Abstract
Importance of the field
Organ failure and tissue loss are challenging health issues due to widespread injury, the lack of organs for transplantation, and limitations of conventional artificial implants. The field of tissue engineering aims to provide alternative living substitutes that restore, maintain or improve tissue function.
Areas covered in this review
In this paper, a wide range of porous scaffolds are reviewed, with an emphasis on phase separation techniques that generate advantageous nanofibrous 3D scaffolds for stem cell-based tissue engineering applications. In addition, methods for presentation and delivery of bioactive molecules to mimic the properties of stem cell niche are summarized. Recent progress in using these bio-instructive scaffolds to support stem cell differentiation and tissue regeneration is also presented.
What the reader will gain
Stem cells have great clinical potential because of their capability to differentiate into multiple cell types. Biomaterials have served as artificial extracellular environments to regulate stem cell behavior. Biomaterials with various physical, mechanical, and chemical properties can be designed to control stem cell development for regeneration.
Take home message
The research at the interface of stem cell biology and biomaterials has made and will continue to make exciting advances in tissue engineering.
Keywords: Drug delivery, growth factor, nanofibrous scaffold, phase separation, stem cell, surface modification, tissue engineering
1. Introduction
The limitations of artificial implants as well as the shortage of organ transplants have intensified the research in the field of tissue engineering to develop biological substitutes that restore, maintain, or improve tissue function1. Through the biomedical translation of multiple fields including chemistry, physics, materials science, biology, and medicine, various technical innovations were created to provide new strategies for tissue engineering. Recent innovations include stem cell harvest2 and transplantation methods, biomaterials for control of physical and chemical cell environment, high-throughput technologies for development of new functional materials, drug delivery strategies for controlled release of growth factors and biomolecules3, as well as sophisticated surgical therapies. In a typical tissue engineering approach, cells are grown in a three-dimensional scaffold, where both cells and materials play a key role in de novo tissue regeneration and therefore are under intense investigation4.
Embryonic stem cells are an attractive cell source for cell replacement therapies and regenerative medicine by virtue of their ability to differentiate into any adult cell type5. Adult stem cells, although limited to certain lineages, are also an attractive cell source for both immunological and ethical reasons6. Other alternatives include amniotic fluid stem cells, which can give rise to multiple lineages and are potentially useful for a variety of therapeutic applications with low risk of tumorigenicity2. Stem cell development is closely related to the natural stem cell niche, which provides mechanical, chemical, and topological cues and initiates a series of complex signaling events to determine stem cell fate (mitotic dormancy, self-renewal, or differentiation into a specific lineage). Extensive research has been dedicated to understanding the molecular mechanisms underlying stem cell fate, in order to better control the homogeneous differentiation of the ES cells prior to transplantation, which is critical to tissue formation, such as to prevent the otherwise teratoma formation in vivo7. Thus, an artificial stem cell niche is critical for exploiting their therapeutic potential in stem cell-based tissue engineering. Researchers are designing biomaterials to mimic the natural stem cell microenvironment that deliver stem cells with precise control to achieve this goal. Biomaterials with various chemical composition and physical properties8 such as mechanical strength, topology etc., have been investigated to control biological response and ES cell differentiation with a bio-instructive extracellular environment. For example, naturally derived or genetically engineered silk has been functionalized to serve as biocompatible scaffold materials to provide excellent mechanical properties and found to promote stem cell adhesion, proliferation, and differentiation in vitro9. Hydrogels with tunable physical and chemical properties in time and space10, as well as spatially-controlled distribution of bio-stimuli11, have been investigated to positively direct and influence stem cell fate7,12. Controlled spatial and temporal gradients of agents were produced by controlled release technology and microfluidic devices in fabricating tissue hierarchical structures3,13. Under the broad range of tissue engineering strategies, we will mainly focus on important technologies for biomaterials fabrication into porous scaffolds as well as recent progress on biomaterial-guided stem cell development, followed by perspectives on the future of stem cell-based tissue engineering.
2. Biomaterials for tissue engineering
Ideally, biomaterials synthesized for tissue engineering should perform the structural and biochemical functions of the natural extracellular matrix (ECM), which provides cells with topological, chemical and mechanical cues via its three-dimensional structure, until the cell-produced ECM takes over14. Thus, materials that can form porous solid-state 3D structures are commonly utilized in tissue engineering to maintain predesigned tissue structure, while at the same time are biodegradable in a fashion that matches the cell modeling/remodeling rate and neo tissue formation process. Biocompatibility and bioactivity, in addition, are desirable to facilitate and enhance cell adhesion, proliferation, migration, and differentiation. Lastly, material stiffness can also direct differentiation, often influencing material choice15.
