Abstract
Purpose
Current Brain Trauma Foundation guidelines recommend avoiding hypoxemia after severe pediatric Traumatic brain injury (TBI). Yet, recent studies on optimum admission oxygenation and ventilation parameters associated with discharge survival in pediatric TBI are lacking.
Materials and Methods
After IRB approval, a retrospective study involving pediatric patients ages ≤ 14 years with severe TBI (head Abbreviated Injury Scale (AIS) score ≥ 3, Glasgow Coma Scale (GCS) score ≤ 8 on admission) admitted to Harborview Medical Center (Level 1 pediatric trauma center), Seattle, WA, during 2003 to 2007 was performed. Admission demographics, clinical data and laboratory characteristics were abstracted. Hypoxemia was defined as PaO2 < 60 mmHg, hypocarbia was defined as PaCO2 ≤ 35 mmHg and hypercarbia was defined as PaCO2 ≥ 46 mmHg.
Results
194 patients met inclusion criteria of which 162 (83.5%) patients survived. Admission hypoxemia occurred in 9 (5.6%) patients who survived and 8 (25%) patients who died (p < 0.001). Children with admission PaCO2 between 36–45 mmHg had greater discharge survival compared to those with both admission hypocarbia (PaCO2 ≤ 35 mmHg) and hypercarbia (PaCO2 ≥ 46 mmHg). Admission PaO2 301–500 mmHg (AOR 8.02 [95% CI 1.73 – 37.10]; p = 0.008) and admission PaCO2 36–45 mmHg (AOR 5.47 [95% CI 1.30 – 23.07]; p = 0.02) were independently associated with discharge survival.
Conclusion
Discharge survival after severe pediatric TBI was associated with admission PaO2 301–500 mmHg and PaCO2 36–45 mmHg. Admission hypocarbia and hypercarbia were each associated with increased discharge mortality.
Keywords: Traumatic Brain Injury, Oxygenation, Ventilation
INTRODUCTION
Traumatic Brain Injury (TBI) is the most common cause of injury related pediatric death and disability [1, 2]. Poor outcome after severe pediatric TBI[3] may be associated with second physiological insults such as hypoxemia[4–6], hypercarbia[5, 6], and hypotension[5–8]. Current Brain Trauma Foundation (BTF) guidelines recommend prevention and early correction of hypoxemia (PaO2<60 mmHg) and avoidance of hypocarbia (PaCO2<35 mmHg) after severe pediatric TBI [9]. However, these recommendations are based primarily on scant older data or on data from the pediatric intensive care unit and not from earlier times after injury (emergency department; ED) after TBI. Moreover, while avoidance of hypoxemia and hypercarbia is recommended, optimal PaO2 and PaCO2 levels associated with improved outcomes after pediatric TBI remain largely unknown.
In one retrospective study of children with severe TBI, Michaud et al. reported that pediatric patients with ED admission PaO2 levels over 350 mm Hg had good discharge outcomes than those with PaO2 levels between 105–350 mmHg and that there was no difference in discharge survival or disability between children with ED PaCO2< 35 mmHg vs. PaCO2 ≥ 35 mmHg [5]. In the other study that examined admission ventilation and outcomes in severe pediatric TBI, hypercarbia which was defined as PaCO2 > 35 mmHg was found to be associated with poor discharge survival[6]. However, both studies grouped all levels of PaCO2 greater than 35 mmHg into one category, thereby lumping normocarbia and hypercarbia. Thus, with the exception of PaCO2 studies in the PICU showing low CBF with hypocarbia[10], the effect of admission ventilation parameters on outcomes remain understudied. To add to our understanding of the relationship between oxygenation and ventilation parameters after severe pediatric TBI and outcome, we aimed to determine the relationship between admission PaO2 and PaCO2 levels and discharge survival.
MATERIALS AND METHODS
Study Design
After Institutional Review Board approval, a retrospective study involving pediatric patients admitted to Harborview Medical Center (Level 1 pediatric trauma center), Seattle, WA, during 2003 to 2007 was performed.
Study Population
Eligibility criteria included patients aged ≤ 14 years; with severe TBI defined as Glasgow Coma Scale (GCS) score ≤ 8 on admission to the ED, head Abbreviated Injury Scale (AIS) score ≥ 3 and TBI ICD codes (800-801.9, 803-804.9, 850-854.1 or 959.01).
