Abstract
FOXM1 is an oncogenic transcription factor of the Forkhead family and it has a well-defined role in cell proliferation and cell cycle progression. Expression of FOXM1 is excluded in quiescent or differentiated cells, but its level is highly elevated in proliferating and malignant cells. Overexpression of FOXM1 has been reported in more than 20 types of human cancer. In recent years, FOXM1 has been implicated in diverse cellular processes and also a growing body of experimental data has underlined the relevance of FOXM1 in tumorigenesis. Although FOXM1 is under the control of three major tumor suppressors (RB, p53, p19ARF), it is still active in the majority of human cancers. The oncogenic potential of FOXM1 is mainly based on its ability to transcriptionally activate genes that are involved in different facets of cancer development. In this review, the contribution of FOXM1 to each of the hallmarks of cancer will be summarized and discussed.
Keywords: FOXM1, tumorigenesis, cancer, apoptosis, proliferation
1. Introduction
Mammalian transcription factor Forkhead Box M1 (FOXM1) belongs to the extensive family of Forkhead transcription factors, whose members are characterized by a 100 amino acid long, evolutionarily conserved DNA binding domain called Forkhead or winged-helix domain (1). Forkhead family members are involved in a wide range of biological processes including embryogenesis, proliferation, differentiation, apoptosis, transformation, tumorigenesis, longevity and metabolic homeostasis (2). FOXM1, previously known in the literature as Trident (mouse), WIN or INS-1 (rat), FKHL-16, MPP-2 (partial human cDNA) or HFH-11 (human) is uniquely proliferation associated among the family members (1). However, over the years the cellular functions of FOXM1 have been extended to cell migration, invasiveness, vascular permeability, angiogenesis, surfactant homeostasis, oxidative stress and inflammation (2). Alternative splicing of two exons gives rise to three isoforms of FOXM1, the transcriptionally active FOXM1b and c, and the transcriptionally inactive FOXM1a splice variants (1). For simplicity we will refer to the different isoforms as FOXM1 in this review.
2. FOXM1 is a bona-fide cell cycle regulator in normal cells
The expression and the transcriptional activity of FOXM1 depend on the progression of the cell cycle (1). FOXM1 mRNA and protein levels are diminished in quiescent cells, but both are upregulated in the late G1-phase of the cell cycle and persist throughout the G2 and M-phases (1). Furthermore, FOXM1 expression correlates with the proliferative state of the cell. During embryonic development FOXM1 is ubiquitously expressed in embryonic tissues (1), especially in proliferating epithelial and mesenchymal cells. In adult tissues, FOXM1 expression is confined to actively dividing cells of the thymus, testis, colon and intestine, but mainly its expression is eliminated in resting or terminally differentiated cells (1). However, FOXM1 expression is induced in adult cell types by mitogenic stimuli, by tissue injury, or by oxidative stress (1). The transcriptional activity of FOXM1 correlates with its phosphorylation level. Both gradually increase as cells progress through the cell cycle reaching maximum levels at the G2/M transition. FOXM1 is initially phosphorylated in G1 and then further phosphorylated in a sequential order by multiple protein kinases including Cdk-cyclin complexes and mitogenic kinases in the S and G2/M phases of the cell cycle generating the hyperphosphorylated and fully active form of FOXM1 by the G2/M-phase (1, 3, 4). Screen for inhibitors of FOXM1 transactivation uncovered a unique mode of its transcriptional regulation. Our group has demonstrated that inhibitors of FOXM1 not only inhibit FOXM1 as a transcription factor, but they also downregulate the mRNA and protein expression of FOXM1. These data suggest that FOXM1 is involved in a positive autoregulatory loop, wherein FOXM1 induces its own transcription (5) and it may bind to its own promoter. However, the functional significance of FOXM1 autoregulation in the context of normal cell cycle progression still remains elusive.
A large cohort of mouse studies provided evidence for a pivotal role of FOXM1 in the regulation of cell proliferation and cell cycle progression. Ectopic expression of FOXM1 in non-regenerating liver cells did not induce aberrant hepatocyte proliferation (6). However, when these FOXM1-overexpressing transgenic mice were subjected to partial hepatectomy earlier onset of hepatocyte DNA-replication and mitosis occurred accompanying by premature expression of cell cycle regulatory genes (6). Complete deletion of FOXM1 is embryonic lethal due to developmental defects in major organs (7, 8). FOXM1−/− mouse embryos exhibited increased polyploidy in cardiomyocytes and hepatocytes suggesting that FOXM1 is required to prevent cells from reentering DNA replication before mitosis (7, 9). Also, FOXM1 knockout mouse embryos showed reduced cell proliferation in the liver (7) and the lung (8) supporting the fact that FOXM1 is not only expressed in dividing cells, but it is essential for normal cell proliferation and cell cycle progression.
Additional studies conducted in cell culture uncovered a network of genes intimately involved in cell cycle progression that are under the direct transcriptional regulation of FOXM1. FOXM1 was shown to be critical for G1/S and G2/M transition, mitotic progression and the maintenance of chromosome stability (10–12). The significance of FOXM1 in cell cycle progression was further supported by depletion studies. FOXM1 is required for the execution of the mitotic program since FOXM1 depleted cells did not advance beyond the prophase stage of mitosis (10). Also, loss of FOXM1 resulted in cell cycle abnormalities including a delay in G2/M progression, chromosome missegregation and failure of cytokinesis (10–12).
Since FOXM1 is such a critical regulator of the cell cycle, its activity is tightly controlled to provide orderly expression of its downstream effectors and to prevent uncontrolled cell proliferation. Subsequent phosphorylation of FOXM1 is the driving force behind its activity during cell cycle progression and not surprisingly rapid dephosphorylation of FOXM1 occurs when cells are exiting mitosis (3). FOXM1 protein is also targeted for proteasomal degradation by the APC/C-Cdh1 complex in late mitosis and early G1 phases of the cell cycle (13). Paradoxically, FOXM1 expression is inhibited by proteasome inhibitors (14). However, inhibition of FOXM1 by proteasome inhibitors contradicts their nature to stabilize the expression of the majority of cellular proteins. To resolve this paradox, we propose that proteasome inhibitors stabilize a negative regulator of FOXM1 (NRFM) (15, 16). In the absence of proteasome inhibitors FOXM1 is expressed due to the positive feedback loop where FOXM1 transcriptional activity is required for its expression. Following proteasomal inhibition the hypothetical NRFM gets stabilized and either directly or indirectly inhibits FOXM1 transcriptional activity leading to the suppression of FOXM1 expression as a result of the positive autoregulatory loop (5, 15). Since, FOXM1 is considered as one of the main targets of proteasome inhibitors (17), it is tempting to speculate that the specificity of proteasome inhibitors to cancer cells is linked to the expression of FOXM1 in cancer, but not in normal cells. Direct interaction with tumor suppressor RB (18), B55α, a regulatory subunit of PP2A phosphatase (19) and between its own N-terminal domain and C-terminal transactivation domain (TAD) (20) in the G1-phase also contributes to the negative modulation of FOXM1 transcriptional activity in the early stages of the cell cycle. However, it needs to be emphasized that FOXM1 is not completely transcriptionally inactive in G1/S phase (20) since it regulates transcription of genes responsible for G1/S transition (1) and it is important for regulated S-phase entry in regenerating hepatocytes (6). In G1/S phase the transcriptional activity of FOXM1 is lower than in G2/M phase as a result of the above discussed regulatory mechanisms (20).
