Abstract
Background and Aims
Progression from steatosis to steatohepatitic lesions is hypothesized to require a second hit. These lesions have been associated with increased oxidative stress, often ascribed to high levels of leptin and other proinflammatory mediators. Here we have examined the role of leptin in inducing oxidative stress and Kupffer cell activation in CCl4-mediated steatohepatitic lesions of obese mice.
Methods
Male C57BL/6 mice fed with a high fat diet (60%kcal) at 16 weeks were administered CCl4 to induce steatohepatitic lesions. Approaches included use of immuno-spin trapping for measuring free radical stress, gene-deficient mice for leptin, p47 phox, iNOS and adoptive transfer of leptin primed macrophages in vivo.
Results
Diet-induced obese (DIO) mice, treated with CCl4 increased serum leptin levels. Oxidative stress was significantly elevated in DIO mice liver but not in OB/OB mice, or in DIO mice treated with leptin antibody. In OB/OB mice, leptin supplementation restored markers of free radical generation. Markers of free radical formation were significantly decreased by the peroxynitrite decomposition catalyst FeTPPS, the iNOS inhibitor 1400W, the NADPH oxidase inhibitor apocynin, or in iNOS or p47 phox-deficient mice. These results correlated with the decreased expression of TNF-alpha and MCP-1. Kupffer cell depletion eliminated oxidative stress and inflammation, whereas in macrophage-depleted mice, the adoptive transfer of leptin-primed macrophages significantly restored inflammation.
Conclusions
These results, for the first time, suggest that leptin action in macrophages of steatotic liver through induction of iNOS and NADPH oxidase caused peroxynitrite-mediated oxidative stress thus activating Kupffer cells.
Keywords: Adipocytokines, Kupffer cell, oxidative stress, tyrosine nitration, NADPH oxidase, OB/OB mice
Introduction
Leptin’s role as a proinflammatory adipocytokine has gained attention in nonalcoholic steatohepatitis. Circulating leptin levels are elevated in obesity and NASH. Leptin-induced cytokine release, especially of IL-1 and TNF-α, has been shown in microglia and monocytes [1, 2]. Leptin acts on Kupffer cells, the resident macrophages, by binding to its functional receptor in the liver and inducing the release of TNF-α, TGF-beta and IL-15. [3] [4] [5]
Despite wide-ranging reports of leptin’s role in inflammation and release of inflammatory mediators, its role in inducing oxidative stress in the liver remains unclear. There are reports regarding leptin-induced reactive oxygen species formation by different cell types including endothelial cells, cardiomyocytes and hepatic stellate cells [6] [7] [8]. These studies focused on reactive oxygen species formation but the mechanisms of free radical species generation and their link to exacerbated inflammation through Kupffer cell activation is not completely understood. Since leptin is known to induce both NADPH oxidase and iNOS, the resultant superoxide and nitric oxide can react at a diffusion-controlled rate to produce peroxynitrite, a strong physiological oxidant. Peroxynitrite can form several free radical species including •OH, •CO3 and •NO2 radicals, depending on the pathophysiological microenvironment [9] [10] [11] Based on the available studies on the role of leptin in oxidative stress induction and inflammation in steatohepatitis, we hypothesized that leptin-induced peroxynitrite and its ensuing free radical formation play a major role in early liver injury in obesity. Here we show that CCl4 administration in diet-induced obese mice increases circulating levels of leptin; we also demonstrate that heightened levels of leptin contribute significantly to the pathogenesis of the resultant liver damage by activating NADPH oxidase, inducing iNOS, and activating release of TNF-α and MCP-1 from Kupffer cells by peroxynitrite-dependent mechanisms. Furthermore, we prove that leptin exerts its free radical formation and proinflammatory effects mainly by acting on macrophages and Kupffer cells of obese mice.
Materials and Methods
Obese mice
Custom DIO adult male, pathogen-free, with a C57BL6/J background (Jackson Laboratories, Bar Harbor, Maine) were used as models of diet-induced obesity. The mice were fed with a high fat diet (60% kcal) from 6 weeks until 16 weeks. All experiments were conducted in the 16-week age group. Age-matched lean controls were fed with a diet having 10% kcal fat. The animals were housed one to a cage before any experimental use. Mice that contained the disrupted OB gene (leptin) (B6.V-Lep<ob>/J) (Jackson Laboratories), disrupted p47 phox (B6.129S2-Ncf1tm1shl N14) (Taconic, Cranbury, NJ) genes, or disrupted NOS2 (B6.129P2-Nos2tm1Lau/J, Jackson Laboratories; C57BL6 background) were fed with a high fat diet and treated identically to DIO obese mice. Mice had ad libitum access to food and water and were housed in a temperature-controlled room at 23–24 °C with a 12-hour light/dark cycle. All animals were treated in strict accordance with the NIH Guide for the Humane Care and Use of Laboratory Animals, and the experiments were approved by the institutional review board.
