Abstract
The basis of hypersexual behavior among patients with dementia is not entirely clear. Hypersexual behavior may be a particular feature of behavioral variant frontotemporal dementia (bvFTD), which affects ventromedial frontal and adjacent anterior temporal regions specialized in interpersonal behavior. Recent efforts to define Hypersexual Disorder indicate an increasing awareness of heightened sexual activity as a source of personal distress and functional impairment, and clarification of hypersexuality in bvFTD could contribute to understanding the neurobiology of this behavior. This study reviewed 47 patients with bvFTD compared to 58 patients with Alzheimer’s disease (AD) for the presence of heightened sexual activity to the point of distress to caregivers and others. Hypersexual behavior occurred in 6 (13%) bvFTD patients compared to none of the AD patients. Caregivers judged all six bvFTD patients with hypersexual behavior as having a dramatic increase in sexual frequency from premorbid levels. All had general disinhibition, poor impulse control, and actively sought sexual stimulation. They had widened sexual interests and experienced sexual arousal from previously unexciting stimuli. One patient, with early and predominant right anterior temporal involvement, was easily aroused by slight stimuli, such as touching her palms. Although previously considered to be predominantly disinhibited sexual behavior as part of generalized disinhibition, these patients with dementia illustrate varying degrees of increased sexual desire. We conclude that bvFTD is uniquely associated with hypersexuality; it is more than just cognitive impairment with frontal disinhibition but also involves alterations in sexual drive, possibly from right anterior temporal-limbic involvement in this disease.
Keywords: hypersexuality, dementia, right temporal lobe, frontotemporal dementia
INTRODUCTION
Hypersexual disorder (HD) may enter the psychiatric nomenclature for the Diagnostic and Statistical Manual-5 (DSM-5) (Kafka, 2010; Marshall & Briken, 2010; Reid, Garos, & Carpenter, 2011). This reflects the increasing awareness among clinicians and investigators of the suffering and functional impairment that can result from excessively increased sexual behavior. Dementia is a common cause of inappropriate or increased sexual behavior and can illuminate the underlying mechanisms for hypersexuality (Black, Muralee, & Tampi, 2005; Lindau et al., 2007). Despite few systematic studies in dementia (de Medeiros, Rosenberg, Baker, & Onyike, 2008; Zeiss, Davies, & Tinklenberg, 1996), existing reports indicate that up to a quarter of patients with Alzheimer’s disease (AD) have some form of inappropriate sexual behavior, including hypersexuality (Black et al., 2005; Derouesné, 2009). These behaviors result in increased caregiver burden, use of psychoactive medications, utilization of health care resources, and early institutionalization (Black et al., 2005). Of the dementias, behavioral variant frontotemporal dementia (bvFTD) appears particularly likely to result in increased sexual activity. The distinguishing features of bvFTD are social and emotional behavioral changes from focal pathology in ventromedial frontal and adjacent anterior temporal lobes (Rascovsky et al., 2011). There are reports of hypersexual behavior among 8-18%% of bvFTD patients (Mendez, Chen, Shapira, & Miller, 2005; Miller, Darby, Swartz, Yener, & Mena, 1995).
For DSM-5, the proposed definition of HD is recurrent and intense sexual fantasies, urges, and behavior over a period of six months or more (Kafka, 2010). This definition includes four or more of the following: (1) excessive time consumed by the sexual symptoms; (2) hypersexuality in response to dysphoric mood states; (3) hypersexuality in response to stressful life events; (4) repetitive but unsuccessful efforts to reduce or control the sexual symptoms; and (5) repetitive engagement in sexual behavior despite the risk for harming themselves or others. The sexual symptoms result in significant personal distress or impairment in social, occupational or other important areas of functioning and are not due to the effects of drugs, medications, or to manic episodes. In addition to a reaction to dysphoric mood states or stressful life events, hypersexual behavior could be due to an addiction, a compulsivity, an impulse control disorder, or to a primary disorder of sexual desire (Kafka, 2010).
Understanding the heightened sexual behavior in bvFTD may shed light on the neurobiology of HD. Dementia patients with frontal lobe dysfunction often react impulsively to tempting environmental situations involving sexual or other objects of interest without concern for the consequences (Mendez, Chow, Ringman, Twitchell, & Hinkin, 2000). Sexual disinhibition as part of a frontally-mediated general disinhibition, however, does not explain hypersexuality due to an increased sexual drive (Baird, Wilson, Bladin, Saling, & Reutens, 2007). We investigated the prevalence of hypersexual behavior among a cohort of patients with bvFTD, compared to patients with AD with a similar severity of dementia. We identified those with increased sexual activity sufficient to be disruptive to caregivers and others. We further examined six patients with bvFTD who met criteria for excessive time consumed in sexual activity and who had a total disregard for the risks of their behavior.
METHOD
Participants
The clinical records of 47 patients with bvFTD and 58 with early-onset AD were reviewed for the presence of sexual behavior that could be characterized as hypersexual. All study participants presented for evaluation to our university-affiliated specialty program in dementia. The patients were community-based patients referred by family or other physicians for assessment of cognitive or behavioral changes. All of the dementia patients included in this study met either criteria for bvFTD or for AD after an extensive evaluation involving clinical examination, neuropsychological tests, and neuroimaging. Most of these patients were participants in a larger project on bvFTD or the UCLA Alzheimer’s Disease Research Center. Institutional Review Board approval was obtained for de-identified review of their medical records.
