Abstract
Purpose
To evaluate safety in an interim analysis of transarterial chemoembolization (TACE) with doxorubicin-eluting beads (DEB) in 13 patients with hepatic metastases from neuroendocrine tumors (NETs) as part of a phase II trial.
Methods
Institutional Review Board approval and informed consent were obtained. Thirteen patients completed preliminary safety analysis. Their mean age was 65 years, Eastern Cooperative Oncology Group status was 0/1, tumor burden range was 4–75 %, and mean targeted tumor size was 5.9 cm. Up to four DEB-TACE sessions (100–300 μm beads loaded with ≤100 mg doxorubicin) within 6 months were allowed. Tumor response was assessed by magnetic resonance imaging 1 month after treatment using contrast-enhancement [European Association for the Study of the Liver (EASL) and size Response Evaluation Criteria in Solid Tumors (RECIST)] criteria. Safety was assessed by National Cancer Institute Common Terminology Criteria.
Results
DEB-TACE was successfully performed in all 13 patients. At 1 month follow-up, there was a mean 12 % decrease in tumor size (p <0.0003) and a 56 % decrease in tumor enhancement (p <0.0001). By EASL criteria, the targeted lesion objective response rate was 78 %. Grade 3 to 4 toxicities were fatigue (23 %), increased alanine amino transferase (15 %), hyperglycemia (15 %), and abdominal pain (8 %). Seven patients developed bilomas (54 %); all of these patients had multiple small (<4 cm) lesions. Subsequently, four underwent percutaneous drainage, three for abscess formation and one for symptoms related to mass effect.
Conclusions
Although biloma and liver abscess are known risks after TACE, the high incidence in our study population was unexpected and forced interruption of the trial. Although this occurred in a small group of patients, we have changed our technique and patient selection as a result of these findings, thus allowing resumption of the trial.
Keywords: Biliary injury, Chemoembolization, Drug-eluting beads, Neuroendocrine metastases
Introduction
Neuroendocrine tumors (NETs) are rare with an incidence of 1 to 2 cases/1,000,000 persons/y in the United States [1]. The term “neuroendocrine tumor” encompasses a diverse range of tumors, the secretion of which arise from the amine precursor uptake and decarboxylation (APUD) system. They typically arise in the gastrointestinal tract but can also originate in the respiratory tract. Carcinoid tumors are considered a well-differentiated subset of NETs. They tend to metastasize to the liver, and those patients with neuroendocrine liver metastases (NELM) have a poorer prognosis (compared with those that do not have metastases) and quality of life [2]. Although surgical resection may be curative, it is possible in only <10 % of patients [3–6].
Although chemoembolization has not shown a survival benefit in randomized controlled trials, encouraging clinical results have made it a preferred therapy in those patients with liver-dominant or liver-only neuroendocrine metastases. Numerous retrospective analyses have consistently shown 5 year survival rates of approximately 50 % [7–11].
Although transarterial chemoembolization (TACE) with doxorubicin-eluting beads (DEB) has been used worldwide to treat patients with liver cancer, most of the reported data have been on the treatment of hepatocellular carcinoma (HCC). Only two series, both of which showed complication rates similar to those of HCC treatment with DEB-TACE, have been published on the treatment of NET liver metastasis with DEB-TACE [12, 13].
Complications after DEB-TACE are not uncommon. In the literature, reported complications include postembolization syndrome (82 %) and liver abscess (2 %). Mortality has been reported in 2 % of patients who underwent DEB-TACE; causes include myocardial infarction (n = 3), progressive liver disease (n = 5), pulmonary embolism (n = 1), sepsis (n = 1), and liver failure (n = 1) [14–21].
The purpose of this study was to evaluate safety in an interim analysis of DEB-TACE in 13 patients with hepatic metastases from NETs as part of a phase II trial.
Methods
Inclusion and Exclusion Criteria
Patients with a diagnosis of unresectable NELM based on histological confirmation were evaluated for this study. Eligibility criteria included Eastern Cooperative Oncology Group (ECOG) performance status 0 to 2, adequate hepatic [alanine amino transferase (ALT) and aspartate aminotransferase (AST) ≤5× the upper limit of normal, total bilirubin ≤3 mg/dL, albumin >2.0], renal (creatinine ≤2.0 mg/dL), and hematologic (leukocyte count ≥1,500 cells/mm3, platelets ≥50,000/mm3) function. Exclusion criteria included previous anticancer therapy for hepatic neuroendocrine metastases (previous resection permitted), hepatic tumor burden >90 %, complete occlusion of the portal venous system, moderate or greater ascites, predominant extrahepatic disease, left-ventricular ejection fraction <50 %, evidence of bleeding diathesis or coagulopathy, or second primary malignancy.
The study was approved by the United States Food and Drug Administration with a physician-sponsored Investigational Device Exemption and our Institutional Review Board. The study was conducted in accordance with the principles of the Declaration of Helsinki. All patients provided informed consent before enrollment.
Study Design
Patients were treated with DEB-TACE, up to four procedures in 6 months (as indicated), for a maximum of six procedures during the course of 2 years. Baseline evaluation included history and physical examination, cross-sectional imaging, and laboratory assessment. Follow-up clinical examinations, imaging, and laboratory assessments took place 1 month after each DEB-TACE treatment and then every 2 to 3 months for a period of 2 years. Treatment continued until the lesions reached ≥90 % necrosis, there was occurrence of unacceptable toxicities, or there was substantial progression of the extrahepatic disease (i.e., spreading to multiple organs or new symptomatic disease).