Polymeric materials for scaffolding can be generally categorized into naturally derived and synthetic polymers. Naturally derived polymers, e.g., protein and polysaccharides, have been used for tissue regeneration with the potential advantage of biological recognition that might support cell development. Collagen, as the main component of extracellular matrix of mammalian tissues, has been employed for tissue repair applications as cell-carrying vehicles and scaffold materials. Collagen offers suitable surface chemistry for cell growth and differentiation16. However, natural collagen brings concern over potential immunogenicity and pathogen transmission, as well as poor mechanical properties, biodegradability and handling. As a result, synthetic polymers receive considerable attention and are employed as biomaterials in tissue engineering by virtue of their flexibility in composition and fabrication for specific needs. For instance, biodegradability and biological activity can be imparted to polymers via chemical, physical or/and processing techniques.
Poly(α-hydroxy acids), including poly(lactic acid) (PLA), poly(glycolic acid) (PGA), and their copolymer poly(lactic-co-glycolic acid) (PLGA), which are among the few Food and Drug Administration (FDA) approved synthetic materials for certain human clinical applications (e.g. degradable sutures, stents, wound dressings), are among the most widely used synthetic polymeric materials in scaffold fabrication17–19. These polyesters degrade via hydrolysis of the ester bonds, releasing acidic breakdown products that lead to autocatalytic degradation20, and eventually breaking into oligomers or monomers that can enter the metabolic pathways. PGA degrades in several weeks due to its hydrophilic nature21,22, resulting in weak mechanical properties. PLA has a much slower degradation rate with the introduction of a methyl group23,24, which makes the polymer more hydrophobic and maintains mechanical strength over a longer period of time in vitro or in vivo. PLGA, with controllable LA/GA ratio in the copolymer, can be tailored to satisfy the needed degradation rate and mechanical properties. Some other linear aliphatic polyesters, including poly(ε-caprolactone) (PCL)25 and poly(hydroxyl butyrate) (PHB)26, are less popular as scaffold materials due to their slower degradation, but more attractive as long-term implants and controlled release vehicles.
Some other synthetic polymers are also utilized in scaffold fabrication with their own advantages. Poly(urethane)s (PU) can offer a wide range of mechanical properties27,28, and the recently developed degradable PU could overcome the issue of PU’s slow degradability29. Poly(phosphazene)s emerge as a promising class of biomaterials with their flexibility in chemical composition and thus the well-controlled properties30. Poly(anhydride)s and poly(ortho esters) are also attractive, especially in controlled drug delivery, by virtue of their surface erosion properties31,32. Polyethers like polyethylene glycol (PEG), a widely used hydrogel, are also used in drug delivery33 and stem cell encapsulation12 applications. With the rapid development of biomaterials, combinatorial methodology has been used to evaluate cell-material interactions to accelerate the development process in finding the optimal materials for specific applications34.
In addition to chemical modification of polymers for desirable characteristics, composite materials are designed to meet the various requirements in tissue engineering that single polymers cannot fulfill. For example, hydroxyapatite (HAP), which mimics natural bone mineral and provides mechanical and osteoconductive properties, has been widely utilized as the mineral phase in composite materials design in combination with polymers including PCL, PLLA, PGA etc, for bone tissue engineering applications35,36.
3. Nanofibrous (NF) biomaterials
Scaffolds with nanofibrous structure mimic the collagen physical structure and were found to enhance cell/matrix interactions37,38. Our group has developed a novel phase separation technique to construct NF scaffolds in a three dimensional fashion from a variety of synthetic and naturally- derived materials, which can be combined with other scaffold processing techniques to introduce additional desirable structures and properties, including predesigned well-controlled macro-pore/channel/tubular structures, designed 3D overall shapes, as well as bioactive molecule delivery capability37,39–41.
