Abstract
Effects of curcuminoids on breast cancer cell secretion of the bone-resorptive peptide parathyroid hormone-related protein (PTHrP) and on lytic breast cancer bone metastasis were evaluated. In vitro, transforming growth factor (TGF)-β-stimulated PTHrP secretion was inhibited by curcuminoids (IC50 = 24 μM) in MDA-MB-231 human breast cancer cells independent of effects on cell growth inhibition. Effects on TGF-β signaling revealed decreases in phospho-Smad2/3 and Ets-1 protein levels with no effect on p-38 MAPK-mediated TGF-β signaling. In vivo, mice were inoculated with MDA-MB-231 cells into the left cardiac ventricle and treated ip every other day with curcuminoids (25 or 50 mg/kg) for 21 days. Osteolytic bone lesion area was reduced up to 51% (p < 0.01). Consistent with specific effects on bone osteolysis, osteoclast number at the bone-tumor interface was reduced up to 53% (p < 0.05) while tumor area within bone was unaltered. In a separate study, tumor mass in orthotopic mammary xenografts was also unaltered by treatment. These data suggest that curcuminoids prevent TGF-β induction of PTHrP and reduce osteolytic bone destruction by blockade of Smad signaling in breast cancer cells.
Of women diagnosed with breast cancer, nearly one third will develop advanced disease characterized by primary tumor metastases to distant organs.1 Seventy percent of women with advanced disease have bone metastases, most of which are osteolytic, or bone destructive.2 Osteolytic bone lesions can result in severe bone pain, hypercalcemia of malignancy and increased risk of fracture, significantly reducing the quality and length of life for cancer patients.3,4 Upon seeding in the bone microenvironment, breast cancer tumor cells secrete osteolytic factors among which parathyroid hormone-related protein (PTHrP) has been recognized as a major contributor to the pathogenesis of skeletal complications in breast cancer.4–6 Tumor-derived PTHrP stimulates osteoblast production of receptor activator of NF-κB ligand (RANKL), which binds to its cognate receptor RANK on osteoclast precursor cells, resulting in cellular differentiation and osteoclastic bone resorption. Release of soluble TGF-β from resorbed bone matrix in turn stimulates tumor cell production of PTHrP and other osteoclast-activating factors via Smad-dependent and Smad-independent pathways, further amplifying metastatic tumor osteolysis.7–9 Disruption of this feed-forward cycle could have significant therapeutic benefits in the prevention of bone loss associated with breast cancer.
Bone-specific therapeutics for the clinical management of bone metastases (bisphosphonates and denosumab) specifically target the osteoclast and are currently only approved for the treatment of established metastases. Since micrometastases are known to seed in the bone marrow of women even at early stages of the disease,10 a preventative treatment that blocks tumor-secreted osteolytic factors in the bone microenvironment prior to the development of clinically evident skeletal metastases may be beneficial, if safe therapeutics can be identified. Targeted treatment to reduce PTHrP secretion from tumor cells via blockade of TGF-β signaling in breast cancer metastasis is a promising therapeutic approach that has been pursued successfully in pre-clinical studies.5,11
In earlier studies, our laboratory demonstrated that blockade of PTHrP production from tumor-like rheumatoid synoviocytes prevented bone loss in a pre-clinical model of rheumatoid arthritis.12,13 Subsequently, it was demonstrated that curcuminoids, a class of phenols isolated from the dried rhizomes of turmeric (Curcuma longa L., Zingiberaceae; Figure 1A), have anti-arthritic and bone-protective effects in the same model and their mechanism of protection against cytokine-mediated bone resorption.14,15 In addition to in vivo evidence of inhibitory effects on RANK pathway activation of osteoclastic bone resorption in animal models,14,16 curcuminoids prevented PTHrP secretion in vitro from tumor-like synoviocytes isolated from the joints of rheumatoid arthritis patients.17 Collectively, these promising effects on metabolic bone diseases led us to hypothesize that PTHrP-mediated breast cancer-induced bone loss could be prevented by curcuminoid treatment.
Figure 1.