The DEB-TACE procedure was performed using a maximum of 100 mg doxorubicin/procedure loaded onto 100–300 μm LC Beads (Biocompatibles UK Ltd.) beads. Each vial of LC Beads was loaded with 50 mg doxorubicin hydrochloride (25 mg/mL; Pharmacia-UpJohn, Bridgewater, NJ) and mixed in a 1:1 ratio of nonionic contrast media (Oxilan; Guerbet, Bloomington, IN). DEBs were administered by alternating injections of small aliquots (3 ml) of DEBs and contrast until either complete delivery of the DEBs or near stasis of the feeding artery (two to five heartbeats to clear the contrast column). The delivery was performed as superselectively as possible, i.e., treating as little normal liver parenchyma while still treating a large volume of tumor. No lobar treatments were performed. This DEB-TACE procedure is described more fully elsewhere [22].
Efficacy
Tumor response of the targeted lesions was evaluated with the use of contrast-enhanced and diffusion-weighted magnetic resonance imaging (MRI) 1 month after each DEB-TACE treatment and then every 2 to 3 months for 2 years. The targeted lesions were those directly targeted by DEB-TACE. Response was assessed by size (Response Evaluation Criteria in Solid Tumors [RECIST; modified to allow for measurement of targeted tumors]) [23] and enhancement (European Association for the Study of the Liver [EASL] criteria) [24]. Tumor size was based on the maximum diameter of the lesion(s) as measured by electronic calipers. For EASL criteria, percent enhancement was based on enhancement seen in the treated tumor(s). Complete response was defined as the absence of enhancement in the targeted tumor. Partial response was defined as ≥50 % decrease from baseline enhancement in the targeted tumor. Progressive disease was defined as ≥25 % increase in the targeted tumor, and stable disease defined all other cases. Our method of response assessment of targeted lesions is described more fully elsewhere [25]. Efficacy assessments were made after 1, 6, and 12 months after initial DEB-TACE.
Radiographic Analysis of Bilomas
A semiautomatic three-dimensional (3D) volume segmentation employing non-Euclidean radial basis functions was used on 20 s contrast-enhanced MRI to segment the biloma [26, 27]. Fisher’s exact test was conducted on biloma volume versus need for drainage. Statistical significance was considered as p < 0.05.
Safety
Safety was assessed at each DEB-TACE procedure and at every follow-up visit thereafter. The safety assessment included parameters from imaging, clinical assessment, and laboratory evaluations. All treatment-emergent toxicities were recorded and assessed using the National Cancer Institute Common Toxicity Criteria version 3.0.
Statistical Considerations
The primary end point was safety, and the secondary end point was efficacy. The objective response rate [ORR (the total of complete and partial responses of targeted lesions)] and the disease control rate [DCR (the total complete, partial, and stable responses of targeted lesions)] were assessed using both RECIST and EASL criteria. Comparisons between baseline and follow-up measurements (tumor size and percentage contrast enhancement) were made using paired Student t tests. All p values were two-sided, and p < 0.05 was considered statistically significant.
The interim efficacy analysis (stage 1) was conducted after the first 10 patients completed initial DEB-TACE. In this stage, if an ORR (using EASL criteria) was observed in ≥5 of the initial 10 patients, then the study would continue to stage 2, and 20 additional patients would be enrolled. Absence of therapeutic efficacy in ≥6 of the initial 10 patients (ORR <50 %) would prompt study termination. The two-stage design yielded a 75 % probability that we would stop the study after the first 10 patients if DEB-TACE was not sufficiently efficacious in this population.
Toxicity profiles were grouped by severity (grades 1–2 vs. grades 3–4). Subgroup analyses of disease presentation at baseline (including history of biliary anastomosis, intrahepatic ductal dilatation, tumor burden) were analyzed using descriptive statistics. Thirty-day treatment mortality was not expected to exceed 4 %. Treatment-related deaths occurring in two patients would prompt study termination.
Results
From May 2009 through August 2010, 13 patients were enrolled in the study (Fig. 1). The study population was predominantly male (69 %) and ECOG status 0 and 1 (54 and 46 %, respectively), and fewer than half were being treated with Sandostatin (38 %, n = 5) (Table 1). Three patients (23 %) had carcinoid syndrome at presentation (all of whom had remittance of their symptoms after the first DEB-TACE procedure). The primary cancer sites of origin were pancreas (39 %), other sites (including rectum, gallbladder, ovary, and small bowel) (31 %), and unknown (31 %). The mean largest index tumor size per patient was 5.9 cm (Table 1). We planned an interim analysis to assess safety and therapeutic efficacy after the initial 10 patients were treated. Therapeutic efficacy was established at that time point, and enrollment continued. However, an unexpected safety concern was found shortly thereafter (at which time 13 patients had been enrolled). These 13 patients are the subject of the current study. The 13 patients underwent a total of 27 DEB-TACE procedures [median per patient = 2 (range 1 to 4)].
Fig. 1.
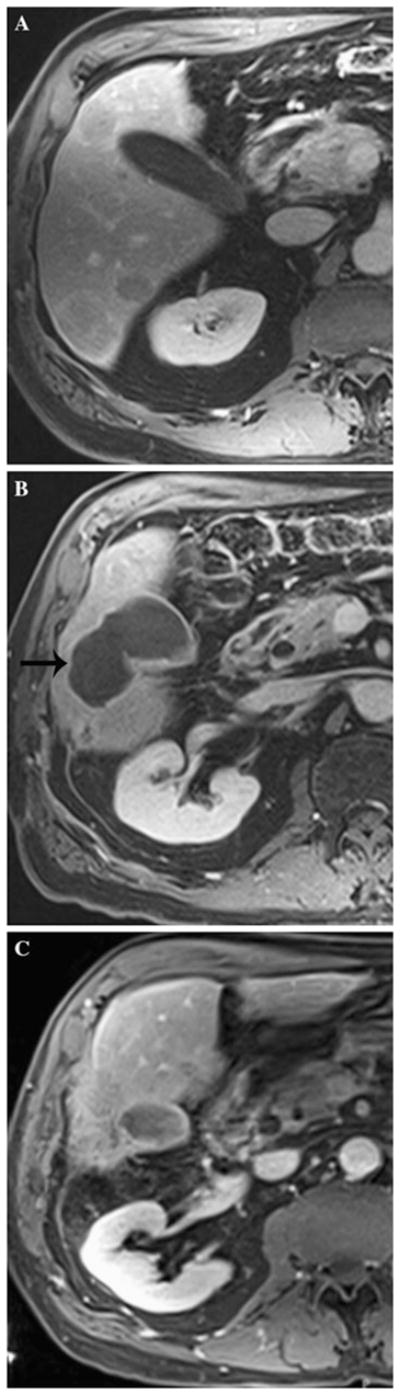
A 68 year-old man with 70 s postcontrast T1-weighted MRI through lower liver showing baseline image with tumor (A), biloma adjacent to tumor (B, black arrow on left), and resolved biloma after drainage, now with mostly necrotic tumor (C)
Table 1.
