Abstract
We previously showed that knockdown of the anaplerotic enzyme pyruvate carboxylase in the INS-1 832/13 insulinoma cell line inhibited glucose-stimulated insulin release and glucose carbon incorporation into lipids. We now show that knockdown of fatty acid synthase (FAS) mRNA and protein also inhibits glucose-stimulated insulin release in this cell line. Levels of numerous phospholipids, cholesterol esters, diacylglycerol, triglycerides and individual fatty acids with C14-C24 side chains were acutely lowered about 20% in glucose-stimulated pyruvate carboxylase knockdown cells over a time course that coincides with insulin secretion. In FAS knockdown cells glucose carbon incorporation into lipids and the levels of the subclasses of phospholipids and cholesterol ester species were lower by 20–30% without inhibition of glucose oxidation. These studies suggest that rapid lipid modification is essential for normal glucose-stimulated insulin secretion.
Keywords: Insulinoma cells, shRNA, pyruvate carboxylase, fatty acid synthase, phospholipids, cholesterol esters, lipid remodeling
INTRODUCTION
Recent studies of pancreatic beta cells suggested that rapid lipid remodeling of cellular lipids might be important for insulin exocytosis. We noticed that stimulation of INS-1 832/13 insulinoma cells with glucose and other insulin secretagogues acutely increased the level of many lipids with C14-C24 chains, including phospholipids (PLs)1, cholesterol esters (CEs), triglycerides (TGs) and free fatty acids (FFAs), by about 20% [1]. Others [2–7] and we [1] have observed that glucose carbon is rapidly incorporated into lipids in pancreatic islets and in insulin cell lines indicating de novo lipid synthesis from glucose carbon occurs over a time course that coincides with insulin secretion. In addition, the enzyme patterns in pancreatic islets and pancreatic beta cell lines suggest they are a lipogenic tissue. Acetyl-CoA carboxylase is a cytosolic enzyme that catalyzes the formation of malonyl-CoA that cells use for fatty acid synthesis as well as possibly for signaling purposes [8, 9]. Of the two isoforms of acetyl-CoA carboxylase (ACC1 or 2) the one that is present in pancreatic islets of humans and rats, as well as the insulinoma INS-1 832/13 cell line, is ACC1 [1] which is the isoform found in lipogenic tissues. In addition, the level of fatty acid synthase is quite high in human pancreatic islets [10] and in the INS-1 832/13 cell line [10].
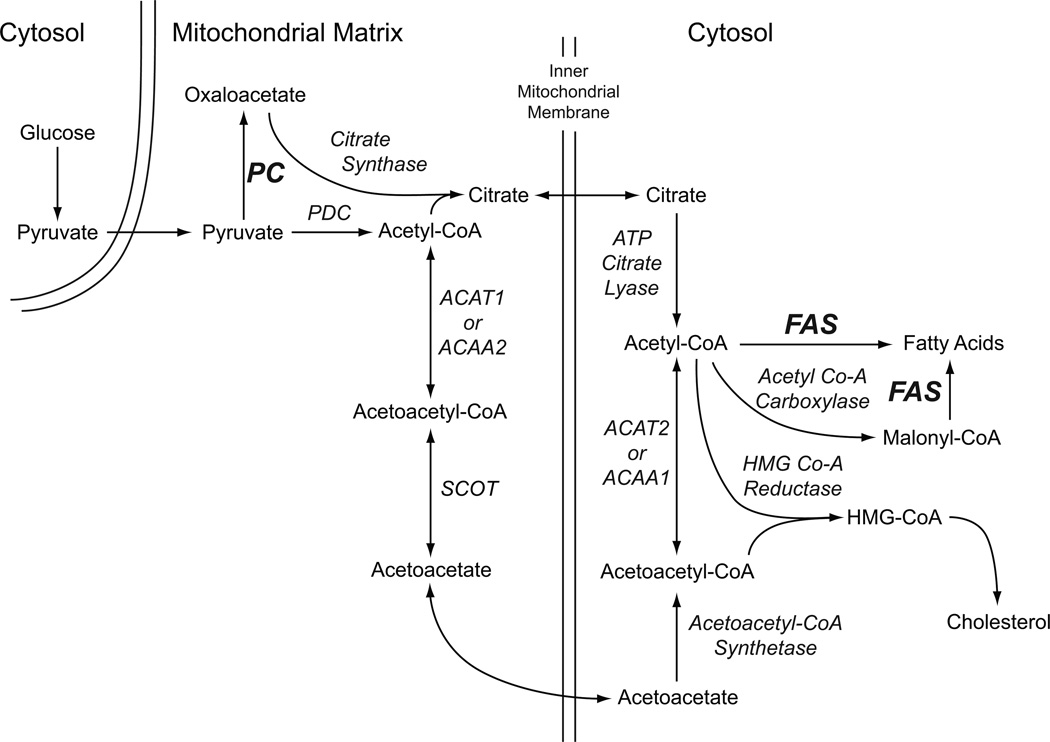
Rodent pancreatic islets [10–13] and various insulin cell lines, such as the INS-1 832/13 cell line [10], contain a high level of the anaplerotic enzyme pyruvate carboxylase. This allows the mitochondria of these cells to synthesize lipid precursors from pyruvate to form malate and citrate. Citrate can be exported from the mitochondria to the cytosol of the beta cell where ATP citrate lyase, which is also abundant in the beta cell [14, 15], can catalyze the conversion of citrate to oxaloacetate and acetyl-CoA. The acetyl-CoA can be converted to malonyl-CoA catalyzed by ACC1 and the acetyl-CoA and malonyl-CoA can both be used for lipid synthesis as shown in Figure 1. Our research [10, 16–20] has provided extensive evidence to suggest that in addition to this classical pathway that provides short chain acyl-CoA precursors to the cytosol for lipid synthesis, pancreatic islets (especially pancreatic islets of humans [10]) possess enzymes for another pathway for the synthesis of short chain acyl-CoA lipid precursors. This pathway also begins in mitochondria, but with acetyl-CoA formed in the reaction catalyzed by the pyruvate dehydrogenase complex. Within the mitochondria the acetyl-CoA can be converted to acetoacetyl-CoA by either acetyl-CoA acetyltransferase 1 (ACAT1) or acetyl-CoA acyltransferase 2 (ACAA2) and then to acetoacetate by succinyl-CoA:3-ketoacid-CoA transferase (SCOT). The acetoacetate can then be exported from the mitochondria to the cytosol where, via the reactions that begin with acetoacetyl-CoA synthetase (AACS), it can be converted into acetyl-CoA and malonyl-CoA for lipid synthesis (Figure 1) [10, 16–20].
Figure 1. Pathways of formation of lipid from glucose-derived pyruvate in the pancreatic beta cell.
Abbreviations: ACAA1 or 2, acetyl-CoA acyltransferase 1 or 2; ACAT1 or 2, acetyl-CoA acetyltransferase 1 or 2; FAS, fatty acid synthase; PC, pyruvate carboxylase; PDC, pyruvate dehydrogenase complex; SCOT, succinyl-CoA:3-ketoacid-CoA transferase.
Human pancreatic islets and the INS-1 832/13 insulinoma cell line, but less so islets of rats, possess a high level of fatty acid synthase [10, 21]. We have found that application of small molecule inhibitors of either acetyl-CoA carboxylase or fatty acid synthase to rat pancreatic islets and INS-1 832-13 insulinoma cells lowers insulin release [1] suggesting rapid lipid synthesis is important for insulin secretion. In contradiction to the idea that lipid synthesis is necessary for insulin secretion, Joseph et al [22] knocked down fatty acid synthase mRNA levels 81% in the INS-1 832/13 cell line and observed a 59% decrease in [U-14C]glucose incorporation into lipid, but did not see a decrease in glucose-stimulated insulin release. They also knocked down fatty acid synthase mRNA levels 52% in rat pancreatic islets without observing a decrease in glucose stimulated insulin release [22]. Neither the Newgard group with adenoviral delivery of siRNA that targeted ATP citrate lyase [21], nor our laboratory with stable transfection of multiple different shRNAs that knocked down ATP citrate lyase, has detected a lowering of glucose-stimulated insulin release in INS-1 832/13 cells [19]. Guay et al [23] did observe inhibition of insulin release with siRNA that targeted ATP citrate lyase mRNA in this cell line. The severe knockdown of ATP citrate lyase activity without inhibition of insulin release suggests that the pathway that uses ATP citrate lyase is redundant with another pathway that exports short chain acyl-CoAs from the mitochondria to the cytosol. The pathway that uses SCOT and AACS is likely the redundant pathway (Figure 1). In further support of this idea, the cell lines we have generated from the INS-1 832/13 cell line with either knocked down SCOT [17] or AACS [19] show decreased glucose-stimulated insulin release.