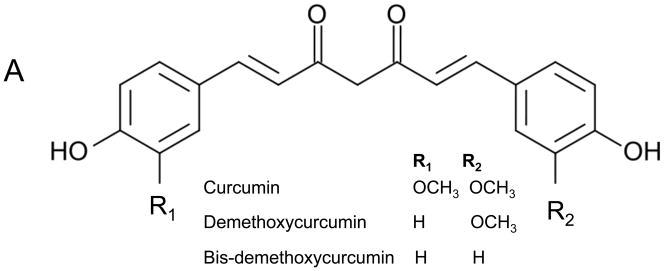
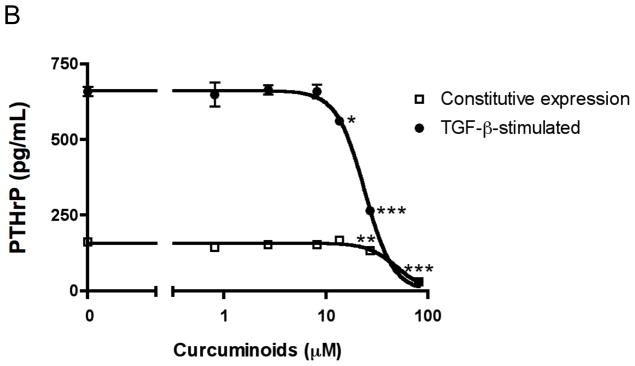
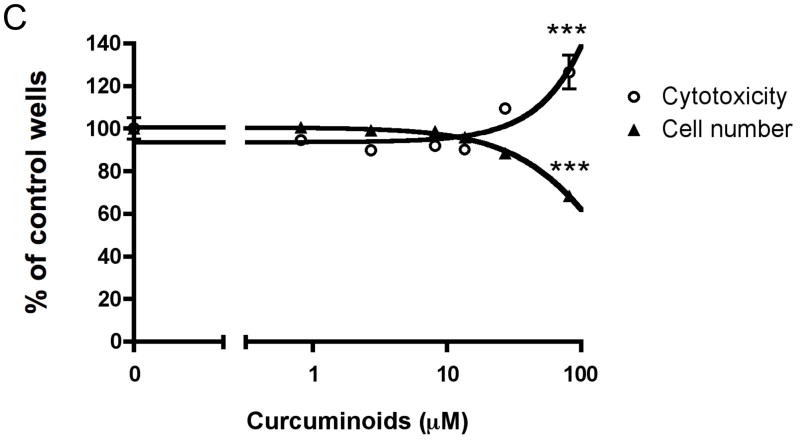
Curcuminoids inhibit MDA-MB-231 human breast cancer cell expression of parathyroid hormone-related protein (PTHrP) separate from effects on cell number and cytotoxicity. A. Chemical structures of curcuminoids are pictured. B. Subconfluent MDA-MB-231 cells were incubated overnight in fresh medium containing 10% serum and antibiotics at 37 °C and 5% CO2 in a humidified atmosphere. Cells (n = 4 wells/group) were pre-incubated with curcuminoids (0.1 – 300 μM) for 4 h followed by treatment with TGF-β (5 ng/mL) or medium alone (2% serum) for 24 h. PTHrP secretion was measured in conditioned media by commercial radioimmunoassay (Diagnostic Systems Laboratories; *p < 0.05, **p < 0.01, ***p < 0.001 relative to vehicle-treated control wells). C. Cell number and cytotoxicity were assessed under identical conditions in the presence of TFG-β by mitochondrial reduction of MTT (ATCC) and conditioned media content of lactate dehydrogenase (LDH; Promega), respectively.
Breast cancer survivors exhibit a strong pattern of usage of complementary and alternative medicinal treatments, and have shown a particular interest in natural product supplements and herbal remedies. Up to 40% of breast cancer survivors use herbal treatments,18,19 despite expressing concerns that their safety and efficacy are not well established. The pre-clinical studies described here aim to test the potential bone-sparing effects of curcuminoids, a polyphenolic mixture analogous to over-the-counter turmeric dietary supplements, in breast cancer metastasis to bone.
RESULTS AND DISCUSSION
Curcuminoids Inhibited PTHrP Secretion from Human Breast Cancer Cells Separate from Effects on Tumor Cell Growth or Cytotoxicity
While curcuminoids are known to prevent bone loss in pre-clinical models of rheumatoid arthritis and postmenopausal osteoporosis,14,16 their effects on cancer osteolysis or cancer cell secretion of osteolytic peptides had not been investigated prior to the studies reported herein. TGF-β-stimulated MDA-MB-231 breast cancer cell secretion of the osteolytic peptide parathyroid hormone-related protein (PTHrP) was inhibited dose-dependently by curcuminoids with a least effective concentration (LEC) of 13 μM (Figure 1B), while constitutive PTHrP secretion was also only decreased at the highest concentrations (Figure 1B). Curcuminoids inhibited TGF-β-stimulated PTHrP secretion with a half maximal inhibitory concentration (IC50 = 24 μM; Figure 1B) that was lower than the LEC for inhibition of cell viability and induction of cytotoxicity (LEC = 82 μM for both; Figure 1C), indicating that inhibitory effects on PTHrP secretion are independent of changes in cancer cell growth at low concentrations previously documented to be achievable in vivo in rodents and humans.20,21