Procedure
The bvFTD patients met the Consensus Criteria for bvFTD and, retrospectively, the International Criteria for bvFTD (Neary et al., 1998; Rascovsky et al., 2011). The latter criteria for bvFTD included progressive deterioration of behavior and/or cognition with at least three of the following: (1) early behavioral disinhibition; (2) early apathy or inertia; (3) early loss of sympathy or empathy; (4) early perseverative, stereotyped or compulsive/ritualistic behavior; (5) hyperorality and dietary changes; and (6) deficits in executive tasks with relative sparing of episodic memory and visuospatial skills (Rascovsky et al., 2011). These clinical criteria were then supported by the presence of regional abnormalities on neuroimaging located in the frontotemporal regions.
As a comparison group, the study evaluated AD patients who were age-matched with the bvFTD patients and, hence, had an earlier age of onset than typical AD. These patients met DSM-IV criteria for AD (American Psychiatric Association, 2000). The study included patients with AD who were comparable in severity of disease with the patients with bvFTD as indicated by disease duration, the Mini-Mental State Examination (MMSE) scores (Folstein, Folstein, & McHugh, 1975), and most neuropsychological measures, which included the 15-item Mini-Boston Naming Test (mBNT) and the verbal learning memory test from the Consortium to Establish a Registry in Alzheimer’s Disease (CERAD) (Welsh et al., 1994).
Measures
The bvFTD or AD patients were reviewed for the presence of hypersexual behavior. The proposed criteria for hypersexual disorder were modified and operationalized in order to apply to patients with dementia. The three inclusion criteria were: (1) the presence of six or more months of heightened sexual behavior from premorbid levels and sufficient to cause significant concern to their caregivers and others; (2) the sexual behavior occurred >30 minutes/day or there were two or more reports/week of known, observed or public manifestations of sexual behavior, either masturbation or partner-related; and (3) the sexual behavior occurred without regard to potential risk to themselves or others.
In the presence of these inclusion criteria, the records were further reviewed for the characteristics of their hypersexual behavior. These included: (1) evidence of sexual disinhibition; (2) evidence of general disinhibition and poor impulse control that could be associated with sexual disinhibition; (3) the active seeking of sexual gratification as opposed to just passive, opportunistic sexual behavior; (4) increased extent of their sexual interests; and (5) increased ease of sexual arousal. The first two criteria point to a disinhibition mechanism for hypersexual behavior and the second two criteria point to increased sexual drive.
The neuroimaging studies included a structured brain scan consisting of magnetic resonance imaging (MRI) and a functional brain scan consisting of either positron emission tomography (PET) or single photon emission tomography (SPECT). The scans were independently reviewed by the neuroimaging specialist and the clinical neurologist. The neurologist was not blinded to the neuroimaging specialist’s interpretation. When discrepant, the two physicians reached consensus on the presence or absence of frontotemporal atrophy on MRI or of hypometabolism on PET or hypoperfusion or SPECT.
RESULTS
All of the bvFTD patients had the first five diagnostic criteria for bvFTD and none of the AD patients had more than two (Rascovsky et al., 2011). On the neuropsychological measures, there were no significant group differences except for memory (see Table 1). The normative values for these measures indicate that both dementia groups were similarly impaired. They both had abnormally low scores for MMSE, word fluency, mBNT, and the CERAD (Welsh et al., 1994). As expected, the bvFTD patients were relatively better than the AD patients on memory (delayed recall).
Table 1.
Patients with Dementia: Demographic and Cognitive Characteristics
| Measure | bvFTD n = 47 |
AD n = 58 |
Significance |
|---|---|---|---|
| Hypersexual behavior | 6 (12.7%) | 0 (0%) | χ2 = 5.67, p < .05 |
| Sex | 22M/25F | 31M/27F | ns |
| Handedness | 41R/6L | 55R/3L | ns |
|
| |||
| M (SD) | M (SD) | ||
|
| |||
| Age at presentation in years | 60.43(8.11) | 57.71 (6.61) | ns |
| Disease durat ion in years | 2.78 (2.11) | 2.95 (2.16) | ns |
| Education in years | 13.76 (3.05) | 14.45 (2.66) | ns |
| MMSEa | 23.02 (5.68) | 21.11 (6.01) | ns |
| Digit span forward | 5.72 (0.72) | 5.43 (1.24) | ns |
| Word fluency | |||
| Animals/minute | 10.12 (5.60) | 11.68 (5.17) | ns |
| “F” words/minute | 6.07 (4.56) | 7.74 (5.19) | ns |
| Mini-Boston Naming Test (15) | 11.94 (3.85) | 11.59 (3.61) | ns |
| CERADb | |||
| Delayed Recall Score (10) | 3.32 (2.85) | 1.28 (1.89) | t(103) = 4.39, p < .001 |
| Recognit ion Score (20) | 14.91 (3.54) | 14.10 (3.68) | ns |
MMSE = Mini-Mental State Examinat ion.
CERAD: Consortium to Establish a Registry in Alzheimer’s Disease Verbal List Learning Test
Among the bvFTD patients, the hypersexual behavior indicated increased sexual arousal as well as disinhibition, and the neuroimaging indicated a more than expected involvement of the right anterior temporal region. Six bvFTD patients, and none of the AD patients, had the three inclusion criteria for hypersexual behavior. All of these six bvFTD patients had all four of the characteristics of hypersexual behavior. On neuroimaging, frontotemporal abnormalities, either atrophy on MRI, hypometabolism on PET or hypoperfusion on SPECT, were present in all bvFTD patients and none of the AD patients, confirming the diagnosis. Among the 47 bvFTD patients with frontotemporal abnormalities, only two had their greatest changes in the right anterior temporal region than in other frontotemporal areas and both of these had hypersexual behavior (Patient 4 and Patient 6).