To further study the idea that rapid lipid synthesis could be important for insulin secretion we measured the levels of glucose-stimulated individual lipids in our previously generated cell lines with knocked down pyruvate carboxylase that were shown to have severely inhibited glucose-stimulated insulin release [24]. Pyruvate carboxylase catalyzes a reaction near the beginning of pathways for lipid synthesis (Figure 1). Inhibition of insulin secretion via knockdown of pyruvate carboxylase could affect processes intermediate between its reaction and the final stages of lipid synthesis. Therefore, to study the effect of knockdown of an enzyme that catalyzes a reaction closer to the end of the pathways for synthesis of individual lipids, we also generated a series of cell lines from INS-1 832/13 cells using stably integrated shRNA constructs that target the fatty acid synthase gene. We studied insulin release and measured insulin content in the cell lines with knocked down fatty acid synthase and measured the levels of lipids with C14 – C24 side chains, such as PLs and CE, as well as the rate of incorporation of glucose carbon into lipid. The results show that the secretagogue-stimulated insulin release and levels of numerous lipids were knocked down proportionate to fatty acid synthase knockdown without a decrease in glucose oxidation. The levels of many lipid species were also lowered proportionate to knockdown of pyruvate carboxylase in the cell lines in which the pyruvate carboxylase gene was targeted. The results suggest rapid remodeling of cellular lipids occurs during insulin exocytosis.
EXPERIMENTAL PROCEDURES
Materials and methods
pSilencerTM hygro was from Ambion (Austin TX). The polyclonal antibody against fatty acid synthase (H-300) was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The INS-1 832/13 cell line [25] was a gift from Chris Newgard. Lipid mixture 1 (Catalog no. L0288) was from Sigma-Aldrich (St. Louis, MO). All other chemicals, in the highest purity available, were from Sigma-Aldrich (St. Louis, MO).
Generation of fatty acid synthase knockdown and control cell lines
The control CHS cell line contained a nontargeting DNA sequence and was described previously [24]. All target DNA sequences were 19 nucleotides long. These were contained within the rat Fasn targeting vector which was a 64-bp DNA insert that codes for shRNAs cloned into the BamHI and the HindIII sites downstream of the U6 promoter of the plasmid pSilencer 2.1-U6/hygro. For stable expression of shRNAs that are processed in vitro to generate siRNA that targets Fasn mRNA, the vectors were transfected into INS-1 832/13 cells and the hygromycin resistant stable cell lines were isolated as described previously [24]. The rat Fasn mRNA (GenBankTM accession number NM_017332) targets used were: Fasn118, GGAAATTGCCCGAGTCAGA (corresponds to nucleotides 113–131); Fasn171, GGACATGGTCACAGACGAT (corresponds to nucleotides 171–189) Fasn 1115, GGTGCTGTTATCCCTAGAA (corresponds to nucleotides 1110–1128); and Fasn 6282, GCGATGGGTACCAATGACA (corresponds to nucleotides 6277–6295). The design of target sequences was performed using Web-based siRNA design engines (Supplemental Table 1).
The transfection of vectors into INS-1 832/13 cells was performed using Lipofectamine 2000 reagent (Invitrogen). Following transfection, the cells were incubated overnight without selection. Subsequently, the cells were grown in the presence of 150 µg/ml hygromycin in INS-1 cell culture medium (RPMI 1640 medium (contains11.1mM glucose) supplemented with 10% fetal calf serum, 1mM sodium pyruvate, 50 µM β-mercaptoethanol, 100 units/ml penicillin and 100 µg/ml streptomycin in 10 mM sodium Hepes buffer, pH 7.3) [19, 26, 27]. Pools of hygromycin resistant cells stably transfected with each shRNA vector directed against one fatty acid synthase mRNA target were subsequently maintained in INS-1 cell culture medium supplemented with 10 ml per liter of Sigma Lipid mixture 1 to enhance the cell growth. Prior to the various measurements, the lipid mixture was withheld for one week and two subcultures in INS-1 medium only. Hygromycin was continuously present in the medium.
Maintenance of cells before experiments
Cells previously maintained in the presence of the Lipid Mixture 1 were subcultured for four days in INS-1 cell culture medium without a lipid supplement and then for an additional three days without the supplement in individual 100 mm or 150 mm tissue culture plates or 24-well tissue culture plates. The cells were then processed for the isolation of total mRNA, insulin release studies or lipid measurements.
Real Time Quantitative Reverse Transcription PCR (qRT-PCR) mRNA quantification
Cells grown on 100 mm plates were harvested at 60–80% confluence as previously described [24]. The cells were washed twice with 10 ml of phosphate-buffered saline and then lysed and the total RNA was prepared using the RNeasy-minikit (Qiagen), with on-column deoxyribonuclease digestion (RNase-free DNase set, Qiagen). cDNA was prepared using 1.5 µg of total RNA using the RETROscriptTM kit (Ambion/Applied Biosystems) with oligo(dT) primers. Real-time PCR was performed on a Bio-Rad MyiQTM single color real-time PCR detection system. A standard curve prepared from the CHS cell cDNA was included in each run for relative quantitation. Quantification of glutamate dehydrogenase mRNA (Glud1) was used as an internal control. Real time quantification of Fasn mRNA was carried out using the forward primer GACAGTGGCAACCTGATAGTGAGC, and the reverse primer ATACTTCTCCCTGCGTCAAGCG. The primers for Glud1 (NM_012570) were forward CGAGAAGCAGTTGACCAAATCC, and reverse CACTCCTCCAGCATTCAGGTAGAG. The primers for Ins1 and Ins2 mRNA were: Ins1 forward ACCATCAGCAAGCAGGTCAT and Ins1 reverse CACTTGTGGGTCCTCCACTT; Ins2 forward CCTGCTCATCCTCTGGGAGCCCCGC and Ins2 reverse CTCCAGTGCCAAGGTCTGAAGGTCA.
Western Blots
For immunoblotting of fatty acid synthase the cells were grown without lipid supplement as described above and were washed twice with PBS and resuspended in KMSH buffer containing protease inhibitor. The protein concentration was measured by the Bradford assay in cells lysed in sample buffer (1% SDS, 5% glycerol, 65 mM Tris-chloride, pH 6.8, 0.01% bromphenol blue and either 100 mM dithiothreitol or 2.5% β-mercaptoethanol) using bovine serum albumin (BSA) as a standard. Proteins were separated by electrophoresis on 7.5% polyacrylamide SDS gels and electrotransfered to nitrocellulose membranes. The membranes were blocked with a mixture of 5% milk in Tris-buffered saline (TBS) and incubated overnight at 4°C with a suitable dilution of rabbit polyclonal anti-fatty acid synthase antiserum in blocking buffer. The membranes were washed and treated with the secondary antibody labeled with goat anti-rabbit horseradish peroxidase in blocking buffer and proteins were visualized with the Supersignal West Pico Chemiluminescent Substrate Kit (Pierce). Luminescence was captured on a Chemi Doc XRS Gel Documentation System (BioRad) or on KSR-B Luc Ultra Autorad X-ray film (ISC Bioexpress). To demonstrate equal loading of proteins per lane, the membranes were washed in TBS containing 0.05% Tween) and incubated in Restore Western Blot Stripping Buffer (Pierce) and reprobed with anti-β-actin antibody (Sigma).
Insulin release experiments
Insulin release and insulin measurements were described previously [24]. Insulin release experiments were performed in 24-well tissue culture plates. One day before an insulin release experiment was to be performed, the glucose concentration in the cell culture medium was reduced to 5 mM. Two hours before the experiment, the medium was replaced with Krebs-Ringer bicarbonate buffer, pH 7.3 (modified to contain 15 mM Hepes and 15 mM NaHCO3 with the NaCl concentration adjusted to maintain osmolarity at 310) containing 3 mM glucose and 0.5% bovine serum albumin (BSA). Cells were washed once with the Krebs-Ringer Hepes BSA solution, and incubated in 1 ml of this same solution in the presence or absence of secretagogues. After 1 h at 37 °C, samples of incubation solution were collected and centrifuged to sediment any cells floating in the incubation solution. An aliquot of the supernatant fraction was removed and saved for insulin measurements by radioimmunoassay. The plates were then washed once with Krebs-Ringer solution containing no BSA, water was added to the plates, and the mixture containing the cells was removed and saved for estimation of total protein by the Bradford method using a dye reagent from Bio-Rad.
