Abstract
Objective
Commonly, patients undergoing craniotomy are admitted to an intensive care setting post-operatively to allow for close monitoring. We aim to determine the frequency with which patients who have undergone elective craniotomies require intensive care unit level interventions or experience significant complications during the post-operative period to identify a subset of patients for whom an alternative to ICU level care may be appropriate.
Methods
Following Institutional Review Board approval, a prospective, consecutive cohort of adult patients undergoing elective craniotomy was established at the Massachusetts General Hospital between the dates of April 2010 and March 2011. Inclusion criteria were intradural operations requiring craniotomy performed on adults (18 years of age or greater). Exclusion criteria were cases of an urgent or emergent nature, patients who remained intubated post-operatively, and patients who had a ventriculostomy drain in place at the conclusion of the case.
Results
400 patients were analyzed. Univariate analysis revealed that diabetics (p = 0.00047), patients who required intra-operative blood product administration (p = 0.032), older patients (p < 0.0001), patients with higher intra-operative blood losses (p = 0.041), and patients who underwent longer surgical procedures (p = 0.021) were more likely to require ICU-level interventions or experience significant post-operative complications. Multivariate analysis only found diabetes (p = 0.0005) and age (p = 0.0091) to be predictive of a patient’s need for post-operative intensive care unit admission.
Conclusions
Diabetes and older age predict the need for ICU-level intervention after elective craniotomy. Properly selected patients may not require post-craniotomy ICU monitoring. Further study of resource utilization is necessary to validate these preliminary findings, particularly in different hospital types.
Keywords: elective, craniotomy, intensive care unit, ICU, post-operative care
Introduction
A 1994 retrospective study of national intensive care unit (ICU) costs in Veterans Affairs’ Hospitals revealed that while ICU beds comprise less than 10% of inpatient hospital beds nationally, they make up 22% of total hospital costs, equating to roughly 1% of the United States gross domestic product.(11,12) Recent estimates suggest that ICU costs may approach 1/3 of total inpatient costs at some hospitals, as the cost of a day in the ICU remains roughly three to five times greater than a day on a general medical/surgical floor.(8,18,19,23) As a result, considerable efforts have been made to reduce ICU over-utilization.(27) This may be achieved by minimizing the number of unnecessary ICU admissions by stratifying postoperative patients by need. Morasch et al. used this approach to ICU utilization reduction in demonstrating that patients could be safely transferred to a surgical floor following carotid endarterectomy (CEA) if they remained neurologically and hemodynamically stable for three hours post-operatively.(20) 79% of the 185 patients who underwent CEA in this study were successfully transferred to a surgical floor without a single patient from this group requiring transfer to an ICU or a return to the operating room. Many neurosurgical patients have been managed successfully postoperatively in a non-ICU setting, although the analysis of such is limited to retrospective review.(3)
While controversial, it must be noted that a few clinicians in Canada, and more recently in the United Kingdom (UK), have performed craniotomies for tumor resection in select patients on a day-surgery basis.(4,5,10) The combined results from an 11-year series from Toronto Western Hospital (Toronto, Canada) and a 1-year series from Wessex Neurological Centre (Southampton, UK) showed that of 177 patients scheduled for outpatient craniotomy for supratentorial tumor resection, only 9 cases required direct post-operative admission and 2 cases required re-admission following discharge.(10) Importantly, none of the patients in either series suffered an adverse outcome as a result of their planned early discharge. 163 of the 177 outpatient craniotomy cases were performed as awake procedures and 14 were performed following the induction of general anesthesia. Both the UK and Canadian groups only included patients as candidates for outpatient craniotomy if they lived with a responsible adult, had “no co-morbidity requiring hospitalization,” and had a case completion time prior to 13:00, allowing for a 6 hour observation period prior to the 19:00 closing time of the surgical units.(10)
Neurosurgeons have generally been reluctant to entertain reductions in post-operative monitoring following craniotomy, with the fear of not being able to recognize complications that would require urgent attention. In particular, the development of a post-operative hematoma is a known complication of any neurosurgical procedure requiring a craniotomy. Historical literature suggests that roughly 2% of patients undergoing an elective craniotomy will develop a post-operative hematoma requiring surgical evacuation. However, it is important to note that the majority of post-operative hematomas will present with clinical signs of deterioration within six hours of surgery and are heralded by a clinical change. (15,24) It is our hope that by further characterizing the patient population undergoing elective craniotomy, paying special attention to patient co-morbidities and the details of the neurosurgical procedure performed, we may be able to identify a subset of patients for whom an alternative to ICU level care in the post-operative period is appropriate.