3. Oncogenic FOXM1 is overexpressed in human cancer cells
Elevated expression of FOXM1 has been detected in a broad range of cancer cell lines and cancer types examined so far, suggesting that FOXM1 is essential for tumor cell proliferation (1, 2, 21). Generally, increased expression of FOXM1 in tumors is associated with advanced tumor stage, high proliferation rate and poor prognosis (22), suggesting that FOXM1 could be a novel prognostic marker for cancer patients.
A mounting body of evidence suggests that FOXM1 contributes to oncogenesis. Overexpression of FOXM1 increased invasion (23), angiogenesis (24, 25) and the anchorage-independent growth potential of cancer cells in vitro (22, 26). Overexpression of FOXM1 in human cancer cell lines also increased their tumorigenicity in xenograft models (22, 25). Conversely, RNAi-mediated knockdown of FOXM1 in cancer cells decreased proliferation (10, 11), migration, invasion, angiogenic capacity (23–25) and anchorage-independent growth in soft agar in vitro (22, 27–29). Moreover, FOXM1 suppression inhibited xenograft tumor growth of human cancer cells in nude mice (22, 24, 25, 28, 29), suggesting that FOXM1 has an essential role in regulating the tumorigenecity of human cancer cells. Overexpression of FOXM1 in TRAMP or LADY transgenic mice, two well-established mouse models of prostate cancer, accelerated the development, proliferation and growth of prostate tumors, suggesting that FOXM1 plays an important role in prostate cancer progression (30). Conditional deletion of FOXM1 in the liver before DEN/PB treatment resulted in diminished proliferation and resistance to HCC (26). A decrease in the number and size of lung tumors as a result of diminished proliferation was observed when FOXM1 was conditionally deleted in all cell types of the lung prior to treatment with urethane (31). Conditional deletion of FOXM1 in respiratory epithelial cells before urethane or MCA/BHT treatment (32), or in pulmonary macrophages prior to treatment with MCA/BHT (33) also compromised lung tumor formation. FOXM1 overexpression is associated with an increase in proliferation and tumorigenecity of cancer cells, while FOXM1 depletion decreases proliferation and inhibits tumorigenesis, suggesting that FOXM1 is involved in the formation, the proliferative expansion and the progression of tumors. However, the data that OCI/AML3 leukemia cell line with inactive, uncharacteristically cytoplasmic FOXM1 is still tumorigenic (29, 34) suggests that FOXM1 might not be universally required for oncogenesis.
The precise underlying mechanism for the broad overexpression of FOXM1 in human malignancies is still the subject of speculation. Since FOXM1 is one of the key cell cycle regulators, overexpression of FOXM1 in cancer cells and all the effects of FOXM1 on cancer could reflect its important role in cell proliferation and could simply be the sign of a passenger effect of the enhanced proliferation capacity of cancer cells. However, it has been shown recently that there are breast cancers with low proliferation and high FOXM1 expression as well as with high proliferation and low FOXM1 expression (35). In addition, certain cancer cells with FOXM1 knockdown are viable and proliferating (28, 36, 37), but their tumorigenicity is severely impaired both in vitro and in vivo (28, 29, 36). All these data suggest that the FOXM1-mediated cancer-related processes may be separated from its cell cycle regulatory functions and rather be explained by the oncogenic upregulation of FOXM1 in cancer cells.
The following mechanisms are currently considered responsible for the elevated expression and activity of FOXM1 in cancer: (a) Amplification of the FOXM1 locus. The human FOXM1 gene is mapped on the chromosomal band 12p13 (38), which is frequently amplified in various cancers including cervical carcinomas, breast adenocarcinomas, nasopharyngeal carcinomas, and head and neck squamous cell carcinomas (1). In addition, integrative genomic profiling of Non-Hodgkin's Lymphoma (NHL) revealed that amplification of the FOXM1 locus is one the most prevailing genetic alterations in NHL entities to promote lymphomagenesis (39). (b) Increased stability or expression of FOXM1. FOXM1 stability or expression in cancer cells can be increased via the interaction with different types of proteins or by different modifications. For example, the Wnt signaling pathway inhibits FOXM1 degradation (40), while direct interaction with NPM (29) and direct phosphorylation by Cdk4,6/cyclinD complexes (4) stabilize FOXM1 protein. (c) Enhanced transcription of FOXM1. E2F (41), c-Myc (42), and HIF-1 (43) directly bind to the FOXM1 promoter and stimulate its expression. (d) Mutations of tumor suppressor p53. p53 negatively regulates FOXM1 expression (44, 45). Mutation or inactivation of p53, often seen in human tumors could contribute to the wide overexpression of FOXM1 in different human cancers (44) (see also section 4.2). (e) Activation of FOXM1 by oncogenic signaling pathways. FOXM1 functions downstream of established oncogenic signaling pathways including PI3/Akt, EGFR, Raf/MEK/MAPK and Hedgehog (via the Gli1 transcription factor (46)), that play crucial roles in cancer development (reviewed in (21)).
4. FOXM1 contributes to all major hallmarks of cancer
4.1. FOXM1 sustains proliferation
The most basic property of cancer cells is their infinite capacity to proliferate. Normal cells obey regulatory mechanisms to enter, advance and exit the cell cycle in an orderly manner. In contrast, cancer cells disregard these regulatory signals and operate in an independent fashion (47). The central role of FOXM1 in the control of cell proliferation under normal conditions is well established, however, it is also evident that FOXM1 is greatly involved in tumor cell proliferation.
The contribution of estrogen receptors to the proliferation of normal and cancerous breast epithelial cells is well-defined. FOXM1 directly binds to the promoter and stimulates the transcription of ERα, thereby promoting breast cancer cell proliferation via inducing the expression of ERα (48). Also, ERα directly regulates FOXM1 and FOXM1 mediates the proliferative effects of ERα in breast cancer cells (49). These data suggest a positive feedback loop between FOXM1 and ERα. The feedback loop is likely to have a critical role in breast cancer development, since it intensifies the mitogenic effects of estrogen (49). ERβ1 directly inhibited ERα-dependent FOXM1 transcription by physically displacing ERα on the FOXM1 promoter. Since overexpression of FOXM1 antagonized the anti-proliferative effects of ERβ1, it was postulated that ERβ1 signaling inhibits breast cancer cell proliferation through the repression of FOXM1 (50). Human epidermal growth factor receptor-2 (HER2) is a member of the EGFR family, which is implicated in cell proliferation. FOXM1 was found as a downstream target of HER2 and a potential downstream effector of HER2 signaling in breast cancer, thereby HER2 may promote breast tumorigenesis by regulating the expression of FOXM1 (51).
In FOXM1 depleted lung tumors and lung cancer cells diminished proliferation correlated with decreased expression of Topoisomerase-2α (TOPO-2α), which plays an important role in proliferation of tumor cells. FOXM1 directly activates the transcription of TOPO-2α, thus contributing to proliferation of cancer cells leading to tumorigenesis (32). c-Myc itself is an important regulator of proliferation, differentiation and apoptosis. Interestingly, c-Myc was found to be a direct transcriptional target of FOXM1, thus it also serves as a mediator of FOXM1-induced proliferation (52). Also, c-Myc directly binds and transcriptionally activates FOXM1 to regulate hepatocyte proliferation only in response to the activation of the nuclear receptor CAR (constitutive adrostane receptor) by TCPOBOP carcinogen (42). These findings suggest that there is a positive feedback loop between FOXM1 and c-Myc. This feedback mechanism potentially amplifies and maintains the expression of c-Myc and FOXM1 target genes in order to promote continuous proliferation (42). Simultaneous activation of AKT and N-Ras in the mouse liver dramatically accelerated the development of liver tumors due to elevated proliferation and angiogenesis. FOXM1/SKP2 and c-Myc signaling were partially responsible for the increased hepatocyte proliferation and liver tumorigenesis (53).