Induction of liver injury in obese mice
DIO mice or high fat-fed gene-specific knockout mice at 16 weeks were administered carbon tetrachloride (0.8 mmoles/kg, diluted in olive oil) through the intraperitoneal route. This model is a free radical-based mechanistic model for nonalcoholic steatohepatitis [12]
Administration of allopurinol, FeTPPS and 1400W
Allopurinol, a specific inhibitor of xanthine oxidase, was administered in a single bolus dose of 35 mg/kg through the i.p. route 30 minutes prior to carbon tetrachloride treatment [13]. In other studies, the iNOS inhibitor 1400W was administered through the intraperitoneal route at a dose of 10 mg/kg 1 h before carbon tetrachloride treatment using an intraperitoneal route[14]. FeTPPS was administered at 30 mg/kg to mice 1 h prior to CCl4 treatment [10, 15]
Administration of mouse recombinant leptin and leptin neutralization
OB/OB mice received recombinant leptin (100 μg/mice) twice daily for 5 days prior to CCl4 administration through the intraperitoneal route. Leptin antibody was used to neutralize the circulating leptin in DIO mice. DIO mice were treated for 2 days prior to CCl4 administration either with 100 μg of control mouse IgM or with mouse leptin Abs intraperitoneally in a total volume of 100 μl of PBS [16]
Isolation of Kupffer cells
Kupffer cells were isolated as per the protocol by Froh et al. [17]. Qualitative screening for Kupffer cells was carried out with immunoreactivity against a CD68 antibody. Cultures with >80% CD68-positive cells were used for experiments.
Enzyme-linked immunosorbent assay
Immuno-spin trapping, a method for detection of free radical formation was used and immunoreactivity for DMPO nitrone adducts and nitrotyrosine was detected in liver homogenates and Kupffer cell lysates using standard ELISA [10].
Western Blot analysis
Liver homogenates were resolved in 4–10% Bis-Tris gels using SDS-PAGE and subjected to western blot analysis.
Histopathology
For each animal, sections of liver were collected and fixed in 10% neutral buffered formalin. For histological examinations, formalin-fixed liver sections were stained with hematoxylin/eosin and observed under a light microscope.
Real-Time Reverse Transcription–Polymerase Chain Reaction Analysis
Gene expression levels in tissue samples were measured by real-time reverse transcription–polymerase chain reaction analysis as described in supplementary material.
Confocal laser scanning microscopy (Zeiss LSM 510 UV Meta)
Frozen tissue sections after formalin fixation were analyzed by confocal microscopy a Zeiss LSM710-UV meta (Carl Zeiss, Inc., Oberkochen, Germany) using a Plan-NeoFluor 40X/1.3/40XxOil DIC objective with different zoom levels.
Macrophage depletion by GdCl3 and liposomal clodronate
Mice were injected with gadolinium chloride (10mg/kg) through the i.v. route 24 h prior to CCl4 treatment as described by Rai. et al [18]. Liposomal clodronate was injected through intravenous injections at a dose of 4 μl/g of mice (Clophosome™; Formumax, Pao Alto, CA) 24 h prior to CCl4 treatment.
Adoptive transfer of leptin primed cells
Mouse non-parenchymal cells (mostly Kupffer cells) were isolated as per Froh et al [17]. Cells were washed and plated in 35mm2 dishes using 10% FBS containing DMEM with mouse recombinant leptin (500ng/ml). The dose was selected on the basis of the concentration used by Wang et al. (10–100nmoles/L) [4]. The cells were harvested at 18 hours and 1×106 cells/mice were injected through the tail vein into macrophage depleted mice. The recipient mice were macrophage-depleted by the administration of the macrophage toxin gadolinium chloride.
Statistical analyses
All in vivo experiments were repeated three times with 3 mice per group (N=3; data from each group of three mice was pooled). The statistical analysis was carried out by analysis of variance (ANOVA) followed by a post-hoc test. Quantitative data from Western blots as depicted by the relative intensity of the bands were analyzed by performing a Student’s t test. P<0.05 was considered statistically significant.
Results
Increased leptin levels cause oxidative and nitrosative stress in DIO-steatohepatitic mice
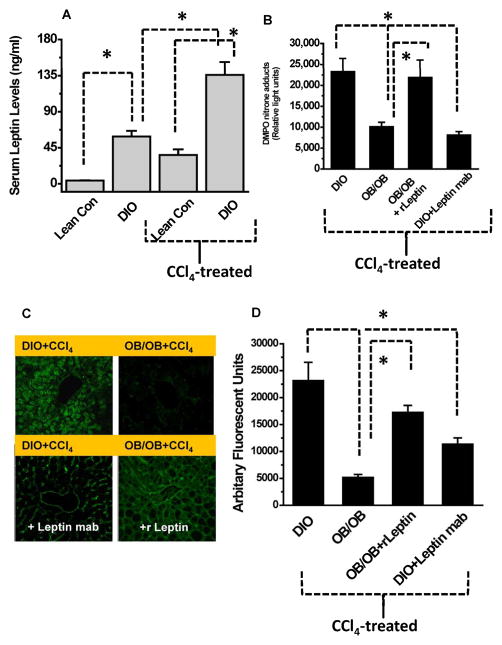
Our results indicated that diet-induced obese mice had significantly higher leptin levels as compared to lean control mice (Fig. 1A), which is in line with human studies [19]. Our previous study established a free radical-based mechanistic model of nonalcoholic steatohepatitis where low dose CCl4 administration induced non alcoholic steatoheaptitis in obese mice [12]. Furthermore, DIO mice treated with CCl4 had significantly higher serum leptin levels when compared to both untreated DIO and CCl4-treated lean control mice (Fig. 1A). The study showed that leptin deficiency significantly decreased protein radical formation. We found that when CCl4-treated leptin-deficient mice and CCl4-treated DIO mice were administered a neutralizing antibody against leptin, they had significantly decreased protein DMPO nitrone adduct formation, a measurement of free radical formation on proteins (Fig. 1B) compared to DIO mice treated with CCl4 only [20]. Treating leptin-deficient mice with a seven-day course of recombinant leptin increased their protein radical formation in response to CCl4 administration to levels that were comparable to those in wild type DIO mice (Fig. 1B).