Insulin Content of Cells
Cells from individual wells of a 24-well plate incubated only in Krebs-Ringer Hepes BSA solution in the absence of secretagogues were suspended in 1 ml of 75% ethanol and 0.17 M HCl and used to determine the insulin content of the sample by radioimmunoassay, as previously described [28].
Measurement of 14C incorporation from glucose
The incubation of cells and the measurement of incorporation of 14C from [U-14C]glucose into CO2, lipid and other anaplerotic products was performed as previously described [1].
Lipid composition
INS-1 832/13 cells (Passage Nos. 10–15) were maintained on 150 mm plates for 22 h in INS-1 tissue culture medium containing 5 mM glucose as described above. Plates containing pyruvate carboxylase-targeted cells were washed twice with warm phosphate-buffered saline and cells were incubated in the presence of the modified Krebs Ringer bicarbonate Hepes buffer, pH 7.3, containing 11.1 mM glucose. After 40 min and cells were scraped from each plate into individual test tubes and 50 µl of the suspension was removed and saved for estimation of total protein. Four milliliters per test tube of chloroform/methanol (2:1) containing 0.01% butylated hydroxytoluene (BHT) was added and cells were vortexed vigorously for 30 min and the mixture allowed to set for 22 h in capped tubes. Lipids were extracted by the method of Bligh and Dyer [29] and separated on silica gel 60 thin layer chromatography plates (EMD). Lipid classes were extracted from the plates in chloroform/methanol (4:1) containing 0.01% BHT. The extract was evaporated to dryness and lipids were methylated by boiling in 14% BF3 in methanol. Hexane and water were added and the mixture was centrifuged. The organic layer was transferred to a test tube and evaporated under nitrogen and resuspended in hexane and analyzed by gas chromatography, as previously described [1, 30, 31]. Cells from fatty acid synthase-targeted cells were harvested after they were maintained for 22 h in INS-1 cell culture medium containing 5 mM glucose the same as for the pyruvate carboxylase-targeted cells except that 1.5 ml instead of 4 ml of chloroform/methanol/BHT was added to each test tube for lipid extraction. Extracts from the pyruvate carboxylase-targeted cells were analyzed in the laboratory of AD and those from the fatty acid synthase-targeted cells were analyzed in the laboratory of JMN.
Data analysis
Statistical significance, when performed, was confirmed with Student’s t-test. Statistical analysis on the measurements of individual lipids is not shown in the figures to increase the clarity. Measured small differences among levels of individual lipids could be biologically relevant whether statistically significant or not. The general consistency in the directions of the changes in most of the lipids within cell lines, or among different cell lines containing a construct that targets the same gene and the positive correlation with inhibited insulin release, suggests the individual and collective changes have biological meaning regardless of the statistical significance of the changes.
RESULTS
Knockdown of pyruvate carboxylase lowers glucose-stimulated levels of lipids
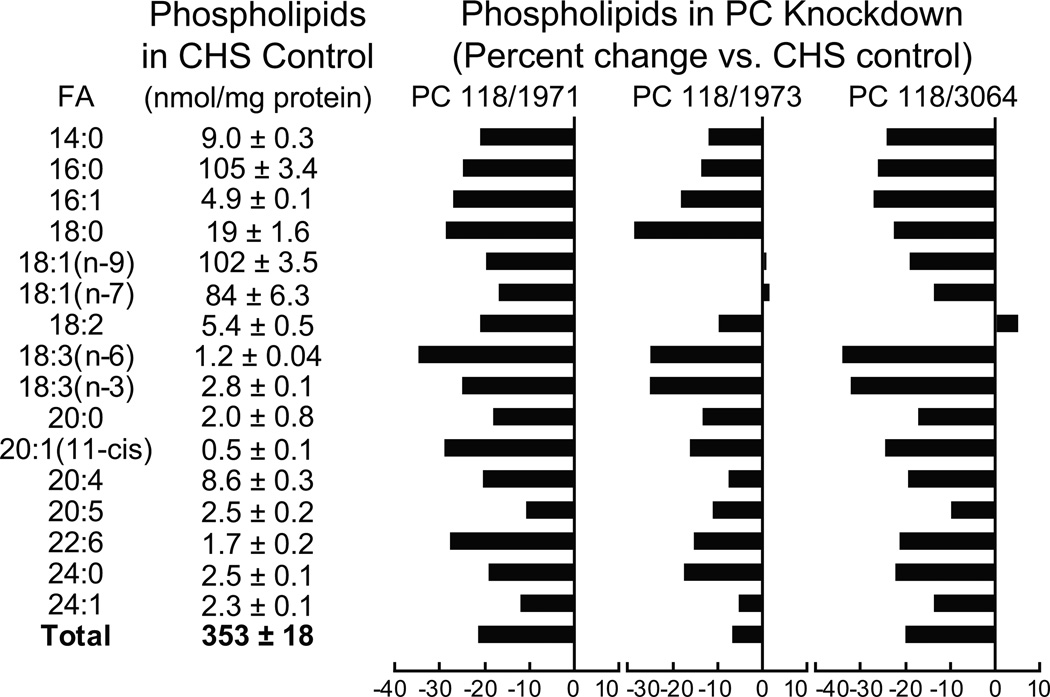
We previously showed, with a series of more than ten INS-1 832/13-derived cell lines that possessed various shRNA-lowered levels of pyruvate carboxylase expression, that glucose-stimulated insulin release was lowered in proportion to the lowering of the pyruvate carboxylase mRNA and enzyme activity [24]. We selected three of the cell lines with severely lowered pyruvate carboxylase mRNA and enzyme activity (PC118/1971, 91% and 88%; PC118/1973, 75% and 60%; and PC118/3064, 87% and 78% (percent decrease in pyruvate carboxylase mRNA and pyruvate carboxylase activity, respectively) compared to the parent INS-1 832/13 cell line) for studies of lipids. Glucose-stimulated insulin release was lowered about 75% in these three cell lines compared to the CHS control cell line) [24]. Figures 2 and 3 show the levels of lipids in three glucose-stimulated (for 40 min) cell lines with knocked down pyruvate carboxylase compared to the levels in the CHS control cell line that contains a nontargeting shRNA. In general, levels of lipids were lowered in proportion to the knockdown of pyruvate carboxylase expression with greater decreases of lipids in the PC118/1971 and PC118/3064 cell lines than in the PC118/1973 cell line which is the cell line with less severe knockdown of pyruvate carboxylase.
Figure 2. Lower levels of many individual and total lipids in five lipid classes in glucose-stimulated INS-1 832/13-derived cell lines with knocked down pyruvate carboxylase.
The contents of lipids in the CHS control cell line are shown in table format and the percent increases or decreases in individual lipids in the pyruvate carboxylase knockdown cell lines are shown in bar graph format. The levels of knockdown of pyruvate enzyme activity in the PC118/1971, PC118/1973 and PC118/3064 cell lines were 88%, 60% and 78%, respectively (from reference 24). Cells were incubated in the presence of 11.1 mM glucose (a concentration of glucose that gives near maximal insulin release in the INS-1 832/13 cell line) in Krebs Ringer bicarbonate solution, pH 7.3, for 40 minutes before cells were harvested for lipid analysis. The number of replicate incubations were 6 per cell line except for PC118/1973 where N = 3. For clarity standard error bars are not shown in the graphs.
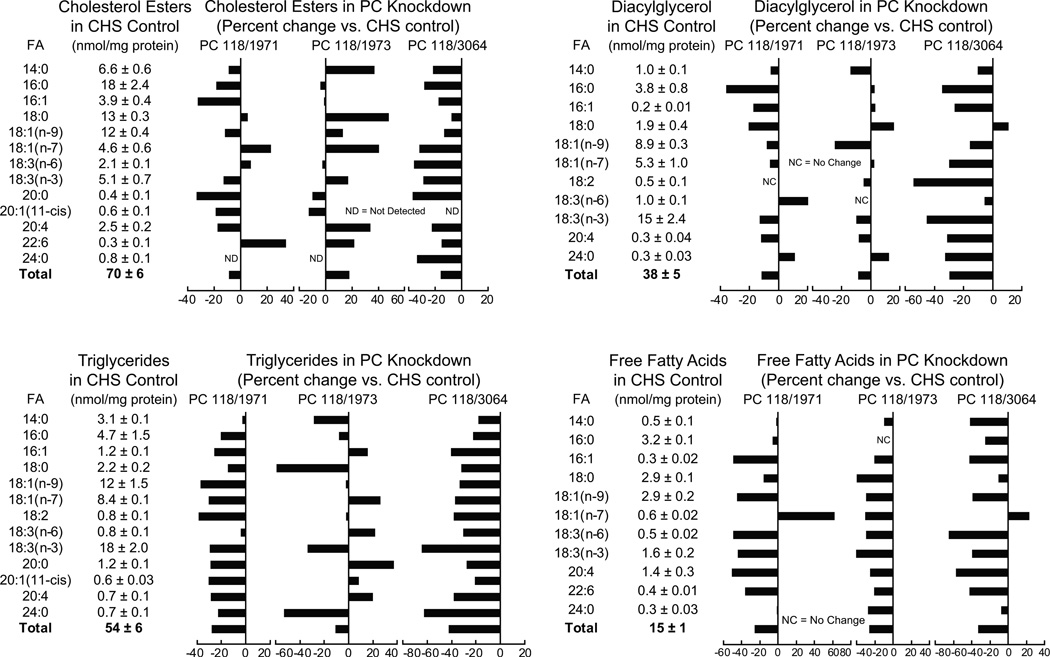
Figure 3. Lower levels of many individual and total lipids in five lipid classes in glucose-stimulated INS-1 832/13-derived cell lines with knocked down pyruvate carboxylase.