Cdk inhibitors p21Cip1 and p27Kip1 halt cell cycle progression by interfering with the activation of Cdks. FOXM1 inhibits p27Kip1 protein expression by multiple mechanisms. FOXM1 promotes the proteasomal degradation of the p27Kip1 protein via direct transcriptional activation of the Skp2 and Cks1 genes (10). In addition, following growth factor stimulation FOXM1 directly activates the transcription of KIS (kinase-interacting stathmin), which in turn phosphorylates p27Kip1 and thus facilitates its nuclear export and proteolysis leading to cell cycle progression (54).
4.2. FOXM1 evades the action of tumor suppressors
Cancer cells not only must maintain mitogenic growth signals, but they also have to escape potent anti-growth signals in order to sustain constant proliferation. Inhibition of proliferation mainly depends on the functions of tumor suppressors. Disruption or loss of tumor suppressor activity is often seen in human cancers (47).
Both major tumor suppressors RB and p53 control FOXM1. RB directly binds to FOXM1 and inhibits its transcriptional activity. Cyclin D1/Cdk4 phosphorylates RB, thus relieving the repression by RB and activating FOXM1-dependent transcription at the G1/S progression (18). Interaction with RB in the G1-phase may also contribute to the negative regulation of FOXM1 in the early stages of the cell cycle. Initially, a study using microarray analysis found that p53 activation leads to a decrease in FOXM1 mRNA (55). Our and Carol Prives’s group independently reported that expression of FOXM1 at both mRNA and protein levels were upregulated upon inactivation or deletion of p53, suggesting that p53 is a negative regulator of FOXM1 (44, 45). This data also implies that p53 mutation or inactivation in human tumors could be one of the underlying mechanisms for the broad overexpression of FOXM1 found in various human cancers. For example, in high-grade serous ovarian cancer (HGS-OvCa) p53 mutations were observed in 96% of tumors, and the FOXM1 pathway was activated in 87% of the cases (56). Following DNA-damage FOXM1 expression increased in the absence or decreased in the presence of its negative regulator p53 (37, 44, 45). Since DNA-damaging agents are key components of commonly used chemotherapeutic treatments, this observation indicates that serious considerations should be taken in regard to the design of treatment strategies for patients with mutated or inactivated p53 in order to prevent potential FOXM1-related drug resistance. DEN/PB treatment induced the expression of tumor suppressor p19ARF in the liver. The p19ARF protein directly binds to the C-terminal region of FOXM1 and inhibits its transcriptional activity by translocating FOXM1 to the nucleolus (26).
4.3. FOXM1 increases resistance of cancer cells to apoptosis
Cancer cells can survive and expand not only by the means of proliferation, but also by resisting cell death signals (57). Apoptosis, perhaps the most common form of cell elimination, is a strictly regulated stepwise process. A variety of signals can trigger apoptosis; thereby cancer cells developed multiple mechanisms to avoid cell destruction. Other forms of cell death such as autophagy and necrosis represent additional obstacles to cancer development (47).
FOXM1 protected from apoptosis induced by proteasome inhibitors (14), Herceptin and paclitaxel (58), cisplatin (59), and epirubicin (41). In addition, FOXM1 was found to mediate endocrine resistance in breast cancer cells (49). However, attenuation of FOXM1 expression by RNAi, by treatment with proteasome inhibitors or with the ARF-derived peptide reintroduced sensitivity of cancer cells to various cell death-inducing stimuli (58–60). Furthermore, knockdown of FOXM1 increased sensitivity to apoptosis induced by proteasome inhibitors (61), DNA-damaging agents (37) and oxidative stress (60). The downstream effectors of FOXM1 that mediate resistance to apoptosis have just started being uncovered. FOXM1-overexpressing breast cancer cells became resistant to Herceptin treatment by keeping p27Kip1 expression low to override the Herceptin-induced G1-arrest. Also, FOXM1-overexpressing breast cancer cells showed insensitivity to cell death induced by paclitaxel as a result of elevated expression and activity of Stathmin, a microtubule-destabilizing protein and direct FOXM1 transcriptional target (58). FOXM1 mediated cisplatin and epirubicin resistance in breast cancer cells by enhancing DNA repair (41, 59). Recently, we have reported that JNK activation and Bcl-2 downregulation after FOXM1 knockdown are potential underlying mechanisms to sensitize cancer cells to DNA-damage-induced cell death (37).
4.4. FOXM1 induces replicative immortality
Senescence is an irreversible, nonproliferative but viable state of cells. Senescence has been viewed as an anticancer defense mechanism since it represents a barrier to proliferation. Consequently, evading cellular senescence could promote infinite replicative capacity leading to tumor development (47).
FOXM1−/− MEFs showed several features of premature senescence, such as expressing high levels of β-galactosidase, increased nuclear levels of senescence markers p19ARF and p16INK4A, suggesting that FOXM1 might protect against senescence (10). Furthermore, ectopic expression of FOXM1 in NIH3T3 cells inhibited oxidative stress-induced premature senescence by up-regulating the expression of Bmi-1 via c-Myc (62). However, FOXM1 knockdown not only contributes to cellular senescence in normal cells, but in malignant cells as well. For example, FOXM1 knockdown in gastric cancer cells increased cellular senescence, which was partially mediated by p27Kip1 (27). CDK4/6 phosphorylation of FOXM1 led to its activation and to protection of cancer cells from senescence (4). Also, FOXM1 overexpression reduced ROS levels and senescence phenotype in mAKT1-expressing cells, while FOXM1 depletion from these cells had the opposite effect. FOXM1 protected cancer cells from oxidative stress-induced premature senescence by reducing ROS levels via the direct activation of the transcription of antioxidant enzymes, catalase and MnSOD (60).
Stem and cancer cells are both characterized by constant proliferation, immortality and the capacity for self-renewal. Recently, FOXM1 has been suggested to contribute to tumorigenesis by promoting stem cell-like properties. According to the recent work of Zhang and colleagues, direct interaction of FOXM1 and β-catenin is required for the maintenance of GIC (GBM-initiating cells) self-renewal (40). Also, FOXM1 promoted the tumorigenecity of neurobalstoma cells by maintaining their undifferentiated state via directly regulating the expression of pluripotency gene Sox2 (28). Ectopic expression of FOXM1 in primary human keratinocytes contributed to the expansion of epithelial stem/progenitor cells by interfering with their terminal differentiation program, consequently generating a hyperproliferative phenotype often seen in human epithelial hyperplasia (63).
4.5. FOXM1 stimulates angiogenesis
Tumor growth and progression highly depends on adequate blood supply for acquiring nutrients and oxygen as well as for disposing metabolic wastes and carbon dioxide. The process of angiogenesis fulfills these requirements. After the completion of embryonic development angiogenesis is downregulated. However, at the onset of tumor development an angiogenic switch is turned on and remains activated to facilitate growth and progression of cancer (47).