General Approach to Severe TBI Management
During the study period, patients were resuscitated according to institutional practice, which is consistent with the 2003 Brain Trauma Foundation Guidelines [9]. Relevant to this study, general approach to severe pediatric TBI management includes sedation, analgesia, mechanical ventilation, ICP monitoring via camino or ventriculostomy, care aimed at maintaining intracranial pressure (ICP) < 20 mmHg, CPP >40 mmHg, PaCO2 35–40 mmHg, SaO2> 90% and maintaining core body temperature between 35 and 37.5°C with antipyretics, cooling/warming blankets, or intravascular cooling devices if needed. Hyperventilation was used only as a temporizing measure in cases of impending brain herniation or refractory intracranial hypertension. During the study period, utilization of the measures of cerebral oxygenation, including brain tissue oxygenation and jugular venous oximetry was patient specific and were at the discretion of the treating physician.
Data Sources
Harborview Medical Center Trauma Registry, electronic medical records and laboratory information system records were used to compile the final data set. Trauma registry records were used to abstract demographics and select clinical characteristics including outcomes.
Data Abstracted
The following patient level characteristics were abstracted: demographic data (age, gender), admission GCS score, Head AIS score, Injury Severity Score (ISS), presence or absence of admission hypotension, presence or absence of chest injuries (ICD codes 860.0 - 860. 5, 861.1- 861.3 or 862.0 - 862.3), discharge GCS score, discharge mortality and discharge disposition. Laboratory data extracted included PaO2, PaCO2, FiO2, PaO2/ FiO2, blood glucose and hematocrit recorded at the time of emergency department (ED) admission.
Outcomes
The main outcome measure was all cause discharge survival.
Definitions/Categories
Hypotension was defined as systolic blood pressure (SBP) less than 5th percentile (Age × 2 + 70) [9]. Hypoxemia as was defined as PaO2< 60 mmHg. We also categorized admission PaO2 into 4 groups [5]; Group 1: PaO2 ≤ 100 mmHg, Group 2: PaO2 101–300 mmHg, Group 3: PaO2 301–500 mmHg and Group 4: PaO2 ≥ 500 mmHg. Hypocarbia was defined as PaCO2 ≤ 35 mmHg, normocarbia was defined as PaCO2 36–45 mmHg, and hypercarbia was defined as PaCO2 ≥ 46 mmHg.
Statistical Analysis
Statistical analysis was performed using IBM SPSS 19 software. Demographic and clinical data (Admission GCS, Head AIS, ISS, admission PaO2, PaCO2, FiO2, blood glucose and hematocrit) were evaluated for normality and then analyzed with non-parametric Mann Whitney rank sum test and differences in these parameters were examined for discharge survival and death (Table 1). Nominal data (gender, presence or absence of chest trauma, and presence or absence of admission hypotension) were analyzed using Chi Square test and are described as n (%). The distribution of admission PaCO2 and PaO2 was also examined. Data are described as median, 25 – 75th percentile and range.
Table 1.
Admission Characteristics and Survival in 194 Pediatric Patients with Severe Traumatic Brain Injury.
| Total n=(194) Median (25%, 75%) Range |
Survived (n=162) Median (25%, 75%) Range |
Died (n=32) Median (25%, 75%) Range |
p | |
|---|---|---|---|---|