Baseline patient demographic characteristics
| Variable | Value (n = 13) |
|---|---|
| Mean (range) age (years) | 64 (47–80) |
| No. (%) male | 9 (69) |
| No. (%) site of primary lesion (%) | |
| Pancreas | 5 (39) |
| Other (rectum, ovary, small bowel) | 4 (31) |
| Unknown | 4 (31) |
| No. (%) ECOG performance status | |
| 0 | 7 (54) |
| 1 | 6 (46) |
| Tumor (%) burden (range) | 4–75 |
| Tumor size (SD) mean largest index (cm) | 5.9 |
| Treated with Sandostatin at baseline (%) | 5 (38) |
| Previous therapy for hepatic neuroendocrine metastases | |
| Chemotherapy | 0 |
| External radiation (%) | 2 (15) |
| Carcinoid syndrome (%) | 3 (23) |
| Chromogranin A: no. diagnostic (ng/mL) | 10 (1,037.46) |
| 5-HIAA: no. (mean) diagnostic (mg/24 h) | 6 (149) |
5-HIAA 5-Hydroxyindoleacetic acid
Safety
Thirteen patients were included in the toxicity assessment. After the initial DEB-TACE procedure, all patients experienced at least one toxicity (Table 2 and Fig. 2). The most common toxicities (occurring in >30 % of the patients) were fatigue, fever, night sweats, anorexia, abnormal liver function, hyperglycemia, abdominal pain, and bilomas. Grade 3 to 4 toxicities consisted of fatigue (23 %), increased ALT (15 %), liver abscess (15 %), hyperglycemia (15 %), abdominal pain (8 %), and bilomas (8 %). Six patients developed bilomas after their initial DEB-TACE procedure, and one patient developed a biloma after her second DEB-TACE procedure, resulting in a total biloma incidence of 54 % (7 of 13).
Table 2.
Treatment-emergent toxicities
| Toxicities | 1 months after initial DEB-TACE 1 (n = 13)
|
||
|---|---|---|---|
| All grades | Grades 1–2 | Grades 3–4 | |
| Any (%) | 13 (100) | ||
| Blood/bone marrow suppression (%) | |||
| Anemia | 1 (8) | 1 (8) | |
| Lymphopenia | 2 (15) | 2 (15) | |
| Cardiac events | |||
| Hypotension | 1 (8) | 1 (8) | |
| Cardiovascular system (%) | |||
| Edema | 2 (15) | 2 (15) | |
| Coagulation (%) | |||
| Increased INR | 2 (15) | 2 (15) | |
| Constitutional symptoms (%) | |||
| Fatigue | 9 (69) | 6 (46) | 3 (23) |
| Fever | 4 (31) | 4 (31) | |
| Night sweats | 6 (46) | 6 (46) | |
| Dermatologic events (%) | |||
| Alopecia | 3 (23) | 3 (23) | |
| Dry skin | 1 (8) | 1 (8) | |
| Gastrointestinal events (%) | |||
| Ascites | 1 (8) | 1 (8) | |
| Anorexia | 5 (38) | 5 (38) | |
| Biloma | 5 (38) | 5 (38) | |
| Constipation | 1 (8) | 1 (8) | |
| Diarrhea | 1 (8) | 1 (8) | |
| Nausea | 1 (8) | 1 (8) | |
| Taste alteration | 1 (8) | 1 (8) | |
| Hemorrhage/bleeding (%) | |||
| Hematoma | 1 (8) | 1 (8) | |
| Hepatic function (%) | |||
| Increased ALT | 5 (38) | 3 (23) | 2 (15) |
| Increased AST | 6 (46) | 6 (46) | |
| Increased AP | 8 (62) | 8 (62) | |
| Hyperbilirubinemia | 1 (8) | 1 (8) | |
| Hypoalbuminemia | 5 (38) | 5 (38) | |
| Infection (%) | |||
| Liver abscess | 1 (15) | 1 (15) | |
| Metabolic (%) | |||
| Hyperglycemia | 5 (38) | 3 (23) | 2 (15) |
| Hyponatremia | 1 (8) | 1 (8) | |
| Neurologic symptoms/events (%) | |||
| Neuropathy | 1 (8) | 1 (8) | |
| Pain (%) | |||
| Abdominal nonspecific | 5 (38) | 4 (31) | 1 (8) |
| Right upper quadrant | 6 (46) | 6 (46) | |
| Other | 1 (8) | 1 (8) | |
| Pulmonary (%) | |||
| Dyspnea | 1 (8) | 1 (8) | |
| Dyspnea on exertion | 1 (8) | 1 (8) | |
Fig. 2.