Fig. 1.
Increased leptin levels cause free radical and nitrosative stress in DIO-steatohepatitic mice. Lean control or high fat fed diet induced obese (DIO) mice were treated with either olive oil (vehicle) or CCl4. A. Serum levels at 24 h post CCl4 B. Liver homogenates from DIO, OB/OB, leptin supplemented and DIO mice injected with leptin antibody were subjected to immuno-spin trapping and anti-DMPO immunoreactivity was measured using ELISA. C. Frozen liver slices were analyzed for 3-nitrotyrosine immunoreactivity. C. Relative fluorescence intensities of 3-nitrotyrosine immunoreactivity from mice livers (n=4). *P<0.05
Since protein 3-nitrotyrosine formation originates from tyrosyl radicals reacting with reactive nitrogen species [21], we probed nitrotyrosine formation as a stable post-translational oxidative modification. Results from laser scanning confocal microscopy showed both sinusoidal and centrolobular 3-nitrotyrosine immunoreactivity in DIO mouse liver treated with CCl4 and in leptin-deficient mice treated with CCl4 and recombinant leptin (Fig. 1C). However, leptin-deficient mice and DIO mice administered leptin monoclonal antibody similarly treated with CCl4 had 3-nitrotyrosine immunoreactivity primarily in the sinusoids.
Finally, quantification of fluorescent intensities of 3-nitrotyrosine immunoreactivity from these groups showed that when CCl4-treated leptin deficient mice and CCl4-treated DIO mice were administered a neutralizing antibody against leptin, they had significantly decreased 3-nitrotyrosine formation (Fig. 1D) compared to DIO mice treated with CCl4. However, nitrotyrosine reactivity increased significantly following a seven-day course of recombinant leptin treatment.
Serum levels of leptin might have originated from both the adipose tissue and liver since western blot analysis of leptin showed significant increases in both liver and adipose tissue following CCl4 - treatment (Suppl Fig. 2A).
Leptin augments proinflammatory cytokines that are markers of Kupffer cell activation
Oxidative stress can play a significant role in Kupffer cell activation [12, 22,23]. Kupffer cell activation can be marked by increased release of TNF-α and monocyte chemoattractant protein-1 (MCP-1). Thus we analyzed leptin-induced effects on TNF-α and MCP-1 in DIO mice treated with CCl4. We found that CCl4-treated leptin-deficient mice and CCl4-treated DIO mice administered neutralizing antibody against leptin had significantly decreased TNF-α and MCP-1 levels (Suppl. Fig. 1A) compared to DIO mice treated with CCl4 alone. However, pre-treating leptin deficient mice with a seven-day course of recombinant leptin restored their post-CCl4 TNF-α and MCP-1 levels to those of wild type DIO mice that were treated with CCl4 (Suppl. Fig. 1B). Liver activation of NFKappaB was also increased in DIO mice treated with CCl4 as seen from increased translocation of p65 unit to the nucleus (Suppl Fig. 2C).