Curcuminoids Inhibited Smad-Dependent TGF-β Signaling in Human Breast Cancer Cells
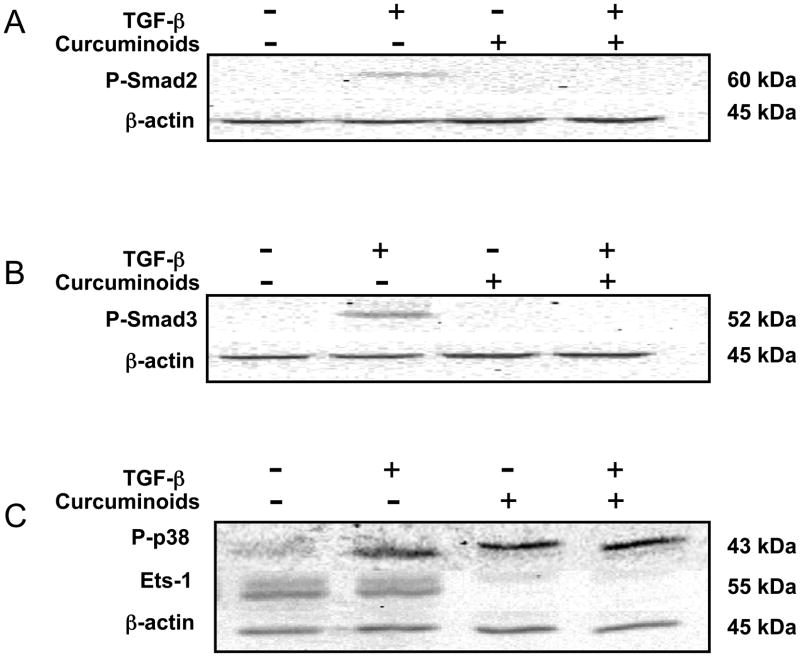
The mechanism by which curcuminoids inhibited TGF-β-stimulated secretion of the osteolytic factor PTHrP was explored. Smad-dependent and Smad-independent (p38 MAPK mediated) TGF-β-signaling pathways previously identified as regulating PTHrP expression in MDA-MB-231 cells were investigated by Western blot analysis.22,23 TGF-β induction of phospho (P)-Smad2/3 (Figure 2A,B; lane 2 vs. lane 1) was inhibited by curcuminoids (Figure 2A,B; lane 4). Ets-1, a proto-oncogene required for P-Smad2/3 activation of the PTHrP P3 promoter23 that is expressed constitutively in MDA-MB-231 cells (Figure 2C; lanes 1–2)24 was also decreased by curcuminoid treatment (Figure 2C; lanes 3–4). In contrast, Smad-independent22 TGF-β-induced phosphorylation of p38 MAPK (P-p38; Figure 2C; lanes 1–2) was not altered by curcuminoid treatment (Figure 2C; lanes 3–4). While TGF-β stimulates PTHrP expression via Smad-dependent and independent pathways (via mitogen-activated protein kinases)22,23 curcuminoids only inhibited the former by reducing both Smad phosphorylation and Ets-1 protein levels. These findings are consistent with previous reports of curcuminoid inhibition of Smad signaling in other non-breast cancer cell lines,25,26 and of curcuminoid inhibition of PKC-α,27,28 for which the constitutive expression in MDA cells drives production of the oncogenic transcription factor Ets-1.24 In addition to regulating PTHrP expression, Smads are involved in the expression of other TGF-β-mediated pro-metastatic and osteolytic genes in breast cancer, including interleukin (IL)-11, matrix metalloproteinases (MMPs) and connective tissue growth factor (CTGF).2 Though not evaluated in this study, it is possible that curcuminoids have inhibitory effects on the expression of other TGF-β-regulated osteolytic genes in breast cancer cells.
Figure 2.
Curcuminoids inhibit TGF-β-induced phosphorylation of Smad2 and Smad3 and constitutive expression levels of Ets-1. Subconfluent MDA-MB-231 cells were pre-incubated with curcuminoids (80 μM) for 12 h before the addition of TGF-β (5 ng/mL) for 1 h. A. Phospho (P)-Smad2, B. P-Smad3, C. P-p38 MAPK and Ets-1 protein contents were determined in 18 μg of whole cell lysates by Western blotting using chemiluminescence. An antibody raised against β-actin was used to verify normalization of protein loading. Data presented are representative of three individual experiments.
Curcuminoid Administration Prevented the Development of Osteolytic Bone Lesions While Not Altering Tumor Area Within Bone in Mice with Breast Cancer Bone Metastases
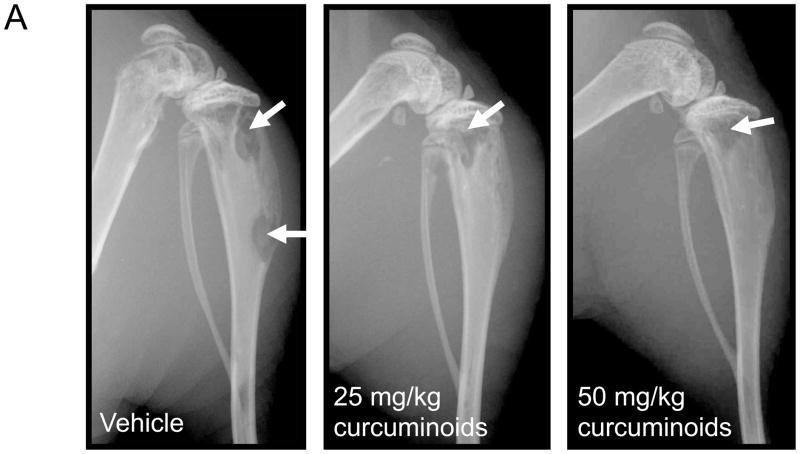
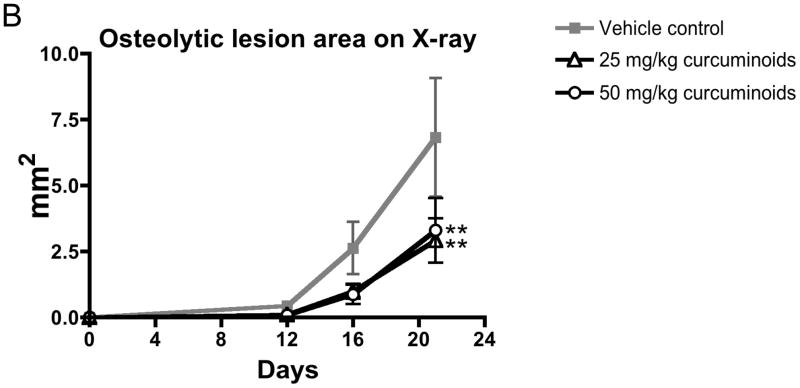