| Age (years) | 8 (3, 13) 0 – 14 |
8.5 (3, 13) 0 – 14 |
1 (1, 12) 0 – 14 |
0.001 |
| Glasgow Coma Scale score | 3 (3, 3) 3 – 6 |
3 (3, 3) 3 – 8 |
3 (3, 3) 3 – 6 |
0.03 |
| Head Abbreviated Injury Scale | 4 (4, 5) 3 – 6 |
4 (4, 5) 3 – 6 |
5 (5, 5) 3 – 6 |
< 0.001 |
| Injury Severity Score | 26 (17, 35) 9 – 75 |
26 (17, 34) 9 – 75 |
35 (26, 64) 9 – 75 |
< 0.001 |
| PaO2 (mmHg) | 303 (129, 425) 31 – 638 |
344 (170, 446) 36 – 638 |
125 (52, 261) 36 – 638 |
< 0.001 |
| PaO2/FiO2 | 332 (150, 448) 31 – 638 |
365 (182, 461) 36 – 638 |
125 (53, 276) 31 – 600 |
< 0.001 |
| PaCO2 (mmHg) | 39 (32, 46) 18 – 114 |
39 (33, 45) 26 – 114 |
35 (31, 50) 18 – 71 |
0.66 |
| Glucose (mg/dL) | 166 (138, 211) 76 – 446 |
163 (135, 200) 76 – 364 |
210 (155, 290) 88 – 446 |
0.004 |
| Hematocrit (%) | 35 (31, 38) 6 – 54 |
35 (32, 38) 14 – 54 |
34 (24, 36) 6 – 48 |
0.04 |
| Chest Trauma (n, %) | 52 (26.8%) | 39 (24.1) | 13 (40.6%) | 0.05 |
| Hypotension (n, %) | 6 (3.1%) | 3 (1.9%) | 3 (9.4%) | 0.03 |
A binomial logistic regression model for discharge survival (main outcome) containing relevant predictors was created based on significant (p < 0.05) and clinically relevant univariate factors. Predictor variables were categorized as follows: A) age, B) ISS, C) presence of admission hypotension, D) admission blood glucose (mg/dl), E) admission PaO2 (Group 1: PaO2 ≤ 100 mmHg, Group 2: PaO2 101–300 mmHg, Group 3: PaO2 301–500 mmHg and Group 4: PaO2 ≥ 500 mmHg) with group 1 as reference and F) admission PaCO2 (Group 1: PaCO2 ≤ 35 mmHg, Group 2: PaCO2 36–45 mmHg and Group 3 PaCO2 ≥ 46 mmHg) with Group 1 as reference. Data are reported as adjusted odds ratio (AOR) and 95% confidence interval (CI). p<0.05 was considered as statistically significant.
RESULTS
Sample Characteristics
Of the initial 201 patients whose data were available for analysis, 7 patients were excluded due to lack of laboratory data, leaving194 patients as the final sample. Of 194 patients, 162 (83.5%) survived.
Cohort characteristics and baseline clinical differences between patients who survived compared to patients who died are described in Table 1. Significant univariate associations for discharge survival were older age, higher admission GCS, lower ISS, higher admission PaO2, higher PaO2/FiO2, lower admission blood glucose, higher admission hematocrit and absence of admission hypotension. There were no differences between those patients who survived and those who died at discharge in terms of presence or absence of chest injury, gender, or admission FiO2.
Admission PaO2 Thresholds and Discharge Survival
Figure 1 shows the distribution of admission PaO2 for survivors and for those who died at discharge. Children who survived had higher admission PaO2 (344 mmHg vs. 125 mmHg, p <0.001) when compared to those who died (Table 1). Admission hypoxemia occurred in 9 (5.6%) patients who survived and 8 (25%) patients who died (p <0.001). Admission PaO2< 100 mmHg was observed in fewer (20; 12.3%) patients who survived than those who died (13; 40.6%; p <0.001). Discharge survival was independently associated with PaO2 301–500 mmHg (AOR 8.02 95% CI 1.73 – 37.10]; p = 0.008; Table 2).
Fig 1.
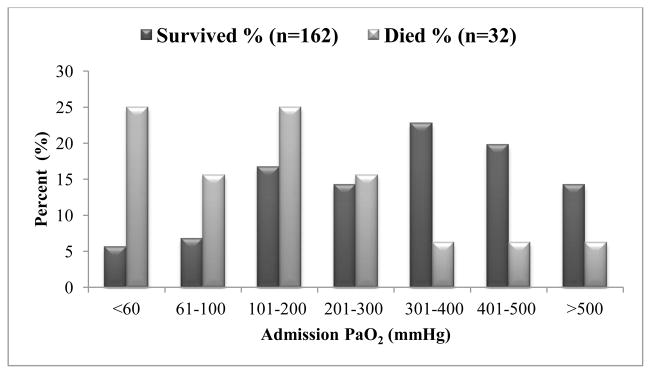
Distribution of Admission PaO2 Values by Discharge Survival. Deaths decrease with higher PaO2 values.
Table 2.