The contents of lipids in the CHS control cell line are shown in table format and the percent increases or decreases in individual lipids in the pyruvate carboxylase knockdown cell lines are shown in bar graph format. The levels of knockdown of pyruvate enzyme activity in the PC118/1971, PC118/1973 and PC118/3064 cell lines were 88%, 60% and 78%, respectively (from reference 24). Cells were incubated in the presence of 11.1 mM glucose (a concentration of glucose that gives near maximal insulin release in the INS-1 832/13 cell line) in Krebs Ringer bicarbonate solution, pH 7.3, for 40 minutes before cells were harvested for lipid analysis. The number of replicate incubations were 6 per cell line except for PC118/1973 where N = 3. For clarity standard error bars are not shown in the graphs.
Phospholipids
The levels of almost all PLs with various fatty acid side chains and total PLs were lower by about 20% in all three of the glucose-stimulated cell lines with knocked down pyruvate carboxylase (Figure 2).
Cholesterol esters (CEs)
In comparison with the glucose-stimulated CHS control cell line, the levels of many glucose-stimulated individual CEs and total CEs were lower in the two cell lines with the more severely knocked down pyruvate carboxylase, PC118/1971 and PC118/3064. The cell line PC118/1973 with a smaller knockdown of pyruvate carboxylase did not show lower levels of most CEs (Figure 3).
Diacylglycerols
The levels of many of the DAG lipids, as well as total DAG lipids, were lower by 10–20% in all three glucose-stimulated pyruvate carboxylase knockdown cell lines (Figure 3).
Triglycerides
Knockdown of pyruvate carboxylase lowered all TGs with various fatty acid side chains and the total triglycerides in the PC118/1971 and PC118/3064 cell lines, typically 20–40%, and some of the triglycerides in the PC118/1973 cell line (Figure 3).
Free fatty acids
In all three glucose-stimulated cell lines with knocked down pyruvate carboxylase, almost all individual FFAs were lowered about 40%. Total free fatty acids were lowered 20–30% in the three cell lines (Figure 3).
There was no consistent change in any of the lipid classes as a percent of total across the three cell lines with knocked down pyruvate carboxylase (Supplemental Tables 2–6 online).
It was previously shown that the incorporation of glucose carbon into anaplerotic products, including the lipid fraction, was decreased in cell lines with knocked down pyruvate carboxylase [24]. In the current study, cells with knocked down pyruvate carboxylase and control cells were incubated with BCH, a nonmetabolizable leucine analog that activates glutamate dehydrogenase, in order to stimulate the conversion of glutamine-derived glutamate into α-ketoglutarate which feeds into mitochondrial anaplerotic pathways at different entry points than glucose-derived carbon. Table 1 shows that the incorporation of 14C-labeled glutamine carbon into lipid, as well as other anaplerotic products, was also lowered in cell lines with knocked down pyruvate carboxylase.
Table 1. Pyruvate carboxylase knockdown lines show decreased incorporation of glutamine carbon into anaplerotic products and into lipid.
Cells were maintained for 24 h in RPMI cell culture medium containing 5 mM glucose and without pyruvate and then harvested and incubated in suspension for 45 min in the presence of 10 mM BCH and 10 mM [U-14C]glutamine (specific radioactivity 2.5 mCi/mmol) followed by extraction of the radioactivity incorporated into anaplerotic products and a lipid fraction. Results are expressed as the mean ± SE from four replicate incubations.
| Cell Line | Glutamine Incorporation | |
|---|---|---|
| (nmol/mg cell protein/h | ||
| Anaplerotic Products | Lipid Fraction | |
| U6 (Control) | 0.64 ± 0.05 | 0.20 ± 0.01 |
| PC118/1973 | 0.49 ± 0.03a | 0.13 ± 0.01b |
| PC118/3064 | 0.34 ± 0.015b | 0.11 ± 0.002c |
p < 0.05,
p < 0.005 and
p < 0.001 vs control
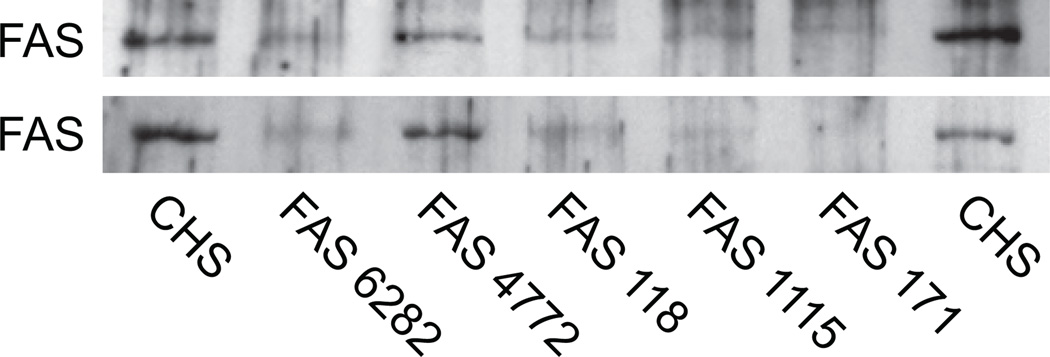
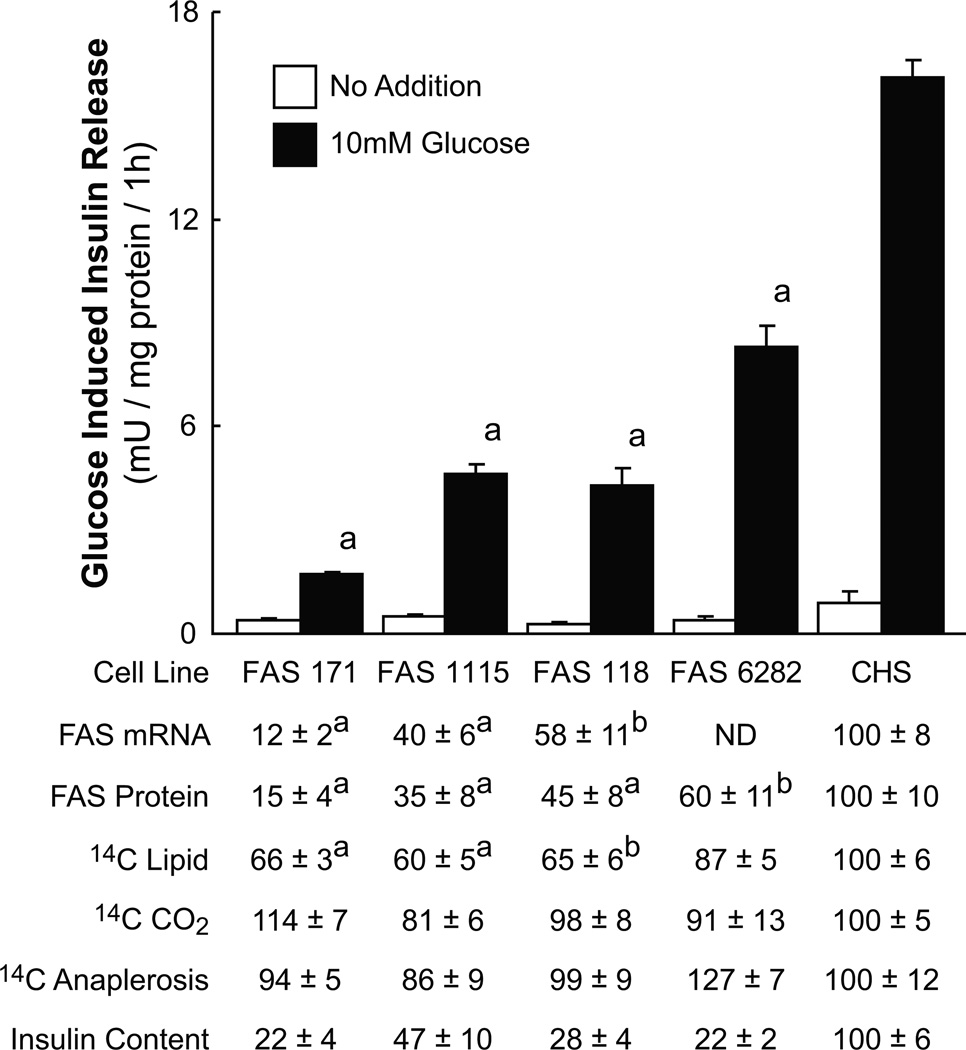
Knockdown of fatty acid synthase lowers insulin release and incorporation of glucose carbon into lipid