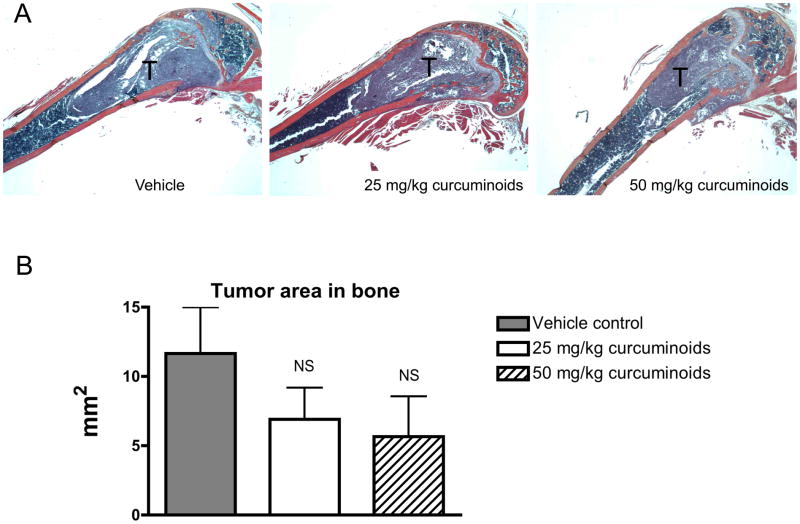
Having demonstrated that curcuminoids inhibited TGF-β-stimulated PTHrP secretion, a well-established murine model of PTHrP-driven breast cancer bone metastasis was used to evaluate effects on osteolytic lesion formation in vivo.5,22 Following intra-cardiac inoculation of MDA-MB-231 human breast cancer cells, osteolytic lesions were detected by X-ray in all treatment groups by day 16, with lesion area increasing by day 21. However, low- and high-dose curcuminoid treatment attenuated bone lysis relative to vehicle-treated controls, decreasing lytic bone lesion area by 57% and 51%, respectively (Figure 3A,B). Survival in cancer-bearing mice, whose body weights were no different between groups (Figure 3C), was improved with curcuminoid treatment relative to vehicle-treated control (Figure 3D). While tumor burden as determined on day 21 by histologic analysis of tumor area within bone (Figure 4A) tended to be lower in the curcuminoid-treated animals, this trend was not statistically significant (Figure 4B), in contrast to the >50% decrease in bone lysis. Consistent with the decrease in bone osteolysis, bone-resorbing osteoclasts at the bone-tumor interface (cell number/mm2 of interface) were reduced by 53% and 48% in the low and high dose curcuminoid-treated mice, respectively (Figure 4C). At the same time, there was no effect of curcuminoid treatment on bone-forming osteoblast cell numbers (data not shown). In toto, these data thus suggest that curcuminoid inhibition of TGF-β-stimulated PTHrP secretion by tumor cells in the bone microenvironment likely contributed to inhibition of osteoclast differentiation at the bone-tumor interface, resulting in decreased bone osteolysis independent of effects on local tumor growth within the bone.
Figure 3.
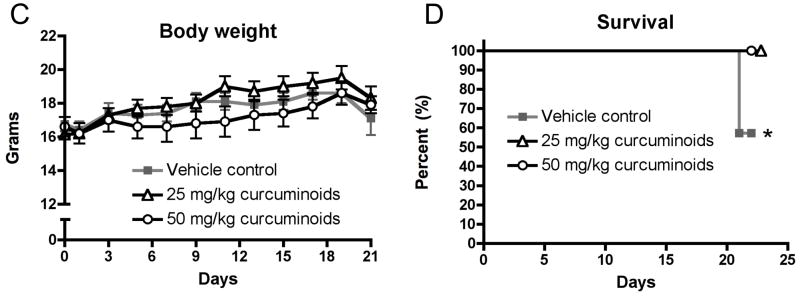
Curcuminoids prevent the development of osteolytic bone metastases. Four-week nude mice were inoculated with MDA-MBA-231 human breast cancer cells in the left cardiac ventricle and treated with vehicle or curcuminoids (25 or 50 mg/kg; n = 11/group) every other day for 3 weeks and followed by X-ray for the development of osteolytic lesion area. A. Representative x-rays are presented. B. Quantitative analysis of lesion area was evaluated on experimental days 12, 16 and 21. Data are expressed as means + SEM and differences were evaluated by two-way ANOVA with Bonferroni post-hoc testing (**p < 0.01 relative to vehicle-treated control). Data presented are representative of 3 separate experiments. C. Body weight is expressed as means + SEM and differences were assessed by two-way ANOVA (p > 0.05). D. Survival was assessed over time in the same mice and expressed in a Kaplan-Meier plot and analyzed by Logrank test for treatment effect (*p = 0.032).
