient in the short LE group, 6 (IQR 3–13) patients in the medium group, and 6 (IQR 3–13) patients in the long group.
Figure 1.
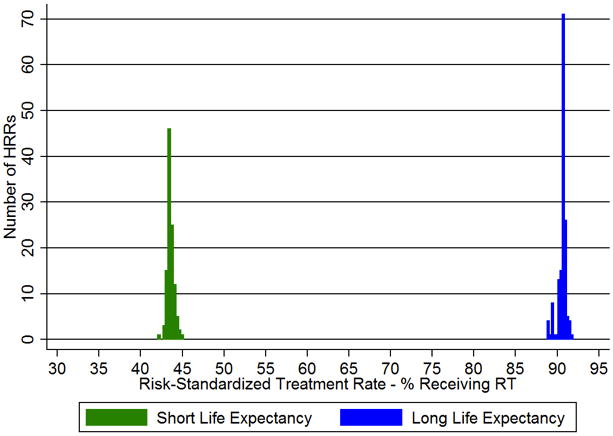
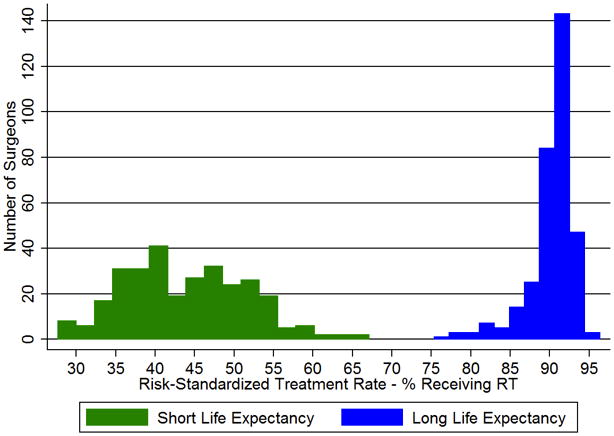
a–b. Risk-standardized treatment rates (RSTRs) for receipt of radiation therapy (RT) after breast-conserving surgery (BCS) according to (a) Hospital Referral Region (HRR) and (b) primary surgeon, limited to surgeons performing ≥ 10 BCS annually.
We found that short LE women were more likely to receive radiation when they lived in areas with a high density of radiation oncologists, with an odds ratio of 1.59 (P = .02; 95% CI, 1.07–2.36). The density of radiation oncologists was significantly related to receipt of RT in short LE, but not long LE women. Other physician measures, including number of physicians per capita and percentage of physicians in primary care, were not significantly associated with receipt of RT among either LE group.
In the sensitivity analysis in which we restricted the sample to estrogen receptor-positive patients, there were no substantive changes, so we do not report the results.
DISCUSSION
We observed significant variation across primary surgeons in adjuvant RT use for women with stage I breast cancer who had a short LE, a group predicted to derive little benefit from such treatment. In contrast, we found substantially less variation in RT use at the regional level, among both short and long LE groups. In fact, the surgeon exerted a stronger influence on receipt of RT after BCS than measures of regional health care infrastructure, practice patterns, resource utilization, and local legal statutes. These results suggest that efforts to understand regional variation in cancer care should incorporate physician-level as well as regional-level variation into hierarchical models, as much of the “action” regarding variation may actually be occurring at the physician level. However, not all relevant factors could be assessed in this study; other factors may indeed have greater influence than either regions or individuals. Prior studies have demonstrated that surgeons play an important role in surgical decision-making; our results suggest that the influence of surgeons on patterns of care extends beyond the initial surgical treatment.35 Efforts to better align use of RT with expected benefits and risks should incorporate surgeons, as they play a major role in patterns of care.