Independent Factors Associated with Discharge Survival in Severe Pediatric Traumatic Brain Injury (n=194). Data as Adjusted Odds Ratio (AOR 95% CI).
| Survival AOR | 95% CI | p | |
|---|---|---|---|
| Older Age (years) | 1.13 | 1.02 – 1.26 | 0.02 |
| Lower Injury Severity Score | 0.94 | 0.91 – 0.97 | < 0.001 |
| PaCO2 36–45 mmHg (n= 73) | 5.47 | 1.30 – 23.07 | 0.02 |
| PaO2 301–500 mmHg (n= 73) | 8.02 | 1.73 – 37.10 | 0.008 |
Admission PaCO2 Thresholds and Discharge Survival
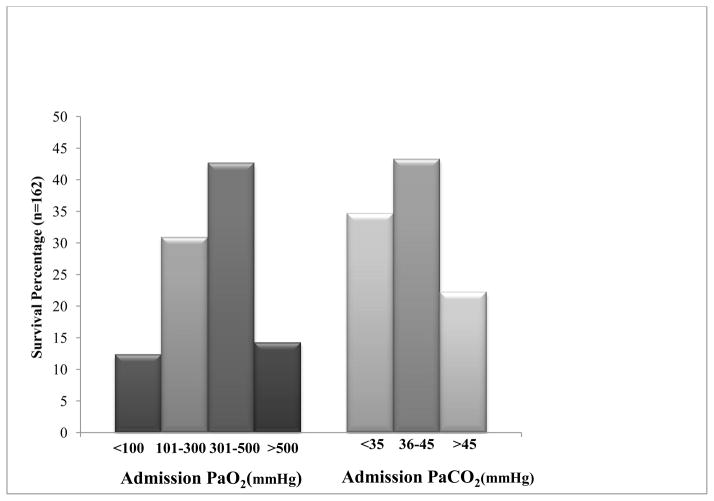
Figure 2 shows the distribution of PaCO2 for survivors and those who died at discharge. There was no statistical difference in admission PaCO2 between children who survived and those who died (39 mmHg vs. 35 mmHg, p= 0.66). However, univariate analysis showed increased discharge survival in children with admission PaCO2 between 36–45 mmHg. There was increased discharge mortality associated with both hypocarbia (PaCO2 ≤ 35 mmHg) and hypercarbia (PaCO2 ≥ 46 mmHg; Figure 2 and 3). Using PaCO2 ≤ 35 mmHg as reference, PaCO2 36–45 mmHg was independently associated with discharge survival advantage (AOR 5.47 [95% CI 1.30 – 23.07]; p = 0.02; Table 2).
Fig 2.
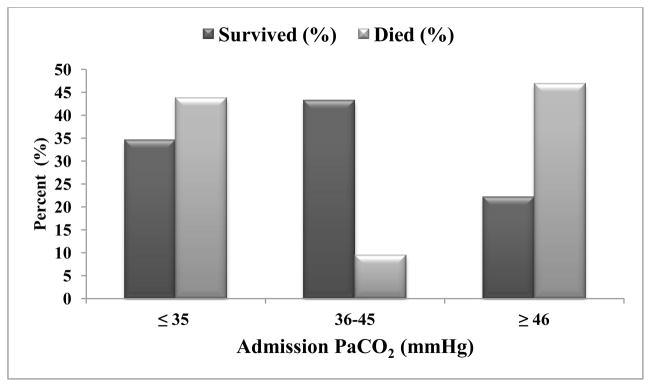
Distribution of Admission PaCO2 Values by Discharge Survival. The lowest deaths are with PaCO2 values between 36 and 45 mmHg.
Fig 3.
Admission PaO2 and PaCO2 Values Associated with Discharge Survival.
Other independent factors associated with discharge survival are older age (AOR = 1.13[95% CI = 1.02 – 1.26]; p=0.02) and lower ISS (AOR 0.94[95% CI = 0.91 – 0.97]; p= < 0.001; Table 2).
DISCUSSION
The main findings of this study are that discharge survival in severe pediatric TBI was associated with 1) Older age, 2) Lower ISS, 3) Admission PaO2 301–500 mmHg, and 4) Admission PaCO2 36–45 mmHg. We also found that admission PaO2< 100 mHg, and PaCO2 < 35 mmHg or > 45 mmHg were associated with higher discharge mortality. Together, these findings suggest optimal discharge outcomes for patients who have PaO2 values greater than historical definitions of normoxemia (PaO2 > 100 mmHg) and with maintenance of normocarbia (PaCO2 35–45 mmHg) early after severe pediatric TBI.