Peroxynitrite from NADPH oxidase and iNOS activity is a key regulator in Kupffer cell activation in steatohepatitic injury
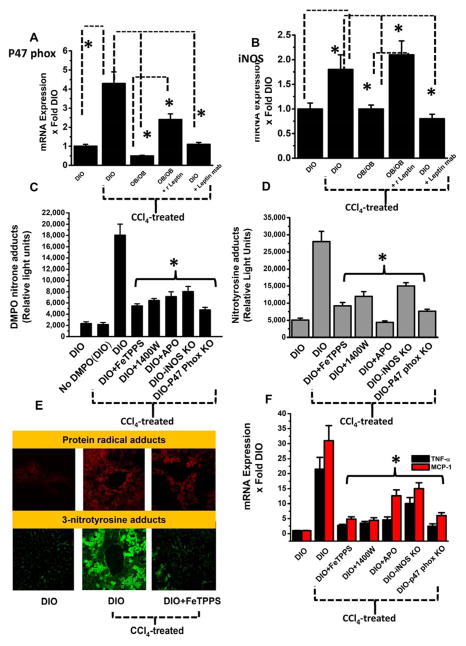
When leptin-deficient mice and DIO mice, administered a monoclonal antibody against leptin were treated with CCl4, their levels of p47 phox and iNOS mRNA expression was significantly less (Fig. 2A and 2B) compared to DIO mice treated with CCl4. When leptin-deficient mice were pretreated with recombinant leptin before CCl4 exposure, expression of p47 phox mRNA increased from the leptin-deficient level significantly, to a point near, but less than of the (hyperleptinemic) DIO mice treated with CCl4 (Fig. 2A), while iNOS mRNA expression also increased significantly when compared to leptin-deficient mice treated identically, and was comparable to that in the DIO mice treated with CCl4 (Fig. 2B). The significant increase in mRNA expression of both p47 phox and iNOS in DIO mice observed 24 hours following treatment with CCl4 thus coincided with the increase in protein radical formation and nitrotyrosine formation. Since we observed a significant leptin-dependent increase in both p47 phox and iNOS mRNA expression, we explored the role of peroxynitrite in formation of protein radicals and tyrosine nitration. Peroxynitrite is a key oxidant species and nitric oxide formed from iNOS reacts in a diffusion controlled rate with superoxide to form peroxynitrite. We used the peroxynitrite decomposition catalyst FeTPPS administered in vivo to assess the role of peroxynitrite as reported by Chatterjee et al. and others [15] [24,25]. In DIO mice treated with CCl4 and administered FeTPPS, protein radical formation and tyrosine nitration were significantly decreased (Fig. 2C and 2D). They also decreased significantly when mice were administered the NADPH oxidase inhibitor apocynin or the iNOS inhibitor 1400 W. When fed with a high fat diet and treated with CCl4, mice that lacked the NADPH oxidase subunit p47 phox or iNOS had significantly decreased protein radical formation and tyrosine nitration compared to DIO mice treated with CCl4 (Fig. 2C and 2D).
Fig. 2.
Peroxynitrite from NADPH oxidase and iNOS activity is a key regulator in Kupffer cell activation in steatohepatitic injury. A. Quantitative real time PCR analysis of p47 phox and (B) iNOS mRNA expression in DIO, DIO+CCl4, OB/OB mice, with or without leptin supplementation and DIO+CCl4 mice treated with leptin monoclonal antibody. C. Liver homogenates from CCl4-treated DIO mice either challenged with peroxynitrite decomposition catalyst FeTPPS or iNOS inhibitor 1400w or NADPH oxidase inhibitor apocynin; or using iNOS and p47 phox knockout mice, were assayed for anti-DMPO immunoreactivity using ELISA, (D) for 3-nitrotyrosine immunoreactivity using ELISA. E. Localization of DMPO nitrone adducts (red, upper panel) or nitrotyrosine adducts (green, lower panel) were analyzed by confocal laser scanning microscopy of liver slices from CCl4-treated mice with or without peroxynitrite decomposition catalyst FeTPPS and is a representative of liver slices collected from 4 mice (n=4). F. Peroxynitrite formation is correlated with increased liver resident macrophage activation. F. Liver homogenates were assayed for TNF-α and MCP-1 mRNA expression. The figure represents data from 3 mice/group and *P<0.05
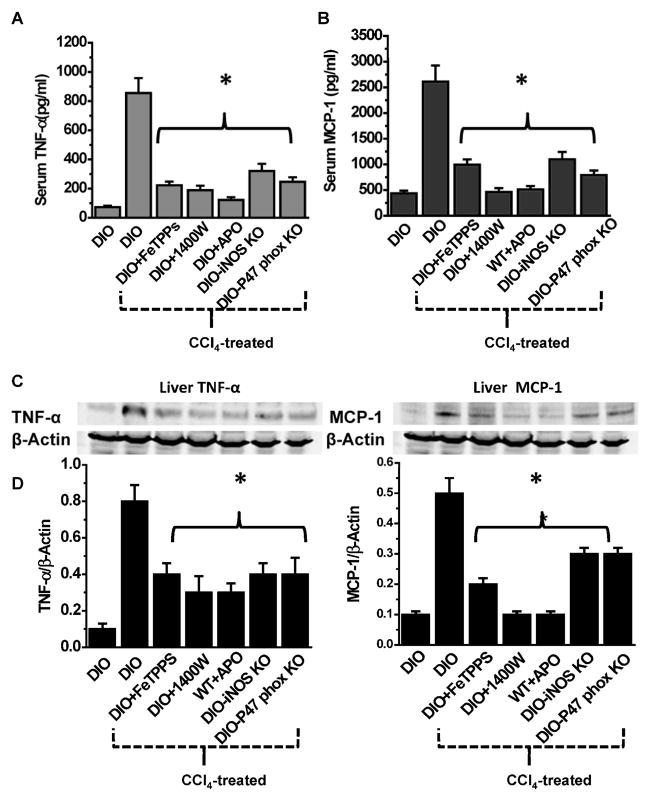
Confocal laser scanning microscopy showed decreased protein radical adducts and nitrotyrosine immunoreactivity in the centrolobular regions of CCl4-treated mouse livers after administration of FeTPPS compared to CCl4-treated DIO mice after vehicle treatment only (without FeTPPS) (Fig. 2E). FeTPPS also had a significant effect in reducing both mRNA and protein levels of proinflammatory cytokines that exacerbate sterile inflammation in steatohepatitis. In CCl4-treated DIO mice administered FeTPPS, both TNF-α and MCP-1 mRNA expressions and protein levels were significantly decreased, compared to DIO mice treated with CCl4 alone (Fig. 2F and 3A–D). Similarly, pharmacologically inhibiting either iNOS or NADPH oxidase or using p47 phox and iNOS gene-deleted mice resulted in significantly decreased TNF-α and MCP-1 mRNA and protein levels post-CCl4 treatment compared to DIO mice treated with CCl4 (2F and 3A–D).