Methods
Following approval by the Massachusetts General Hospital (MGH) Institutional Review Board (Protocol #2010P000126) consecutive, adult patients (age > 17 years) undergoing elective craniotomy by all providers were prospectively collected and observed from April 2010 to March 2011. Consent was not obtained from patients for this observational study and the MGH Institutional Review Board approved this methodology. Inclusion criteria included intradural cranial operations, both supratentorial and infratentorial, for indications including tumor resection, open brain biopsy, vascular malformation obliteration, temporal lobectomy for epilepsy, microvascular decompression for neurovascular compression syndromes, and Chiari malformation decompressions. As an institutional practice pattern, these patients are routinely admitted to the neurological sciences ICU, regardless of neurological status or clinical complexity at the conclusion of the procedure. In the ICU, the patient:nursing ratio is either 1:1 or 2:1. Neurological exams are performed by nursing every 1 or 2 hours. Blood pressure is continually monitored with an arterial pressure line for at least the first 24 hours. Continuous cardiac telemetry and oxygen saturation are monitored. This differs from the regular neuroscience floor bed, where neurological checks and vital signs can be obtained every 4 hours. Also, on the floor, the institution of IV drips requires transfer to the ICU. In rare circumstances because of bed availability, elective craniotomy patients spent their first post-operative night in the post-operative care unit (PACU) or on the neurosciences floor and were excluded from the study. Additionally, any craniotomy that was performed as an urgent or emergent case, defined in the official anesthesia booking record as requiring a start time in less than four hours or 30 minutes, respectively, was excluded from this study. Furthermore, patients who remained intubated post-operatively or had a ventriculostomy drain in place for intracranial pressure monitoring at the conclusion of the surgery were excluded from the study as these ongoing interventions would always necessitate an ICU admission.
Details of the operative procedure were recorded from the anesthesia and surgical records including the type of procedure performed, estimated intra-operative blood loss, use of blood transfusions, and length of surgery. Tumor volume was calculated with the use of Vitrea Advanced software (Vital Images, Minnetonka, Minnesota, USA). There was insufficient power in our pre-study analysis to divide neoplastic lesions into sub-categories based on pathology. The ICU courses of patients meeting criteria were retrospectively studied; each patient’s electronic inpatient medical record (including daily progress notes, event notes, medication records, radiology records, and physician orders) was extracted for data. Interventions that required an ICU setting, i.e., were not feasible on a general medical/surgical floor unit, were defined as the use of hemodynamic medications delivered by intravenous infusion (other than low dose anti-hypertensives: nicardipine at a rate ≤ 5 mg/hr; cumulative total dose ≤ 20 mg or labetalol at a rate ≤ 10 mg/hr; cumulative total dose ≤ 40 mg), the use of hyperosmolar therapy (defined as the use of 3% NaCl delivered at a rate of greater than 30 cc/hr or the use of mannitol), the use of an insulin drip, and the use of an ICP monitor or ventriculostomy drain. Significant adverse events were defined as the development of a clinically significant post-operative hematoma, ischemic stroke, myocardial infarction, the need to return to the operating room from the ICU, the need for re-intubation, goals of patient care being established as comfort measures only (CMO), and death.
Statistical analysis was performed using SAS software (version 9.3). Fisher’s Exact test was used to the determine the association between the need for post-operative intensive care unit admission and binary risk factors, Chi-Square test was used for categorical risk factors, and Wilcoxon Rank-Sum tests for continuous factors. A multivariable logistic regression model was used to test the predictive power of these the significant risk factors on the need for post-operative intensive care unit admission. Significant variables in the model were selected according to the stepwise selection process. Significance was defined as p ≤ 0.05.
Results
432 consecutive adult patients underwent elective craniotomy during the study timeframe. 31 cases were excluded as the patients spent the first post-operative nights in the PACU or on the neurosciences floor, rather than being admitted to the neurological sciences ICU. One patient died intra-operatively from complications of air emboli during a craniotomy for tumor and was also excluded from the study. Thereby, 400 elective craniotomy cases were included in the study population.
There were 166 males and 234 females. The mean age was 53.0, the mean BMI was 28.2, and the mean American Society of Anesthesiologists (ASA) physical status classification system score was 2.37. 58 patients were active smokers and 37 had a known pre-operative diagnosis of diabetes mellitus type II. 123 of the patients studied were admitted to MGH as inpatients in the immediate pre-operative period, 47 of the patients had been admitted to an ICU of some kind during the 12-month period prior to their surgery, and 61 of the cases were repeated craniotomy (“re-do”) cases. The majority (n=298) of patients underwent supratentorial procedures. Most commonly, general anesthesia was employed (n=386), as compared to awake craniotomies (n=14). The average surgery length was 263 minutes. The average intra-operative estimated blood loss (EBL) was 352.5 ml. 46 patients required intra-operative blood products. (Table 1)
Table 1.
Patient and Operative Characteristics.
| Average Age | 53.01 |
| Male/Female | 166 (41.5%)/234 (58.5%) |
| Average ASA status | 2.37 (n = 395) |
| Average BMI | 28.20 (n = 287) |
| Diabetes | 37 (9.25%) |
| Active smoker | 58 (14.5%) |
| Pre-operative inpatient admission | 123 (30.75%) |
| ICU admission over prior 12-months | 47 (11.75%) |
| Re-do cases | 61 (15.25%) |
| Supra-/Infratentorial | 298 (74.5%)/102 (25.5%) |
| General anesthesia/Awake | 386 (96.5%)/14 (3.5%) |
| Average surgery length (minutes) | 263.36 |
| Average EBL (mLs) | 352.50 |
| Received intra-operative blood products | 46 (11.5%) |
n = 400 unless otherwise noted.
Tumor resection was the most common indication for surgery (n=260), followed by microsurgical aneurysm obliteration (n=62), microvascular decompression for neurovascular compression syndrome (n=21), temporal lobectomy for epilepsy (n=19), Chiari malformation decompression (n=8), cavernous malformation resection (n=5), arteriovenous malformation resection (n=5), and open tumor biopsy (n=4). Additional categories of procedures were performed on less than one percent of the study population. (Table 2)
Table 2.