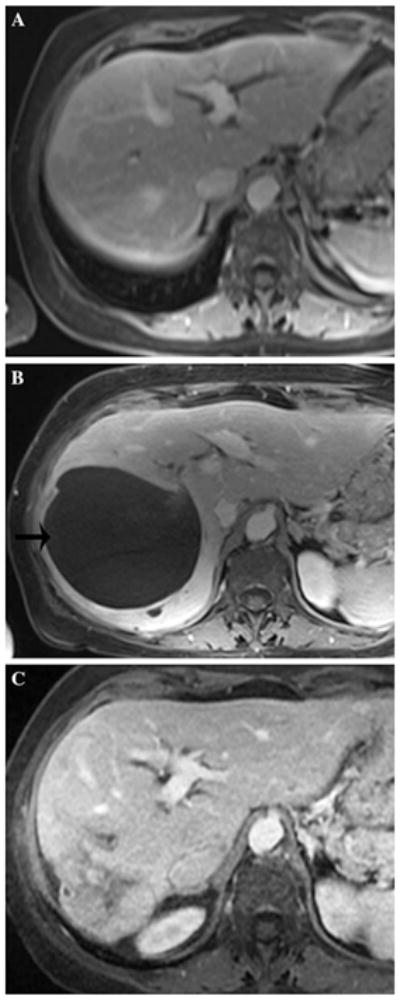
A 69 year-old woman with 70 s postcontrast T1-weighted MRI through the mid-liver showing baseline image (A), biloma adjacent to tumor after second DEB-TACE (B, black arrow on left), and resolved biloma after drainage (C)
Once a biloma was identified on MRI imaging, this imaging was repeated with every clinic visit (every 2 months) to assess whether the bilomas were growing in size. If the bilomas were symptomatic (associated fevers or pain), then they were drained percutaneously. Four of the seven patients with bilomas eventually underwent drainage, three for abscess formation and one for symptoms related to mass effect (Fig. 3). Although biloma and liver abscess are a known risks after TACE, the high incidence in our study population was unexpected (see Figs. 1–3). Aside from the bilomas, the toxicity profile was limited and predominantly lower grade (Fig. 2). There were no deaths within 30 days of treatment with DEB-TACE.
Fig. 3.
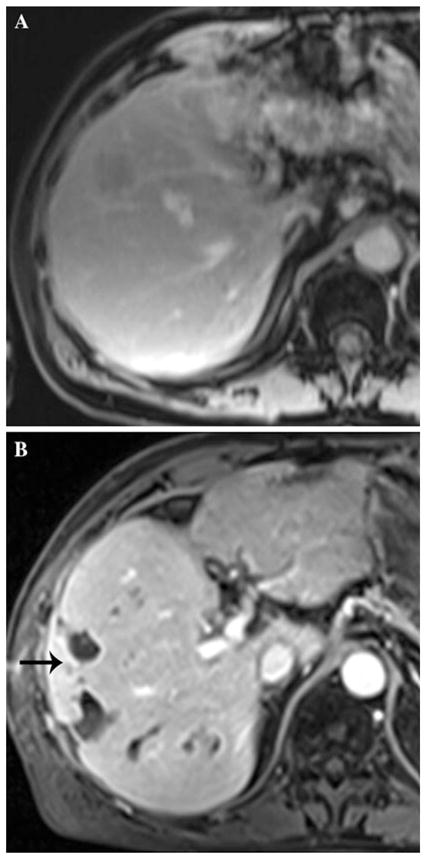
A 66 year-old man with 70 s postcontrast T1-weighted MRI through the mid-liver showing baseline image (A). A biloma formed after the second DEB-TACE procedure, and the patient had a prolonged hospital course and developed multiple small abscesses adjacent to original biloma (B, arrow)
Radiographic Analysis of Bilomas
3D segmentation took 5 to 10 mouse clicks and was completed in <2 min. The biloma volumes [32.4 ± 35.3 cm3 (range 1.7–83.2 cm3)] are listed in Table 3. Patients with larger bilomas (>7.3 cm3) had statistically greater need for drainage (p = 0.029).
Table 3.
Patient characteristics of those with Biloma
| Mean DEB dose (mg) | Mean tumor burden (%) | |
|---|---|---|
| All (n = 13) | 80 | 29 |
| No biloma (n = 6) | 79 | 44 |
| Biloma (n = 7) | 85 | 12 |
| Patient no. | Disease presentationa | Biliary anastomosis | Ductal dilitation before DEB-TACE | Tumor burden | Time point biloma developed | Biloma volume (cm3) | Drainage/reason | Outcome from biloma/abscess |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | No | No | 9 | DEB-TACE no. 1 | 1.7 | No | No sequelae |
| 2 | 1 | No | No | 30 | DEB-TACE no. 2 | 2.3 | No | No sequelae |
| 3 | 2 | No | Yes | 20 | DEB-TACE no. 1 | 394.42 | Yes, mass effect | Resolved |
| 4 | 1 | No | No | 17 | DEB-TACE no. 1 | 68.6 | Yes, symptomatic for infection | Resolved |
| 5 | 0 | No | No | 10 | DEB-TACE no. 1 | 7.3 | No | Unknown: hospice; extrahepatic disease progression |
| 6 | 2 | Yes | No | 10 | DEB-TACE no. 1 | 55.3 | Yes, symptomatic for infection | Prolonged hospital course and infection; subsequent death |
| 7 | 0 | No | Yes | 4 | DEB-TACE no. 1 | 83.2 | Yes, symptomatic for infection | Prolonged hospital course and infection; subsequent death |
0 single lesion with or without satellites, 1 multiple lesions (smallest 3.5 cm), 2 diffuse, multiple lesions (largest 3.4 cm)
Subgroup Analysis: Groups 1 (Biloma) and 2 (No Biloma)
The patients who developed bilomas (group 1) had approximately one third of the hepatic tumor burden than those in group 2 [14 vs. 45 %, n = 0.06 (Table 3)]. However, the mean dose of doxorubicin used in for DEB-TACE was not significantly different between both groups (82 and 77 mg, p = 0.64).
Three patients had intrahepatic ductal dilatation at baseline, two of whom developed bilomas after treatment. The only patient with a previous reconstructive biliary enteric anastomosis also developed a biloma after treatment. In group 1 (those who developed bilomas) at baseline presentation, two patients had a single lesion; three patients had diffuse disease; and two patients had multiple lesions. In the six patients who did not develop a biloma (group 2), all had multiple lesions.