3.1 Preparation of NF matrix
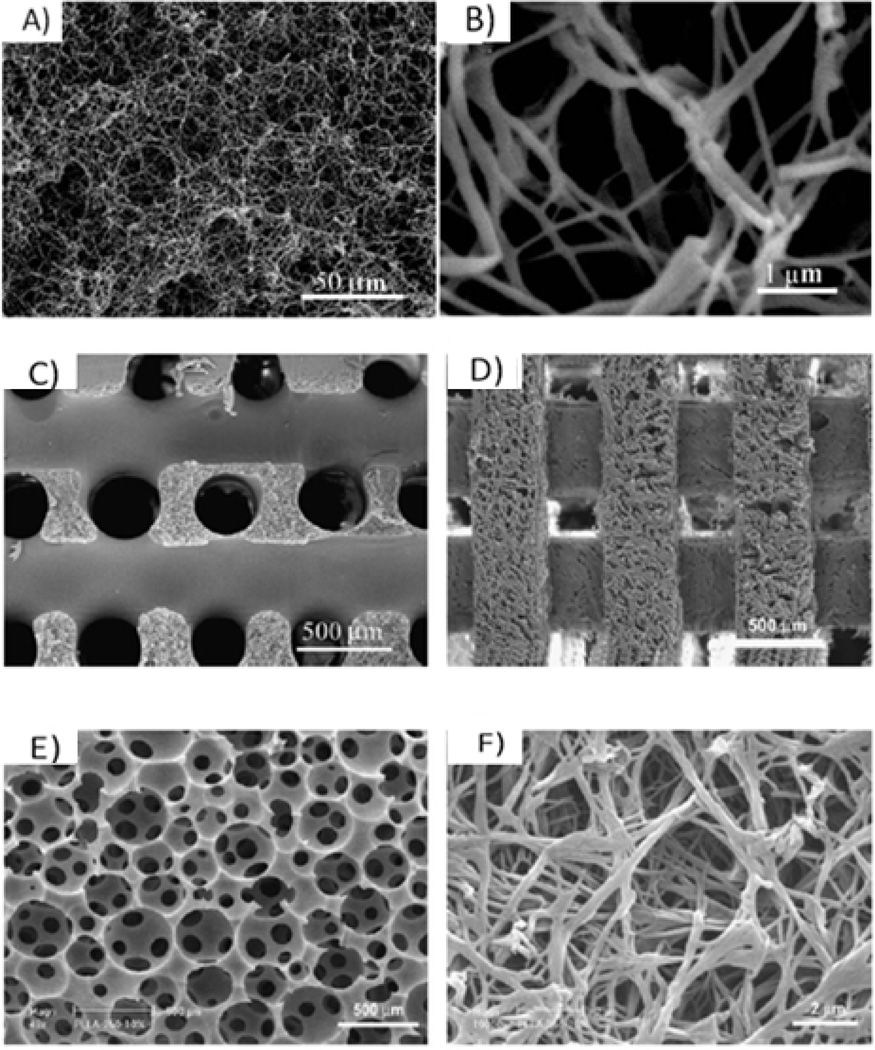
Phase separation is a thermodynamic process in which a homogeneous multi-component system separates into multiple phases to reach lower system free energy. In the case of polymer solution, phase separation can be induced either thermally or by adding a non-solvent, resulting in the formation of a polymer-rich and a polymer-lean phase. Upon solvent sublimation, polymer-rich phase solidifies to form the polymer foam while the polymer-lean phase becomes the void space14. A novel thermally induced phase separation (TIPS) has been developed by our group to generate NF structures40–43. In one example, PLLA solution was thermally induced to phase separate into nanofibers42 (Figure 1A and B). With the removal of the solvent by extraction, sublimation or evaporation, highly porous (such as 98%) PLLA foam was generated with continuous 3D NF structure42. The fiber diameter ranges from 50 to 500 µm, similar to natural collagen fibers. By virtue of the much higher surface to volume ratio as compared to control porous PLLA materials, the degradation rate is significantly faster, indicating the nanofiber effect on hydrolytic degradation of the polymer scaffolds44.
Figure 1.
scanning electron micrographs (SEM) of: a PLLA NF scaffold prepared from 2.5% PLLA/THF solution at a phase separation temperature of 8 °C with magnification of 500× (A) and 20 000× (B); 3D macroporous NF PLLA scaffolds; (C) prepared from sugar fiber template leaching and phase separation; (D) prepared from SFF and phase separation; and (E), (F) prepared from sugar sphere template leaching and phase separation. (A) (B) From Ma and Zhang42, Copyright © 1999 by John Wiley & Sons. Reprinted with permission of John Wiley & Sons. (C) From Zhang and Ma39, Copyright © 2000 by John Wiley & Sons. (D) From Chen et al.30, Copyright © 2006 by Elsevier. Reprinted with permission of Elsevier. (E), (F) From Wei and Ma49, Copyright © 2006 by John Wiley & Sons. Reprinted with permission of John Wiley & Sons.