Fig. 3.
Leptin induced oxidative stress and peroxynitrite formation activates Kupffer cells. Serum and liver tissue TNF-α (A &C) and MCP-1 (B&D) analyzes following blockade of peroxynitrite formation. The figure represents data from 3 mice/group and *P<0.05
Leptin-mediated protein radical and 3-nitrotyrosine formation and release of the proinflammatory cytokines TNF-α and MCP-1 are Kupffer cell and macrophage-dependent
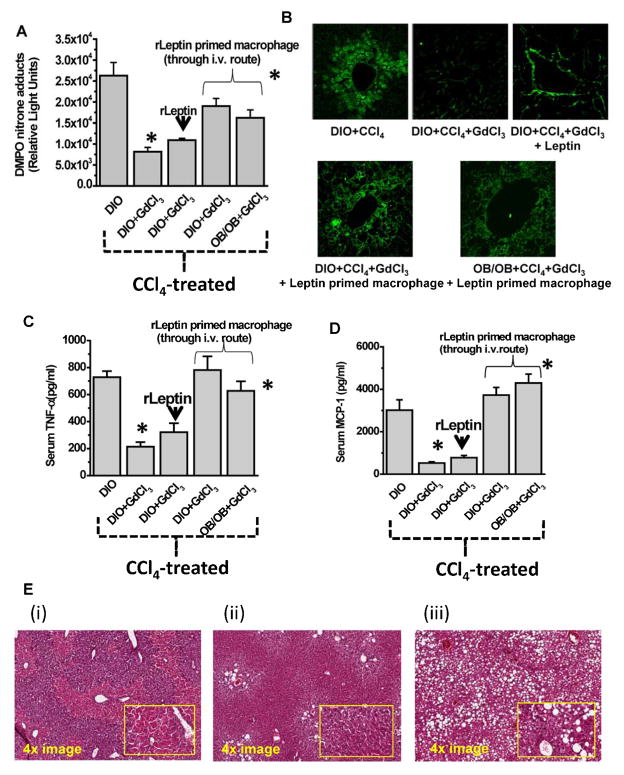
Resident liver macrophages (primarily Kupffer cells) and infiltrating neutrophils and other immune cells that exhibit macrophage-like function are found to express both the vascular form of NADPH oxidase and iNOS. Consistent with that concept, we found that protein radical formation and tyrosine nitration were significantly reduced upon administration of the macrophage toxin GdCl3 or liposomal clodronate to DIO mice before treatment with CCl4 (Fig. 4A and 4B; Suppl Fig. 6.), and they were not restored upon further supplementation of recombinant leptin. However, they were significantly increased after adoptive transfer of leptin-primed Kupffer cells into DIO mice treated with CCl4 or OB/OB mice (Fig. 4A and 4B; Suppl Fig. 6.). Since TNF-α and MCP-1 release from macrophages contribute to steatohepatitic lesions in obesity following treatment with CCl4, it was important to explore whether the leptin effect on release of the cytokines was also macrophage dependent. Results indicated that administration of macrophage toxin GdCl3 to DIO mice before treatment with CCl4, significantly decreased TNF-α and MCP-1 release (Fig. 4C and 4D; Suppl. Fig. 6.). These results thus clearly suggested that leptin was acting through macrophages in the CCl4-treated DIO mice liver in exacerbating the steatohepatitic lesions in these mice.
Fig. 4.
Leptin action through liver macrophages and Kupffer cells is responsible for peroxynitrite chemistry and subsequent free radical formation in steatohepatitc injury. Macrophage toxin gadolinium chloride was used to deplete liver macrophages and was followed by CCl4 treatment in DIO mice. In certain experimental groups which had their macrophages depleted, either only leptin or leptin primed macrophages were infused through tail vein. A. Whole liver homogenates from these mice were then analyzed for anti-DMPO immunoreactivity by ELISA. B. Liver slices of mice from the above experimental setup and adoptive transfer of leptin or leptin primed macrophages were subjected to confocal laser scanning microscopy for analyzing 3-nitrotyrosine immunoreactivity and localization. Analysis of serum TNF-α (C) and MCP-1 (D) was assayed by ELISA. The figure represents data from 3 mice/group and *P<0.05 was considered statistically different. E. Eosin-Hematoxylin staining of liver of obese, CCl4-treated mice either administered saline (i) or FeTPPS (ii) or use OB/OB mice. The images represent both a 4x magnification and a 20x magnification (inset).