Figure 4.
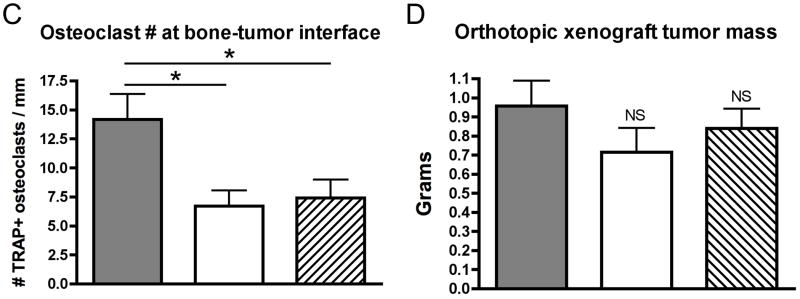
Effects of curcuminoid treatment on tumor area in bone, osteoclast cell number and orthotopic xenograft tumor growth are presented. A–C. Four-week nude mice were inoculated into the left cardiac ventricle with MDA-MB-231 human breast cancer cells and treated every other day for three weeks with vehicle or curcuminoids (25 or 50 mg/kg; n = 11/group). A. Representative histologic sections of the distal femur are presented and B. quantitative assessment of tumor area in the femur and tibia in mice treated with curcuminoids relative to vehicle-treated mice was evaluated at the time of harvest (d 21). C. TRAP+ multi-nucleated (>3) osteoclast cells were quantified at the bone-tumor interface (osteoclasts/mm) in the femur and tibia. D. In a separate experiment, the abdominal mammary fat pad of four-week nude mice (n = 7/group) was surgically visualized and inoculated with MDA-MB-231 cells. After tumor growth was established by palpitation, mice were randomly divided into groups and treated for three weeks with curcuminoids at 25 and 50 mg/kg or vehicle every other day ip. At the end of treatment, tumors were excised and weighed. Data are presented as means + SEM with significance determined by one-way ANOVA with Bonferroni post-hoc testing (*p < 0.05 relative to vehicle-treated control; NS = not significant).
Administration of Curcuminoids Did Not Decrease Orthotopic Xenograft Breast Cancer Tumor Mass in Mice
In order to confirm that effects of curcuminoids on osteolytic breast cancer metastasis were bone microenvironment-specific and likely independent of direct effects on tumor cell proliferation, the effect of the same dose and duration of curcuminoid treatment on tumor xenograft growth in the mammary fat pad was also examined. Using an identical dosing regimen as in the intra-cardiac studies, orthotopic tumor mass was not significantly different in curcuminoid-treated animals relative to vehicle-treated controls (Figure 4D). Results from these experiments, in combination with the lack of effect of this same dosing regimen on tumor burden in bone, thus confirm the hypothesis that local levels of curcuminoids achieved in vivo were sufficient to inhibit PTHrP secretion while not significantly altering cancer cell growth.
Curcuminoid Treatment Was Non-Toxic and May Hold Promise for the Prevention of Breast Cancer Osteolysis
As previously documented in other rodent models,14–16 doses of 25 and 50 mg/kg curcuminoids administered ip every other day had no toxic effects on the renal, hepatic or hemopoietic systems of mice as assessed by body weight (Figure 3C), liver weight, alanine aminotransferase (ALT), spleen weight, number of white blood cells or platelets, and hematocrit (Table 1).
Table 1.
Assessment of Treatment Toxicitya
| naïve-control | vehicle-control | 25 mg/kg curcuminoids | 50 mg/kg curcuminoids | |
|---|---|---|---|---|
| liver weight (g) | 1.06 ± 0.17 | 1.00 ± 0.10 | 0.987 ± 0.07 | 1.10 ± 0.03 |
| spleen weight (g) | 0.143 ± 0.03 | 0.134 ± 0.02 | 0.129 ± 0.01 | 0.139 ± 0.01 |
| hematocrit (%) | 35.1 ± 3.3 | 30.2 ± 1.5 | 30.6 ± 2.2 | 31.1 ± 1.7 |
| white blood cells (K/μL) | 4.56 ± 0.58 | 5.84 ± 1.3 | 4.69 ± 0.67 | 6.83 ± 0.77 |
| platelets (K/μL) | 503 ± 38 | 513 ± 54 | 485 ± 77 | 589 ± 43 |
| alanine aminotransferase (U/L) | 3.13 ± 1.4 | 7.04 ± 2.2 | 7.13 ± 1.4 | 5.02 ± 1.3 |
Effects of 3 weeks of vehicle and curcuminoid treatment on organ weights, cell counts and ALT in non-tumor-bearing mice are presented as means ±SEM with differences assessed by one-way ANOVA (p > 0.05).