FOXM1 is required for the development of pulmonary vasculature during embryogenesis (8), but it was identified as one of the transcription factors that directly regulate the promoter activity of the pivotal angiogenesis inducer, vascular endothelial growth factor (VEGF) (24, 25). FOXM1 depletion correlated with reduced expression and activity of VEGF leading to decreased angiogenesis (24, 25), while overexpression of FOXM1 resulted in the transactivation of VEGF and increased angiogenesis in vitro (24, 25). Suppression of FOXM1 compromised the tumorigenecity of glioma (24) and gastric cancer cells (25) in orthotopic mouse models and led to decreased tumor vascularization (24). All these data suggest that through the direct transcriptional regulation of VEGF, FOXM1 induces angiogenesis in human cancers (24, 25). Tumor suppressor FOXO3a inhibits VEGF expression in breast cancer cells by directly displacing FOXM1 on the VEGF promoter, thus counteracting the activating function of FOXM1 (64). It is interesting to note, that though both FOXM1 and FOXO3a are members of the Forkhead family of transcription factors that contain a conserved DNA-binding domain and target the same promoter sequences (1), but FOXM1 induces, while FOXO3a inhibits tumorigenesis. In addition, FOXO3a can neutralize FOXM1 activity by inhibiting the expression of FOXM1 (65).
4.6. FOXM1 contributes to invasion and metastasis
Metastasis is a multistep and complex process involving local invasion, intravasation, extravasation, formation of micrometastasis and colonization (47). A mounting body of work suggests that epithelial-mesenchymal transition (EMT), by which epithelial cells acquire mesenchymal characteristics leading to increased migratory and invasive potential of the cancer cells has a critical role in metastasis (47). Emerging evidence suggests that FOXM1 might actively participate in the metastatic processes. FOXM1 expression was upregulated in metastatic prostate cancer samples (66) and in portal vein tumor thrombosis (PVTT), a special type of HCC metastasis (36). Overexpression of FOXM1 increased, while knockdown of FOXM1 decreased metastasis of gastric cancer cells to the liver in an orthotropic mouse model of gastric cancer (25). Mice with transgenic overexpression of FOXM1 in Arf-null background developed highly aggressive form of HCC and lung metastasis after treatment with DEN/PB. FoxM1b Tg;Arf−/− HCC cells isolated from the double transgenic animals were greatly tumorigenic in vitro and in nude mice in vivo, and also efficiently metastasized to the lungs after tail vein injection (67).
Although recent works have just started defining the functional contribution of FOXM1 to metastasis, FOXM1 has already been described as the master regulator of tumor metastasis, since it affects several aspects of the metastatic process including EMT, migration, invasion and pre-metastatic niche formation (67). Matrix metalloproteinases (MMPs) have a well established and pivotal role in the processes of tumor cell invasion and metastasis by degrading the basement membrane collagene (21). RNAi-mediated downregulation of FOXM1 correlated with decreased expression and activity of MMP-2 and MMP-9 leading to reduced migration and invasion of glioma and osteosarcoma cells in vitro (23, 68). FOXM1 directly activates the transcription of MMP-2 (23), and indirectly regulates MMP-9 through its direct transcriptional target JNK1 (68) to promote migration and invasion of cancer cells. FOXM1 also directly stimulates the Stathmin promoter to increase the destabilization of microtubules hence to promote tumor cell migration (67). FOXM1 was essential for the Ras/MKK3/p38-induced invasion and anchorage-independent growth in vitro (69).
FOXM1 overexpression in ASPC-1 pancreatic cancer cells resulted in the acquirement of EMT and cancer stem cell (CSC) phenotype, which correlated with increased expression of EMT and CSC markers (70). FOXM1 also regulated pancreatic cancer EMT and metastasis via direct transcriptional activation of Caveolin-1 (Cav-1), a key structural protein in the caveolae that has been strongly implicated in the development of cancer (71). FoxM1b Tg;Arf−/− HCC cells displayed EMT-like features such as expressing lower levels of E-cadherin, but higher levels of vimentin and showed elevated Akt activity suggesting that FOXM1 promotes EMT partially via the activation of Akt (67). FOXM1 has also been associated with the formation of pre-metastatic niche at distant organ sites by directly stimulating the transcription of LOX and LOXL2 (67).
4.7. FOXM1 and genomic instability (Enabling characteristic)
Genomic instability is the driving force of random mutations, thus enabling cancer cells to acquire favorable genetic alterations that facilitate tumor progression. However, the genome maintenance system keeps the incidence of spontaneous mutations very low by detecting and repairing DNA damages. Consequently, it is not surprising that cancer cells harbor defective components of this genome maintenance machinery in order to promote tumor growth (47).
FOXM1 depletion was associated with the accumulation of polyploidy cells (7, 10). FOXM1 has been demonstrated to play an essential role in the maintenance of appropriate chromosomal segregation and genomic stability (11, 12). FOXM1 was among the top ranking genes, whose expression correlated with chromosomal instability in different human cancers (72). Elevated expression of FOXM1 alone was adequate to increase the occurrence of genomic instability as measured by loss of heterozygosity (LOH) and copy number variations (CNV) in human oral (73) and epidermal keratinocytes (74). In addition, nicotine or UVB exposure further amplified the FOXM1-mediated genomic instability (73, 74). These data suggest that FOXM1 upregulation represents the first hit for cells to gain genomic instability, thus predisposing cells to a subsequent insult such as nicotine or UVB radiation in order to facilitate proliferation of damaged cells and acquisition of further genetic mutations and abnormalities, ultimately fueling the development of cancer (73, 74). Although, FOXM1 is implicated in DNA repair via direct transcriptional regulation of XRCC1 and BRCA2 DNA repair genes (75), it seems that its aberrant expression rather aids the acquisition of genomic instability than increases DNA repair (73, 74).
4.8. FOXM1 and inflammation (Enabling characteristic)
Inflammation is well recognized as a predisposing factor for cancer development, for example many inflammatory diseases increase the risk for developing different types of cancer. Inflammation is involved in tumorigenesis from tumor initiation, through tumor promotion, to metastasis (76). Cancer-related inflammation promotes proliferation, migration, invasion and metastasis of tumor cells, induces angiogenesis and can alter therapeutic response to anticancer agents (76).
FOXM1 overexpressing Rosa26-FOXM1 transgenic mice after treatment with MCA/BHT not only exhibited an increase in lung tumor formation, but also showed an elevated inflammatory response. FOXM1 directly activated the transcription of cyclooxygenase-2 (Cox-2), a well-known marker of inflammation, which in turn mediated the FOXM1-induced inflammation and tumorigenesis in the MCA/BHT lung tumors (77). The role of FOXM1 in tumor-associated macrophage recruitment during lung injury was also examined. FOXM1 contributed to lung tumorigenesis by regulating macrophage recruitment to tumor sites via its direct transcriptional target CX3CR1 chemokine receptor during lung inflammation and tumor growth (33). Tumor-associated endothelial cell-specific deletion of FOXM1 in the lung promoted urethane-induced tumorigenesis by increasing pulmonary inflammation and proliferation. FOXM1 kept inflammation under control by directly activating the transcription of Flk-1 and Foxf1, which are well-established regulators of lung inflammation. Depletion of FOXM1 in endothelial cells stimulated the proliferation of epithelial cells by activating the canonical Wnt signaling pathway. Sfrp1, a recognized inhibitor of Wnt signaling was identified as a direct target of FOXM1. FOXM1 influences the crosstalk between epithelial and endothelial cells, thus endothelial cell-specific expression of FOXM1 is necessary to inhibit inflammation and the Wnt signaling pathway in lung epithelial cells in order to suppress lung tumor growth (78). These data suggest that in certain cell context FOXM1 may act as a tumor suppressor by indirectly suppressing the Wnt pathway.