Leptin contributes to steatohepatitic lesions in CCl4-treated DIO mice
To examine the effect of leptin in inducing steatohepatitic lesions following CCl4 administration in DIO mice, we compared the histopathology of steatohepatitis in leptin-deficient mice and mice fed with a high fat diet. DIO mice with higher leptin levels, treated with CCl4 had centrolobular necrosis and showed markers of early steatohepatitic injury (4Ei). When DIO mice that received FeTPPS (4Eii), or leptin deficient mice (OB/OB) (4Eiii) were administered CCl4, they showed steatosis, periportal necrosis and occasional necrotic areas around the central vein (Zone III) with little or no leukocyte accumulation compared to wild type DIO mice treated with CCl4 (Fig. 4Ei) which had centrilobular necrosis, infiltration of leukocytes and ballooned hepatocytes. This result suggests that leptin contributes significantly in inducing early inflammatory lesions in DIO mice treated with CCl4. Consistent with this result was a significant increase observed in serum ALT levels in DIO mice compared to leptin-deficient mice administered CCl4 (Suppl Fig. 2B). Kupffer cell aggregation near the perivenular regions, a hallmark of early steatohepatitic lesions was also evident by confocal microscopy of CD68 positive cells (Suppl Fig. 7). Histological Activity Index score was found to be significantly higher for DIO mice treated with CCl4 when compared to OB/OB mice (Suppl. Table 1.)
Discussion
This is the first report that shows that leptin-mediated protein radical formation, tyrosine nitration and activation of Kupffer cells are caused by peroxynitrite formation, and demonstrates that this process exacerbates CCl4-induced steatohepatitic lesions in diet-induced obesity. We found that exposing mice with DIO to a potential “2nd hit” from CCl4 caused a two-fold increase in circulating leptin levels (Fig. 1). This result was central to our subsequent investigations, which established that leptin-induced formation of peroxynitrite was key to driving the ensuing inflammatory processes. Higher leptin levels as a result of CCl4 administration in DIO mice might be due to the release of IL-beta as shown previously by others [26]. Since diet-induced obese mice that were treated with CCl4 had higher leptin levels (2- fold higher than vehicle-treated obese mice), and leptin is known to cause oxidative stress [27] we investigated the contribution of leptin to the protein radical formation and tyrosine nitration in early steatohepatitis. In OB/OB mice and DIO mice, treatment with a neutralizing antibody against leptin significantly reduced protein radical formation, whereas leptin supplementation for 7 days restored higher protein radical formation and tyrosine nitration, suggesting that leptin is responsible for the redox imbalance in the liver in early steatohepatitic injury (Fig. 1B–D). This result assumes significance since leptin is known to cause oxidative stress in different tissues and cells in response to inflammatory stress [6] [7] [8]. Furthermore, leptin-mediated reactive oxygen species generation was found to activate stellate cell proliferation, a known phenomenon in steatohepatitic injury [8]. However, little is known with regard to the redox mechanisms by which Kupffer cells promote liver inflammation and fibrosis in obesity. Our results indicated that in addition to leptin’s involvement in protein radical formation and tyrosine nitration, it is also involved in activating Kupffer cell release of TNF-α and MCP-1(Suppl. Fig. 1), but how the cytokine release and Kupffer cell activation are related to the redox processes initiated by leptin remained unknown at this point.
Therefore, to establish the mechanism of free radical formation by leptin action, we analyzed the mRNA expression of enzymes that are significant contributors to production of free radical species in inflammatory microenvironments. We found that levels of expression of inducible NOS and p47 phox mRNA were significantly elevated in early steatohepatitic injury and leptin-supplemented Ob/Ob mice (Fig. 2A–B). In addition, OB/OB mice had less induction of mRNA for these enzymes. This data suggested that nitric oxide and superoxide radicals play a significant role in the protein radical formation process by leptin. Furthermore, since protein radical formation and tyrosine nitration were localized in Kupffer cells [12], there was a possibility that Kupffer cells, which are known to express both NADPH oxidase and inducible NOS, might form peroxynitrite from superoxide and nitric oxide respectively at a diffusion-controlled rate. Such a reaction is feasible since both species are found in the same cell at the same time. Our results showed that the peroxynitrite decomposition catalyst FeTPPs, the NADPH oxidase inhibitor apocynin and the iNOS inhibitor 1400W all significantly inhibited protein radical formation and tyrosine nitration (Fig. 2C–D). High fat-fed mice depleted of iNOS and the NADPH oxidase subunit p47 phox and treated with CCl4 showed significant decreases in protein radical formation and tyrosine nitration, thus confirming the role of these enzymes in the formation of peroxynitrite.
The role of peroxynitrite formation in the activation of Kupffer cells was established by examining subsequent TNF-α and MCP-1 mRNA expression in the presence of FeTPPS and inhibitors of iNOS and NADPH oxidase, as well as the use of gene-deficient mice for both iNOS and p47 phox (Fig. 2F). The radicals that are generated from peroxynitrite form stable post-translational modifications of proteins that can affect their function and contribute to the activation of inflammatory pathways [15]. In the present model of early steatohepatitic injury, Kupffer cell activation (there was an increase in both CD68 and F4/80 immunoreactivity in liver tissues of CCl4-administered DIO mice as compared to DIO and OB/OB mice; Suppl. Fig. 3.) and production of the inflammatory cytokines TNF-α and MCP-1 might have resulted from loss or gain of function of the proteins that modulated the induction response of these genes; however, this point remains speculative at present. Though there may be multiple sources of these cytokines, including stellate cells, Kupffer cells were found to be a principal source of MCP-1 as shown in Suppl Fig. 4B.