Case Reports
Patient 1
A 69-year-old man presented with a 5-year history of personality and behavioral changes with sexually promiscuous behavior. The patient searched for women to have sex with, including strangers and recent acquaintances. He had extramarital affairs and engaged in “free sex” several times per week. He actively sought varied sexual partners and appeared preoccupied with sex throughout much of the day. When confronted with these behaviors and their emotional impact on his wife and children, he dismissed the significance of his sexual activity or the risk to his home and marriage.
In addition to the above, the patient became disinhibited and had declines in his ability to perform complex activities. He developed the new and previously uncharacteristic use of profanity, lost his overall sense of propriety, and neglected his personal hygiene. He became rigid in his routines, lost empathy for family members, and stopped doing his prior activities and avocations. Psychiatric history was positive for a remote history of depression. On medical history, he had idiopathic, generalized tonic-clonic epilepsy since age 25 treated with topiramate with his last seizure six years prior to presentation. He also had a remote history of hypertension.
The patient’s diagnosis was bvFTD. His cognitive testing showed impairments with preservation of visuospatial constructions and abnormal executive tasks. His neurological examination was normal except for slight increased motor tone. His MRI showed bilateral frontotemporal atrophy. In order to help control any component of sexual disinhibition, management recommendations included switching topiramate, his anti-epileptic, to valproate or lamotrigine, and considering a switch from atenolol, his anti-hypertensive, to propranolol. His treatment included selective serotonin reuptake inhibitors (SSRI); however, the patient eventually declined clinical follow-up and his wife could not get him to return.
Patient 2
A 48-year-old man presented with a 5-year history of a progressive personality change exhibited by heightened sexual behavior for over a six month period. He would say sexually inappropriate things and touch others inappropriately. He began to actively seek sexual encounters at his residential facility where he would find female staff members, push them into rooms, and try to have sex with them. He was observed seeking sex several times a day and masturbating. At one point, he was found lying on top of an elderly, impaired woman with his pants down attempting to have intercourse. The patient had little insight into his behavior and continued it despite the risk of being expelled from the facility.
His personality change originally began with difficulty completing his work, attending to his affairs, and keeping up his personal appearance and home. He developed disinhibited stealing and hoarding of minor items. In addition, he had particular food desires, including ice cream cones at all hours and peanut butter and jelly sandwiches. On past history, he had an episode of depression 20 years previously with manic behavior on instituting fluoxetine. He was started on lithium and other medications without any other episodes of depression or mania. The patient had a negative family history for major neurological or psychiatric illnesses.
On examination, he had multiple cognitive impairments with preservation of visuospatial constructions and abnormal executive tasks, such as the Luria alternating tasks and proverb interpretation. His neurologic examination showed slightly increased tone in an extrapyramidal fashion. On MRI scan, this patient had frontotemporal atrophy with corresponding white matter involvement and compensatory enlargement of the frontal horns. PET showed decreased metabolic activity in the frontal lobes and anterior temporal lobes. At one point, aripiprazole was used to control of his sexual behavior, which eventually diminished as his dementia progressed.
Patient 3
A 51-year-old man with a 5-year history of personality and behavioral changes developed increased and inappropriate sexual activity for over six months. He would make several attempts at sexual encounters/day and he was observed to engage in overt masturbation at least twice/ week. He frequently talked about breasts and penises, tried to solicit sex on the internet, and asked a 17-year-old girl to have sex in front of his wife and children. His residential facility called the police when he kept trying to enter women’s rooms to proposition them. He had been caught touching a woman’s breast at the facility while touching himself. The woman had advanced dementia, was immobile in a wheelchair and, according to his wife, would not have previously aroused sexual interest in the patient.
The patient had decreased judgment and general disinhibition. He had become socially tactless and would laugh at inappropriate times and say inappropriate things. He showed a decreased ability to read social cues and to understand anything requiring sympathy or empathy. For example, when his wife was hospitalized for her breast cancer, he presented to the hospital only once, looking or asking for the location of some food item. His wife described him as having “a Ph.D. in social insensitivity or a degree in foot-and-mouth disease.” Further aspects of his history included decreased personal hygiene and stereotypical behaviors. His past psychiatric and medical history was unremarkable, but his family history was significant for a similar illness in his father and, probably, his paternal grandmother. His father’s autopsy showed a frontotemporal lobar degeneration with ubiquitin-positive, tau-negative inclusion bodies as well as spongiosis and gliosis.
This bvFTD patient had an autosomal dominant inheritance pattern suggestive of the progranulin gene mutation or, given his short stature, a valosin gene mutation. His cognitive testing showed impairments with preservation of 2-dimensional and 3-dimensional constructions but he was concrete on the interpretations of unfamiliar proverbs. On the rest of the neurologic examination, cranial nerves were normal, gait was slightly broad-based with evidence of decreased sensation in his distal lower extremities. Although his MRI scan did not show any clear abnormalities, his PET scan showed frontotemporal hypometabolism involving the right hemisphere. His heightened and inappropriate sexual behavior was successfully managed on a regimen of sertraline 150 mg QD, quetiapine 300 mg TID, and oxcarbazine 300 mg BID. Patient 4 (Mendez & Shapira, 2011)
A 55-year-old man had a 2-year history of a personality change with the development of an intense interest in pornography. The patient would seek and view pornography throughout the day. His preferred source was the internet both at home and on his laptop in public places, such as restaurants. This behavior was very distressing to his family. In addition, he developed frequent and overt masturbation, even in front of his elderly mother. She eventually managed to get him to do this in his room with the door closed.