Efficacy Analysis (Per Patient)
At the time of analysis, by EASL criteria the ORR was 70 % (7 of 10) whereas 30 % (3 of 10) of patients had stable disease (SD). Our total population included in this report (n = 13) had a per-patient ORR of 62 % (8 of 13) and SD in 38 % (5 of 13), which is consistent with the interim analysis results.
Efficacy: Radiographic End Points (Per Lesion)
Thirteen patients (n = 32 lesions) were evaluable for response by imaging. At 1 month after the initial DEB-TACE procedure, there was a modes, but statistically significant decrease in tumor size (12 %, p < 0.0003) and a marked decrease in tumor enhancement (56 %, p < 0.0001). By EASL criteria, the ORR of the targeted lesions was 78 %, and the DCR was 97 % by both EASL and RECIST criteria (Tables 4, 5, 6, 7).
Table 4.
Summary of patient characteristics, Biloma vs. No Biloma
| Mean DEB dose (mg) | Mean tumor burden (%) | No. (%) biliary anastomosis before DEB-TACE | No. (%) ductal dilitation before DEB-TACE | No. (%) disease presentation
|
|||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | |||||
| All (n = 13) | 80 | 29 | 1/13 (8) | 3/13 (23) | 2/13 (15) | 3/13 (23) | 8/13 (62) |
| No biloma (n = 6) | 79 | 44 | 0 (0) | 1/6 (17) | 0/6 (0) | 0/6 (0) | 6/6 (100) |
| Biloma (n = 7) | 85 | 12 | 1/7 (14) | 2/7 (29) | 2/7 (29) | 3/7 (43) | 2/7 (29) |
Table 5.
Efficacy: tumor response by imaging
| Features | Baseline: before DEB-TACE) (n = 32 lesions in 13 patients) | 1 month after initial DEB-TACE (n = 32 lesions in 13 patients) | 6 months after initial DEB-TACE (n = 26 lesions in 10 patients) | 12 months after initial DEB-TACE (n = 19 lesions in 6 patients) |
|---|---|---|---|---|
| Tumor size ± SD (cm) | 4.5 ± 2.5 | 4.1 ± 2.7 | 3.1 ± 2.6 | 3.4 ± 3 |
| % change | −12 | −30 | −40 | |
| p | p = 0.0003 | p < 0.0001 | p < 0.0001 | |
| Tumor enhancement ± SD (%) | 88 ± 21.8 | 37 ± 22 | 40 ± 35 | 33 ± 33 |
| % change | −56 | −58 | −65 | |
| p | p < 0.0001 | p < 0.0001 | p < 0.0001 |
Table 6.
Efficacy: response by RECIST/EASL criteria per lesion
| Response | 1 months after initial DEB-TACE (n = 32 lesions)
|
6 months after initial DEB-TACE (n = 26 lesions)
|
12 months after initial DEB-TACE (n = 19 lesions)
|
|||
|---|---|---|---|---|---|---|
| No. RECIST (%) | No. EASL (%) | No. RECIST (%) | No. EASL (%) | No. RECIST (%) | No. EASL (%) | |
| Complete response | – | 2/32 (6) | 3/26 (12) | 6/26 (23) | 4/19 (21) | 7/19 (37) |
| Partial response | 5/32 (16) | 23/32 (72) | 11/26 (42) | 10/26 (38) | 5/19 (26) | 3/19 (16) |
| Stable disease | 26/32 (81) | 7/32 (22) | 11/26 (42) | 10/26 (38) | 9/19 (47) | 9/19 (47) |
| Progressive disease | 1/32 (3) | – | 1/26 (4) | – | 1/19 (5) | – |
Table 7.
Efficacy: response by RECIST/EASL criteria per lesion
| Response | 1 months after initial DEB-TACE (n = 13 patients) | 6 months after initial DEB-TACE (n = 10 patients) | 12 months after initial DEB-TACE (n = 6 patients) | |||
|---|---|---|---|---|---|---|
| No. RECIST (%) | No. EASL (%) | No. RECIST (%) | No. EASL (%) | No. RECIST (%) | No. EASL (%) | |
| Complete response | – | – | – | 1/10 (10) | – | 2/6 (33) |
| Partial response | – | 8/13 (62) | 1/10 (10) | 3/10 (30) | 1/6 (17) | 1/6 (17) |
| Stable disease | 13/13 (100) | 5/13 (38) | 8/10 (80) | 6/10 (60) | 4/6 (67) | 3/6 (50) |
| Progressive disease | – | – | 1/10 (10) | – | 1/6 (17) | – |
Ten patients (n = 26 lesions) were assessable for imaging response at 6 months after initiation of therapy. By EASL criteria, the ORR of the targeted lesions was 61 %, and the DCR was 96 % by both EASL and RECIST criteria. At 12 months after initiation of therapy, 6 patients (n = 19 lesions) were assessable for imaging response. The ORR of the targeted lesions was 53 %, and the DCR was 95 % by both EASL and RECIST criteria (Table 4).
Discussion
We found that DEB-TACE was effective in treating NELM with a good response rate. Indeed, at 1 month after DEB-TACE, 62 % of the patients had imaging response according to EASL criteria, and 92 % had stable disease using RECIST and EASL criteria. At 6 months after treatment, there was 40 % objective response by EASL and 90 % disease-control rate using RECIST. However, we found the safety profile of DEB-TACE to be concerning given the high rate of bilomas in this patient population. Indeed, 7 of the total 13 patients developed a biloma after DEB-TACE treatment (54 %), 4 of which eventually required percutaneous drainage. The other toxicities were minor and similar to those reported in the literature [14, 15].