4.9. FOXM1 and metabolism (Emerging Hallmark)
There is a growing body of evidence that altered nutrient pathways can contribute to cancer development. Cancer cells need high amount of glucose to fulfill their energy needs (47). While the majority of glucose is being processed through glycolysis in cancer cells, a small amount enters alternative metabolic pathways such as the hexosamine biosynthetic pathway (HBP). The HBP pathway regulates the O-GlcNAc modifications of various proteins, which serves as a regulatory switch mechanism similarly to phosphorylation. O-GlcNAc transferase (OGT) catalyzes the addition of O-GlcNAc on cytosolic and nuclear proteins (79).
The metabolic sensor OGT was found to be overexpressed in breast (79) and prostate (80) cancer cells. Attenuation of OGT levels inhibited growth, invasive potential and angiogenic ability of breast and prostate cancer cells, and associated with the decreased protein expression of FOXM1 and its downstream targets (79, 80). OGT appeared to promote breast and prostate tumorigenesis in part by modulating the expression of FOXM1 via interfering with its proteasomal degradation (79, 80). Another feature of cancer cell adaptation is the ability to grow under hypoxic (O2 deprived) conditions. Hypoxia induced FOXM1 expression via direct binding of HIF-1 to the FOXM1 promoter. FOXM1 was required for survival of cancer cells under hypoxia since FOXM1 depletion compromised the proliferation capacity of hypoxic cancer cells (43). A potential interplay is being speculated between proliferation and cellular metabolism (60). From this respect, FOXM1 could serve as a messenger between proliferation and cellular metabolism by allowing cells to proliferate when the circumstances regarding energy metabolism are beneficial (60).
Concluding remarks
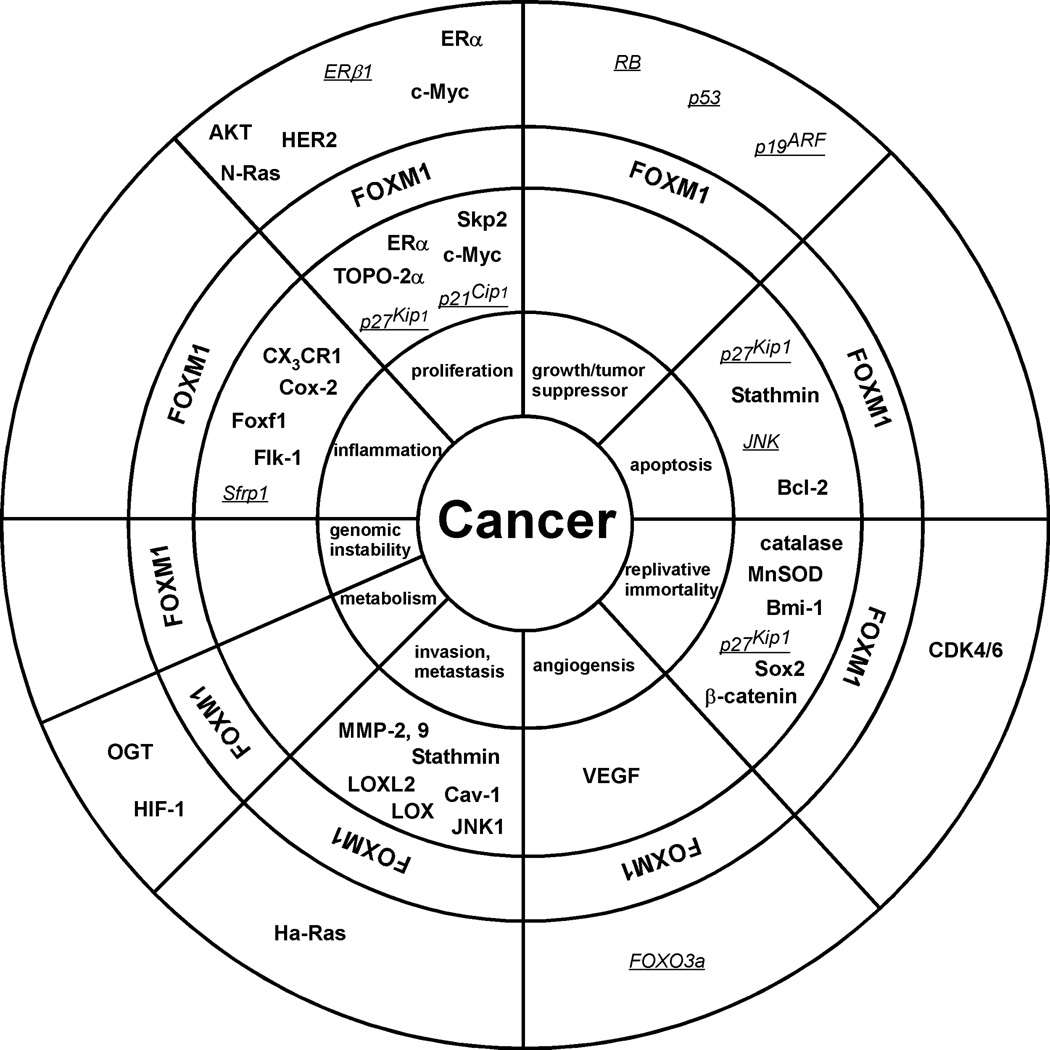
Over the years FOXM1 has become a subject of intense research. Originally, FOXM1 was characterized as a proliferation associated mammalian transcription factor, but recently it has emerged as a pivotal contributor to cancer development. FOXM1 aids cancer cells to obtain tumorigenic features and it plays a critical role in promoting their oncogenic phenotype. FOXM1 has been implicated in tumor initiation, expansion and progression. A growing body of evidence also suggests that FOXM1 actively participates in drug resistance and evasion of cell death. In the near future, we expect to learn more compelling details about the additional roles FOXM1 may play in cancer and about the regulatory mechanisms involving FOXM1 in respect to cancer development and progression. We envision the identification of miRNAs (81) regulated by FOXM1 and miRNAs controlling FOXM1. For example, miR-135a was recently identified as a direct target of FOXM1 and shown to be a significant contributor to the development of portal vein tumor thrombosis (PVTT) (36). Other potential oncogenic miRNAs induced by FOXM1 could be targets of therapeutic intervention in future cancer treatments. In addition, miRNAs that negatively regulate FOXM1 could be utilized as FOXM1 inhibitors. Collectively, the above-summarized findings strongly advocate exploring the potentials of FOXM1 targeting. We proposed 4 years ago that FOXM1 may be the “Achilles’ heel” of cancer (82) and the published data since then support the fact that FOXM1 represents a valid target for therapeutic intervention. Since FOXM1 is involved in multiple hallmarks of cancer (Fig. 1), targeting this single oncogene holds the promise to inhibit tumor development.
Figure 1. FOXM1 is involved in all hallmarks of cancer.
The schematic depicts the contribution of FOXM1 to the hallmarks of cancer. Negative (in italics and underlined) and positive (in bold) downstream targets or upstream regulators of FOXM1 are shown.