Other aspects of his progressive personality change included decreased goal-directed activities and loss of social tact and propriety. For example, he would tend to have flatulence, eructation, and even urination in public without excusing himself and without becoming embarrassed. He had a craving for sweets and gained 100 pounds over the prior 1 to 2 years. He became emotionally disengaged, which was exemplified particularly when his father was dying from cancer and the patient did not respond to him, call to inquire about him, or appear emotionally involved. In addition, the patient had a decrease in his personal self-care, tending to wear the same clothes over and over again. When confronted with his behavior, he denied any changes in his personality. He had otherwise negative past psychiatric, medical, and family histories except for late dementia in a grandmother.
His evaluation was consistent with bvFTD. He had multiple cognitive impairments and abnormalities on bedside executive function tasks but he could do the visuospatial constructions without difficulty. The rest of his examination was normal. His MRI scan was unremarkable, but his PET scan showed decreased brain metabolism involving medial and anterior temporal lobes as well as frontal lobes. His PET scan was prominently worse in the right anterior temporal region. This patient’s sexual behavior decreased slightly with the SSRI escitalopram. The patient’s illness progressed and he eventually died. Autopsy revealed a frontotemporal lobar degeneration with prominent tau positive inclusions in the frontal and temporal cortex and moderate to severe spongiosis of superficial cortex.
Patient 5
A 62-year-old man had over 5 years a progressive personality change with heightened sexual preoccupation. He approached women for sex, including his female relatives. He attempted sexual intercourse with his wife several times a day and groped or fondled her constantly, even in the presence of others. The heightened sexual behavior lasted for years and was characterized by actively seeking sexual contacts several times per day despite the consequences to himself and his family. He displayed little concern or understanding of the consequences of his behavior.
His personality changes had begun with a decline in work performance and evolved to disinhibited behavior, such as making inappropriate and overly familiar personal comments about others. He had compulsive behavior, such as spending hours picking up and dropping papers on the floor, and he developed a voracious appetite. His past psychiatric and medical history was otherwise unremarkable, but, on family history, his father and other members of his paternal family had a similar illness with inappropriate sexual behavior and a cognitive decline.
The patient met criteria for familial bvFTD with a similar disorder involving family members on his father’s side. His cognitive testing showed multiple deficits with preservation of constructions and poor executive tasks. His examination was otherwise normal. His MRI revealed cortical atrophy involving the frontotemporal regions, especially on the right. SPECT scans showed frontotemporal hypoperfusion, more extensive in the right hemisphere. The patient’s behavior was treated with haloperidol and behavioral management and he was admitted to a long-term care facility.
Patient 6
A 42-year-old woman had a 3 to 4-year history of a major personality change characterized by increased sexual behavior. For months, she had frequent sexual conversations with strangers, even asking them to go on sexual encounters. She expressed a need for more attention from men and began wearing tight, provocative clothing. Her family brought her for a neurological assessment when she asked a store clerk to go on a date with her despite the presence of her children. She had become quite open about masturbating several times a day and, most dramatically, she become visibly sexually aroused with touching or stroking of her palms.
There were other personality changes. She had changed from a proper and restrained lady to using “gutter language” and decreased social concern or awareness of social manners. Her interests changed from easy listening to rock music and her dress went from the conservative to the risqué. There were compulsive behaviors from repetitive checking to going into a store and buying six or more of each item and she developed a greater tendency to eat sweets. Her judgment was impaired. For example, when she was given the wrong car by a valet, she tried to drive off in that automobile. Past psychiatric history was negative but medical history was positive for meningitis when she was 5-years old. Her family history was negative for any known familial or neurodegenerative illnesses.
This patient had bvFTD, especially affecting the right anterior temporal lobe. Her cognitive testing showed deficits in multiple domains but she was able to do 3-dimensional constructions. The rest of her examination was normal. MRI showed right-sided greater than left-sided frontotemporal atrophy with prominent right anterior temporal involvement (see Fig. 1). A SPECT scan showed asymmetric hypoperfusion in the right frontal and right temporal lobes with the most prominent changes in the inferomedial aspects of the right temporal lobe (see Fig. 2). After diagnosis, caregivers were recruited to supervise her behavior and she was started on sertraline and memantine. Her sexual behavior abated over time as her disease progressed.
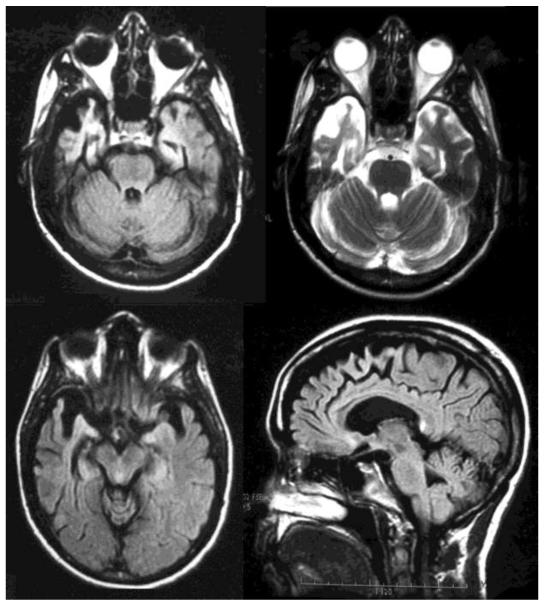
Figure 1.