Although the use of RT did not vary across HRRs as much as it did across surgeons, we did observe some variation. This further emphasizes the impact that geographic variation has on the intensity and cost of care.36 Others have described variation related to clinical factors and geographic region, but did not evaluate the impact of individual physicians.37 Our results suggest that further assessment of individual physicians is warranted when considering variation across regions. In addition, while our analysis was structured around surgeons, it is possible that these individuals represent the decisions of local networks of care, carrying out the decisions of groups such as hospital tumor boards, referring physicians, or professional networks. One regional characteristic, the number of radiation oncologists per 100,000 people in each HRR, was associated with receipt of RT use in short LE women but did not significantly affect RT use in long LE women. Among short LE women, this association between radiation oncologist density and receipt of RT is a small effect given the high baseline prevalence of RT use, while in long LE women the lack of an association indicates the broad agreement on RT’s clinical utility among patients expected to live many years. Furthermore, there was a strong association between recent visit to a PCP and receipt of RT, which may reflect individuals’ access to care or potentially unmeasured attitudes toward medical treatment. The adjusted analysis also demonstrated that those in the lowest income group were less likely to receive therapy.
Our categorization of patients into LE groups does not indicate that patients in the short LE group derive no benefit from therapy; indeed, some patients categorized in the short LE group may eventually benefit from therapy. However, LE exerts a strong influence on the expected benefit from RT. For instance, the number needed to treat among women aged 70–79 without comorbidities to prevent one locoregional recurrence or mastectomy has been estimated to be 21–22 patients, while for women 80 or older with moderate to severe comorbidity the number needed to treat was between 61–125 patients.38 An ideal system would allow physicians to better present the risks and benefits to patients alongside the cost to help individuals make choices that align with their preferences.
We noted that within LE groups, rates of RT use were similar across race and socioeconomic status. This finding was similar to a recent survey of patients with nonmetastatic breast cancer between the ages of 20–79.9 However, others have demonstrated important racial disparities regarding breast cancer diagnosis, treatment and mortality.39, 40 Radiation use may be an exception to the more frequently observed sources of disparity, though our small sample of non-white individuals precludes definitive conclusions.
The strengths of this study include rigorous determination of RT utilization, use of a validated measure of LE, and a large sample size. There are also several limitations: although SEER-Medicare provides extensive information on the primary tumor, treatment and survival outcomes, some patient characteristics were extrapolated from U.S. Census data rather than individually recorded. LE prediction is not perfect; some women in the short LE group may live more than 5 years, and some in the long LE group may live less than 10 years, but it is a useful framework for risk stratification. We have used validated measures of resource use and quality from the Dartmouth Atlas, but not all relevant measures could be analyzed. In particular, no single agency is charged with tracking RT equipment, and numbers of devices per HRR were not assessed here. Additionally, we focused our analysis on the Medicare fee-for-service program; the impact of local differences in the under-65 population on the care of Medicare patients was not examined, and local rates of uninsurance and underinsurance were not included.41–43
Our study demonstrates that the effect of the patient’s primary surgeon was between 15 to 51 times greater than measures of local health care infrastructure on the use of RT after BCS. We show that the number of women receiving RT is far higher than expected, as there has been clear Level I evidence against the use of RT in low-risk older women for many years. Future studies will need to identify the key drivers, such as patient preference, defensive medicine and fee-for-service incentives, behind the ongoing delivery of RT in this population. Reducing the use of RT among women who are unlikely to benefit may offer substantial gains in the diminished burden of morbidity and lower costs. Additionally, recognition of the key role of surgeons in the decision to utilize RT provides a potential leverage point, and future research should explore strategies to better integrate them into shared decision-making. Incorporating age and comorbidity burden, the main determinants of LE, into decisions regarding the course of treatment is critical, and may serve to better align patterns of care with patient preferences and the likelihood of clinical benefit.
Appendix 1.
Sample selection figure.
Acknowledgments
Funding: This work was supported by the National Cancer Institute at the National Institutes of Health (5R01CA149045); and a grant from the Doris Duke Charitable Foundation to Yale University to fund Clinical Research Fellow Aaron Feinstein.