Procedures Performed.
| Procedure Performed | Number of Cases (%) |
|---|---|
| Tumor resection | 260 (65.0%) |
| Open aneurysm obliteration | 62 (15.5%) |
| Microvascular decompression | 21 (5.25%) |
| Temporal lobectomy for epilepsy | 19 (4.75%) |
| Chiari malformation decompression | 8 (2.0%) |
| Cavernoma resection | 5 (1.25%) |
| AVM resection | 5 (1.25%) |
| Open biopsy (tumor) | 4 (1.0%) |
| Arachnoid cyst fenestration | 3 (0.75%) |
| EC-IC bypass | 3 (0.75%) |
| Subdural grids and strips | 2 (0.5%) |
| EDAS | 1 (0.25%) |
| Cortical recording | 1 (0.25%) |
| Vestibular nerve sectioning | 1 (0.25%) |
| Corpus callostomy | 1 (0.25%) |
| SCD repair | 1 (0.25%) |
| Encephalocele repair | 1 (0.25%) |
| Open biopsy (autoimmune) | 1 (0.25%) |
| Open biopsy (infectious) | 1 (0.25%) |
n = 400.
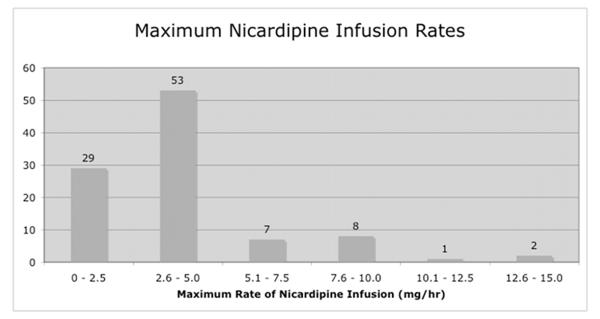
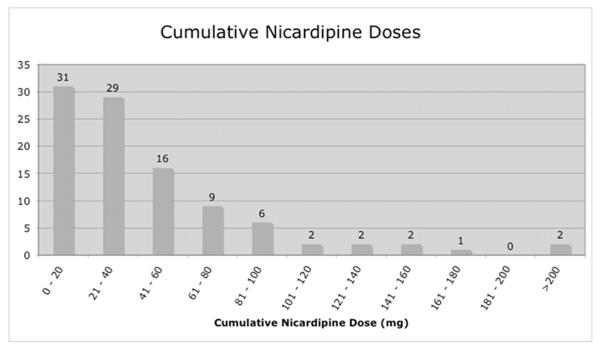
140/400 patients required an ICU level intervention or experienced a significant adverse event during their post-operative ICU course. The most commonly performed ICU-level intervention involved continuous intravenous delivery of blood pressure medication. In our series, 155/400 patients received continuous intravenous blood pressure medications for a portion of their ICU stay, with the majority of these patients requiring low rate, low cumulative dose nicardipine, as defined in the methods section. (Figure 1) Assuming that the short-term use of anti-hypertensive medication by IV drip could be replaced by bolus antihypertensives administered in the recovery room or on the floor, we performed a subset analysis by eliminating those patients requiring “minimal” anti-hypertensive therapy. Removing these cases left 123/400 patients in whom ICU-level intravenous blood pressure medication drips were used during the post-operative period. Of these 123 patients, 108 required anti-hypertensives (70 nicardipine, 25 labetalol, 13 multiple agents) and 15 required vasopressors (13 phenylephrine, 1 norephinephrine, 1 multiple agents) to comply with blood pressure goals.
Fig. 1.
The majority of the 123 patients treated in the ICU with an intravenous blood pressure medication received only nicardipine (n= 100). Generally, this medication was delivered at a low rate (a) and resulted in a small cumulative dose (b) in most patients.
17 patients required ICU-level hyperosmolar therapy, eight required an insulin drip, three required intubation (for post-ictal apnea in two cases and significant desaturation thought to be related to aspiration in one case), two had to return to the OR from the ICU (for epidural hematoma in one case and intraparenchymal hematoma in one case), and only one patient required the placement of an external ventricular drain (for hydrocephalus in the context of cerebellar edema following acoustic neuroma resection). In addition to ICU-level interventions, we recorded significant post-operative complications, assuming that these patients were well-served by the monitoring capacity with which an ICU is equipped. Seven patients experienced a post-operative ischemic stroke, four patients experienced a clinically significant hematoma (two of the four resulted in return to the OR), one patient experienced a myocardial infarction, and there were no patients who died or had therapeutic care withdrawn (CMO) during their post-operative ICU stay. (Table 3)
Table 3.
ICU-level Interventions and Post-Operative Complications Necessitating ICU Care.
| Measured Outcome | Number of Cases (%) |
|---|---|
| IV blood pressure medication drip | 123 (30.75%) |
| Hyperosmolar therapy | 17 (4.25%) |
| IV insulin drip | 8 (2.0%) |
| Ischemic stroke | 7 (1.75%) |
| Clinically significant hematoma | 4 (1.0%) |
| Intubation | 3 (0.75%) |
| Return to OR | 2 (0.5%) |
| ICP monitor placement | 1 (0.25%) |
| MI | 1 (0.25%) |
| Death/CMO | 0 (0.0%) |
| TOTAL* | 140 (35.0%) |
n = 400.