Magnetic resonance imaging (MRI) of the brain for Patient No. 6. The upper left is an axial flair image showing anterior temporal areas of atrophy and gliosis, with the right side involved to a much greater degree than the left. The upper right is an axial T2 imaging of the same findings. The lower right is an axial flair image at a slightly higher level. The lower left is a saggital FLAIR image showing atrophy extending to the medial frontal region consistent with bvFTD.
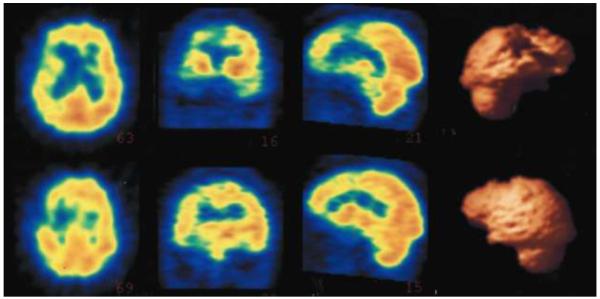
Figure 2.
Single photon emission tomography (SPECT) imaging of the brain for Patient No. 6. The images are, left to right, axial, coronal, saggital, and 3-dimensional (3-D) reconstructions of the SPECT images. They show disproprtionate hypoperfusion of the right frontotemporal region with relative sparing of the left. There is clear involvement of the right anterior temporal area extending to adjacent frontal areas. The 3-D reconstructions illustrate a lateral view of the right hemisphere in the upper right and a corresponding lateral view of the left hemisphere in the lower right.
DISCUSSION
Hypersexual behavior is more common among patients with bvFTD than in those with AD. Among patients with bvFTD, this behavior significantly increases the burden and emotional impact on their caregivers and family and can lead to early institutionalization (Black et al., 2005; Mendez, Lauterbach, Sampson, & Committee on Research, 2008; Miller et al., 1995). Understanding the origin of hypersexual behavior among patients with bvFTD could clarify HD and help lead to targeted interventions. Possible mechanisms for heightened sexual behavior include sexual desire/arousal dysregulation, sexual addiction, and sexual compulsivity (Bancroft, 2008; Kafka, 2010). This study suggests that patients with bvFTD have hypersexual behavior that goes beyond frontal disinhibition and which may relate to temporal lobe-limbic involvement.
Many neurological disorders can result in alterations of sexual behavior. Investigators report hypersexuality among patients with dementia, head trauma, cerebral anoxia, multiple sclerosis, Parkinsonian disorders, and other brain diseases (Alkhalil, Tanvir, Alkhalil, & Lownethal, 2004; Fairweather, 1947; Gorman & Cummings, 1992; Mendez et al., 2008; Miller et al., 1995; Müller, 2011; Ortego, Miller, Itabashi, & Cummings, 1993; Stein, Hugo, Oosthuizen, Hawkridge, & van Heerden, 2000). Dementia, in particular, is a common cause of disordered sexual behavior, including hypersexuality (Black et al., 2005; Lindau et al., 2007). Of the dementias, bvFTD appears most likely to result in hypersexual behavior (Mendez et al., 2000, 2005; Miller et al., 1995).
The prominence of bvFTD as a cause of hypersexuality suggests an origin in the frontal lobes. Sexual arousal results in activation of the right prefrontal, orbitofrontal, and anterior cingulate cortices (Baird et al., 2007; Ferretti et al., 2005; Karama et al., 2002; Redouté et al., 2000; Tiihonen et al., 1994) and sexually inappropriate behavior can result from dysfunction of these areas. Classical writings on frontal lobe dysfunction describe behavioral disinhibition with sexual content, such as “Witzelsücht,” or inappropriate facetiousness often of a sexual nature (Oppenheim, 1889). Frontal infarctions, prefrontal lobotomies, and orbitofrontal trauma can increase sexual behavior as part of behavioral disinhibition (Baird et al., 2007; Levine & Albert, 1951; Miller, Cummings, McIntyre, Ebers, & Grode, 1986). Furthermore, many dementia patients have impulsive sexual acts as their disorder affects the frontal lobes (Alkhalil et al., 2004; Black et al., 2005; Nagaratnam & Gayagay, 2002; Tucker, 2010). For example, one patient in our bvFTD cohort tried to touch and kiss female staff when he encountered them, another had Witzelsücht and made sexually-related comments, and a third would touch his crouch in the presence of women. These patients with bvFTD had predominantly disinhibited and opportunistic sexual acts in the absence of hypersexuality.