We used stable transfection with shRNAs to generate a series of cell lines with various levels of fatty acid synthase mRNA and fatty acid synthase protein as judged from qRT-PCR and western analysis (Figures 4 and 5). Figure 5 shows that the glucose-stimulated insulin release from these cell lines was lowered roughly in proportion to the knockdown of fatty acid synthase mRNA and fatty acid synthase protein. In these cell lines the incorporation of 14C from [U-14C]glucose into lipid was also lowered roughly in proportion to the extent of decreases in fatty acid synthase (Figure 5). Consistent with a specific effect on lipid synthesis, the glucose oxidation to CO2 and incorporation of 14C into products of anaplerosis was not consistently affected by knockdown of fatty acid synthase (Figure 5). The glycerol backbone of PL and TG is derived from a 3-carbon glycolytic intermediate. The fact that glucose carbon must pass through 3-carbon glycolytic intermediates before incorporation into CO2 and anaplerotic products and carbon incorporation into CO2 and anaplerotic products was not lowered in the Fasn knockdown cells suggests the lower carbon incorporation into lipids was due to decreased carbon into fatty acids of the lipids, rather decreased incorporation into the glycerol backbone of PLs and TGs. The normal enzyme activities of ATP citrate lyase measured as a control in the cell lines with knocked down fatty acid synthase were also consistent with the specificity of the knockdown of fatty acid synthase. These activities ranged from 93% to 113% of the activity of the CHS control cell line (Supplemental Table 7 online). As we have previously seen in other cell lines with inhibited lipid synthesis [28], the insulin content of the cells with lowered fatty acid synthase was also low (Figure 5). The lowered insulin content is likely due to decreased insulin synthesis as judged from the lower rat insulin mRNA levels measured with qPCR in the cells with knocked down fatty acid synthase; also similar to other of our cell lines with impaired lipid synthesis [28]. The levels of rat Ins1 and Ins2 mRNAs in the fatty acid synthase targeted cell lines compared to the CHS control cell line were (Ins1: CHS, 100 ± 5% (4); FAS118, 19 ± 3% (8); FAS171, 23 ± 4% (5); FAS1115, 23 ± 5% (5); and FAS6282, 38 ± 4% (4) and INS2: CHS, 100 ± 2% (4); FAS118, 13 ± 1% (8); FAS171, 14% (2); FAS1115, 36 ± 2% (4); and FAS6282, 41 ± 8% (4)), whereas the levels of isocitrate dehydrogenase 1 mRNA (Ich1) levels were not consistently different from levels in the CHS cell line (CHS, 100 ± 16% (4); FAS118, 80 ± 7% (4); and FAS1115, 107 ± 7% (4) (means ± SE (N)).
Figure 4. Immunoblots showing lower levels of fatty acid synthase protein in INS-1 832/13-derived cell lines containing shRNA targeting the Fasn mRNA.
There was 20 and 22 µg whole cell protein/lane in the upper and lower panels, respectively.
Figure 5. Lower insulin release in INS-1 832/13 cell lines is proportional to knockdown of fatty acid synthase (FAS) enzyme activity and lowered lipid synthesis.
Insulin release is the average ± SE of 12–24 replicate incubations (in the presence of 11.1 mM glucose for 1 h). mRNA levels were estimated by qPCR on 6–9 replicate plates of each cell line and FAS protein levels were estimated by western analysis and densitometry and are the average ± SE of measurements on 4–5 separate cell preparations. Except for the insulin release values, results are expressed as a percent ± SE of the CHS cell line set at 100%. The values for incorporation of 14C into lipid, CO2 or anaplerotic products (14C anaplerosis) from incubating cells (Passage numbers 4–13) in the presence of 16.7 mM glucose [U-14C]glucose (specific radioactivity 0.2 µCi/mmol) for 1 h and are the averages of three experiments with 4–6 replicate incubations for each cell line per experiment. The average glucose incorporation into lipid, CO2 and the anaplerotic products for the CHS control cell line were 2.3 ± 0.3, 10.8 ± 0.7 and 3.5 ± 0.5 nmol glucose/mg whole cell protein, respectively. The insulin contents are expressed as a percent of the control CHS cell line (70 ± 4 mUnits insulin/mg whole cell protein) set at 100%. The incorporation of 14C into lipid was lower in the cell lines with targeted FAS expression, but the 14C incorporation into CO2 or anaplerotic products, which indicate glucose oxidation and anaplerosis, respectively, were not lower. This pattern is consistent with knockdown of FAS exerting a specific, and not generalized, effect on cell metabolism.
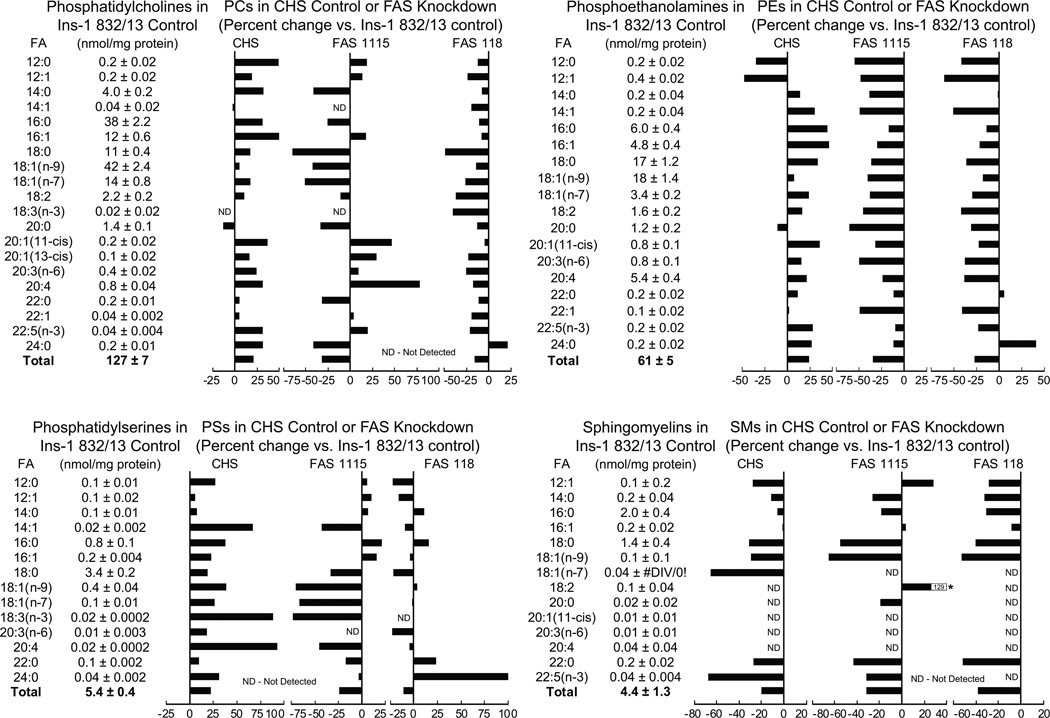
Fatty acid synthase knockdown lowers levels of various lipids
Figures 6 and 7 show the levels of lipids in the cell lines with knocked down fatty acid synthase mRNA and protein (FAS1115 and FAS118). In general, the results showed that the levels of many of the lipids were lower in the cell lines with knocked down fatty acid synthase expression, especially the levels of the more abundant lipids that can be more accurately measured.
Figure 6. Lower levels of individual and total lipids in INS-1 832/13-derived cell lines with knocked down fatty acid synthase.