Some patients required more than 1 ICU-level intervention or experienced more than 1 significant complication necessitating ICU care and these patients were only counted once when calculating the total. Therefore, the sum of all ICU-level interventions and significant complications is more than the total.
Univariate analysis performed on the entire dataset revealed that diabetics (p = 0.00047; odds ratio [OR] = 3.45, 95% confidence interval [95% CI] = 1.72 – 6.94), patients who required intra-operative blood product administration (p = 0.032; OR = 2.02, 95% CI = 1.09 – 3.76), older patients (p < 0.0001; OR per year of age = 1.03, 95% CI = 1.015 – 1.045), patients with higher intra-operative blood losses (p = 0.041; OR per CC of blood loss = 1.001, 95% CI = 1.000 – 1.001), and patients who underwent longer surgical procedures (p = 0.021; OR per minute of operative time = 1.001, 95% CI = 1.000 – 1.003) were more likely to require intensive care unit level interventions or experience significant post-operative complications. (Table 4) Multivariate analysis identified diabetes (p = 0.0005) and age (p = 0.0091) as predictors of a patient’s need for post-operative intensive care unit admission.
Table 4.
Patient and Operative Variables Predictive of a Need for ICU Care – Univariate Analysis.
| Variable | Test Performed | P Value | Odds Ratios (95% Conf. Int.) |
|---|---|---|---|
| Inpatient Status | Fisher’s Exact Test | 1.00 | Inpatient: 0.997 (0.639 – 1.558) |
| Sex | Fisher’s Exact Test | 0.17 | Male: 1.364 (0.901 – 2.067) |
| Type (tumor, vascular, functional, congenital, autoimmune, or infectious) |
Chi-Square | 0.12 | Tumor (against all others): 0.836 (0.545 – 1.285) Vascular (against all others): 1.781 (1.072 – 2.958) Functional (against all others): 0.603 (0.302 – 1.202) Congenital (against all others): 0.820 (0.248 – 2.713) |
| Supra- vs. Infra-tentorial | Fisher’s Exact Test | 0.23 | Supratentorial: 0.740 (0.466 – 1.177) |
| Anesthesia (General vs. Awake) | Fisher’s Exact Test | 0.40 | General: 2.017 (0.553 – 7.355) |
| Re-do cases | Fisher’s Exact Test | 0.66 | Re-do: 1.148 (0.653 – 2.020) |
| ASA Status | Chi-Square | 0.28 | Classes 3 & 4 (against 1 & 2): 1.305 (0.854 – 1.994) |
| Diabetes | Fisher’s Exact Test | 0.00047 | Diabetes: 3.448 (1.715 – 6.944) |
| Active tobacco smoking | Fisher’s Exact Test | 0.55 | Smoker: 0.812 (0.446 – 1.477) |
| OR blood products | Fisher’s Exact Test | 0.032 | OR blood product use: 2.024 (1.091 – 3.759) |
| ICU stay over past 12-months | Fisher’s Exact Test | 0.33 | Prior ICU stay: 0.680 (0.347 – 1.337) |
| Age | Wilcoxon Rank-Sum Test | <0.0001 | Per year of age: 1.030 (1.015 – 1.045) |
| EBL | Wilcoxon Rank-Sum Test | 0.041 | Per CC of blood loss: 1.001 (1.000 – 1.001) |
| BMI | Wilcoxon Rank-Sum Test | 0.093 | Per integer increase in BMI: 1.040 (1.002 – 1.079) |
| Surgery length | Wilcoxon Rank-Sum Test | 0.021 | Per minute of surgery time: 1.001 (1.000 – 1.003) |
| Tumor Volume | Wilcoxon Rank-Sum Test | 0.16 | Per CC of tumor volume: 1.007 (0.998 – 1.017) |
Of the 140 patients who required ICU-level-of-care interventions or had significant post-operative complications, 107 (76.4%) of them required an intravenous blood pressure medication drip as their sole intervention/complication. Therefore, only 33 of the 400 patients in the series (8.25%) required an ICU-level-of-care intervention or had significant post-operative complication beyond requiring an intravenous blood pressure medication drip. Additional univariate and multivariate analyses were performed in order to determine if there were patient or operative variables which were predictive of a patient’s need for an ICU intervention (or significant post-operative complication) other than administration of intravenous blood pressure medications. This univariate analysis revealed that diabetics (p = 0.0014; OR = 4.55, 95% CI = 1.93 – 10.75), patients who required intra-operative blood product administration (p = 0.0019; OR = 4.00, 95% CI = 1.76 – 9.09), patients with higher ASA status values (p = 0.0008; OR of ASA classes 3 & 4 compared against classes 1 & 2 = 4.636, 95% CI = 2.08 – 10.32), patients with higher intra-operative blood losses (p = 0.015; OR per CC of blood loss = 1.001, 95% CI = 1.000 – 1.002), and patients who underwent longer surgical procedures (p = 0.0066; OR per minute of operative time = 1.003, 95% CI = 1.001 – 1.005) were more likely to require intensive care unit level interventions other than requiring intravenous blood pressure medications. (Table 5) Within this subset of patients, multivariate analysis identified diabetes (p = 0.0002) the use of intra-operative blood products (p = 0.0014), and age (p = 0.0215) to be predictive of a patient’s need for post-operative intensive care unit admission.