Recently a series of biodegradable amphiphilic poly(hydroxyalkyl (meth)acrylate)-graft-poly(L-lactic acid) (PHAA-g-PLLA) copolymers has been synthesized and phase-separated into NF scaffolds45. These copolymers can be further functionalized with bioactive moieties to enhance bioactivity. In addition, these copolymers are more hydrophilic, thus degrade faster than the PLLA homopolymer, showing potential advantages in tissue engineering. In general, this technique expands the applicability of the phase separation techniques to materials with different chemistry in fabricating an NF network.
Gelatin, derived from collagen, has similar chemical composition as collagen and has also been phase-separated into highly porous NF matrix in a recent work41. These scaffolds mimic the natural collagen both in physical structure and chemical composition and might provide better environments for cells in a variety of tissue engineering applications.
Besides 3D NF structures, phase separation technique has also been employed to engineer other desirable micro-structures for tissue engineering. In one case, by phase separation of PLLA/PDLLA or PLLA/PLGA blends and particulate leaching, we have developed macroporous polymer scaffolds with partially NF pore wall architectures46. These partially NF scaffolds can be potentially utilized in studying cellular responses to different pore surface architectures co-presented in a 3D environment, which might provide insights in guiding cell/matrix interactions, and are potential candidates for various tissue engineering scenarios, such as co-culturing multiple cells that have different preferences of surface architectures. By creating a temperature gradient during phase separation process, scaffolds with oriented tubular architecture at the micro-scale and fibrous structure at the nano-scale were obtained and are excellent biomimetic scaffolds in regeneration of tissues with oriented cellular structure, such as dentin, blood vessels etc47,48.
3.2 Preparation of NF matrix with designed macro-pore structure
Several fabrication techniques, including particulate leaching and reverse solid free form (SFF) methods, have been combined with TIPS techniques to build pre-designed macro-/micro-pore networks within the NF matrix39,40,42,43,49. The pore network facilitates cell seeding process as well as mass transport, vascularization, and tissue organization. For example, highly porous, biodegradable polymer scaffolds seeded with myoblasts, embryonic fibroblasts and endothelial cells facilitate the induction of vessel networks50. Porous structure could be obtained by the assembly of sugar fibers as a 3D geometric porogen structure, where polymer solution was cast and phase separated39 (Figure 1C). As another example, solid free form technique has been developed to construct wax mold with designed micro-structure used as a negative replica for NF scaffold fabrication to build micro-channels40 (Figure 1D). Likewise, sugar spheres with desired diameter can be assembled into negative replica of the scaffold for polymer solution casting and phase separation to control pore size49. The resulting spherical macro-pore structure can reach different inter-pore opening sizes via manipulating the assembly conditions (Figure 1E and F). Therefore, various processing techniques could be combined with phase separation technique to generate NF scaffolds with diverse pre-designed micro-environment for different tissue engineering scenarios.
Electrospinning has also been applied to fabricate natural51,52 and synthetic materials53–55 into nanofibers, as well as microfibers (>1 µm) in a quick and simple fashion56. Additionally, biomolecules such as proteins and hydroxyapatite57 can be incorporated into the fibrous materials during the electrospinning process. Thus, electrospun NF biomaterials have been widely investigated in tissue engineering applications, while challenges still exist in constructing complex 3D scaffolds.