One limitation of this study was the use of ip vs po curcuminoid dosing. Even so, correcting for body surface area and assuming 10% bioavailability, the human dose equivalent (HED) of curcuminoids found to be effective and safe in this pre-clinical study was 1.25 g every other day, a dose well within the range of doses previously documented to be safe in human trials corresponding to three capsules of a curcuminoid-enriched turmeric dietary supplement.14
In conclusion, bone is the most common site of breast cancer metastasis in women. Once breast cancer cells infiltrate the bone microenvironment, growth factors in bone, including TGF-β, stimulate tumor cell secretion of osteolytic factors, such as PTHrP, which drive localized osteoclastogenesis and bone resorption leading to pathologic fractures and bone pain. Despite evidence for multiple benefits of blockade of the osteoclast-activating peptide PTHrP in breast cancer metastasis,4 no agents targeting PTHrP are currently available for clinical use. The data presented in this study offer evidence for bone-protection conferred by curcuminoids by demonstrating for the first time their potent inhibitory effect on human breast cancer cell secretion of the osteolytic peptide PTHrP via inhibition of Smad-dependent TGF-β signaling in MDA-MB-231 cells. In vivo, curcuminoids inhibited osteolytic lesion formation in a PTHrP-mediated murine model of breast cancer bone metastasis. Taken together, these novel and encouraging findings justify the need for further studies to determine whether curcuminoids may hold promise for the clinical management of bone metastases in women with breast cancer.
EXPERIMENTAL SECTION
Chemical Analysis of Curcuminoids
A curcuminoid-enriched turmeric product, sold as “>98% curcumin,” but composed of 89.6% of the three major curcuminoids by weight (Figure 1A) was purchased from Fisher Scientific (#218580100, Lot A019754401). Chemical content of the curcuminoid product, which is analogous to commercially available turmeric dietary supplements14 was assessed as previously described using an Agilent 1100 series high performance liquid chromatography (HPLC) system (Agilent, Palo Alto, CA) and stock solutions of pure curcumin, demethoxycurcumin, and bis-demethoxycurcumin (curcuminoid content by weight: 76.9% curcumin, 17.6% demethoxycurcumin and 5.5% bis-demethoxycurcumin).15
Cell Cultures
Sub-confluent human breast cancer MDA-MB-231 cells were plated at 5 × 104 cells/cm2 and incubated overnight in fresh DMEM medium containing 10% fetal bovine serum (FBS) and antibiotics at 37°C and 5% CO2 in a humidified atmosphere. Cells were pre-incubated in DMEM medium containing 2% FBS with or without curcuminoids (0.1 – 100 μM) for 4 h, and the medium was then refreshed with the addition of recombinant human TGF-β1 (5 ng/mL; R&D Systems) for 24 h. PTHrP expression was measured in conditioned media by commercial radioimmunoassay (Diagnostic Systems Laboratories) and cell number and cytotoxicity were assessed by mitochondrial reduction of MTT (ATCC) and conditioned media content of lactate dehydrogenase (LDH; Promega), respectively, as per manufacturer’s protocol.
Western Blots
Whole cell extracts were prepared from subconfluent T-75 flasks of MDA-MB-231 cells treated for 12 h with curcuminoids (80 μM) prior to 1 h treatment with TGF-β (5 ng/mL) using radio- immunoprecipitation assay (RIPA) buffer (Sigma Aldrich) at 4 °C. Protein concentrations of each lysate were assayed by one-step colorimetric method (Bio-Rad) and 18 μg protein were resolved by SDS-PAGE. After electrophoresis, proteins were transferred to a polyvinylidene fluoride (PVDF) membrane (BioRad). Membranes were placed in blocking solution (1x Tris-buffered saline with 0.1% Tween 20 and 5% nonfat milk) for 1 h and immunoblotted with rabbit anti-phospho-Smad2 (Cell Signaling), anti-phospho-Smad3 (Cell Signaling), anti-Ets-1 (Santa Cruz Biotechnology, Inc) and anti-phospho-p38 kinase (Cell Signaling) at a 1:1000 dilution in 1x Tris-buffered saline with 0.1% Tween 20 and 5% bovine serum albumin overnight on a rocking platform at 4 °C. A rabbit anti-β-actin (Cell Signaling) antibody at a 1:2000 dilution in 1x Tris-buffered saline with 0.1% Tween 20 and 5% bovine serum albumin was used as a control to verify equivalent protein loading. After incubation, membranes were washed and incubated with appropriate horseradish peroxidase-conjugated secondary antibodies (Cell Signaling) for 1 h at room temperature in 1x Tris-buffered saline with 0.1% Tween 20 and 5% nonfat milk. Membranes were then washed and developed according to chemiluminescence protocols (Cell Signaling).