References
- 1.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. New Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 2.Abe O, Abe R, Enomoto K, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 3.Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. New Engl J Med. 2004;351:971–977. doi: 10.1056/NEJMoa040587. [DOI] [PubMed] [Google Scholar]
- 4.Schonberg MA, Marcantonio ER, Ngo L, et al. Does life expectancy affect treatment of women aged 80 and older with early stage breast cancers? J Ger Oncol. 2012;3:8–16. doi: 10.1016/j.jgo.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White J, Joiner MC. Toxicity from radiation in breast cancer. Cancer Treat Res. 2006;128:65–109. doi: 10.1007/0-387-25354-8_5. [DOI] [PubMed] [Google Scholar]
- 6.Smith IE, Ross GM. Breast radiotherapy after lumpectomy - No longer always necessary. New Engl J Med. 2004;351:1021–1023. doi: 10.1056/NEJMe048173. [DOI] [PubMed] [Google Scholar]
- 7.Buchholz TA. Radiation therapy for early-stage breast cancer after breast-conserving surgery. New Engl J Med. 2009;360:63–70. doi: 10.1056/NEJMct0803525. [DOI] [PubMed] [Google Scholar]
- 8.Carlson RW, Allred DC, Anderson BO, et al. Invasive breast cancer: Clinical practice guidelines in oncology. J Nat Compr Cancer Netw. 2011;9:136–222. doi: 10.6004/jnccn.2011.0016. [DOI] [PubMed] [Google Scholar]
- 9.Jagsi R, Abrahamse P, Morrow M, et al. Patterns and correlates of adjuvant radiotherapy receipt after lumpectomy and after mastectomy for breast cancer. J Clin Oncol. 2010;28:2396–2403. doi: 10.1200/JCO.2009.26.8433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hollingsworth JM, Zhang Y, Krein SL, et al. Understanding the variation in treatment intensity among patients with early stage bladder cancer. Cancer. 2010;116:3587–3594. doi: 10.1002/cncr.25221. [DOI] [PubMed] [Google Scholar]
- 11.Lu-Yao GL, Albertsen PC, Moore DF, et al. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA. 2008;300:173–181. doi: 10.1001/jama.300.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper GS, Koroukian SM. Geographic variation among medicare beneficiaries in the use of colorectal carcinoma screening procedures. Am J Gastroenterol. 2004;99:1544–1550. doi: 10.1111/j.1572-0241.2004.30902.x. [DOI] [PubMed] [Google Scholar]
- 13.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28:1117–1123. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sirovich B, Gallagher PM, Wennberg DE, et al. Discretionary decision making by primary care physicians and the cost of U.S. health care. Health Aff. 2008;27:813–823. doi: 10.1377/hlthaff.27.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grytten J, Sørensen R. Practice variation and physician-specific effects. J Health Econ. 2003;22:403–418. doi: 10.1016/S0167-6296(02)00105-4. [DOI] [PubMed] [Google Scholar]
- 16.Epstein AJ, Nicholson S. The formation and evolution of physician treatment styles: An application to cesarean sections. J Health Econ. 2009;28:1126–1140. doi: 10.1016/j.jhealeco.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Davis P, Gribben B, Lay-Yee R, et al. How much variation in clinical activity is there between general practitioners? A multi-level analysis of decision-making in primary care. J Health Serv Res Policy. 2002;7:202–208. doi: 10.1258/135581902320432723. [DOI] [PubMed] [Google Scholar]
- 18.Hayward RA, Manning WG, Jr, McMahon LF, Jr, et al. Do attending or resident physician practice styles account for variations in hospital resource use? Med Care. 1994;32:788–794. doi: 10.1097/00005650-199408000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Krein SL, Hofer TP, Kerr EA, et al. Whom should we profile? Examining diabetes care practice variation among primary care providers, provider groups, and health care facilities. Health Serv Res. 2002;37:1159–1180. doi: 10.1111/1475-6773.01102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wennberg JE. Unwarranted variations in healthcare delivery: Implications for academic medical centres. BMJ. 2002;325:961–964. doi: 10.1136/bmj.325.7370.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aneja S, Smith BD, Gross CP, et al. A Geographic Analysis of the Radiation Oncology Workforce. Int J Radiat Oncol Biol Phys. 2011 doi: 10.1016/j.ijrobp.2011.01.070. article-in-press. [DOI] [PubMed] [Google Scholar]
- 22.Ballas LK, Elkin EB, Schrag D, et al. Radiation therapy facilities in the United States. Int J Radiat Oncol Biol Phys. 2006;66:1204–1211. doi: 10.1016/j.ijrobp.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 23.Fisher ES, Wennberg DE, Stukel TA, et al. The implications of regional variations in Medicare spending. Part 1: The content, quality, and accessibility of care. Ann Intern Med. 2003;138:273–287. doi: 10.7326/0003-4819-138-4-200302180-00006. [DOI] [PubMed] [Google Scholar]
- 24.Makarov DV, Loeb S, Landman AB, et al. Regional Variation in Total Cost per Radical Prostatectomy in the Healthcare Cost and Utilization Project Nationwide Inpatient Sample Database. J Urol. 2010;183:1504–1509. doi: 10.1016/j.juro.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 25.Ross JS, Ho V, Wang Y, et al. Certificate of need regulation and cardiac catheterization appropriateness after acute myocardial infarction. Circulation. 2007;115:1012–1019. doi: 10.1161/CIRCULATIONAHA.106.658377. [DOI] [PubMed] [Google Scholar]
- 26.Short MN, Aloia TA, Ho V. Certificate of need regulations and the availability and use of cancer resections. Ann Surg Oncol. 2008;15:1837–1845. doi: 10.1245/s10434-008-9914-1. [DOI] [PubMed] [Google Scholar]
- 27.Wennberg JE, Cooper MM. The Dartmouth Atlas of Health Care in the United States. 1996. [PubMed] [Google Scholar]
- 28.The Dartmouth Atlas of Health Care. [Accessed April 28, 2011]. Available at: http://www.dartmouthatlas.org/tools/downloads.aspx.