The patients with bvFTD and hypersexuality had more than just sexual disinhibition; they also had evidence of increased sexual desire. They actively sought sexual stimulation, had widened sexual interests, and tended to experience sexual arousal from previously unexciting stimuli, such as frail and elderly partners. One patient was easily aroused with slight touch of her palms. Although bvFTD is associated with increased compulsive behavior and their sexual behaviors may decrease with SSRI medications (Anneser, Jox, & Borasio, 2007), an increase in sexual behavior from a compulsive preoccupation or from sexual addiction does not explain increased sexual arousal (Baird et al., 2007). Brain areas involved in sexual arousal include the globus pallidus and ansa lenticularis through dopaminergic pathways, the hypothalamus through endocrine and autonomic systems, and the septal nuclei through pleasurable responses (Baird et al, 2007; Ferretti et al, 2005; Hamann, Herman, Nolan, & Wallen, 2004; Heath, 1972; Redouté et al., 2000). Lesions in these areas can result in hypersexuality (Gorman & Cummings, 1992; Mendez, O’Connor, & Lim, 2004; Miller & Cummings, 1991; Miller et al., 1986; Poeck & Pilleri, 1965). Patients with bvFTD, however, do not usually have disease in these neuroanatomical structures.
A more likely affected region for increased sexual arousal in bvFTD is the right temporolimbic area (Black et al., 2005). The right temporal lobe with its embedded amygdalar nuclei participate in the perception of sexual behavior and in the inhibition of sexual thoughts and impulses (Ozkara et al., 2006; Tiihonen et al., 1994). Although simple viewing of sexual stimuli is associated with amygdalar activation (Hamann et al., 2004), there is deactivation of temporal areas, including the amygdalae during erection and orgasm (Holstege et al., 2003; Redouté et al, 2000). A sexual preoccupation may result because lesions of the right amygdala and/or temporal neocortical areas are prevented from normally inhibiting other limbic and subcortical areas involved in sexual arousal and expression (Devinsky, Sacks, & Devinsky, 2009; Müller, 2011; Ozmen, Erdogan, Duvenci, Ozyurt, & Ozkara, 2004). Temporal-amygdalar damage may lead to a release of the sexual appetite and hypersexual behavior has occurred with strokes and surgical resections for epilepsy, tumors, or other lesions of the right temporal lobe (Baird, Wilson, Bladin, Saling, & Reutens, 2004; Blumer, 1970; Braun, Dumont, Duval, Hamel, & Godbout, 2003; Gorman & Cummings, 1992; Levine & Albert, 1951; Monga, Monga, Raina, & Hardjasudarma, 1986; Ortego et al., 1993). Hypersexual behavior is also part of the Klüver-Bucy syndrome from bilateral anterior temporal-amygdalar disease (Baird et al., 2004; Lilly, Cummings, Benson, & Frankel, 1983). Heightened or altered sexual behavior may particularly occur in the right temporal variant of bvFTD (Edwards-Lee et al., 1997; Mendez et al., 2000; Miller et al., 1986).
There were potential limitations of this study. First, this was a retrospective investigation reliant on clinical reports and testing. This methodology, however, allowed an evaluation of significant numbers of patients with bvFTD in comparison to AD. Second, the criteria for hypersexuality were specified for this study. The requirement for disruptive increased sexual behavior depended on caregiver reports. This was necessary as the caregivers were the ones most impacted from the behavior; the patient’s themselves were usually unconcerned or lacked insight. Third, only two of the six patients had their greatest frontotemporal abnormalities in the right anterior temporal region; however, none of the other bvFTD patients had this finding. Finally, the interpretation of the presence of increased sexual desire depended on an analysis of only six patients; nevertheless, their manifestations indicated the presence of more than just sexual disinhibition.
In sum, patients with bvFTD have a tendency to increased sexual arousal. These findings support the use of treatments to reduce sexual arousal in dementia (Ozkan, Wilkins, Muralee, & Tampi, 2011; Tucker, 2010). Among those with HD, these findings suggest developmental or genetic differences in the ability of the right anterior temporal lobe to inhibit limbic and subcortical areas for sexual arousal. Undetected right anterior lesions or epileptic activity could be present in some patients with HD. Future research on hypersexual behavior from brain disorders like bvFTD may also help clarify the underlying mechanisms for HD.
Acknowledgement
Funding/Support: NIA Grant #R01AG034499-03 (M. F. Mendez, P.I.).