Intra-Cardiac Inoculation Procedure
All animal protocols described were approved by the Institutional Animal Care and Use Committee at The University of Arizona in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Four-week female athymic nude mice were purchased from Harlan Laboratories and housed in plastic cages in laminar flow isolated hoods with access to water and autoclaved mouse chow ad libitum. Mice were inoculated in the left cardiac ventricle with MDA-MB-231 tumor cells as previously described.5 Briefly, tumor cell inoculation was performed percutaneously into the left cardiac ventricle on anesthetized mice in a supine position with a 26-gauge needle attached to a 1 mL syringe containing 1 × 105 cells suspended in 0.1 mL sterile PBS. Visualization of bright red blood entering the hub of the needle in pulsatile fashion was indicative of correct needle placement into the left cardiac ventricle. After tumor inoculation, mice were divided randomly into three groups (n = 11), treated by intraperitoneal (ip) injection with 25 or 50 mg/kg curcuminoids or vehicle alone every other day beginning on day 1 for 21 days and followed prospectively for the development of bone lesions by radiography on days 12, 16 and 21 of the experiment. Mice were X-rayed in a prone position using a Digital Faxitron MS-20 with a digital camera at 4x magnification. Lytic lesion area, reported as total lesion area per animal in the hind limb long bones, was analyzed in a blinded fashion by three independent investigators using ImageJ 1.43u software (National Institutes of Health).
Bone Histology
Hindlimbs were removed from mice 21 days after intra-cardiac cell inoculation. Tissues were fixed in 10% neutral-buffered formalin for 48 h, decalcified in 10% EDTA for 2 weeks, processed in an automated tissue processor (Tissue-Tek® VIP®5, Sakura), and embedded in paraffin. Mid-sagittal 5 μm sections were stained with hematoxylin and eosin (H&E) with orange G and phloxine to visualize new bone, or for TRAP activity as previously described to visualize osteoclast cells.7 Sections were viewed on a Nikon Eclipse E600 compound microscope outfitted with a RS Photometrics CoolSNAP digital color camera and analyzed using ImageJ 1.43u software (National Institutes of Health). Total tumor area was measured in the mid-sections of tibiae and femora on H&E-stained sections at 10x magnification without knowledge of experimental groups, as previously described.7 Osteoclast cells were quantified in the mid-sagittal sections of tibiae and femora in tumor-bearing hindlimbs as previously described.7 Briefly, TRAP-positive multinucleated cells were quantified at 40x magnification along the perimeter of the tumor where the cancer cells interfaced directly with bone surfaces, and data were expressed as number of osteoclasts per mm of tumor-bone interface.
Orthotopic Tumor Xenograft Procedure
Tumor cell inoculation into the right abdominal mammary fat pad in the inguinal region was performed on anesthetized mice in a supine position. Briefly, a 5 mm incision was made to visualize the mammary fat tissue and a suspension of tumor cells (1 × 106 in 0.1 mL sterile PBS) was slowly injected into the mammary fat tissue using a 26-gauge needle attached to a 1 mL syringe. After palpable a tumor was formed, the mice were randomly divided into three groups (n = 7) and treated ip with 25 or 50 mg/kg curcuminoids or vehicle alone every other day for 21 days. Tumor mass was measured ex vivo at the time of harvest on day 21 of treatment.
Evaluation of Treatment Toxicity
In order to evaluate potential curcuminoid treatment toxicities, four-week nude mice were dosed with vehicle or curcuminoids (25 or 50 mg/kg every other day for 21 days) and compared to naïve control mice (n = 6). At the termination of 3 weeks of dosing, the liver and spleen were weighed and circulating white blood cell counts were determined using a Hemavat 880 analyzer (CDC Technologies, Oxford, CT). Cell differentials were determined by manual counting. Serum alanine aminotransferase (ALT) levels were measured using Endocheck Plus chemistry analyzer (Hemagen Diagnostics, Columbia, MD) to monitor for possible treatment effects on liver function.
Statistical Analysis
The Kaplan-Meier survival plot was analyzed by Logrank Test with post-hoc Logrank Test for trend. Differences between means were determined by one- or two-way ANOVA, as appropriate, with Bonferroni post hoc test using Instat software (Graphpad; San Diego, CA). Half maximal inhibitory concentrations (IC50) were obtained by analyzing concentration-response data using a four-parameter logistic equation without assignment of a minimal value (Prism 4.0 software, GraphPad).
Acknowledgments
This work was supported by the National Institutes of Health grants F31AT004875 to L.E.W., and R21AT005145 to J.L.F. from the National Center for Complementary and Alternative Medicine and the Office of Dietary Supplements, R01CA69158 and U01CA143057 to T.A.G. from the National Cancer Institute, as well as grants from the Indiana Economic Development Fund to T.A.G., and the Susan G. Komen Foundation (T.A.G.). The content presented is solely the responsibility of the authors and does not necessarily represent the official views of our funding agencies. In loving memory of Marlin and Sandy Neil.
Footnotes
Notes
The authors declare no competing financial interest.