3.3 Preparation of NF matrix with designed 3D shape
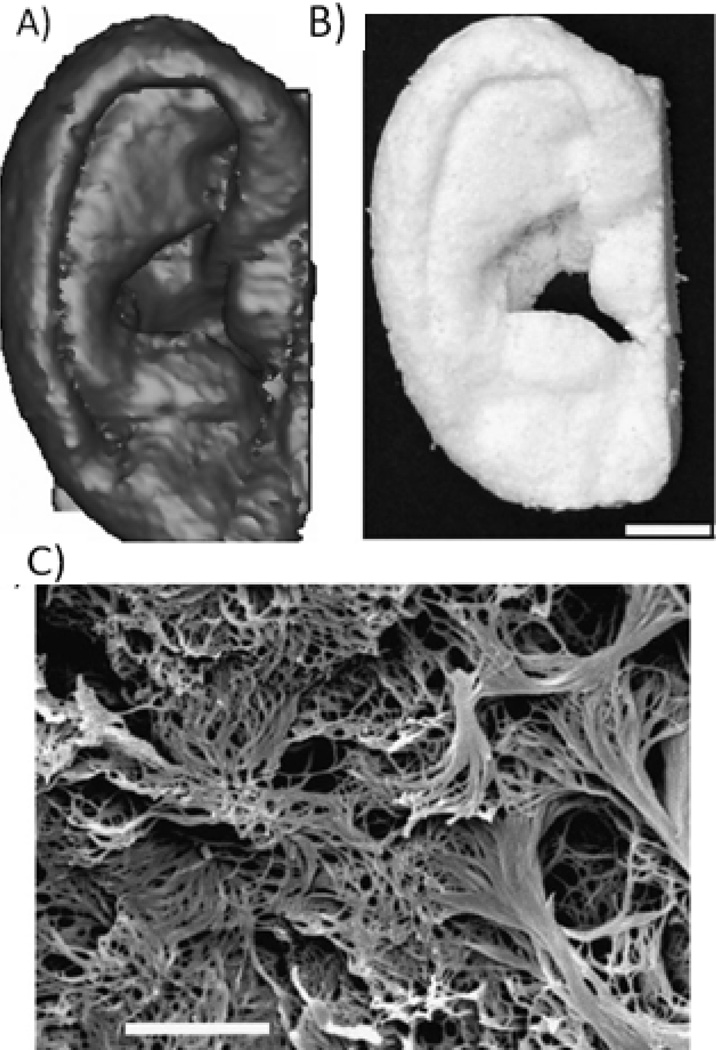
2D culture of cells in vitro provides therapeutic solutions to several types of tissues, such as skin and corneal tissues. In one example, cell sheets composed of autologous oral mucosal epithelium were engineered using a temperature-sensitive surface for the reconstruction of the corneal surface58. However, for engineering complex 3D tissues, a 3D scaffold is needed. In these cases, the 3D overall shapes of the fabricated scaffolds are of significant importance especially in certain types of tissue regeneration, e.g., vascular engineering, as well as in clinical trials where patient-specific scaffold designs are needed. However, many current scaffolding methods lack the control over the 3D shapes due to the restriction caused by the characteristics of the methods themselves, such as electrospinning. This problem was tackled by combining phase separation techniques and computer assisted design and computer assisted manufacture (CAD/CAM) techniques40. Computed-tomography scans of histological sections of human anatomical parts were utilized by CAD in designing and constructing the 3D shapes of the wax mold, thus controlling the external shape of the resulting scaffolds. NF Scaffolds with several 3D shapes, e.g., ear (Figure 2), a human mandible segment and hand digit bone, as well as pre-designed pore structure were successfully fabricated using the CAD/CAM techniques, phase separation and particulate leaching methodologies40,59.
Figure 2.
NF scaffolds created from 3D medical images and a phase-separation technique. A) human ear template reconstructed from histological sections; B) resulting NF scaffold of the human ear (scale bar: 10 mm); C) the nanofibrous pore wall morphology (scale bar: 5µm). From Chen et al.40, Copyright © 2006 by Elsevier. Reprinted with permission of Elsevier.
In vascular tissue engineering, tissue-engineered vascular grafts that resemble the natural vascular tissue structure and function are desirable, especially in small-diameter blood vessel engineering (e.g., coronary and peripheral vessels), where the non-degradable polymer-based grafts successfully used in large-diameter blood vessel replacement might cause poor patency due to thrombosis and hyperplasia60–62. TIPS technique has been employed together with porogen leaching techniques to create biodegradable highly porous NF PLLA scaffolds with tubular 3D shapes for vascular tissue engineering47. By using materials with different conductivities to construct the tubular mold for scaffolding, a temperature gradient was thus created during solid-liquid phase separation process that led to the creation of gradient micro-tube structure on the micrometer scale with controllable orientation direction (axially or radially oriented)48. NF structure could be maintained by using a mixed solvent system.
4. Composite scaffolds with nano-structure biomaterials
Mineralized tissues, e.g., bone and dentin, are organic/inorganic nanocomposites. In natural bone, the nano-structure of collagen fibers and apatite crystals give rise to a mechanically strong and tough nanocompostite. Thus, in developing biomaterials for bone tissue regeneration, nanocomposites are advantageous to mimic both the structure and composition of natural bone.
Hydroxyapatite (HAP) and calcium phosphate are inorganic compounds similar to the inorganic component in natural bone and are often employed in composite scaffold for bone regeneration, which provide osteoconductivity and bone bonding capability63–65. Polymers, on the other hand, usually served as the organic component to construct a continuous 3D structure with high surface area and porosity36. By blending and phase separation techniques, PLLA/HAP and PLGA/HAP composite scaffolds were created with improved mechanical properties and osteoconductivity23,66. Osteoblastic cell culture showed enhanced cell seeding uniformity and expression of bone markers osteocalcein (OCN) and bone sialoprotein (BSP) in the composite scaffolds67.