The contents of the lipids in the INS-1 832/13 parent cell line are shown in table format and the percent increases or decreases in the CHS cell line that contains a nontargeting DNA sequence and the two cell lines, FAS115 and FAS118, containing a stably integrated DNA sequence that targets fatty acid synthase (FAS) mRNA, are shown in bar graph format. Cells were maintained in RPMI 1640 medium containing 10% fetal bovine serum and 5 mM glucose for 24 h before harvesting for lipid analysis. Results are the mean ± SE for 3 replicate incubations for each cell line. To increase clarity standard error bars are not shown in the graphs.
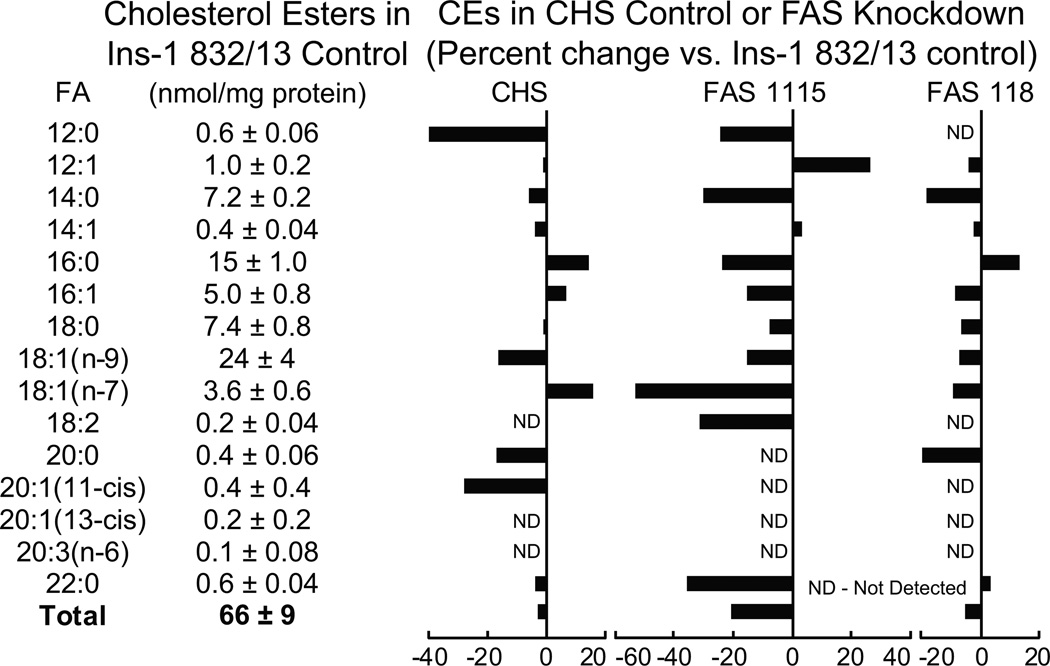
Figure 7. Lower levels of individual and total lipids in INS-1 832/13-derived cell lines with knocked down fatty acid synthase.
The contents of the lipids in the INS-1 832/13 parent cell line are shown in table format and the percent increases or decreases in the CHS cell line that contains a nontargeting DNA sequence and the two cell lines, FAS115 and FAS118, containing a stably integrated DNA sequence that targets fatty acid synthase (FAS) mRNA, are shown in bar graph format. Cells were maintained in RPMI 1640 medium containing 10% fetal bovine serum and 5 mM glucose for 24 h before harvesting for lipid analysis. Results are the mean ± SE for 3 replicate incubations for each cell line. To increase clarity standard error bars are not shown in the graphs.
Phosphatidylcholines
PCs are very abundant in beta cells [1–7]. The levels of most PCs were slightly higher in the control CHS cell line that contains a nontargeting vector, in comparison with the INS-1 832/13 parent cell line. In contrast, the levels of almost all of the PCs were lower in the FAS1115 and FAS118 cell lines. Although the sum total of the PCs was slightly higher in the control CHS cell line than in the parent INS-1 832/13 cell line, the total PC levels in the FAS1115 and FAS118 cell lines were lower (Figure 6).
Phosphatidylethanolamines (PEs)
The levels of many PEs in INS-1 832/13 cells are quite high. While the levels of most of the PEs were unchanged or increased in the CHS cell line in comparison with the INS-1 832/13 cell line, the levels of most of the PEs with various fatty acid side chains, as well as the sum total of the PE levels were lower in the FAS1115 and FAS118 cell lines (Figure 6).
Phosphatidylserines (PSs)
Although the levels of PSs were much lower than those of the PCs and the PEs the levels of the PSs were slightly higher in the CHS cell line in comparison with the INS-1 832/13 cell line, the levels of most of the lipids as well as the sum total levels, were lower in the FAS1115 and FAS118 cell lines (Figure 6).
Sphingomyelins
The level of all sphingomyelins were very low in the parent INS-1 832/13 cell line and the cell lines derived from it. Thus the accuracy of the sphingomyelin measurements might be low. The levels of most of the sphingomyelin lipids were not significantly lower in the two fatty acid synthase knockdown cell lines than the control CHS cell line. However, the levels of the sum total of the sphingomyelin levels were lower in the two cell lines with knocked down fatty acid synthase (Figure 6).
Cholesterol esters
The levels of CEs with abundant fatty acid side chains, such as 14:0, 16:0, 18:1 (n-9) and 18:1 (n-7) in the CHS control cell line were slightly lower or were slightly increased in comparison with the parent INS-1 832/13 cell line. In contrast, the levels of most of the CE lipids with the more abundant side chains were lowered more in cell lines FAS1115 and FAS118 with knocked down fatty acid synthase. The level of the sum totals of the lipids in the FAS1115 cell line was significantly lower than that of the CHS cell line (Figure 7).
The levels of lipids across the cell lines with knocked down fatty acid synthase, when expressed as a percent of the total, were not significantly different from the control cell line (Supplemental Table 8–12 online).
DISCUSSION
The results of the current study in which multiple shRNA constructs were used to target the pyruvate carboxylase and fatty acid synthase genes support the idea that rapid lipid remodeling is necessary for normal insulin secretion. The use of four or more constructs to successfully knockdown the level of each enzyme in general proportion to the degree of inhibition of insulin release and lipid synthesis mitigates against off target effects as an explanation for the results. We previously showed that numerous individual phospholipids, sphingomyelins and cholesterol esters were increased in INS-1 832/13 cells after a 30 minute period of stimulation with glucose [1]. Others [2–7] and we [1] have shown that glucose carbon is incorporated into lipid in glucose-stimulated pancreatic islets and/or insulin cell lines over short time intervals indicating rapid de novo synthesis of lipid from glucose carbon can occur in the beta cell concurrent with the time course of insulin secretion. De novo lipid synthesis from glucose requires mitochondrial anaplerosis catalyzed by the classic pathway starting with pyruvate carboxylase in the mitochondria and culminating with ATP citrate lyase in the cytosol or via an alternative pathway (as discussed below) (Figure 1). One of the apparent mysteries of the beta cell is that it contains a high level of the anaplerotic enzyme pyruvate carboxylase. Pyruvate carboxylase is often thought of as a gluconeogenic enzyme and its level in the islet beta cells [11, 32] is equal to that in gluconeogenic tissues, such as liver. Since the beta cell has no need for and is unable to synthesize glucose from pyruvate [12, 33–35], pyruvate carboxylase has to be present in the beta cell for other anaplerotic purposes. A requirement for rapid lipid remodeling and synthesis may be one of the reasons for the high level of pyruvate carboxylase in the beta cell.
We previously showed that lowering pyruvate carboxylase with shRNA technology in the INS-1 832/13 cell line lowers insulin release stimulated by glucose and other secretagogues [24]. Knockdown of pyruvate carboxylase should inhibit lipid synthesis near the beginning of pathways for lipid synthesis (Figure 1). In the current study, the levels of many individual phospholipids and cholesterol esters with fatty side chains from 12 to 24 carbon side chains were lower by about 20% in glucose-stimulated cell lines with knocked down pyruvate carboxylase (Figures 2 and 3). Virtually all PLs with various fatty acid side chain lengths were decreased by pyruvate carboxylase knockdown, while lowering of CEs, DAGs and TGs was less consistent and slightly less pronounced. Among the PL, CE, DAG, TG and FFA individual lipids that were decreased, the ones that were lowered the most and/or most often were often those with unsaturated fatty acid chains 16:1, 18:0, 18:3 (n-6), 18:3 (n-3), 18:1, 22:6 (in PL and FFA) and 20:4 and also 24:0 (Although because of the intrinsic low levels of the lipids containing very long carbon chain fatty acids, the accuracy of the measurements of the very long chain lipid species might be low.). These data might suggest that low pyruvate carboxylase activity is accompanied by reduced activity of intracellular desaturases and/or changes in partitioning of fatty acids into different intracellular compartments, including intracellular membranes, such as membranes in the Golgi and insulin secretory granules.