References
- 1.Hortobagyi GN, Piccart-Gebhart MJ. Semin Oncol. 1996;23:1–5. [PubMed] [Google Scholar]
- 2.Wielbaecher KN, Guise TA, McCauley LK. Nat Rev Cancer. 2011;11:411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jimenez-Andrade JM, Mantyh WG, Bloom AP, Ferng AS, Geffre CP, Mantyh; PW. Ann N Y Acad Sci. 2010;1198:173–181. doi: 10.1111/j.1749-6632.2009.05429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mundy GR. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 5.Guise TA, Yin JJ, Taylor SD, Kumagai Y, Dallas M, Boyce BF, Yoneda T, Mundy GR. J Clin Invest. 1996;98:1544–1549. doi: 10.1172/JCI118947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guise TA. Cancer. 2000;88(12 Suppl):2892–2898. doi: 10.1002/1097-0142(20000615)88:12+<2892::aid-cncr2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 7.Yin J, Selander K, Chirgwin JM, Dallas M, Grubbs BG, Wieser R, Massague J, Mundy GR, Guise TA. J Clin Invest. 1999;103:97–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang Y, He W, Tulley S, Gupta GP, Serganova I, Chen CR, Manova-Tordorova K, Blasberg R, Gerald WL, Massague J. Proc Natl Acad Sci U S A. 2005;102:13909–13914. doi: 10.1073/pnas.0506517102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacLeod RJ, Chattopadhyay N, Brown EM. Am J Physiol Endocrinol Metab. 2003;284:E435–442. doi: 10.1152/ajpendo.00143.2002. [DOI] [PubMed] [Google Scholar]
- 10.Hussein O, Komarova SV. J Cell Commun Signal. 2011;5:85–99. doi: 10.1007/s12079-011-0117-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Karaplis AC, Huang DC, Siegel PM, Camirand A, Yang XF, Muller WJ, Kremer R. J Clin Invest. 2011;121:4655–4669. doi: 10.1172/JCI46134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Funk JL, Cordaro LA, Wei H, Benjamin JB, Yocum DE. J Clin Invest. 1998;101:1362–1371. doi: 10.1172/JCI484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Funk JL, Chen J, Downey KJ, Davee SM, Stafford G. Arthritis Rheum. 2003;48:1721–1731. doi: 10.1002/art.10985. [DOI] [PubMed] [Google Scholar]
- 14.Funk JL, Frye JB, Oyarzo JN, Kuscuoglu N, Wilson J, McCaffrey G, Stafford G, Chen G, Lantz RC, Jolad SD, Solyom AM, Kiela PR, Timmermann BN. Arth Rheum. 2006;54:3452–3464. doi: 10.1002/art.22180. [DOI] [PubMed] [Google Scholar]
- 15.Funk JL, Oyarzo JN, Frye JB, Chen G, Lantz RC, Jolad SD, Solyom AM, Timmermann BN. J Nat Prod. 2006;69:351–355. doi: 10.1021/np050327j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright LE, Frye JB, Timmermann BN, Funk JL. J Agric Food Chem. 2010;8:9498–9504. doi: 10.1021/jf101873f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frye JB, Timmermann BN, Funk JL. BMC Complementary and Alternative Medicine. 2012;12(Supplement):p55. [Google Scholar]
- 18.Boon HS, Olatunde F, Zick SM. BMC Womens Health. 2007;7:4. doi: 10.1186/1472-6874-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthews AK, Sellergren SA, Huo D, List M, Fleming G. J Altern Complement Med. 2007;13:555–556. doi: 10.1089/acm.2007.03-9040. [DOI] [PubMed] [Google Scholar]
- 20.Begum AN, Jones MR, Lim GP, Morihara T, Kim P, Heath DD, Rock CL, Pruitt MA, Yang F, Hudspeth B, Hu S, Faull KF, Teter B, Cole GM, Frautschy SA. J Pharmacol Exp Ther. 2008;326:196–208. doi: 10.1124/jpet.108.137455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vareed SK, Kakarala M, Ruffin MT, Crowell JA, Normolie DP, Djuric Z, Brenner DE. Cancer Epidemiol Biomarkers Prev. 2008;17:1411–1417. doi: 10.1158/1055-9965.EPI-07-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kakonen SM, Selander KS, Chirgwin JM, Yin JJ, Burns S, Rankin WA, Grubbs BG, Dallas M, Cui Y, Guise TA. J Biol Chem. 2002;277:24571–24578. doi: 10.1074/jbc.M202561200. [DOI] [PubMed] [Google Scholar]
- 23.Lindemann RK, Ballschmeiter P, Nordheim A, Dittmer J. J Biol Chem. 2001;276:46661–46670. doi: 10.1074/jbc.M105816200. [DOI] [PubMed] [Google Scholar]
- 24.Lindemann RK, Braig M, Ballschmeiter P, Guise TA, Nordheim A, Dittmer J. Int J Oncol. 2003;22:799–805. [PubMed] [Google Scholar]
- 25.Smith MR, Gangireddy SR, Narala VR, Hogaboam CM, Standiford TJ, Christensen PJ, Kondapi AK, Reddy RC. Am J Physiol Lung Cell Mol Physiol. 2010;5:L616–625. doi: 10.1152/ajplung.00002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu Y, Liang H, Du Y, Zhu Y, Wang X. Am J Nephrol. 2010;31:332–341. doi: 10.1159/000287230. [DOI] [PubMed] [Google Scholar]
- 27.Semsri S, Krig SR, Kotelawala L, Sweeney CA, Anuchapredda S. FEBS Lett. 2011;585:2235–2242. doi: 10.1016/j.febslet.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 28.Perez-Lara A, Corbalan-Garcia S, Gomez-Fernandez JC. Arch Biochem Biophys. 2011;513:36–41. doi: 10.1016/j.abb.2011.06.010. [DOI] [PubMed] [Google Scholar]