In addition to mimicking the chemistry of the bone matrix, polymer/nano-HAP scaffolds have been developed to mimic the nano-size mineral features, which improved not only the mechanical properties and protein absorption, but also cell adhesion and function consequently, as compared to the micro-sized HAP/polymer scaffolds68. In addition, the introduction of nano-HAP did not alter the scaffold structure compared to using micro-sized HAP.
In order to further improve cell-matrix interactions, which occur on the surface of the scaffolds, a biomimetic approach has been developed to grow bone-like apatite nano-particles on porous polymer scaffolds in a simulated body fluid (SBF) while maintaining the scaffold bulk structures and properties35,69,70. The grown HAP particles have nano sizes and a more similar chemical composition to natural bone apatite (Ca/P ~ 1.5) than the stoichiometric HAP crystals (Ca/P ~ 1.67). These partially carbonated apatites degrade faster to facilitate new bone modeling and remodeling. The growth of HAP particles is affected by the scaffold morphology as well as the ionic concentration and pH value of the SBF71.
To further mimic the NF structure of collagen in bone tissues, NF PLLA scaffolds with predesigned macro-pore structure developed by phase separation and particulate leaching were investigated for bone-like apatite deposition in SBF49. Large amounts of apatite were found on the surface of scaffold without compromising the porous structure using a suitable incubation time in SBF. Recently, with the development of NF gelatin porous scaffolds, apatite was also grown onto the surface of the gelatin NF scaffolds, resulting in NF gelatin/HAP porous scaffolds that mimic both the physical and chemical properties of natural bone ECM72. Additionally, electrodeposition is developed recently as another straightforward and versatile method for accelerated mineral deposition onto scaffold surfaces73.
5. Bioactive biomaterials
5.1 Surface modification
While improved surface bioactivity of the synthetic scaffolds is desirable to facilitate cell recognition and ultimately affect the tissue formation, there are currently limited effective surface modification methods, especially for 3D scaffolds. Surface patterning can be used to control cell shape and therefore their fate74. Plasma treatment was employed to modify poly(3-hydroxybutyrate) (PHB) thin films using low pressure ammonia, which had enhanced hydrophilicity and could be further conjugated to protein via the introduced amine groups75. However, this method could only be applied to 2D films or very thin 3D constructs because of the limited plasma penetration.
A few techniques were developed to introduce bioactive molecules onto the internal surfaces of scaffolds with 3D porous structure, along with the apatite modification mentioned above. In one method, a pre-fabricated scaffold was immersed in a gelatin solution in a solvent mixture (e.g., dioxane as the solvent of the scaffold material, and water as the non-solvent)76. After the pore surfaces of the scaffold swelled to a certain degree, so that the gelatin molecules were at least able to partially penetrate into the pore surface layer, the scaffold was moved to water (non-solvent) to solidify, resulting in the permanent entrapment of gelatin onto the scaffold that are stable in aqueous medium.
A porogen-induced surface modification technique was also developed77. The modifying agent, chosen as gelatin, was prepared as porogen. The surface modification was carried out at the same time while the scaffold was fabricated. First, gelatin spheres were assembled into a 3D negative replica of the scaffold, followed by polymer solution casting and phase separation processes. Polymer solution was prepared using a solvent mixture, which contained the solvent for gelatin (water in this case), so as to physically entrap gelatin molecules during the casting procedure.
An electrostatic layer-by-layer self-assembly process has also been developed by our group to modify the pore surface of 3D NF scaffolds78. Polymer scaffolds with macro-porous NF structure were fabricated in advance, which were then activated in an aqueous poly(diallyldimethylammonium chloride) (PDAC) solution to build positively-charged pore surfaces. The activated scaffolds were then immersed into the negatively charged biomolecules (e.g., gelatin) solution. After repeating these two steps several times, the surface modification layer with a controlled thickness was built on the pore surfaces of the scaffold, and found to improve scaffold hydrophilicity and enhance cell adhesion and proliferation within the scaffold.
5.2 Bioactive molecule delivery
Biological signaling is a key component for regulating tissue regeneration, especially in the repair of defects of a large size, where endogenous signaling molecules are often not sufficient in terms of type or quantity, making the addition of exogenous signaling molecules necessary4. Due to the short half-lives of proteins and peptide drugs, as well as cells’ sensitivity to the protein/peptide concentration, controlled delivery of bioactive molecules is desirable. In addition, growth factor delivery systems may be employed to induce the differentiation of stem cells and subsequent neo tissue morphogenesis79.