Acknowledgments
We would like to thank Dr. Jessica J. Gierut (Harvard Medical School) for proofreading the manuscript and for her valuable comments. A.L. Gartel is supported by NIH grant 1RO1CA129414.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Laoukili J, Stahl M, Medema RH. FoxM1: At the crossroads of ageing and cancer. Biochimica et biophysica acta. 2007;1775:92–102. doi: 10.1016/j.bbcan.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Kalin TV, Ustiyan V, Kalinichenko VV. Multiple faces of FoxM1 transcription factor: lessons from transgenic mouse models. Cell cycle. 2011;10:396–405. doi: 10.4161/cc.10.3.14709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen YJ, Dominguez-Brauer C, Wang Z, Asara JM, Costa RH, Tyner AL, et al. A conserved phosphorylation site within the forkhead domain of FoxM1B is required for its activation by cyclin-CDK1. The Journal of biological chemistry. 2009;284:30695–30707. doi: 10.1074/jbc.M109.007997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anders L, Ke N, Hydbring P, Choi YJ, Widlund HR, Chick JM, et al. A systematic screen for CDK4/6 substrates links FOXM1 phosphorylation to senescence suppression in cancer cells. Cancer cell. 2011;20:620–634. doi: 10.1016/j.ccr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halasi M, Gartel AL. A novel mode of FoxM1 regulation: positive auto-regulatory loop. Cell cycle. 2009;8:1966–1967. doi: 10.4161/cc.8.12.8708. [DOI] [PubMed] [Google Scholar]
- 6.Ye H, Holterman AX, Yoo KW, Franks RR, Costa RH. Premature expression of the winged helix transcription factor HFH-11B in regenerating mouse liver accelerates hepatocyte entry into S phase. Molecular and cellular biology. 1999;19:8570–8580. doi: 10.1128/mcb.19.12.8570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krupczak-Hollis K, Wang X, Kalinichenko VV, Gusarova GA, Wang IC, Dennewitz MB, et al. The mouse Forkhead Box m1 transcription factor is essential for hepatoblast mitosis and development of intrahepatic bile ducts and vessels during liver morphogenesis. Developmental biology. 2004;276:74–88. doi: 10.1016/j.ydbio.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 8.Kim IM, Ramakrishna S, Gusarova GA, Yoder HM, Costa RH, Kalinichenko VV. The forkhead box m1 transcription factor is essential for embryonic development of pulmonary vasculature. The Journal of biological chemistry. 2005;280:22278–22286. doi: 10.1074/jbc.M500936200. [DOI] [PubMed] [Google Scholar]
- 9.Korver W, Schilham MW, Moerer P, van den Hoff MJ, Dam K, Lamers WH, et al. Uncoupling of S phase and mitosis in cardiomyocytes and hepatocytes lacking the winged-helix transcription factor Trident. Curr Biol. 1998;8:1327–1330. doi: 10.1016/s0960-9822(07)00563-5. [DOI] [PubMed] [Google Scholar]
- 10.Wang IC, Chen YJ, Hughes D, Petrovic V, Major ML, Park HJ, et al. Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase. Molecular and cellular biology. 2005;25:10875–10894. doi: 10.1128/MCB.25.24.10875-10894.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wonsey DR, Follettie MT. Loss of the forkhead transcription factor FoxM1 causes centrosome amplification and mitotic catastrophe. Cancer research. 2005;65:5181–5189. doi: 10.1158/0008-5472.CAN-04-4059. [DOI] [PubMed] [Google Scholar]
- 12.Laoukili J, Kooistra MR, Bras A, Kauw J, Kerkhoven RM, Morrison A, et al. FoxM1 is required for execution of the mitotic programme and chromosome stability. Nature cell biology. 2005;7:126–136. doi: 10.1038/ncb1217. [DOI] [PubMed] [Google Scholar]
- 13.Park HJ, Costa RH, Lau LF, Tyner AL, Raychaudhuri P. Anaphase-promoting complex/cyclosome-CDH1-mediated proteolysis of the forkhead box M1 transcription factor is critical for regulated entry into S phase. Molecular and cellular biology. 2008;28:5162–5171. doi: 10.1128/MCB.00387-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhat UG, Halasi M, Gartel AL. FoxM1 is a general target for proteasome inhibitors. PLoS ONE. 2009;4:e6593. doi: 10.1371/journal.pone.0006593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gartel AL. A new target for proteasome inhibitors: FoxM1. Expert opinion on investigational drugs. 2010;19:235–242. doi: 10.1517/13543780903563364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gartel AL. Thiostrepton, proteasome inhibitors and FOXM1. Cell cycle. 2011;10:4341–4342. doi: 10.4161/cc.10.24.18544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crawford LJ, Walker B, Irvine AE. Proteasome inhibitors in cancer therapy. J Cell Commun Signal. 2011;5:101–110. doi: 10.1007/s12079-011-0121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wierstra I, Alves J. Transcription factor FOXM1c is repressed by RB and activated by cyclin D1/Cdk4. Biological chemistry. 2006;387:949–962. doi: 10.1515/BC.2006.119. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez-Fernandez M, Halim VA, Aprelia M, Laoukili J, Mohammed S, Medema RH. Protein phosphatase 2A (B55alpha) prevents premature activation of forkhead transcription factor FoxM1 by antagonizing cyclin A/cyclin-dependent kinase-mediated phosphorylation. The Journal of biological chemistry. 2011;286:33029–33036. doi: 10.1074/jbc.M111.253724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park HJ, Wang Z, Costa RH, Tyner A, Lau LF, Raychaudhuri P. An N-terminal inhibitory domain modulates activity of FoxM1 during cell cycle. Oncogene. 2008;27:1696–1704. doi: 10.1038/sj.onc.1210814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Ahmad A, Li Y, Banerjee S, Kong D, Sarkar FH. Forkhead box M1 transcription factor: a novel target for cancer therapy. Cancer treatment reviews. 2010;36:151–156. doi: 10.1016/j.ctrv.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu M, Dai B, Kang SH, Ban K, Huang FJ, Lang FF, et al. FoxM1B is overexpressed in human glioblastomas and critically regulates the tumorigenicity of glioma cells. Cancer research. 2006;66:3593–3602. doi: 10.1158/0008-5472.CAN-05-2912. [DOI] [PubMed] [Google Scholar]
- 23.Dai B, Kang SH, Gong W, Liu M, Aldape KD, Sawaya R, et al. Aberrant FoxM1B expression increases matrix metalloproteinase-2 transcription and enhances the invasion of glioma cells. Oncogene. 2007;26:6212–6219. doi: 10.1038/sj.onc.1210443. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Zhang N, Dai B, Liu M, Sawaya R, Xie K, et al. FoxM1B transcriptionally regulates vascular endothelial growth factor expression and promotes the angiogenesis and growth of glioma cells. Cancer research. 2008;68:8733–8742. doi: 10.1158/0008-5472.CAN-08-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Q, Zhang N, Jia Z, Le X, Dai B, Wei D, et al. Critical role and regulation of transcription factor FoxM1 in human gastric cancer angiogenesis and progression. Cancer research. 2009;69:3501–3509. doi: 10.1158/0008-5472.CAN-08-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalinichenko VV, Major ML, Wang X, Petrovic V, Kuechle J, Yoder HM, et al. Foxm1b transcription factor is essential for development of hepatocellular carcinomas and is negatively regulated by the p19ARF tumor suppressor. Genes & development. 2004;18:830–850. doi: 10.1101/gad.1200704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng J, Wang L, Li Q, Li W, Bjorkholm M, Jia J, et al. FoxM1 is up-regulated in gastric cancer and its inhibition leads to cellular senescence, partially dependent on p27 kip1. The Journal of pathology. 2009;218:419–427. doi: 10.1002/path.2530. [DOI] [PubMed] [Google Scholar]