PaO2 and Survival in pediatric TBI
Current practice includes the use of 100% oxygen during resuscitation [9] and empiric supplemental use of oxygen in pediatric patients after severe TBI is common, regardless of documented presence of hypoxemia. However, high fraction of inspired oxygen has been associated oxygen toxicity to a number of organ systems [11–13] including the development of retinopathy of prematurity [14], diffuse damage to the alveolar-capillary barrier leading to edema, impaired gas exchange, atelectasis, respiratory failure, and death [13, 15]. Specifically problematic for patients with TBI may be hyperoxic cerebral vasoconstriction [16] which may reduce cerebral blood flow in the presence of normal cerebral metabolic rate, thereby worsening cerebral ischemia and poor outcomes [13, 17].
The practice of maintaining high PaO2 by supplemental oxygen appears to be based on data from adult TBI[18] or from experimental studies[19] and possibly due to concern for hypoxemia contributing to poor outcomes in pediatric TBI patients. While mitochondrial function may be impaired in patients with severe TBI, with subsequent decreased ATP production [20], CMRO2 has been shown to remain unchanged with hyperoxia in TBI patients indicating lack of advantage of the use of 100% oxygen in patients with TBI to improve brain oxygen metabolism [11, 21]. Although we did not have cerebral metabolism data in this study, we found a survival benefit with admission PaO2 301–500 mmHg after adjusting for other factors known to impact outcomes after TBI such as age and ISS.
Our findings that PaO2< 100 mmHg was associated with mortality is similar to a previously reported study in severe pediatric TBI [5]. However, older studies either examined the effect of hypoxemia alone (PaO2< 60 mmHg)[6, 7] or failed to detect a PaO2 threshold associated with survival/mortality[5]. In their study on children with severe pediatric TBI, Michaud et al. reported lower ED PaO2 to be associated with increased discharge mortality and discharge disability. They found that patients with ED admission PaO2 levels ≥ 350 mm Hg had lower discharge disability than those with PaO2 levels between 105–350 mmHg [5]. In adult TBI, Davis et al. analyzed the relationship between admission hypoxemia and hyperoxemia on discharge survival and they, like the present study, reported peak discharge survival benefit to be associated with admission PaO2 between 110 and 487 mmHg flanked by discharge mortality below PaO2 110 mm Hg and PaO2> 487 mmHg. [22]. Together, these findings suggest a U shaped effect of PaO2 on discharge mortality in both adult and pediatric severe TBI.
PaCO2 and Survival in pediatric TBI
In addition to oxygenation, PaCO2 may impact outcome after severe TBI because cerebral blood flow varies directly with PaCO2. Within physiological range, for each 1 mmHg change in PaCO2, cerebral blood flow changes by 1–2 ml/100 mg/min. Hypoventilation can therefore lead to increase in PaCO2 with subsequent increase in cerebral blood flow and cerebral blood volume. Increase in PaCO2 can worsen raised ICP in TBI but at same time, can counter balance reduce cerebral blood flow due to hyperoxemia induced cerebral vasoconstriction. Similarly, while hyperventilation can lead to decrease in PaCO2 with subsequent decrease in cerebral blood flow and cerebral blood volume contributing to lower ICP, it may worsen cerebral blood flow in patients with hyperoxemia induced cerebral vasoconstriction.
Skippen et al studied the effect of hyperventilation on regional CBF in severe pediatric TBI [10]. They found a modest decrease in CBF but a much larger decrease in cerebral oxygen consumption. Absolute hyperemia was uncommon at any time, but measured CBF rates were still above the metabolic requirements of most children with TBI. They also demonstrated the increase in frequency of one or more regions of ischemia (CBF < 18 mL/min/100g) from 28.9% during normocapnia to 73.1% with hyperventilation (PaCO2<25 mmHg). Muizelaar et al, in their randomized control trial on hyperventilation in severe adult TBI, demonstrated deleterious effect of prophylactic hyperventilation in severe TBI with motor scores 4 to 5[23]. Despite these two studies, there is insufficient data to conclusively establish the effect of early PaCO2 on outcome in severe pediatric TBI. Michaud et al [5], examined the effect of PaCO2< 35 mmHg vs. PaCO2 ≥ 35 mmHg on survival and found no association between these two groups. In another study, hypercarbia was defined as PaCO2 > 35 mmHg and was associated with poor survival. However, both studies grouped all levels of PaCO2 greater than 35 mmHg into one category and there was no differentiation between normocarbia and hypercarbia. To overcome these study limitations, and to determine PaCO2 threshold associated with survival/mortality, we defined hypocarbia as PaCO2 ≤ 35 mmHg, normocarbia as PaCO2 36–45 mmHg and hypercarbia as PaCO2 ≥ 46 mmHg.