Although much of our attention in this study was focused on the ability of either resident macrophages or infiltrating leukocytes to promote leptin-induced formation of peroxynitrite, the data so far is incomplete without further confirmatory evidence of their involvement in leptin-induced redox changes, inflammation and progression of steatohepatitic injury. To establish the site of leptin action, we first depleted DIO mice of macrophages by pre-treating them with GdCl3 and then adoptively transferred leptin-primed Kupffer cells into both DIO mice and leptin-deficient ob/ob mice. Results showed that protein radical formation, tyrosine nitration and release of proinflammatory cytokines were restored in macrophage-depleted mice only when leptin primed Kupffer cells were adoptively transferred (Fig. 4). Furthermore, supplementation with leptin only (without macrophages) in these mice had no effect. Similarly, macrophage-depleted mice that were leptin deficient exhibited increased protein radical formation, tyrosine nitration and release of proinflammatory cytokines following adoptive transfer of leptin-primed macrophages, suggesting the essential roles of both leptin and macrophages in potentiating redox-mediated steatohepatitic injury (Fig. 4). Our results also establish that leptin and macrophages are symbiotic in their actions in promoting inflammation in the fatty liver and it might be speculated that the process involves multiple signaling mechanisms and intermediary molecules [28].
Finally, we report a leptin-mediated oxidative stress mechanism for inflammatory events promoted by the formation of peroxynitrite in the macrophages in the steatohepatitic liver.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge James Clark, Tiwanda Marsh, Jeoffrey Hurlburt, Jeff Tucker and Ralph Wilson for excellent technical assistance. We also sincerely thank Dr. Ann Motten and Mary Mason for help in the careful editing of this manuscript.
Grant Support: This work has been supported by a K99-R00, NIH pathway to Independence Award (4R00ES019875-02 to Saurabh Chatterjee) and the Intramural Research Program of the National Institutes of Health and the National Institute of Environmental Health Sciences (Z01 ES050139-13 to Ronald P. Mason)
Footnotes
Conflict of interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lafrance V, Inoue W, Kan B, Luheshi GN. Leptin modulates cell morphology and cytokine release in microglia. Brain Behav Immun. 2010;24:358–365. doi: 10.1016/j.bbi.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Zarkesh-Esfahani H, Pockley AG, Wu Z, Hellewell PG, Weetman AP, Ross RJ. Leptin indirectly activates human neutrophils via induction of TNF-alpha. J Immunol. 2004;172:1809–1814. doi: 10.4049/jimmunol.172.3.1809. [DOI] [PubMed] [Google Scholar]
- 3.Shen J, Sakaida I, Uchida K, Terai S, Okita K. Leptin enhances TNF-alpha production via p38 and JNK MAPK in LPS-stimulated Kupffer cells. Life Sci. 2005;77:1502–1515. doi: 10.1016/j.lfs.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Leclercq I, Brymora JM, Xu N, Ramezani-Moghadam M, London RM, et al. Kupffer cells mediate leptin-induced liver fibrosis. Gastroenterology. 2009;137:713–723. doi: 10.1053/j.gastro.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Z, Lin H, Yang S, Diehl AM. Murine leptin deficiency alters Kupffer cell production of cytokines that regulate the innate immune system. Gastroenterology. 2002;123:1304–1310. doi: 10.1053/gast.2002.35997. [DOI] [PubMed] [Google Scholar]
- 6.Cao Q, Mak KM, Lieber CS. Leptin represses matrix metalloproteinase-1 gene expression in LX2 human hepatic stellate cells. J Hepatol. 2007;46:124–133. doi: 10.1016/j.jhep.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 7.Xu FP, Chen MS, Wang YZ, Yi Q, Lin SB, Chen AF, et al. Leptin induces hypertrophy via endothelin-1-reactive oxygen species pathway in cultured neonatal rat cardiomyocytes. Circulation. 2004;110:1269–1275. doi: 10.1161/01.CIR.0000140766.52771.6D. [DOI] [PubMed] [Google Scholar]
- 8.De Minicis S, Seki E, Oesterreicher C, Schnabl B, Schwabe RF, Brenner DA. Reduced nicotinamide adenine dinucleotide phosphate oxidase mediates fibrotic and inflammatory effects of leptin on hepatic stellate cells. Hepatology. 2008;48:2016–2026. doi: 10.1002/hep.22560. [DOI] [PubMed] [Google Scholar]
- 9.Ferrer-Sueta G, Radi R. Chemical biology of peroxynitrite: kinetics, diffusion, and radicals. ACS Chem Biol. 2009;4:161–177. doi: 10.1021/cb800279q. [DOI] [PubMed] [Google Scholar]