- 28.Wang Z, Park HJ, Carr JR, Chen YJ, Zheng Y, Li J, et al. FoxM1 in tumorigenicity of the neuroblastoma cells and renewal of the neural progenitors. Cancer research. 2011;71:4292–4302. doi: 10.1158/0008-5472.CAN-10-4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhat UG, Jagadeeswaran R, Halasi M, Gartel AL. Nucleophosmin interacts with FOXM1 and modulates the level and localization of FOXM1 in human cancer cells. The Journal of biological chemistry. 2011;286:41425–41433. doi: 10.1074/jbc.M111.270843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalin TV, Wang IC, Ackerson TJ, Major ML, Detrisac CJ, Kalinichenko VV, et al. Increased Levels of the FoxM1 Transcription Factor Accelerate Development and Progression of Prostate Carcinomas in both TRAMP and LADY Transgenic Mice. Cancer research. 2006;66:1712–1720. doi: 10.1158/0008-5472.CAN-05-3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim IM, Ackerson T, Ramakrishna S, Tretiakova M, Wang IC, Kalin TV, et al. The Forkhead Box m1 transcription factor stimulates the proliferation of tumor cells during development of lung cancer. Cancer research. 2006;66:2153–2161. doi: 10.1158/0008-5472.CAN-05-3003. [DOI] [PubMed] [Google Scholar]
- 32.Wang IC, Meliton L, Ren X, Zhang Y, Balli D, Snyder J, et al. Deletion of Forkhead Box M1 transcription factor from respiratory epithelial cells inhibits pulmonary tumorigenesis. PLoS ONE. 2009;4:e6609. doi: 10.1371/journal.pone.0006609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balli D, Ren X, Chou FS, Cross E, Zhang Y, Kalinichenko VV, et al. Foxm1 transcription factor is required for macrophage migration during lung inflammation and tumor formation. Oncogene. 2012;31:3875–3888. doi: 10.1038/onc.2011.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quentmeier H, Martelli MP, Dirks WG, Bolli N, Liso A, Macleod RA, et al. Cell line OCI/AML3 bears exon-12 NPM gene mutation-A and cytoplasmic expression of nucleophosmin. Leukemia. 2005;19:1760–1767. doi: 10.1038/sj.leu.2403899. [DOI] [PubMed] [Google Scholar]
- 35.Yau C, Wang Y, Zhang Y, Foekens JA, Benz CC. Young age, increased tumor proliferation and FOXM1 expression predict early metastatic relapse only for endocrine-dependent breast cancers. Breast cancer research and treatment. 2011;126:803–810. doi: 10.1007/s10549-011-1345-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu S, Guo W, Shi J, Li N, Yu X, Xue J, et al. MicroRNA-135a contributes to the development of portal vein tumor thrombus by promoting metastasis in hepatocellular carcinoma. Journal of hepatology. 2012;56:389–396. doi: 10.1016/j.jhep.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Halasi M, Gartel AL. Suppression of FOXM1 Sensitizes Human Cancer Cells to Cell Death Induced by DNA-Damage. PLoS ONE. 2012;7:e31761. doi: 10.1371/journal.pone.0031761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Korver W, Roose J, Heinen K, Weghuis DO, de Bruijn D, van Kessel AG, et al. The human TRIDENT/HFH-11/FKHL16 gene: structure, localization, and promoter characterization. Genomics. 1997;46:435–442. doi: 10.1006/geno.1997.5065. [DOI] [PubMed] [Google Scholar]
- 39.Green MR, Aya-Bonilla C, Gandhi MK, Lea RA, Wellwood J, Wood P, et al. Integrative genomic profiling reveals conserved genetic mechanisms for tumorigenesis in common entities of non-Hodgkin's lymphoma. Genes, chromosomes & cancer. 2011;50:313–326. doi: 10.1002/gcc.20856. [DOI] [PubMed] [Google Scholar]
- 40.Zhang N, Wei P, Gong A, Chiu WT, Lee HT, Colman H, et al. FoxM1 Promotes beta-Catenin Nuclear Localization and Controls Wnt Target-Gene Expression and Glioma Tumorigenesis. Cancer cell. 2011;20:427–442. doi: 10.1016/j.ccr.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Millour J, de Olano N, Horimoto Y, Monteiro LJ, Langer JK, Aligue R, et al. ATM and p53 regulate FOXM1 expression via E2F in breast cancer epirubicin treatment and resistance. Molecular cancer therapeutics. 2011;10:1046–1058. doi: 10.1158/1535-7163.MCT-11-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blanco-Bose WE, Murphy MJ, Ehninger A, Offner S, Dubey C, Huang W, et al. C-Myc and its target FoxM1 are critical downstream effectors of constitutive androstane receptor (CAR) mediated direct liver hyperplasia. Hepatology (Baltimore, Md. 2008;48:1302–1311. doi: 10.1002/hep.22475. [DOI] [PubMed] [Google Scholar]
- 43.Xia LM, Huang WJ, Wang B, Liu M, Zhang Q, Yan W, et al. Transcriptional upregulation of FoxM1 in response to hypoxia is mediated by HIF-1. Journal of cellular biochemistry. 2009;106:247–256. doi: 10.1002/jcb.21996. [DOI] [PubMed] [Google Scholar]
- 44.Pandit B, Halasi M, Gartel AL. p53 negatively regulates expression of FoxM1. Cell cycle. 2009;8:3425–3427. doi: 10.4161/cc.8.20.9628. [DOI] [PubMed] [Google Scholar]
- 45.Barsotti AM, Prives C. Pro-proliferative FoxM1 is a target of p53-mediated repression. Oncogene. 2009;28:4295–4305. doi: 10.1038/onc.2009.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teh MT, Wong ST, Neill GW, Ghali LR, Philpott MP, Quinn AG. FOXM1 is a downstream target of Gli1 in basal cell carcinomas. Cancer research. 2002;62:4773–4780. [PubMed] [Google Scholar]
- 47.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 48.Madureira PA, Varshochi R, Constantinidou D, Francis RE, Coombes RC, Yao KM, et al. The Forkhead box M1 protein regulates the transcription of the estrogen receptor alpha in breast cancer cells. The Journal of biological chemistry. 2006;281:25167–25176. doi: 10.1074/jbc.M603906200. [DOI] [PubMed] [Google Scholar]
- 49.Millour J, Constantinidou D, Stavropoulou AV, Wilson MS, Myatt SS, Kwok JM, et al. FOXM1 is a transcriptional target of ERalpha and has a critical role in breast cancer endocrine sensitivity and resistance. Oncogene. 2010;29:2983–2995. doi: 10.1038/onc.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Horimoto Y, Hartman J, Millour J, Pollock S, Olmos Y, Ho KK, et al. ERbeta1 represses FOXM1 expression through targeting ERalpha to control cell proliferation in breast cancer. The American journal of pathology. 2011;179:1148–1156. doi: 10.1016/j.ajpath.2011.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Francis RE, Myatt SS, Krol J, Hartman J, Peck B, McGovern UB, et al. FoxM1 is a downstream target and marker of HER2 overexpression in breast cancer. International journal of oncology. 2009;35:57–68. doi: 10.3892/ijo_00000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wierstra I, Alves J. FOXM1c transactivates the human c-myc promoter directly via the two TATA boxes P1 and P2. The FEBS journal. 2006;273:4645–4667. doi: 10.1111/j.1742-4658.2006.05468.x. [DOI] [PubMed] [Google Scholar]
- 53.Ho C, Wang C, Mattu S, Destefanis G, Ladu S, Delogu S, et al. AKT (v-akt murine thymoma viral oncogene homolog 1) and N-Ras (neuroblastoma ras viral oncogene homolog) coactivation in the mouse liver promotes rapid carcinogenesis by way of mTOR (mammalian target of rapamycin complex 1), FOXM1 (forkhead box M1)/SKP2, and c-Myc pathways. Hepatology. 2012;55:833–845. doi: 10.1002/hep.24736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petrovic V, Costa RH, Lau LF, Raychaudhuri P, Tyner AL. FoxM1 regulates growth factor-induced expression of kinase-interacting stathmin (KIS) to promote cell cycle progression. The Journal of biological chemistry. 2008;283:453–460. doi: 10.1074/jbc.M705792200. [DOI] [PubMed] [Google Scholar]