In the present study, there was survival benefit with normocapnia (admission PaCO2 36–45 mmHg) and increased mortality with both hypocapnia and hypercapnia (PaCO2 beyond this range). These findings show the deleterious effects of both hypoventilation and hyperventilation in severe pediatric TBI and advocates for monitoring of ventilation with either PaCO2 with capnogram or arterial blood gas early after severe TBI.
The main limitations of our study are the factors associated with a single center retrospective study and the lack of brain tissue oxygenation and metabolic monitoring. As a result, we cannot comment if higher PaO2 values resulted in higher cerebral oxygenation and increased survival in severe pediatric TBI. However, the use of these advanced neuromonitoring technique are currently not standard of care nor available at all clinical sites, leaving clinicians with questions as to how to use systemic oxygenation data to guide or modify TBI outcomes. While we cannot attribute the relationship of higher oxygen levels to brain tissue oxygenation thereby missing the mechanistic basis behind this observation, nevertheless, this preliminary would suggest that further examination of this association is warranted. Similarly we are unable to comment on the effects of PaCO2 on hyperoxemia induced cerebral vasoconstriction during hypocarbia or hypercarbia. Our study examined admission clinical and laboratory characteristics and survival/mortality in severe pediatric TBI but we are unable to comment on the duration of these admission clinical and laboratory characteristics which remained at or above/below described levels. We were not able to examine interactions between PaO2 and PaCO2 given the number of patients in this study and only a few patients had admission hypotension in this series. Larger studies will be needed to fully understand the interrelationships between PaO2, PaCO2 and blood pressure on outcomes. Despite these limitations, we provide new information on admission oxygenation and ventilation parameters on patient level outcomes after severe TBI.
In summary, there are two new findings in this study. The first new finding is the association between discharge survival with admission PaO2 301–500 mmHg and PaCO2 36–45 mmHg in severe pediatric TBI. Second, both, hypocarbia (PaCO2< 35 mmHg) and hypercarbia (PaCO2> 46 mmHg) were associated with increased discharge mortality. Together, these findings suggest the need for larger studies examining ways to optimize outcomes after pediatric TBI with PaO2 and PaCO2.
Acknowledgments
Funding: R01NS072308-03
References
- 1.Tepas JJ, 3rd, DiScala C, Ramenofsky ML, Barlow B. Mortality and head injury: the pediatric perspective. J Pediatr Surg. 1990;25:92–95. doi: 10.1016/s0022-3468(05)80170-8. discussion 96. [DOI] [PubMed] [Google Scholar]
- 2.Langlois JA, Rutland-Brown W, Thomas KE. The incidence of traumatic brain injury among children in the United States: differences by race. J Head Trauma Rehabil. 2005;20:229–238. doi: 10.1097/00001199-200505000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Feickert HJ, Drommer S, Heyer R. Severe head injury in children: impact of risk factors on outcome. J Trauma. 1999;47:33–38. doi: 10.1097/00005373-199907000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Miller JD, Sweet RC, Narayan R, Becker DP. Early insults to the injured brain. JAMA. 1978;240:439–442. [PubMed] [Google Scholar]
- 5.Michaud LJ, Rivara FP, Grady MS, Reay DT. Predictors of survival and severity of disability after severe brain injury in children. Neurosurg. 1992;31:254–264. doi: 10.1227/00006123-199208000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Mayer TA, Walker ML. Pediatric head injury: the critical role of the emergency physician. Ann Emerg Med. 1985;14:1178–1184. doi: 10.1016/s0196-0644(85)81025-8. [DOI] [PubMed] [Google Scholar]
- 7.Pigula FA, Wald SL, Shackford SR, Vane DW. The effect of hypotension and hypoxia on children with severe head injuries. J Pediatr Surg. 1993;28:310–314. doi: 10.1016/0022-3468(93)90223-8. discussion 315–316. [DOI] [PubMed] [Google Scholar]