REFERENCES
- Alkhalil C, Tanvir R, Alkhalil B, Lowenthal DT. Treatment of sexual disinhibit ion in dement ia: Case reports and review of the literature. American Journal of Therapeutics. 2004;11:231–235. doi: 10.1097/00045391-200405000-00013. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Associat ion . Diagnostic and statistical manual of mental disorders (4th ed. text rev.) Author; Washington, DC: 2000. [Google Scholar]
- Anneser JM, Jox RJ, Borasio GD. Inappropriate sexual behaviour in a case of ALS and FTD: Successful treatment with sertraline. Amyotrophic Lateral Sclerosis. 2007;8:189–190. doi: 10.1080/17482960601073543. [DOI] [PubMed] [Google Scholar]
- Baird AD, Wilson SJ, Bladin PF, Saling MM, Reutens DC. Neurological control of human sexual behaviour: Insights from lesion studies. Journal of Neurology, Neurosurgery, and Psychiatry. 2007;78:1042–1049. doi: 10.1136/jnnp.2006.107193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird AD, Wilson SJ, Bladin PF, Saling MM, Reutens DC. The amygdala and sexual drive: Insights from temporal lobe surgery. Annals of Neurology. 2004;55:87–96. doi: 10.1002/ana.10997. [DOI] [PubMed] [Google Scholar]
- Bancroft J. Sexual behavior that is “out of control”: A theoretical conceptual approach. Psychiatric Clinics of North America. 2008;31:593–601. doi: 10.1016/j.psc.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Black B, Muralee S, Tampi RR. Inappropriate sexual behaviors in dement ia. Journal of Geriatric Psychiatry and Neurology. 2005;18:155–62. doi: 10.1177/0891988705277541. [DOI] [PubMed] [Google Scholar]
- Blumer D. Hypersexual episodes in temporal lobe epilepsy. American Journal of Psychiatry. 1970;126:1099–1106. doi: 10.1176/ajp.126.8.1099. [DOI] [PubMed] [Google Scholar]
- Braun CM, Dumont M, Duval J, Hamel I, Godbout L. Opposed left and right brain hemisphere contribut ions to sexual drive: A mult iple lesion case analysis. Behavioural Neurology. 2003;14:55–61. doi: 10.1155/2003/123757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Medeiros K, Rosenberg PB, Baker AS, Onyike CU. Improper sexual behaviors in elders with dement ia living in residential care. Dementia and Geriatric Cognitive Disorders. 2008;26:370–377. doi: 10.1159/000163219. [DOI] [PubMed] [Google Scholar]
- Derouesne C. Comportements dits d’hypersexualite et demences. Psychologie et NeuroPsychiatrie du Vieillissement. 2009;7:101–108. doi: 10.1684/pnv.2009.0164. [DOI] [PubMed] [Google Scholar]
- Devinsky J, Sacks O, Devinsky O. Klüver-Bucy syndrome, hypersexuality, and the law. Neurocase. 2009;18:1–6. doi: 10.1080/13554790903329182. [DOI] [PubMed] [Google Scholar]
- Edwards-Lee T, Miller BL, Benson DF, Cummings JL, Russell GL, Boone K, Mena I. The temporal variant of frontotemporal dement ia. Brain. 1997;120:1027–1040. doi: 10.1093/brain/120.6.1027. [DOI] [PubMed] [Google Scholar]
- Fairweather DS. Psychiatric aspects of the post-encephalit ic syndrome. Journal of Mental Science. 1947;391:200–254. [PubMed] [Google Scholar]
- Ferretti A, Caulo M, Del Gratta C, Di Matteo R, Merla A, Montorsi F, et al. Dynamics of male sexual arousal: Dist inct components of brain act ivat ion revealed by fMRI. Neuroimage. 2005;26:1086–1096. doi: 10.1016/j.neuroimage.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, Mchugh PR. “Mini-mental state”: A practical method for grading the cognit ive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gorman DG, Cummings JL. Hypersexuality following septal injury. Archives of Neurology. 1992;49:308–310. doi: 10.1001/archneur.1992.00530270128029. [DOI] [PubMed] [Google Scholar]
- Hamann S, Herman RA, Nolan CL, Wallen K. Men and women differ in amygdala response to visual sexual stimuli. Nature Neuroscience. 2004;7:411–416. doi: 10.1038/nn1208. [DOI] [PubMed] [Google Scholar]
- Heath RG. Pleasure and brain act ivity in man. Journal of Nervous and Mental Disease. 1972;154:3–18. doi: 10.1097/00005053-197201000-00002. [DOI] [PubMed] [Google Scholar]
- Holstege G, Georgiadis JR, Paans AM, Meiners LC, Van Der Graaf FH, Reinders AA. Brain activation during human male ejaculat ion. Journal of Neuroscience. 2003;23:9185–9193. doi: 10.1523/JNEUROSCI.23-27-09185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafka MP. Hypersexual disorder: A proposed diagnosis for DSM-V. Archives of Sexual Behavior. 2010;39:377–400. doi: 10.1007/s10508-009-9574-7. [DOI] [PubMed] [Google Scholar]
- Karama S, Lecours AR, Leroux JM, Bourgouin P, Beaudoin G, Joubert S, Beauregard M. Areas of brain act ivat ion in males and females during viewing of erotic film excerpts. Human Brain Mapping. 2002;16:1–13. doi: 10.1002/hbm.10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J, Albert H. Sexual behavior after lobotomy. Journal of Nervous and Mental Disease. 1951;113:323–341. [PubMed] [Google Scholar]
- Lilly R, Cummings JL, Benson F, Frankel M. The human Klüver-Bucy syndrome. Neurology. 1983;33:1141–1145. doi: 10.1212/wnl.33.9.1141. [DOI] [PubMed] [Google Scholar]