Table 5.
Patient and Operative Variables Predictive of a Need for ICU Care Other than Intravenous Blood Pressure Medication Drips – Univariate Analysis.
| Variable | Test Performed | P Value | Odds Ratio (95% Conf. Int.) |
|---|---|---|---|
| Inpatient Status | Fisher’s Exact Test | 0.17 | Inpatient: 1.745 (0.844 – 3.610) |
| Sex | Fisher’s Exact Test | 0.064 | Male: 2.031 (0.987 – 4.178) |
| Type (tumor, vascular, functional, congenital, autoimmune, or infectious) |
Chi-Square | 0.99 | Tumor (against all others): 1.232 (0.569 – 2.668) Vascular (against all others): 0.943 (0.375 – 2.371) Functional (against all others): 0.734 (0.215 – 2.506) Congenital (against all others): 0.925 (0.116 – 7.340) |
| Supra- vs. Infra-tentorial | Fisher’s Exact Test | 1.00 | Supratentorial: 1.076 (0.469 – 2.467) |
| Anesthesia (General vs. Awake) | Fisher’s Exact Test | 1.00 | General: 1.175 (0.149 – 9.275) |
| Re-do cases | Fisher’s Exact Test | 0.61 | Re-do: 1.261 (0.498 – 3.195) |
| ASA Status | Chi-Square | 0.0008 | Classes 3 & 4 (against 1 & 2): 4.636 (2.083 – 10.318) |
| Diabetes | Fisher’s Exact Test | 0.0014 | Diabetes: 4.545 (1.927 – 10.753) |
| Active tobacco smoking | Fisher’s Exact Test | 1.00 | Smoker: 0.800 (0.270 – 2.365) |
| OR blood products | Fisher’s Exact Test | 0.0019 | OR blood product use: 4.000 (1.764 – 9.091) |
| ICU stay over past 12-months | Fisher’s Exact Test | 1.00 | Prior ICU stay: 1.040 (0.239 – 3.096) |
| Age | Wilcoxon Rank-Sum Test | 0.060 | Per year of age: 1.026 (1.002 – 1.051) |
| EBL | Wilcoxon Rank-Sum Test | 0.015 | Per ml of blood loss: 1.001 (1.000 – 1.002) |
| BMI | Wilcoxon Rank-Sum Test | 0.35 | Per integer increase in BMI: 1.067 (1.013 – 1.123) |
| Surgery length | Wilcoxon Rank-Sum Test | 0.0066 | Per minute of surgery time: 1.003 (1.001 – 1.005) |
| Tumor Volume | Wilcoxon Rank-Sum Test | 0.44 | Per CC of tumor volume: 0.998 (0.981 – 1.016) |
In order to better understand the ability of the patient/operative variables identified in our two univariate analyses to predict a patient’s need for ICU-level care, we tabulated how many risk-factor free patients ultimately required ICU-level care. In our initial univariate analysis of the whole patient data set we found that diabetes, intra-operative blood product administration, greater age, higher intra-operative blood losses, and longer surgical procedure times were predictive of a patient’s need for the ICU. Selecting for patients who did not have these risk factors, we identified 239 patients (59.8% of our dataset). Of these 239 patients, 66 patients (or 27.6%) ultimately required ICU-level care.
Similarly, we analyzed patients who required ICU-level care other than the administration of hemodynamic intravenous drips. Selecting for patients who were not diabetic, did not require intra-operative blood products, who were ASA classes 1 or 2, had intra-operative blood losses less that 1L, and had surgical procedure times of 8 hours or less, we identified 190 patients (47.5% of our dataset). Of these 190 patients, five (2.6%) required ICU-level care aside from administration of hemodynamic intravenous blood pressure drips. (Table 6)
Table 6.
Predictive Need for ICU-Level Care Depending on Univariate Analysis Risk Factors.