- 8.White JR, Farukhi Z, Bull C, Christensen J, Gordon T, Paidas C, Nichols DG. Predictors of outcome in severely head-injured children. Crit Care Med. 2001;29:534–540. doi: 10.1097/00003246-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Adelson PD, Bratton SL, Carney NA, Chesnut RM, du Coudray HE, Goldstein B, Kochanek PM, Miller HC, Partington MD, Selden NR. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 4. Resuscitation of blood pressure and oxygenation and prehospital brain-specific therapies for the severe pediatric traumatic brain injury patient. Pediatric critical care medicine: a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2003;4:S12–18. [PubMed] [Google Scholar]
- 10.Skippen P, Seear M, Poskitt K, Kestle J, Cochrane D, Annich G, Handel J. Effect of hyperventilation on regional cerebral blood flow in head-injured children. Crit Care Med. 1997;25:1402–1409. doi: 10.1097/00003246-199708000-00031. [DOI] [PubMed] [Google Scholar]
- 11.Diringer MN. Hyperoxia: good or bad for the injured brain? Curr Opin Crit Care. 2008;14:167–171. doi: 10.1097/MCC.0b013e3282f57552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bostek CC. Oxygen toxicity: an introduction. AANA J. 1989;57:231–237. [PubMed] [Google Scholar]
- 13.Jenkinson SG. Oxygen toxicity. New Horiz. 1993;1:504–511. [PubMed] [Google Scholar]
- 14.Tasman W, Patz A, McNamara JA, Kaiser RS, Trese MT, Smith BT. Retinopathy of prematurity: the life of a lifetime disease. Am J Ophthalmol. 2006;141:167–174. doi: 10.1016/j.ajo.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 15.Demchenko IT, Welty-Wolf KE, Allen BW, Piantadosi CA. Similar but not the same: normobaric and hyperbaric pulmonary oxygen toxicity, the role of nitric oxide. Am J Physiol Lung Cell Mol Physiol. 2007;293:L229–238. doi: 10.1152/ajplung.00450.2006. [DOI] [PubMed] [Google Scholar]
- 16.Floyd TF, Clark JM, Gelfand R, Detre JA, Ratcliffe S, Guvakov D, Lambertsen CJ, Eckenhoff RG. Independent cerebral vasoconstrictive effects of hyperoxia and accompanying arterial hypocapnia at 1 ATA. J Appl Physiol. 2003;95:2453–2461. doi: 10.1152/japplphysiol.00303.2003. [DOI] [PubMed] [Google Scholar]
- 17.Bitterman N. CNS oxygen toxicity. Undersea Hyperb Med. 2004;31:63–72. [PubMed] [Google Scholar]
- 18.Menzel M, Doppenberg EM, Zauner A, Soukup J, Reinert MM, Clausen T, Brockenbrough PB, Bullock R. Cerebral oxygenation in patients after severe head injury: monitoring and effects of arterial hyperoxia on cerebral blood flow, metabolism and intracranial pressure. J Neurosurg Anesthesiol. 1999;11:240–251. doi: 10.1097/00008506-199910000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Rossi S, Longhi L, Balestreri M, Spagnoli D, deLeo A, Stocchetti N. Brain oxygen tension during hyperoxia in a swine model of cerebral ischaemia. Acta neurochirurgica Supplement. 2000;76:243–245. doi: 10.1007/978-3-7091-6346-7_49. [DOI] [PubMed] [Google Scholar]
- 20.Verweij BH, Muizelaar JP, Vinas FC, Peterson PL, Xiong Y, Lee CP. Impaired cerebral mitochondrial function after traumatic brain injury in humans. J Neurosurg. 2000;93:815–820. doi: 10.3171/jns.2000.93.5.0815. [DOI] [PubMed] [Google Scholar]
- 21.Diringer MN, Aiyagari V, Zazulia AR, Videen TO, Powers WJ. Effect of hyperoxia on cerebral metabolic rate for oxygen measured using positron emission tomography in patients with acute severe head injury. J Neurosurg. 2007;106:526–529. doi: 10.3171/jns.2007.106.4.526. [DOI] [PubMed] [Google Scholar]
- 22.Davis DP, Meade W, Sise MJ, Kennedy F, Simon F, Tominaga G, Steele J, Coimbra R. Both hypoxemia and extreme hyperoxemia may be detrimental in patients with severe traumatic brain injury. J Neurotrauma. 2009;26:2217–2223. doi: 10.1089/neu.2009.0940. [DOI] [PubMed] [Google Scholar]
- 23.Muizelaar JP, Marmarou A, Ward JD, Kontos HA, Choi SC, Becker DP, Gruemer H, Young HF. Adverse effects of prolonged hyperventilation in patients with severe head injury: a randomized clinical trial. J Neurosurg. 1991;75:731–739. doi: 10.3171/jns.1991.75.5.0731. [DOI] [PubMed] [Google Scholar]