Numerous reports discuss biliary necrosis and biloma formation after conventional lipiodol-based transarterial chemoembolization (c-TACE) [28–31]. In the largest series of 972 patients, 35 patients (3.6 %) developed intrahepatic bilomas after TACE treatment. The mechanism is still unclear but is likely related to the deposition of chemotherapeutic agents into the peribiliary arterial plexus [30]. According to Sakamoto’s study, the prognostic factors for biloma formation include the presence of liver metastatic tumors measuring <5 cm in size, pre-existing biliary ductal dilatation, proximal chemoembolization injection site, and repeat chemoembolization procedures [30].
Our 54 % rate of biloma formation, although in a small patient population (n = 13), is much greater than the 9.6 % reported in patients with metastatic liver tumor in the c-TACE literature [30]. The reason for such a high rate of bilomas is likely multifactorial as explained later in the text. DEB has many theoretical advantages over c-TACE, such as improved drug delivery to the tumor, thus resulting in less systemic exposure and greater potency. Recent studies have shown that DEB increases patient survival in select populations while decreasing the incidence of postembolization syndrome compared with c-TACE [22, 32–37]. As described previously, the reported rate of biloma formation after c-TACE was much greater when treating metastatic disease compared with HCC, likely because of the protective effect of cirrhosis and portal hypertension [30], which promotes the development of a more developed arterial network supplying the biliary ductal system. In our series, one patient was cirrhotic and notably did not develop a biloma; no one had portal hypertension.
In addition, when these patients were treated, DEBs were mixed with contrast in a 1:1 ratio. The beads could not be seen when injected into the hepatic artery. The embolization end point was determined on the basis of tactile response by the operator, which may have led to overembolization, with subsequent bead deposition outside the tumor. In addition, because the doxorubicin beads elute chemotherapy slowly, drug exposure to the tissue is prolonged. Such extended exposure, in turn, could have caused profound damage to the peribiliary plexus. It is interesting to note that technical recommendations regarding the use of the DEBs have been made by leading experts in the field, and an article summarizing these recommendations was recently published. One of these recommendations is to dilute the DEBs with contrast to minimize the deposition of highly concentrated DEBs in one area of the liver [38]. This will help to better determine the appropriate end point to embolization and avoid nontarget embolization.
In our patient population, those who developed bilomas had either single lesions (with or without satellite lesions) or diffuse disease. Only two of the seven patients who developed bilomas had multiple lesions. In contrast, all six patients who did not develop bilomas had multiple lesions with the smallest measuring 3.5 cm. These findings suggest it is possible that the presence of multiple lesions conferred protection to these patients. In addition, those patients who developed bilomas had a mean hepatic tumor burden of 12 %, whereas those who did not develop bilomas had a mean tumor burden more >3 times greater (44 %). One possible hypothesis is that the tumor burden in those patients with multiple lesions was large enough to allow deposition of the entire quantity of doxorubicin beads into the tumor with little bead deposition into healthy liver parenchyma.
One of the patients who developed a biloma had a pre-existing surgical biliary enteric anastamosis. Patients with a reconstructive biliary enteric anastomosis who undergo TACE are known to have a much greater risk for hepatic abscess formation [39, 40]. In this setting, the biliary tree is chronically colonized by bacteria from the small bowel and is likely the cause of increased incidence of abscess formation. In this particular case, before treating the patient, we believed that the potential benefits of chemoembolization would outweigh its risks.
De Baere et al. [12], Gaur [13], and Guiu et al. [41] have published the only papers to date describing the results of DEB-TACE in patients with NETs. In de Baere’s preliminary study in 2008, no patients had biloma formation on 1- and 3-month posttreatment MRIs, although 5 of 20 patients (25 %) had peripheral wedge-shaped hypointensities that were consistent with hepatic infarcts. Two patients in the study by Gaur et al. [13] had “biliary injuries” but did not have bilomas needing drainage. The large discrepancy between the rate of biloma formation in our population (54 %) versus that of De Baere’s population (0 %) could be explained by (1) the fact that the tumors treated were quite different (70 % of our population had a tumor burden <30 %, whereas 32 % of De Baere’s population had a tumor burden <30 %) and (2) the different bead sizes used (100–300 μm beads in our study vs. 500–700 μm particles in De Baere’s study). The smaller bead size in this study could certainly be one of the predisposing factors for biloma formation by causing more profound ischemic changes, which ultimately affect the peribiliary plexus. This could be a topic of future study. In addition, because our patients had more widespread disease and liver involvement, selective placement of the micro-catheter was often not feasible.
Guiu et al. [41] has published the most extensive patient experience comparing biliary injuries (defined as bilomas and liver infarcts) in multiple patient populations: those with HCC versus neuroendocrine metastases and those receiving DEB-TACE treatment versus c-TACE [41]. They concluded that biliary injuries are independently associated with DEB-TACE and neuroendocrine metastases. In their retrospective study, approximately 30 % of patients developed biliary injuries, none of which required drainage (they were all managed conservatively). They also classified the database on the location of treatment and size of DEB-TACE beads, both of which were not related to the formation of bilomas. We believe that even though our study population is much smaller, it is prospective in nature, and patient information is presented in much more detail. In addition, as described previously, the incidence of biliary complications is much greater (54 vs. 30 %). The reasons for this are described in preceding text.
Recommendations
On the basis of the safety findings of our interim analysis, we modified our patient selection criteria and changed our DEB-TACE technique. We decided to include only patients with multiple large lesions (smallest lesion >4 cm) and extensive tumor burden. After the interim analysis, we no longer consider small multifocal disease (largest lesion <4 cm) for DEB-TACE treatment. The DEB-TACE procedure was also modified. The DEBs are now mixed with four times the amount of contrast to improve visualization of the DEBs, thereby preventing potential misadministration of the DEBs. We understand that these changes in technique/patient selection are based on a small number of patients and are therefore speculative. This certainly would be a good topic for further investigation.