| Risk Factors Overall | |||
|---|---|---|---|
| Risk Factor | Patients with Risk Factor in the Entire Series |
Patients with Risk Factor Requiring ICU-Level Care |
Given Risk Factor What is Likelihood of Requiring the ICU? |
| Diabetes | 37/400 (9.3%) | 20/140 (14.3%) | 20/37 (54.1%) |
| OR blood products | 46/400 (11.5%) | 22/140 (15.7%) | 22/46 (47.8%) |
| Age > 65 | 86/400 (21.5%) | 43/140 (30.7%) | 43/86 (50.0%) |
| EBL ≥ 1000 | 20/400 (5.0%) | 9/140 (6.4%) | 9/20 (45.0%) |
| Surgery time > 8hrs | 37/400 (9.3%) | 13/140 (9.3%) | 13/37 (35.1%) |
| NO RISK FACTORS | 239/400 (59.8%) | 66/140 (47.1%) | 66/239 (27.6%) |
| Risk Factors For Reasons Other than IV BP Medication Infusion | |||
| Risk Factor | Patients with Risk Factor in the Entire Series |
Patients with Risk Factor Requiring ICU-Level Care |
Given Risk Factor What is Likelihood of Requiring the ICU? |
| Diabetes | 37/400 (9.3%) | 8/33 (24.2%) | 8/37 (21.6%) |
| OR blood products | 46/400 (11.5%) | 8/33 (24.2%) | 8/46 (17.4%) |
| ASA Classes 3 & 4 | 151/395 (38.2%) | 23/33 (69.7%) | 23/151 (15.2%) |
| EBL ≥ 1000 | 20/400 (5.0%) | 5/33 (15.2%) | 5/20 (25.0%) |
| Surgery time > 8hrs | 37/400 (9.3%) | 3/33 (9.1%) | 3/37 (8.1%) |
| NO RISK FACTORS | 190/400 (47.5%) | 5/33 (15.2%) | 5/190 (2.6%) |
Discussion
The findings in this study support previous research findings that better risk assessment may be able to predict the need for ICU-level care following craniotomy. In a retrospective study of 113 patients undergoing spine surgery or craniotomy for tumor at a single institution, Nitahara et al. actually found that no patient required active intervention during their ICU stay.(21) Another study by Ziai et al. attempted to determine variables predictive of the length of stay and need for ICU intervention in a similar group of 158 patients.(28) Curiously, they defined IV analgesic use as an ICU level intervention. This accounted for the majority of their ICU resource utilization. At our institution, IV analgesic use is common practice on the floor setting and is not an indication for ICU admission. An additional retrospective study by Bui et al. analyzed 394 patients who underwent elective craniotomy over a 54-month timeframe (five times the study duration as our study) to assess the need for ICU admission.(6) The majority of the patients in this study were admitted to the floor (n=344). Of the 51 patients admitted to the ICU, they found that long operation times, extensive blood loss, and high anesthesic risks were predictive of ICU admission. Finally, Rhondali et al. described the occurrence of postoperative complications in 358 patients admitted to the ICU following elective craniotomy.(22) However, their study design only defined a postoperative complication as neurological deterioration triggering imaging or treatment. The results of our study uniquely contribute to a growing body of evidence that specific risk factors identify the need for ICU-level intervention.
In this study, we have delineated ICU resource utilization by post-operative elective craniotomy patients in the hope of identifying those for whom ICU-level care may not be needed. While institutional practice may vary, patterns of care are fairly similar amongst major medical centers with high-volume neurosurgical practices. Regardless, it should be emphasized that this is a single institution study and the results may not be generalizable to other institutions, such as community-based hospitals or other academic institutions. Key factors, such as hospital size, infrastructure, case volume, proportion of pathological conditions, local practice standards (including the use of intra-operative and post-operative imaging), and the level of nursing care available may influence the relative need for post-operative critical care.
This study is limited by its purely observational nature. Without controlled randomization it is impossible to assess the true value of post-operative ICU admission for elective craniotomy patients. There are intangible aspects of ICU care that could not be quantified in this study, but may impact patient outcomes. For example, the aggressive nursing monitoring afforded in an ICU setting may have contributed to the low post-operative complication rates observed in this series. In the ICU, the patient:nurse ratio is either 1:1 or 2:1. Neurological exams are performed by nursing every one or two hours. Our neurological sciences ICU is equipped with specialized resources: a team of neuro-intensivists that provides continuous in-unit critical care coverage(25), continuous in-house neurosurgery coverage, and the resources consistent with status as both an American Heart Association certified stroke center and an American College of Surgeons level 1 trauma center.(9,16,17)
The most common ICU-level-of-care intervention observed was the delivery of blood pressure medication by continuous intravenous infusion. At our institution, all postoperative craniotomies have arterial line monitoring of continuous blood pressure. A systolic blood pressure goal of 100-140 mmHg is recorded in the electronic order set for all patients. This institutional practice pattern is grounded on the understanding that the perioperative course of patients undergoing intracranial surgery can be complicated by acute hypertensive episodes, which are associated with intracranial bleeds, resultant morbidity, and prolonged hospital stays.(11) The high rate of intravenous blood pressure medication drip usage reflects our institution’s commonly used post-operative systolic blood pressure parameter goal of 100-140 mmHg. Interestingly, the majority of medication usage could be interpreted as low rate and low cumulative dose, indicative of a relative degree of hemodynamic stability. There are no randomized controlled trials to support strict adherence to these blood pressure parameters; however, tight blood pressure control may have contributed to the low rate of clinically significant post-operative hematomas (1.0%) observed in this study.(1) While it would be inappropriate to infer the clinical value of maintaining the aforementioned systolic blood pressure parameters in the post-operative period based on the findings of an observational study, it is clear that once intravenous blood pressure medication drips are excluded, relatively few patients in the study population required an ICU-level intervention or suffered a significant post-operative complication. It is conceivable that patients whose blood pressure falls slightly outside of these guidelines and are otherwise hemodynamically stable could be treated in an intermediate care unit.