Polymer particulate carriers have been shown to effectively release the encapsulated drugs in a controlled fashion and maintain their bioactivity post administration80–82. These carriers are often made from biodegradable polyesters such as PLLA and PLGA due to their excellent biocompatibility and controllable biodegradability as discussed earlier. A double emulsion technique has been utilized to fabricate growth factor encapsulated micro/nano-spheres81. The size of the spheres was controlled by varying the double emulsion parameters such as surfactant amount and emulsion strength, while the release kinetics was controlled by adjusting the copolymer (PLGA) composition and molecular weight. Sustained protein release over days to months was achieved while the bioactivity was maintained in a high level. From our study, recombinant human platelet-derived growth factor BB (rhPDGF-BB) released from PLGA nanospheres was biologically active and was able to stimulate the proliferation of human gingival fibroblasts83.
To introduce these nanospheres as drug delivery vehicles into the 3D scaffolds during tissue regeneration, a novel technique has been developed to immobilize biological factor- encapsulated nanospheres onto prefabricated NF scaffolds to achieve in situ delivery83,84 (Figure 3). This approach circumvents the issues of uncontrolled spatial delivery by using micro/nano-spheres alone due to undesired coalescence or migration. Single or multiple biological factors delivery could be spatially and temporally controlled. The release kinetics could be manipulated via varying nanosphere formulation. In one study, bone morphogenetic protein-7 (BMP-7) was incorporated into nanospheres and subsequently immobilized onto NF scaffolds for a rat subcutaneous implantation model study84. BMP-7 was released in a controlled fashion with high biological activity and induced ectopic bone formation, confirmed by H&E staining, von Kossa staining and radiographic measurements. In another study, platelet-derived growth factor (PDGF) was chosen to demonstrate its effect on angiogenesis in the nanosphere-immobilized NF scaffolds85. Following implantation in rats, the released PDGF was biologically active, which induced angiogenesis and the corresponding pericyte formation in a PDGF-dose-dependent manner, evaluated using Factor VIII staining85. Recently, the highly soluble antibiotic drug, doxycycline (DOXY), was also successfully incorporated into PLGA nanospheres and subsequently immobilized onto the NF scaffolds to achieve in situ 3D release86. A reduced initial burst release was observed as compared to using PLGA nanospheres alone. Common bacterial growth (S. aureus and E. coli) was inhibited for a prolonged duration, which was beneficial for dental, periodontal or bone treatments. These results demonstrate that this delivery system within the 3D scaffold is applicable to not only proteins but also other large biological molecules and small molecule drugs, making it possible to design more complex drug delivery strategies for tissue engineering.
Figure 3.
SEM micrographs at two different magnifications (A, B) and laser scanning confocal micrograph (C) of PLGA nanosphere-immobilized PLLA nanofibrous scaffolds. FITC-labeled bovine serum albumin was encapsulated in PLGA nanospheres, showing green emission under confocal microscopy (C). From Wei et al.83, Copyright©2006 by Elsevier. Reprinted with permission of Elsevier.
6. Scaffold-guided stem cell development
Scaffolds fabricated using various techniques as stated above, including phase separation, particulate leaching, SFF techniques etc, can be highly useful in regenerative medicine applications when combined with several types of stem cells. Embryonic, mesenchymal, embryonic-derived mesenchymal, amniotic-fluid derived, and dental pulp stem cells have been used to produce various tissues. The NF PLLA scaffolds provide an advantageous matrix environment for tissue development due to its macro- and nano-scale features. The designed macro-pores allow for cell aggregation within each pore, as well as, cell migration and uniform nutrient/waste exchange throughout the scaffold. Secondly, the nanofibers mimic the extracellular matrix to provide the physical cues that stem cells require for adherence, proliferation, and differentiation87. Bone, cartilage, dentin, bladder, and vascular tissues have been developed from several stem cell types, as discussed below.