Besides a pathway involving pyruvate carboxylase and ATP citrate lyase, we have found evidence to support the presence of another mitochondrial-to-cytosol pathway for the synthesis of lipid precursors that does not involve ATP citrate lyase [16–20] in beta cells and especially in human pancreatic islets [10]. Although this other pathway uses pyruvate dehydrogenase to form acetyl-CoA, the cell needs pyruvate carboxylase to prevent decreases in the levels of citric acid cycle intermediates. The acetyl-CoA is converted to acetoacetyl-CoA and then to acetoacetate by succinyl-CoA:3-ketoacid-CoA transferase (SCOT) in the mitochondria. The acetoacetate is exported to the cytosol where it is converted to acetoacetyl-CoA and then to the precursors for lipid synthesis acetyl-CoA and malonyl-CoA (Figure 1).
Knockdown of fatty acid synthase, which acts nearer to the end of lipid synthesis pathways, would be expected to lower lipid formed from precursors derived from either the classical or the “alternate and redundant” pathway (Figure 1). In the cell lines with lowered fatty acid synthase mRNA and fatty acid synthase protein, glucose-stimulated insulin release and 14C incorporation from [U-14C]glucose into lipid was lowered in proportion to the amount of fatty acid synthase knockdown (Figure 5). In addition, the levels of various individual phospholipids with side chains of from 12 to 20 carbons, including PC, PE, PS and sphingomyelins that are components of cell membranes were lowered in cell lines with knocked down fatty acid synthase in comparison with the CHS control cell line that contains a nontargeting shRNA transcript or with the parental INS-1 832/13 cell line that was used to derive the cell lines with lowered fatty acid synthase (Figure 6). The levels of CEs were also lower in the cell lines with low fatty acid synthase levels (Figure 7), but similar to the cell lines with knocked down pyruvate carboxylase, CEs were lowered less consistently and slightly less extensively in the cells with knocked down fatty acid synthase. Among the lipids that showed decreased levels in the cell lines with low fatty acid synthase activity, ones containing fatty acid chains 18:0, 18:1, 18:1 (n-7) and 18:1 (n-9) were lowered the most and/or most consistently. It is interesting that mice lacking stearoyl-CoA desaturase, an enzyme involved in the synthesis of 18:1 fatty acid, showed decreased glucose-stimulated insulin secretion and a pool of beta cells displayed hallmarks of saturated fatty acid-induced lipotoxicity [36]. It is possible that the low secretagogue-stimulated insulin release in cells with knocked down fatty acid synthase is in part related to a lower content of monounsaturated fatty acids. The fact that the beta cell increases lipid synthesis in response to high glucose [1–7] coupled with the acute inhibition of PL formation in glucose-stimulated cells with knocked down pyruvate carboxylase (Figures 2 and 3) or fatty acid synthase (Figures 6 and 7) is of heuristic value in respect to insulin secretion, as well as the glucolipotoxicity that has been proposed to damage the beta cell in type 2 diabetes. In this regard, an increase in PLs should be healthy for insulin secretion, but increases in CEs and TGs that are believed to be harmful to cells, should be detrimental to the beta cell.
CONCLUSIONS
The current study suggests that one of the purposes of pyruvate carboxylase and fatty acid synthase in the beta cell is to allow rapid glucose-stimulated lipid synthesis especially of membrane lipids. In general, PLs, which are found in cellular membranes, were lowered more and more uniformly in the glucose-stimulated cell lines with knocked down pyruvate carboxylase and fatty acid synthase. We recently generated cell lines with knocked down sphingosine kinase 1. Similar to the cell lines with knocked down fatty acid synthase used in the current study that showed lower levels of many individual lipids, the sphingosine kinase 1 knockdown cell lines showed lowered insulin mRNA and insulin content, as well as lowered glucose-stimulated insulin release [28]. A requirement for lipid synthesis seems likely during the process of insulin synthesis and exocytosis after proinsulin is synthesized and converted to insulin as it traverses the cis- and trans-Golgi networks, is packaged into secretory granules and the granule membranes are modified as the granules mature, until eventually the granule membranes fuse with the plasma membrane where insulin is finally extruded into the circulation [33]. The lower level of insulin mRNA and lower insulin content in the cells with impaired lipid synthesis suggest that the composition of lipids in the organelles involved with processing of proinsulin and the packaging of insulin into secretory granules can influence the rate of insulin synthesis. The similar effects seen with the multiple shRNA constructs that were used to target the Fasn gene, as well as with other shRNA constructs that target sphingosine kinase 1 [28], argue against a nonspecific off target effect and suggest that the lower levels of lipids is causally related to a lower level of insulin synthesis. Consistent with the results of previous studies [1–7], the results of the current study show that many of the lipids whose levels change are membrane lipids (PLs and sphingomyelins) and the changes occur over a time course that coincides with insulin secretion. This indicates that lipid synthesis and remodeling is often rapid in the beta cell and necessary for normal insulin secretion.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by NIH grant DK28348 and the Nowlin Family Trust administered by the Lutheran Community Foundation. The authors thank Mindy A. Kendrick and Melissa J. Longacre for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: AACS, acetoacetyl-CoA synthetase; ACAA1 or 2, acetyl-CoA acyltransferase 1 or 2; ACAT1 or 2, acetyl-CoA acetyltransferase 1 or 2; ACC1 or 2, acetyl-CoA carboxylase 1 or 2; CE, cholesterol ester; DAG, diacylgycerol; FFA, free fatty acid; PC, phosphatidyl choline; PE, phosphatidylethanolamine; PL, phospholipid; PS, phosphatidylserine; SCOT, succinyl-CoA:3-ketoacid-CoA transferase; TG, triglyceride
REFERENCES
- 1.MacDonald MJ, Dobrzyn A, Ntambi J, Stoker SW. The role of rapid lipogenesis in insulin secretion: Insulin secretagogues acutely alter lipid composition of INS-1 832/13 cells. Arch. Biochem. Biophys. 2008;470:153–162. doi: 10.1016/j.abb.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turk J, Wolf BA, Lefkowith JB, Stump WT, McDaniel ML. Glucose-induced phospholipid hydrolysis in isolated pancreatic islets: quantitative effects on the phospholipid content of arachidonate and other fatty acids. Biochim. Biophys. Acta. 1986;879:399–409. doi: 10.1016/0005-2760(86)90232-8. [DOI] [PubMed] [Google Scholar]
- 3.Berne C. The metabolism of lipids in mouse pancreatic islets. The biosynthesis of triacylglycerols and phospholipids. Biochem. J. 1975;152:667–673. doi: 10.1042/bj1520667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berne C, Andersson A. Lipid metabolism of isolated mouse pancreatic islets maintained in culture at different glucose concentrations. Ups. J. Med. Sci. 1981;86:55–61. doi: 10.3109/03009738109179210. [DOI] [PubMed] [Google Scholar]
- 5.Hallberg A, Andersson A. Effects of starvation on phospholipid metabolism of pancreatic islets. Diabetes Res. 1984;1:105–110. [PubMed] [Google Scholar]
- 6.Vara E, Tamarit-Rodriguez J. Glucose stimulation of insulin secretion in islets of fed and starved rats and its dependence on lipid metabolism. Metabolism. 1986;35:266–271. doi: 10.1016/0026-0495(86)90212-x. [DOI] [PubMed] [Google Scholar]
- 7.Martins EF, Miyasaka CK, Newsholme P, Curi R, Carpinelli AR. Changes of fatty acid composition in incubated rat pancreatic islets. Diabetes. Metab. 2004;30:21–27. doi: 10.1016/s1262-3636(07)70085-x. [DOI] [PubMed] [Google Scholar]