- 29.American Health Planning Association. National Directory * State Certificate of Need Programs * Health Planning Agencies. Falls Church, VA: American Health Planning Association; 1998–2007. [Google Scholar]
- 30.National Conference of State Legislatures. Certificate of Need: State Health Laws and Programs. [Accessed May 26, 2011]. Available at: http://www.ncsl.org/IssuesResearch/Health/CONCertificateofNeedStateLaws/tabid/14373/Default.aspx.
- 31.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. New York: Springer; 2002. [Google Scholar]
- 33.Snijders TAB, Bosker R. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. London: Sage; 1999. [Google Scholar]
- 34.Normand S-T, Glickman ME, Gatsonis CA. Statistical methods for profiling providers of medical care: Issues and applications. J Am Stat Assoc. 1997;92:803–814. [Google Scholar]
- 35.Katz SJ, Hawley ST, Abrahamse P, et al. Does it matter where you go for breast surgery?: Attending surgeon’s influence on variation in receipt of mastectomy for breast cancer. Med Care. 2010;48:892–899. doi: 10.1097/MLR.0b013e3181ef97df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keyhani S, Falk R, Bishop T, et al. The relationship between geographic variations and overuse of healthcare services: A systematic review. Med Care. 2012;50:257–261. doi: 10.1097/MLR.0b013e3182422b0f. [DOI] [PubMed] [Google Scholar]
- 37.Tuttle TM, Jarosek S, Habermann EB, et al. Omission of radiation therapy after breast-conserving surgery in the United States: A population-based analysis of clinicopathologic factors. Cancer. doi: 10.1002/cncr.26505. article-in-press. [DOI] [PubMed] [Google Scholar]
- 38.Smith BD, Gross CP, Smith GL, et al. Effectiveness of radiation therapy for older women with early breast cancer. J Natl Cancer Inst. 2006;98:681–690. doi: 10.1093/jnci/djj186. [DOI] [PubMed] [Google Scholar]
- 39.Levy DE, Byfield SD, Comstock CB, et al. Underutilization of BRCA1/2 testing to guide breast cancer treatment: Black and Hispanic women particularly at risk. Gen Med. 2011;13:349–355. doi: 10.1097/GIM.0b013e3182091ba4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med. 2003;163:49–56. doi: 10.1001/archinte.163.1.49. [DOI] [PubMed] [Google Scholar]
- 41.Ayanian JZ, Kohler BA, Abe T, et al. The relation between health insurance coverage and clinical outcomes among women with breast cancer. New Engl J Med. 1993;329:326–331. doi: 10.1056/NEJM199307293290507. [DOI] [PubMed] [Google Scholar]
- 42.Ayanian JZ, Weissman JS, Schneider EC, et al. Unmet health needs of uninsured adults in the United States. JAMA. 2000;284:2061–2069. doi: 10.1001/jama.284.16.2061. [DOI] [PubMed] [Google Scholar]
- 43.Institute of Medicine. Care Without Coverage: Too Little, Too Late. 2002. [Google Scholar]