Conclusion
The findings of our interim analysis highlight the importance and usefulness of smaller studies. We found that DEB-TACE was efficacious in the treatment of unresectable hepatic neuroendocrine metastases, and our safety analysis showed that the toxicity profile was limited and predominantly lower grade. However, it alerted us that there was an unusually high incidence of bilomas (54 %) after DEB-TACE. Although the cause of the biloma formation was likely multifactorial, we believe the two most likely predisposing factors were patient- (number and size of tumors) and procedure-related (drug delivery technique). This finding led us to intervene early and modify our patient selection and drug delivery technique. These changes could be a subject for further investigation.
Acknowledgments
This study was partially funded by National Institutes of Health Grant No. R01 CA160771-2 and BioCompatibles UK Ltd. Clinical Trials.gov trial no. NCT00730483. Previous presentations include the following: (1) American Society of Clinical Oncology, Gastrointestinal Cancers Symposium, San Francisco, CA, January 20 to 22, 2011; (2) Cardiovascular and Interventional Radiological Society of Europe, 2011, Sept 10–14, and (3) Society of Interventional Radiology, 36th Annual Scientific Meeting, Chicago, IL, 2011.
Footnotes
Conflict of interest Nikhil Bhagat, Mingde Lin, and Jean-Francois Geschwind were partially funded by National Institutes of Health Grant No. R01 CA 160771-2. Jean-Francois Geschwind was partially funded by Biocompatibles UK Ltd.
Contributor Information
Nikhil Bhagat, Email: nbhagat1@jhmi.edu, Division of Vascular and Interventional Radiology, Department of Radiology, Johns Hopkins University Medical Center, The Johns Hopkins Hospital, Sheikh Zayed Tower, Suite 7203, Baltimore, MD 21287, USA.
Diane K. Reyes, Email: dreyes@jhmi.edu, Division of Vascular and Interventional Radiology, Department of Radiology, Johns Hopkins University Medical Center, The Johns Hopkins Hospital, Sheikh Zayed Tower, Suite 7203, Baltimore, MD 21287, USA
Mingde Lin, Email: ming.lin@philips.com, Clinical Informatics, Interventional, and Translational Solutions (CIITS), Philips Research North America, Briarcliff, NY 10510, USA.
Ihab Kamel, Division of Vascular and Interventional Radiology, Department of Radiology, Johns Hopkins University Medical Center, The Johns Hopkins Hospital, Sheikh Zayed Tower, Suite 7203, Baltimore, MD 21287, USA.
Timothy M. Pawlik, Department of Surgery, Johns Hopkins University Medical Center, Baltimore, MD 21287, USA
Constantine Frangakis, Email: cfrangak@jhsph.edu, Department of Biostatistics, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD 21287, USA.
J. F. Geschwind, Email: jfg@jhmi.edu, Division of Vascular and Interventional Radiology, Department of Radiology, Johns Hopkins University Medical Center, The Johns Hopkins Hospital, Sheikh Zayed Tower, Suite 7203, Baltimore, MD 21287, USA
References
- 1.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97(4):934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 2.Kress O, Wagner HJ, Wied M, Klose KJ, Arnold R, Alfke H. Transarterial chemoembolization of advanced liver metastases of neuroendocrine tumors—a retrospective single-center analysis. Digestion. 2003;68(2–3):94–101. doi: 10.1159/000074522. [DOI] [PubMed] [Google Scholar]
- 3.Moertel CG. Treatment of the carcinoid tumor and the malignant carcinoid syndrome. J Clin Oncol. 1983;1(11):727–740. doi: 10.1200/JCO.1983.1.11.727. [DOI] [PubMed] [Google Scholar]
- 4.Saltz L, et al. A phase II trial of alpha-interferon and 5-fluorouracil in patients with advanced carcinoid and islet cell tumors. Cancer. 1994;74(3):958–961. doi: 10.1002/1097-0142(19940801)74:3<958::aid-cncr2820740326>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 5.Schnirer I, Yao JC, Ajani JA. Carcinoid—a comprehensive review. Acta Oncol. 2003;42(7):672–692. doi: 10.1080/02841860310010547. [DOI] [PubMed] [Google Scholar]
- 6.Mayo SC, de Jong MC, Pawlik TM. Surgical management and emerging therapies to prolong survival in metastatic neuroendocrine cancer. Ann Surg Oncol. 2011;18(53):S220–221. doi: 10.1245/s10434-010-1343-2. [DOI] [PubMed] [Google Scholar]
- 7.Gupta S, et al. Hepatic arterial embolization and chemo-embolization for the treatment of patients with metastatic neuroendocrine tumors: variables affecting response rates and survival. Cancer. 2005;104(8):1590–1602. doi: 10.1002/cncr.21389. [DOI] [PubMed] [Google Scholar]
- 8.Touzios JG, et al. Neuroendocrine hepatic metastases: does aggressive management improve survival? Ann Surg. 2005;241(5):776–783. doi: 10.1097/01.sla.0000161981.58631.ab. discussion 783–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruutiainen AT, et al. Chemoembolization and bland embolization of neuroendocrine tumor metastases to the liver. J Vasc Interv Radiol. 2007;18(7):847–855. doi: 10.1016/j.jvir.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 10.Vogl TJ, et al. Liver metastases of neuroendocrine tumors: treatment with hepatic transarterial chemotherapy using two therapeutic protocols. AJR Am J Roentgenol. 2009;193(4):941–947. doi: 10.2214/AJR.08.1879. [DOI] [PubMed] [Google Scholar]
- 11.Mayo SC, et al. Surgery versus intra-arterial therapy for neuroendocrine liver metastasis: a multicenter international analysis. Ann Surg Oncol. 2011;18(13):3657–3665. doi: 10.1245/s10434-011-1832-y. [DOI] [PubMed] [Google Scholar]
- 12.de Baere T, et al. Transarterial chemoembolization of liver metastases from well differentiated gastroenteropancreatic endocrine tumors with doxorubicin-eluting beads: preliminary results. J Vasc Interv Radiol. 2008;19(6):855–861. doi: 10.1016/j.jvir.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 13.Gaur SK, et al. Hepatic arterial chemoembolization using drug-eluting beads in gastrointestinal neuroendocrine tumor metastatic to the liver. Cardiovasc Intervent Radiol. 2011;34(3):566–572. doi: 10.1007/s00270-011-0122-1. [DOI] [PubMed] [Google Scholar]