- Lindau ST, Schumm LP, Laumann EO, Levinson W, O’muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. New England Journal of Medicine. 2007;357:762–774. doi: 10.1056/NEJMoa067423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall LE, Briken P. Assessment, diagnosis, and management of hypersexual disorders. Current Opinion in Psychiatry. 2010;23:570–573. doi: 10.1097/YCO.0b013e32833d15d1. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Chen AK, Shapira JS, Miller BL. Acquired sociopathy and frontotemporal dementia. Dementia and Geriatric Cognitive Disorders. 2005;20:99–104. doi: 10.1159/000086474. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Chow T, Ringman J, Twitchell G, Hinkin C. Pedophilia and disturbances of the temporal lobes. Journal of Neuropsychiatry and Clinical Neuroscience. 2000;12:71–76. doi: 10.1176/jnp.12.1.71. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Lauterbach EC, Sampson SM, Committee On Research An evidence-based review of the psychopathology of frontotemporal dement ia: A report of the ANPA Committee on Research. Journal of Neuropsychiatry and Clinical Neuroscience. 2008;20:130–149. doi: 10.1176/jnp.2008.20.2.130. [DOI] [PubMed] [Google Scholar]
- Mendez MF, O’connor SM, Lim GT. Hypersexuality after right pallidotomy for Parkinson’s disease. Journal of Neuropsychiatry and Clinical Neuroscience. 2004;16:37–40. doi: 10.1176/jnp.16.1.37. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Shapira JS. Internet pornography and frontotemporal dement ia. Journal of Neuropsychiatry and Clinical Neuroscience. 2011;23:E3. doi: 10.1176/jnp.23.2.jnpe3. [DOI] [PubMed] [Google Scholar]
- Miller BL, Cummings JL, Mcintyre H, Ebers G, Grode M. Hypersexuality or altered sexual preference following brain injury. Journal of Neurology, Neurosurgery, and Psychiatry. 1986;49:867–873. doi: 10.1136/jnnp.49.8.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BL, Cummings JL. How brain injury can change sexual behavior. Medical Aspects of Human Sexuality. 1991;1:54–62. [Google Scholar]
- Miller BL, Darby AL, Swartz JR, Yener GG, Mena I. Dietary changes, compulsions and sexual behavior in frontotemporal degenerat ion. Dementia. 1995;6:195–199. doi: 10.1159/000106946. [DOI] [PubMed] [Google Scholar]
- Monga TN, Monga M, Raina MS, Hardjasudarma M. Hypersexuality in stroke. Archives of Physical Medicine and Rehabilitation. 1986;67:415–417. [PubMed] [Google Scholar]
- Müller JL. Are sadomasochism and hypersexuality in aut ism linked to amygdalohippocampal lesion? Journal of Sexual Medicine. 2011;8:3241–3249. doi: 10.1111/j.1743-6109.2009.01485.x. [DOI] [PubMed] [Google Scholar]
- Nagaratnam N, Gayagay G. Hypersexuality in nursing care facilit ies--a descript ive study. Archives of Gerontology and Geriatrics. 2002;35:195–203. doi: 10.1016/s0167-4943(02)00026-2. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degenerat ion: A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Oppenheim H. Zur patholologie der grosshirngeschwülste. Archives für Psychiatrie. 1889;21:560–578. [Google Scholar]
- Ortego N, Miller BL, Itabashi H, Cummings JL. Altered sexual behavior with mult iple sclerosis: A case report. Neuropsychiatry, Neuropsychology and Behavioral Neurology. 1993;6:260–264. [Google Scholar]
- Ozkan B, Wilkins K, Muralee S, Tampi R. Pharmacotherapy for inappropriate sexual behaviors in dementia: A systemat ic review of literature. American Journal of Alzheimer Disease and Other Dementias. 2008;23:344–354. doi: 10.1177/1533317508318369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkara C, Ozdemir S, Yilmaz A, Uzan M, Yeni N, Ozmen M. Orgasm-induced seizures: A study of six pat ients. Epilepsia. 2006;47:2193–2197. doi: 10.1111/j.1528-1167.2006.00648.x. [DOI] [PubMed] [Google Scholar]
- Ozmen M, Erdogan A, Duvenci S, Ozyurt E, Ozkara C. Excessive masturbat ion after epilepsy surgery. Epilepsy & Behavior. 2004;5:133–136. doi: 10.1016/j.yebeh.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Poeck K, Pilleri G. Release of hypersexual behaviour due to lesion in the limbic system. Acta Neurologica Scandinavica. 1965;41:233–244. doi: 10.1111/j.1600-0404.1965.tb04295.x. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensit ivity of revised diagnostic criteria for the behavioural variant of frontotemporal dement ia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redoute J, Stoleru S, Gregoire MC, Costes N, Cinotti L, Lavenne F, et al. Brain processing of visual sexual stimuli in human males. Human Brain Mapping. 2000;11:162–177. doi: 10.1002/1097-0193(200011)11:3<162::AID-HBM30>3.0.CO;2-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid RC, Garos S, Carpenter BN. Reliability, validity, and psychometric development of the hypersexual behavior inventory in an outpatient sample of men. Sexual Addiction & Compulsivity. 2011;18:30–51. [Google Scholar]
- Stein DJ, Hugo F, Oosthuizen P, Hawkridge SM, Van Heerden B. Neuropsychiatry of hypersexuality. CNS Spectrums. 2000;5:36–46. doi: 10.1017/s1092852900012657. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Kuikka J, Kupila J, Partanen K, Vainio P, Airaksinen J, et al. Increase in cerebral blood flow of right prefrontal cortex in man during orgasm. Neuroscience Letters. 1994;170:241–243. doi: 10.1016/0304-3940(94)90328-x. [DOI] [PubMed] [Google Scholar]
- Tucker I. Management of inappropriate sexual behaviors in dement ia: A literature review. International Psychogeriatrics. 2010;22:683–692. doi: 10.1017/S1041610210000189. [DOI] [PubMed] [Google Scholar]
- Welsh KA, Butters N, Mohs RC, Beekly D, Edland S, Fillenbaum G, Heyman A. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology. 1994;44:609–614. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]
- Zeiss AM, Davies HD, Tinklenberg JR. An observat ional study of sexual behavior in demented male patients. Journal of Gerontology. 1996;51:M325–329. doi: 10.1093/gerona/51a.6.m325. [DOI] [PubMed] [Google Scholar]