- 10.Chatterjee S, Ehrenshaft M, Bhattacharjee S, Deterding LJ, Bonini MG, Corbett J, et al. Immuno-spin trapping of a post-translational carboxypeptidase B1 radical formed by a dual role of xanthine oxidase and endothelial nitric oxide synthase in acute septic mice. Free Radic Biol Med. 2009;46:454–461. doi: 10.1016/j.freeradbiomed.2008.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zielonka J, Sikora A, Joseph J, Kalyanaraman B. Peroxynitrite is the major species formed from different flux ratios of co-generated nitric oxide and superoxide: direct reaction with boronate-based fluorescent probe. J Biol Chem. 2010;285:14210–14216. doi: 10.1074/jbc.M110.110080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatterjee S, Rana R, Corbett J, Kadiiska MB, Goldstein J, Mason RP. P2x7 Receptor-NADPH Oxidase-Axis Mediates Protein radical Formation And Kupffer Cell Activation in Carbon Tetrachloride-Mediated Steatohepatitis in Obese Mice. Free Radic Biol Med. 2012 doi: 10.1016/j.freeradbiomed.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatterjee S, Lardinois O, Bhattacharjee S, Tucker J, Corbett J, Deterding L, et al. Oxidative stress induces protein and DNA radical formation in follicular dendritic cells of the germinal center and modulates its cell death patterns in late sepsis. Free Radic Biol Med. 2011;50:988–999. doi: 10.1016/j.freeradbiomed.2010.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garvey EP, Oplinger JA, Furfine ES, Kiff RJ, Laszlo F, Whittle BJ, et al. 1400W is a slow, tight binding, and highly selective inhibitor of inducible nitric-oxide synthase in vitro and in vivo. J Biol Chem. 1997;272:4959–4963. doi: 10.1074/jbc.272.8.4959. [DOI] [PubMed] [Google Scholar]
- 15.Chatterjee S, Lardinois O, Bonini MG, Bhattacharjee S, Stadler K, Corbett J, et al. Site-specific carboxypeptidase B1 tyrosine nitration and pathophysiological implications following its physical association with nitric oxide synthase-3 in experimental sepsis. J Immunol. 2009;183:4055–4066. doi: 10.4049/jimmunol.0900593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Rosa V, Procaccini C, Cali G, Pirozzi G, Fontana S, Zappacosta S, et al. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–255. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Froh M, Zhong Z, Walbrun P, Lehnert M, Netter S, Wiest R, et al. Dietary glycine blunts liver injury after bile duct ligation in rats. World J Gastroenterol. 2008;14:5996–6003. doi: 10.3748/wjg.14.5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rai RM, Yang SQ, McClain C, Karp CL, Klein AS, Diehl AM. Kupffer cell depletion by gadolinium chloride enhances liver regeneration after partial hepatectomy in rats. Am J Physiol. 1996;270:G909–918. doi: 10.1152/ajpgi.1996.270.6.G909. [DOI] [PubMed] [Google Scholar]
- 19.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 20.Mason RP. Using anti-5,5-dimethyl-1-pyrroline N-oxide (anti-DMPO) to detect protein radicals in time and space with immuno-spin trapping. Free Radic Biol Med. 2004;36:1214–1223. doi: 10.1016/j.freeradbiomed.2004.02.077. [DOI] [PubMed] [Google Scholar]
- 21.Nakai K, Mason RP. Immunochemical detection of nitric oxide and nitrogen dioxide trapping of the tyrosyl radical and the resulting nitrotyrosine in sperm whale myoglobin. Free Radic Biol Med. 2005;39:1050–1058. doi: 10.1016/j.freeradbiomed.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 22.Wheeler MD, Kono H, Yin M, Nakagami M, Uesugi T, Arteel GE, et al. The role of Kupffer cell oxidant production in early ethanol-induced liver disease. Free Radic Biol Med. 2001;31:1544–1549. doi: 10.1016/s0891-5849(01)00748-1. [DOI] [PubMed] [Google Scholar]
- 23.Cohen JI, Chen X, Nagy LE. Redox signaling and the innate immune system in alcoholic liver disease. Antioxid Redox Signal. 2011;15:523–534. doi: 10.1089/ars.2010.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Remessy AB, Tawfik HE, Matragoon S, Pillai B, Caldwell RB, Caldwell RW. Peroxynitrite mediates diabetes-induced endothelial dysfunction: possible role of Rho kinase activation. Exp Diabetes Res. 2010;2010:247861. doi: 10.1155/2010/247861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuzzocrea S, Mazzon E, Di Paola R, Esposito E, Macarthur H, Matuschak GM, et al. A role for nitric oxide-mediated peroxynitrite formation in a model of endotoxin-induced shock. J Pharmacol Exp Ther. 2006;319:73–81. doi: 10.1124/jpet.106.108100. [DOI] [PubMed] [Google Scholar]
- 26.Faggioni R, Fantuzzi G, Fuller J, Dinarello CA, Feingold KR, Grunfeld C. IL-1 beta mediates leptin induction during inflammation. Am J Physiol. 1998;274:R204–208. doi: 10.1152/ajpregu.1998.274.1.R204. [DOI] [PubMed] [Google Scholar]
- 27.Parola M, Marra F. Adipokines and redox signaling: impact on fatty liver disease. Antioxid Redox Signal. 2011;15:461–483. doi: 10.1089/ars.2010.3848. [DOI] [PubMed] [Google Scholar]
- 28.Vaughan T, Li L. Molecular mechanism underlying the inflammatory complication of leptin in macrophages. Mol Immunol. 2010;47:2515–2518. doi: 10.1016/j.molimm.2010.06.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.