- 14.El-Serag HB, et al. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139(10):817–823. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]
- 15.Davila JA, et al. Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: a population-based study. Gastroenterology. 2004;127(5):1372–1380. doi: 10.1053/j.gastro.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 16.Parkin DM, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 17.Bosch FX, et al. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2005;9(2):191–211. v. doi: 10.1016/j.cld.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Srivatanakul P, Sriplung H, Deerasamee S. Epidemiology of liver cancer: an overview. Asian Pac J Cancer Prev. 2004;5(2):118–125. [PubMed] [Google Scholar]
- 19.Ahmed F, et al. National trends and disparities in the incidence of hepatocellular carcinoma, 1998–2003. Prev Chronic Dis. 2008;5(3):A74. [PMC free article] [PubMed] [Google Scholar]
- 20.Jemal A, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 21.Reuter NP, et al. Radiofrequency ablation vs. resection for hepatic colorectal metastasis: therapeutically equivalent? J Gastrointest Surg. 2009;13(3):486–491. doi: 10.1007/s11605-008-0727-0. [DOI] [PubMed] [Google Scholar]
- 22.Reyes DK, et al. Single-center phase II trial of transarterial chemoembolization with drug-eluting beads for patients with unresectable hepatocellular carcinoma: initial experience in the United States. Cancer J. 2009;15(6):526–532. doi: 10.1097/PPO.0b013e3181c5214b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Therasse P, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 24.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 25.Kamel IR, et al. Unresectable hepatocellular carcinoma: serial early vascular and cellular changes after transarterial chemoembolization as detected with MR imaging. Radiology. 2009;250(2):466–473. doi: 10.1148/radiol.2502072222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gasnier A, et al. IEEE international symposium on biomedical imaging: from nano to macro; Austin, TX, USA. 13–18 June 2010.2010. [Google Scholar]
- 27.Mory B, et al. Non-Euclidean image-adaptive radial basis functions for 3D interactive segmentation. Twelfth IEEE international conference on computer vision (ICCV); Kyoto, Japan. 2009. [Google Scholar]
- 28.Miyayama S, et al. Main bile duct stricture occurring after transcatheter arterial chemoembolization for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2010;33(6):1168–1179. doi: 10.1007/s00270-009-9781-6. [DOI] [PubMed] [Google Scholar]
- 29.Kim HK, et al. Ischemic bile duct injury as a serious complication after transarterial chemoembolization in patients with hepatocellular carcinoma. J Clin Gastroenterol. 2001;32(5):423–427. doi: 10.1097/00004836-200105000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Sakamoto I, et al. Intrahepatic biloma formation (bile duct necrosis) after transcatheter arterial chemoembolization. AJR Am J Roentgenol. 2003;181(1):79–87. doi: 10.2214/ajr.181.1.1810079. [DOI] [PubMed] [Google Scholar]
- 31.Yu JS, et al. Predisposing factors of bile duct injury after transcatheter arterial chemoembolization (TACE) for hepatic malignancy. Cardiovasc Intervent Radiol. 2002;25(4):270–274. doi: 10.1007/s00270-001-0049-z. [DOI] [PubMed] [Google Scholar]
- 32.Liapi E, Geschwind JF. Transcatheter and ablative therapeutic approaches for solid malignancies. J Clin Oncol. 2007;25(8):978–986. doi: 10.1200/JCO.2006.09.8657. [DOI] [PubMed] [Google Scholar]
- 33.Liapi E, Geschwind JF. Intra-arterial therapies for hepatocellular carcinoma: where do we stand? Ann Surg Oncol. 2010;17(5):1234–1246. doi: 10.1245/s10434-010-0977-4. [DOI] [PubMed] [Google Scholar]
- 34.Lammer J, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33(1):41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malagari K, et al. Transarterial chemoembolization of un-resectable hepatocellular carcinoma with drug eluting beads: results of an open-label study of 62 patients. Cardiovasc Intervent Radiol. 2008;31(2):269–280. doi: 10.1007/s00270-007-9226-z. [DOI] [PubMed] [Google Scholar]
- 36.Malagari K, et al. Prospective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with BeadBlock for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2010;33(3):541–551. doi: 10.1007/s00270-009-9750-0. [DOI] [PubMed] [Google Scholar]
- 37.Varela M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46(3):474–481. doi: 10.1016/j.jhep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 38.Lencioni R, et al. Transcatheter treatment of hepatocellular carcinoma with doxorubicin-loaded DC Bead (DEBDOX): technical recommendations. Cardiovasc Intervent Radiol. 2011 Oct; doi: 10.1007/s00270-011-0287-7. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geschwind JF, et al. Influence of a new prophylactic antibiotic therapy on the incidence of liver abscesses after chemoembolization treatment of liver tumors. J Vasc Interv Radiol. 2002;13(11):1163–1166. doi: 10.1016/s1051-0443(07)61959-9. [DOI] [PubMed] [Google Scholar]
- 40.De Jong MC, et al. Liver-directed therapy for hepatic metastases in patients undergoing pancreaticoduodenectomy: a dual-center analysis. Ann Surg. 2010;252(1):142–148. doi: 10.1097/SLA.0b013e3181dbb7a7. [DOI] [PubMed] [Google Scholar]
- 41.Guiu B, et al. Liver/biliary injuries following chemoembolisation of endocrine tumours and hepatocellular carcinoma: lipiodol vs. drug-eluting beads. J Hepatol. 2012;56(3):609–617. doi: 10.1016/j.jhep.2011.09.012. [DOI] [PubMed] [Google Scholar]