Close patient monitoring is clearly desirable in the elective craniotomy post-operative period. However, the low rate of need for ICU interventions other than intravenous blood pressure medication drips and the even lower rate of significant complications suggest that these patients rarely utilize the full armamentarium of ICU resources. It may be possible to compartmentalize post-operative elective craniotomy patients into a lower acuity neurological ICU or step-down unit where the focus of care can remain on close neurological monitoring and rapid response rates for patients who experience neurologic or physiologic deterioration. The benefits of ICU care must be weighed against some of the disadvantages of being in the ICU postoperatively. For example, physical therapy and occupational therapy evaluations may be deferred while a patient is in the ICU, sometimes delaying mobilization. Prompt post-operative mobilization may expedite patient discharge to home.(26) Additionally, in busy neurosurgical centers with a significant trauma volume, allocation of ICU beds remains a constant challenge, which could be alleviated by stratifying post-craniotomy patients into categories of risk and distributing them postoperatively according to anticipated need.(13,14)
The financial implications of reducing the inappropriate utilization of intensive care are significant. This holds true even when considering the anticipated “short” intensive care stays in the immediate post-operative period. For non-ventilated patients, daily costs in the intensive care unit are highest in the first two days following admission, averaging over $15,000 total in that period. (7) In another study analyzing a neurosurgery specific population, immediate transfer to the floor following craniotomy decreased average hospitalization length by three days and provided an estimated cost savings of $4,026 per patient.(2) Patients will continue to benefit from further efforts at optimizing resource utilization both financially and clinically.
Conclusions
Post-operative ICU admission following elective craniotomy is a resource intensive practice. Diabetes and older age predict the need for ICU-level intervention after elective craniotomy. It may be reasonable to assign certain post-craniotomy patients to alternative levels of care, utilizing hospital resources more efficiently. Further study of resource utilization is necessary to validate these preliminary findings, particularly in different hospital types.
Acknowledgements
Dr. Walcott is supported by the Council of State Neurological Societies Socioeconomic fellowship (2012-2013).
Funding- This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (NIH Award #UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the National Institutes of Health. Funding was also provided by the Council of State Neurosurgical Societies – Congress of Neurological Surgeons (Medical Student Summer Fellowship in Socioeconomic Research). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions- All authors made substantial contribution to conception and design, or acquisition of data, or analysis and interpretation of data; participated in drafting the article or revising it critically for important intellectual content; and final approval of the version submitted.
Competing Interests- None
References
- 1.Basali A, Mascha EJ, Kalfas I, Schubert A. Relation between perioperative hypertension and intracranial hemorrhage after craniotomy. Anesthesiology. 2000;93(1):48–54. doi: 10.1097/00000542-200007000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Beauregard CL, Friedman WA. Routine use of postoperative ICU care for elective craniotomy: a cost-benefit analysis. Surg Neurol. 2003;60(6):483–489. doi: 10.1016/s0090-3019(03)00517-2. dicussion 489. [DOI] [PubMed] [Google Scholar]
- 3.Beauregard CL, Friedman WA. Routine use of postoperative ICU care for elective craniotomy: a cost-benefit analysis. Surgical Neurology. 2003;60(6):483–489. doi: 10.1016/s0090-3019(03)00517-2. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein M. Outpatient craniotomy for brain tumor: a pilot feasibility study in 46 patients. The Canadian Journal of Neurological Sciences. Le Journal Canadien Des Sciences Neurologiques. 2001;28(2):120–124. doi: 10.1017/s0317167100052781. [DOI] [PubMed] [Google Scholar]
- 5.Boulton M, Bernstein M. Outpatient brain tumor surgery: innovation in surgical neurooncology. Journal of Neurosurgery. 2008;108(4):649–654. doi: 10.3171/JNS/2008/108/4/0649. [DOI] [PubMed] [Google Scholar]
- 6.Bui JQH, Mendis RL, van Gelder JM, Sheridan MMP, Wright KM, Jaeger M. Is postoperative intensive care unit admission a prerequisite for elective craniotomy? Journal of Neurosurgery. 2011;115(6):1236–1241. doi: 10.3171/2011.8.JNS11105. [DOI] [PubMed] [Google Scholar]
- 7.Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33(6):1266–1271. doi: 10.1097/01.ccm.0000164543.14619.00. [DOI] [PubMed] [Google Scholar]
- 8.Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Critical Care Medicine. 2005;33(6):1266–1271. doi: 10.1097/01.ccm.0000164543.14619.00. [DOI] [PubMed] [Google Scholar]
- 9.DuBose JJ, Browder T, Inaba K, Teixeira PGR, Chan LS, Demetriades D. Effect of Trauma Center Designation on Outcome in Patients With Severe Traumatic Brain Injury. Arch Surg. 2008;143(12):1213–1217. doi: 10.1001/archsurg.143.12.1213. [DOI] [PubMed] [Google Scholar]
- 10.Grundy PL, Weidmann C, Bernstein M. Day-case neurosurgery for brain tumours: the early United Kingdom experience. British Journal of Neurosurgery. 2008;22(3):360–367. doi: 10.1080/02688690801961858. [DOI] [PubMed] [Google Scholar]
- 11.Halpern NA, Bettes L, Greenstein R. Federal and nationwide intensive care units and healthcare costs: 1986-1992. Critical Care Medicine. 1994;22(12):2001–2007. [PubMed] [Google Scholar]
- 12.Hartman M, Kornfeld R, Catlin A. [Accessed August 8, 2011];Health Care Expenditures in the National Health Expenditures Accounts and in Gross Domestic Product: A Reconciliation. 2010 http://ideas.repec.org/p/bea/wpaper/0060.html.