- 55.Spurgers KB, Gold DL, Coombes KR, Bohnenstiehl NL, Mullins B, Meyn RE, et al. Identification of cell cycle regulatory genes as principal targets of p53-mediated transcriptional repression. The Journal of biological chemistry. 2006;281:25134–25142. doi: 10.1074/jbc.M513901200. [DOI] [PubMed] [Google Scholar]
- 56.Bell D, Berchuck A, Birrer M, Chien J, Cramer D, Dao F, et al. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 58.Carr JR, Park HJ, Wang Z, Kiefer MM, Raychaudhuri P. FoxM1 mediates resistance to herceptin and paclitaxel. Cancer research. 2010;70:5054–5063. doi: 10.1158/0008-5472.CAN-10-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kwok JM, Peck B, Monteiro LJ, Schwenen HD, Millour J, Coombes RC, et al. FOXM1 confers acquired cisplatin resistance in breast cancer cells. Mol Cancer Res. 2010;8:24–34. doi: 10.1158/1541-7786.MCR-09-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park HJ, Carr JR, Wang Z, Nogueira V, Hay N, Tyner AL, et al. FoxM1, a critical regulator of oxidative stress during oncogenesis. The EMBO journal. 2009;28:2908–2918. doi: 10.1038/emboj.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pandit B, Gartel AL. FoxM1 knockdown sensitizes human cancer cells to proteasome inhibitor-induced apoptosis but not to autophagy. Cell cycle. 2011;10:3269–3273. doi: 10.4161/cc.10.19.17735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li SK, Smith DK, Leung WY, Cheung AM, Lam EW, Dimri GP, et al. FoxM1c counteracts oxidative stress-induced senescence and stimulates Bmi-1 expression. The Journal of biological chemistry. 2008;283:16545–16553. doi: 10.1074/jbc.M709604200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gemenetzidis E, Elena-Costea D, Parkinson EK, Waseem A, Wan H, Teh MT. Induction of human epithelial stem/progenitor expansion by FOXM1. Cancer research. 2010;70:9515–9526. doi: 10.1158/0008-5472.CAN-10-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karadedou CT, Gomes AR, Chen J, Petkovic M, Ho KK, Zwolinska AK, et al. FOXO3a represses VEGF expression through FOXM1-dependent and -independent mechanisms in breast cancer. Oncogene. 2011;31:1845–1858. doi: 10.1038/onc.2011.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Delpuech O, Griffiths B, East P, Essafi A, Lam EW, Burgering B, et al. Induction of Mxi1-SR alpha by FOXO3a contributes to repression of Myc-dependent gene expression. Molecular and cellular biology. 2007;27:4917–4930. doi: 10.1128/MCB.01789-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chandran UR, Ma C, Dhir R, Bisceglia M, Lyons-Weiler M, Liang W, et al. Gene expression profiles of prostate cancer reveal involvement of multiple molecular pathways in the metastatic process. BMC cancer. 2007;7:64. doi: 10.1186/1471-2407-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park HJ, Gusarova G, Wang Z, Carr JR, Li J, Kim KH, et al. Deregulation of FoxM1b leads to tumour metastasis. EMBO molecular medicine. 2011;3:21–34. doi: 10.1002/emmm.201000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang IC, Chen YJ, Hughes DE, Ackerson T, Major ML, Kalinichenko VV, et al. FoxM1 regulates transcription of JNK1 to promote the G1/S transition and tumor cell invasiveness. The Journal of biological chemistry. 2008;283:20770–20778. doi: 10.1074/jbc.M709892200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Behren A, Muhlen S, Acuna Sanhueza GA, Schwager C, Plinkert PK, Huber PE, et al. Phenotype-assisted transcriptome analysis identifies FOXM1 downstream from Ras-MKK3-p38 to regulate in vitro cellular invasion. Oncogene. 2010;29:1519–1530. doi: 10.1038/onc.2009.436. [DOI] [PubMed] [Google Scholar]
- 70.Bao B, Wang Z, Ali S, Kong D, Banerjee S, Ahmad A, et al. Over-expression of FoxM1 leads to epithelial-mesenchymal transition and cancer stem cell phenotype in pancreatic cancer cells. Journal of cellular biochemistry. 2011;112:2296–2306. doi: 10.1002/jcb.23150. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71.Huang C, Qiu Z, Wang L, Peng Z, Jia Z, Logsdon CD, et al. A novel FoxM1-caveolin signaling pathway promotes pancreatic cancer invasion and metastasis. Cancer research. 2012;72:655–665. doi: 10.1158/0008-5472.CAN-11-3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carter SL, Eklund AC, Kohane IS, Harris LN, Szallasi Z. A signature of chromosomal instability inferred from gene expression profiles predicts clinical outcome in multiple human cancers. Nature genetics. 2006;38:1043–1048. doi: 10.1038/ng1861. [DOI] [PubMed] [Google Scholar]
- 73.Gemenetzidis E, Bose A, Riaz AM, Chaplin T, Young BD, Ali M, et al. FOXM1 upregulation is an early event in human squamous cell carcinoma and it is enhanced by nicotine during malignant transformation. PLoS ONE. 2009;4:e4849. doi: 10.1371/journal.pone.0004849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Teh MT, Gemenetzidis E, Chaplin T, Young BD, Philpott MP. Upregulation of FOXM1 induces genomic instability in human epidermal keratinocytes. Molecular cancer. 2010;9:45. doi: 10.1186/1476-4598-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tan Y, Raychaudhuri P, Costa RH. Chk2 mediates stabilization of the FoxM1 transcription factor to stimulate expression of DNA repair genes. Molecular and cellular biology. 2007;27:1007–1016. doi: 10.1128/MCB.01068-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang IC, Meliton L, Tretiakova M, Costa RH, Kalinichenko VV, Kalin TV. Transgenic expression of the forkhead box M1 transcription factor induces formation of lung tumors. Oncogene. 2008;27:4137–4149. doi: 10.1038/onc.2008.60. [DOI] [PubMed] [Google Scholar]
- 78.Balli D, Zhang Y, Snyder J, Kalinichenko VV, Kalin TV. Endothelial cell-specific deletion of transcription factor FoxM1 increases urethane-induced lung carcinogenesis. Cancer research. 2011;71:40–50. doi: 10.1158/0008-5472.CAN-10-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Caldwell SA, Jackson SR, Shahriari KS, Lynch TP, Sethi G, Walker S, et al. Nutrient sensor O-GlcNAc transferase regulates breast cancer tumorigenesis through targeting of the oncogenic transcription factor FoxM1. Oncogene. 2010;29:2831–2842. doi: 10.1038/onc.2010.41. [DOI] [PubMed] [Google Scholar]
- 80.Lynch TP, Ferrer CM, Jackson SR, Shahriari KS, Vosseller K, Reginato MJ. Critical Role of O-Linked beta-N-Acetylglucosamine Transferase in Prostate Cancer Invasion, Angiogenesis, and Metastasis. The Journal of biological chemistry. 2012;287:11070–11081. doi: 10.1074/jbc.M111.302547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gartel AL, Kandel ES. miRNAs: Little known mediators of oncogenesis. Semin Cancer Biol. 2008;18:103–110. doi: 10.1016/j.semcancer.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 82.Radhakrishnan SK, Gartel AL. FOXM1: the Achilles' heel of cancer? Nat Rev Cancer. 2008;8 doi: 10.1038/nrc2223-c1. c1; author reply c2. [DOI] [PubMed] [Google Scholar]