- 8.Farfari S, Schulz V, Corkey B, Prentki M. Glucose-regulated anaplerosis and cataplerosis in pancreatic beta-cells: possible implication of a pyruvate/citrate shuttle in insulin secretion. Diabetes. 2000;49:718–726. doi: 10.2337/diabetes.49.5.718. [DOI] [PubMed] [Google Scholar]
- 9.Flamez D, Berger V, Kruhøffer M, Orntoft T, Pipeleers D, Schuit FC. Critical role for cataplerosis via citrate in glucose-regulated insulin release. Diabetes. 2002;51:2018–2024. doi: 10.2337/diabetes.51.7.2018. [DOI] [PubMed] [Google Scholar]
- 10.MacDonald MJ, Longacre MJ, Stoker SW, Kendrick MA, Thonpho A, Brown LJ, Hasan NM, Jitrapakdee S, Fukao T, Hanson MS, Fernandez LA, Odorico J. Differences between human and rodent pancreatic islets: low pyruvate carboxylase, ATP citrate lyase and pyruvate carboxylation; high glucose-stimulated acetoacetate in human pancreatic islets. J. Biol. Chem. 2011;286:18383–18396. doi: 10.1074/jbc.M111.241182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacDonald MJ. Feasibility of a mitochondrial pyruvate malate shuttle in pancreatic islets. Further implication of cytosolic NADPH in insulin secretion. J. Biol. Chem. 1995;270:20051–20058. [PubMed] [Google Scholar]
- 12.MacDonald MJ, McKenzie DI, Walker TM, Kaysen JH. Lack of glyconeogenesis in pancreatic islets: Expression of gluconeogenic enzyme genes in islets. Horm. Metab. Res. 1992;24:158–160. doi: 10.1055/s-2007-1003284. [DOI] [PubMed] [Google Scholar]
- 13.Ashcroft SJ, Randle PJ. Enzymes of glucose metabolism in normal mouse pancreatic islets. Biochem. J. 1970;119:5–15. doi: 10.1042/bj1190005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berne C. Nicotinamide adenine dinucleotide phosphate-converting enzymes and adenosine triphosphate citrate lyase in some tissues and organs of New Zealand obese mice with special reference to the enzyme pattern of the pancreatic islets. J. Histochem. Cytochem. 1975;23:660–665. doi: 10.1177/23.9.240882. [DOI] [PubMed] [Google Scholar]
- 15.MacDonald MJ, Longacre MJ, Warner TF, Thonpho A. High level of ATP citrate lyase expression in human and rat pancreatic islets. Horm. Metab. Res. 2012 doi: 10.1055/s-0032-1329987. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasan NM, Longacre MJ, Seed Ahmed M, Kendrick MA, Gu H, Ostenson C-G, Fukao T, MacDonald MJ. Lower succinyl-CoA:3-ketoacid-CoA transferase (SCOT) and ATP citrate lyase in pancreatic islets of a rat model of type 2 diabetes: knockdown of SCOT inhibits insulin release in rat insulinoma cells. Arch. Biochem. Biophys. 2010;499:62–68. doi: 10.1016/j.abb.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacDonald MJ, Hasan NM, Longacre MJ. Studies with leucine, β-hydroxybutyrate and ATP citrate lyase-deficient cells support the acetoacetate pathway of insulin secretion. Biochim. Biophys. Acta. 2008;1780:966–972. doi: 10.1016/j.bbagen.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacDonald MJ, Longacre MJ, Stoker SW, Brown LJ, Hasan NM, Kendrick MA. Acetoacetate and β-hydroxybutyrate in combination with other metabolites release insulin from INS-1 cells and provide clues about pathways in insulin secretion. Am. J. Physiol. Cell. Physiol. 2008;294:C442–C450. doi: 10.1152/ajpcell.00368.2007. [DOI] [PubMed] [Google Scholar]
- 19.MacDonald MJ, Smith AD, III, Hasan NM, Sabat G, Fahein LA. Feasibility of pathways for transfer of acyl groups from mitochondria to the cytosol to form short chain acyl CoAs in the pancreatic beta cell. J. Biol. Chem. 2007;282:30596–30606. doi: 10.1074/jbc.M702732200. [DOI] [PubMed] [Google Scholar]
- 20.MacDonald MJ, Stoker SW, Hasan NM. Anaplerosis from glucose, α-ketoisocaproate, and pyruvate in pancreatic islets, INS-1 cells and liver mitochondria. Mol. Cell. Biochem. 2008;313:195–202. doi: 10.1007/s11010-008-9757-x. [DOI] [PubMed] [Google Scholar]
- 21.Brun T, Roche E, Assimacopoulos-Jeannet F, Corkey BE, Kim KH, Prentki M. Evidence for an anaplerotic/malonyl-CoA pathway in pancreatic beta-cell nutrient signaling. Diabetes. 1996;45:190–198. doi: 10.2337/diab.45.2.190. [DOI] [PubMed] [Google Scholar]
- 22.Joseph JW, Odegaard ML, Ronnebaum SM, Burgess SC, Muehlbauer J, Sherry AD, Newgard CB. Normal flux through ATP-citrate lyase or fatty acid synthase is not required for glucose-stimulated insulin secretion. J. Biol. Chem. 2007;282:31592–31600. doi: 10.1074/jbc.M706080200. [DOI] [PubMed] [Google Scholar]
- 23.Guay C, Madiraju SR, Aumais A, Joly E, Prentki M. A role for ATP-citrate lyase, malic enzyme, and pyruvate/citrate cycling in glucose-induce insulin secretion. J. Biol. Chem. 2007;282:35657–35665. doi: 10.1074/jbc.M707294200. PMID 17928289. [DOI] [PubMed] [Google Scholar]
- 24.Hasan NM, Longacre MJ, Stoker SW, Boonsaen T, Jitrapakdee S, Kendrick MA, Wallace JC, MacDonald MJ. Impaired anaplerosis and insulin secretion in insulinoma cells caused by siRNA-mediated suppression of pyruvate carboxylase. J. Biol. Chem. 2008;283:28048–28059. doi: 10.1074/jbc.M804170200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and –independent glucose-stimulated insulin secretion. Diabetes. 2000;49:424–430. doi: 10.2337/diabetes.49.3.424. [DOI] [PubMed] [Google Scholar]
- 26.Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheim CB. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology. 1992;130:167–178. doi: 10.1210/endo.130.1.1370150. [DOI] [PubMed] [Google Scholar]
- 27.MacDonald MJ. Synergistic potent insulin release by combinations of weak secretagogues in pancreatic islets and INS-1 cells. J. Biol. Chem. 2007;282:6043–6052. doi: 10.1074/jbc.M606652200. [DOI] [PubMed] [Google Scholar]
- 28.Hasan NM, Longacre MJ, Stoker SW, Kendrick MA, Druckenbrod NR, Laychock SG, Mastrandrea LD, MacDonald MJ. Sphingosine kinase 1 knockdown reduces insulin synthesis and secretion in a rat insulinoma cell line. Arch. Biochem. Biophys. 2012;518:23–30. doi: 10.1016/j.abb.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biol. Chem. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 30.Igal RA, Coleman RA. Acylglycerol recycling from triacylglycerol to phospholipid, not lipase activity, is defective in neutral lipid storage disease fibroblasts. J. Biol. Chem. 1996;271:16644–16651. doi: 10.1074/jbc.271.28.16644. [DOI] [PubMed] [Google Scholar]
- 31.Dobrzyn A, Dobrzyn P, Miyazaki M, Sampath H, Chu K, Ntambi JM. Stearoyl-CoA desaturase 1 deficiency increases CTP:choline cytidylyltransferase translocation into the membrane and enhances phosphatidylcholine synthesis in liver. J. Biol. Chem. 2005;280:23356–23362. doi: 10.1074/jbc.M502436200. [DOI] [PubMed] [Google Scholar]
- 32.Schuit F, De Vos A, Farfari S, Moens K, Pipeleers D, Brun T, Prentki M. Metabolic fate of glucose in purified islet cells. Glucose-regulated anaplerosis in beta cells. J. Biol. Chem. 1997;272:18572–18579. doi: 10.1074/jbc.272.30.18572. [DOI] [PubMed] [Google Scholar]
- 33.MacDonald MJ, Fahien LA, Brown LJ, Hasan NM, Buss JD, Kendrick MA. Perspective: emerging evidence for signaling roles of mitochondrial anaplerotic products in insulin secretion. Am. J. Physiol. Endocrinol. Metab. 2005;288:E1–E15. doi: 10.1152/ajpendo.00218.2004. [DOI] [PubMed] [Google Scholar]
- 34.Hedeskov CJ, Capito K. Pancreatic islet metabolism of pyruvate and other potentiators of insulin release. Effects of starvation. Horm. Metab. Res. Suppl. 1980;(Suppl 10):8–13. [PubMed] [Google Scholar]
- 35.MacDonald MJ, Chang CM. Do pancreatic islets contain significant amounts of phosphoenolpyruvate carboxykinase or ferroactivator activity? Diabetes. 1985;34:246–250. doi: 10.2337/diab.34.3.246. [DOI] [PubMed] [Google Scholar]
- 36.Flowers JB, Rabaglia ME, Schueler KL, Flowers MT, Lan H, Keller MP, Ntanmbi JM, Attie AD. Loss of stearoyl-CoA desaturase-1 improves insulin sensitivity in lean mice but worsens diabetes in leptin-deficient obese mice. Diabetes. 2007;56:1228–1239. doi: 10.2337/db06-1142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.