- 13.Iapichino G, Radrizzani D, Pezzi A, Assi E, Di Mauro P, Mistraletti G, Porta F. Evaluating daily nursing use and needs in the intensive care unit: a method to assess the rate and appropriateness of ICU resource use. Health Policy (Amsterdam, Netherlands) 2005;73(2):228–234. doi: 10.1016/j.healthpol.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Iwashyna TJ, Kramer AA, Kahn JM. Intensive Care Unit Occupancy and Patient Outcomes. Critical care medicine. 2009;37(5):1545–1557. doi: 10.1097/CCM.0b013e31819fe8f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khaldi A, Prabhu VC, Anderson DE, Origitano TC. The clinical significance and optimal timing of postoperative computed tomography following cranial surgery. Journal of Neurosurgery. 2010;113(5):1021–1025. doi: 10.3171/2009.11.JNS081048. [DOI] [PubMed] [Google Scholar]
- 16.Lattimore SU, Chalela J, Davis L, DeGraba T, Ezzeddine M, Haymore J, Nyquist P, Baird AE, Hallenbeck J, Warach S. Impact of Establishing a Primary Stroke Center at a Community Hospital on the Use of Thrombolytic Therapy. Stroke. 2003;34(6):e55-e57–e55-e57. doi: 10.1161/01.STR.0000073789.12120.F3. [DOI] [PubMed] [Google Scholar]
- 17.MacKenzie EJ, Rivara FP, Jurkovich GJ, Nathens AB, Frey KP, Egleston BL, Salkever DS, Scharfstein DO. A national evaluation of the effect of trauma-center care on mortality. The New England journal of medicine. 2006;354(4):366–378. doi: 10.1056/NEJMsa052049. [DOI] [PubMed] [Google Scholar]
- 18.Milbrandt EB, Kersten A, Rahim MT, Dremsizov TT, Clermont G, Cooper LM, Angus DC, Linde-Zwirble WT. Growth of intensive care unit resource use and its estimated cost in Medicare*. Critical Care Medicine. 2008;36(9):2504–2510. doi: 10.1097/CCM.0b013e318183ef84. [DOI] [PubMed] [Google Scholar]
- 19.Moerer O, Plock E, Mgbor U, Schmid A, Schneider H, Wischnewsky M, Burchardi H. A German national prevalence study on the cost of intensive care: an evaluation from 51 intensive care units. Critical Care. 2007;11(3):R69–R69. doi: 10.1186/cc5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morasch MD, Hirko MK, Hirasa T, Burke K, Greisler HP, Littooy FN, Baker WH. Intensive care after carotid endarterectomy: a prospective evaluation. Journal of the American College of Surgeons. 1996;183(4):387–392. [PubMed] [Google Scholar]
- 21.Nitahara JA, Valencia M, Bronstein MA. Medical case management after laminectomy or craniotomy: do all patients benefit from admission to the intensive care unit? Neurosurgical Focus. 1998;5(2):e4–e4. doi: 10.3171/foc.1998.5.2.7. [DOI] [PubMed] [Google Scholar]
- 22.Rhondali O, Genty C, Halle C, Gardellin M, Ollinet C, Oddoux M, Carcey J, Francony G, Fauvage B, Gay E, Bosson J-L, Payen J-F. Do patients still require admission to an intensive care unit after elective craniotomy for brain surgery? Journal of Neurosurgical Anesthesiology. 2011;23(2):118–123. doi: 10.1097/ANA.0b013e318206d5f8. [DOI] [PubMed] [Google Scholar]
- 23.Shorr AF. An update on cost-effectiveness analysis in critical care. Current Opinion in Critical Care. 2002;8(4):337–343. doi: 10.1097/00075198-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Taylor WA, Thomas NW, Wellings JA, Bell BA. Timing of postoperative intracranial hematoma development and implications for the best use of neurosurgical intensive care. Journal of Neurosurgery. 1995;82(1):48–50. doi: 10.3171/jns.1995.82.1.0048. [DOI] [PubMed] [Google Scholar]
- 25.Varelas PN, Schultz L, Conti M, Spanaki M, Genarrelli T, Hacein-Bey L. The Impact of a Neuro-Intensivist on Patients with Stroke Admitted to a Neurosciences Intensive Care Unit. Neurocritical Care. 2008;9(3):293–299. doi: 10.1007/s12028-008-9050-6. [DOI] [PubMed] [Google Scholar]
- 26.Wagner AK, Fabio T, Zafonte RD, Goldberg G, Marion DW, Peitzman AB. Physical medicine and rehabilitation consultation: relationships with acute functional outcome, length of stay, and discharge planning after traumatic brain injury. American Journal of Physical Medicine & Rehabilitation/Association of Academic Physiatrists. 2003;82(7):526–536. doi: 10.1097/01.PHM.0000073825.09942.8F. [DOI] [PubMed] [Google Scholar]
- 27.Ward NS, Teno JM, Curtis JR, Rubenfeld GD, Levy MM. Perceptions of cost constraints, resource limitations, and rationing in United States intensive care units: Results of a national survey. Critical Care Medicine. 2008;36(2):471–476. doi: 10.1097/CCM.0B013E3181629511. [DOI] [PubMed] [Google Scholar]
- 28.Ziai WC, Varelas PN, Zeger SL, Mirski MA, Ulatowski JA. Neurologic intensive care resource use after brain tumor surgery: an analysis of indications and alternative strategies. Critical Care Medicine. 2003;31(12):2782–2787. doi: 10.1097/01.CCM.0000098860.52812.24. [DOI] [PubMed] [Google Scholar]