6.1 Bone
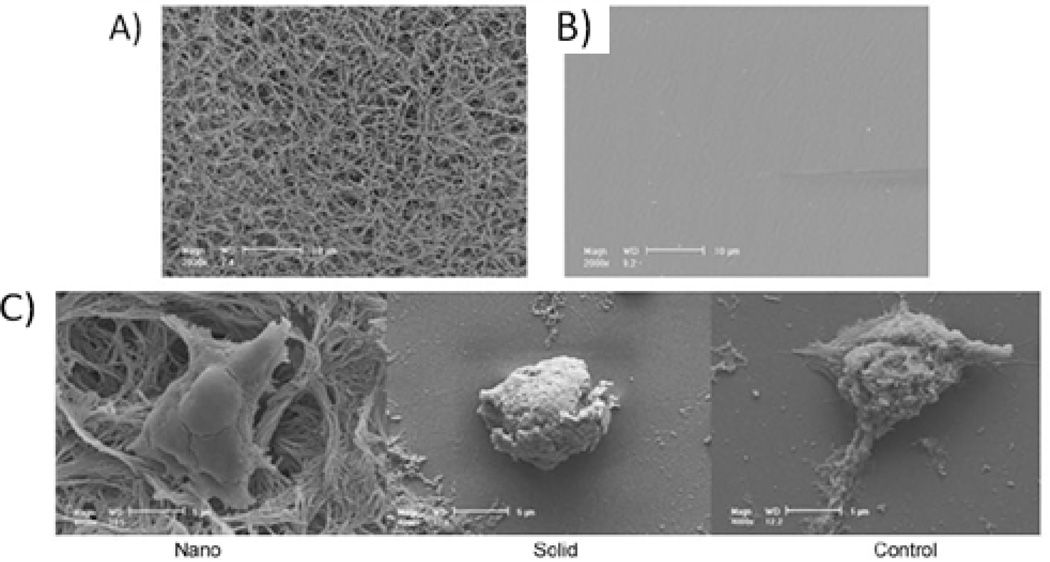
Embryonic stem (ES) cells can be differentiated into any tissue due to their pluripotency88. This advantageous property of ES cells has been utilized for bone formation in tissue engineering. By seeding mouse ES cells on NF matrices (Figure 4A) and comparing these results to solid, flat films (Figure 4B), we deduced the NF effect on ES cell differentiation to an osteogenic lineage. The positive effect of nanofibers was even seen after only 12 hours, as the ES cells formed protrusions to adhere to the nanofibers, compared to the rounded morphology on solid films (Figure 4C)89. When ES cells were seeded on 3D NF scaffolds, the nanofibers increased osteogenic differentiation, shown by gene expression of osteogenic markers and calcium staining for bone mineralization content. This work demonstrates that nanofibers enhance osteogenic differentiation of ES cells.
Figure 4.
SEM micrographs of (A) NF matrix, scale bar-10µm; (B) solid film, scale bar=10µm; and (C) D3 mouse ES cells on NF matrix and solid film 12 hours after seeding, scale bar=5um. From Smith et al.89, Copyright©2009 by Mary Ann Liebert. Reprinted with permission of Mary Ann Liebert.
Another stem cell source that has promise for bone regeneration is human embryonic stem cell-derived mesenchymal stem cells. These cells are attractive because they are more homogenous than ES cells, have a higher proliferative capacity than mesenchymal stem cells (MSCs), and can be induced to chondrogenic and osteogenic lineages90,91. In order to better understand the chemical and physical cues that control human embryonic stem cell-derived mesenchymal stem cell (hESC-MSC) osteogenic differentiation, Hu et al. studied the effects of dexamethasone (Dex), bone morphogenetic protein (BMP)-7, and nanofibrous features. Dex and BMP-7 synergistically improved differentiation, as alkaline phosphatase (ALP) and calcium content was significantly lower when each osteogenic factor was used alone. Secondly, two-dimensional NF matrices increased ALP and calcium contents versus flat films, similar to results with mouse ES cells mentioned previously. By combining these results to form an optimized osteogenic system, hESC-MSC were cultured on three-dimensional NF scaffolds with Dex and BMP-7, which greatly enhanced osteogenesis at 6 weeks of culture. This study reveals that the chemical factors of Dex and BMP-7 and physical architecture of nanofibers improved osteogenic differentiation of hESC-MSCs, another viable cell source for bone tissue regeneration applications92.
A third cell type that has been successfully used for bone formation is human amniotic fluid-derived stem cells (hAFSCs). Human AFSCs are multipotent and highly proliferative but little is known about their responsiveness to growth factors and synthetic scaffolds for osteoblastic differentiation93. Similarly to the other stem cell types discussed previously, BMP-7 and NF scaffolds (versus solid walled scaffolds) facilitated osteoblastic differentiation by mimicking in vivo BMP signaling and natural ECM.
6.2 Cartilage
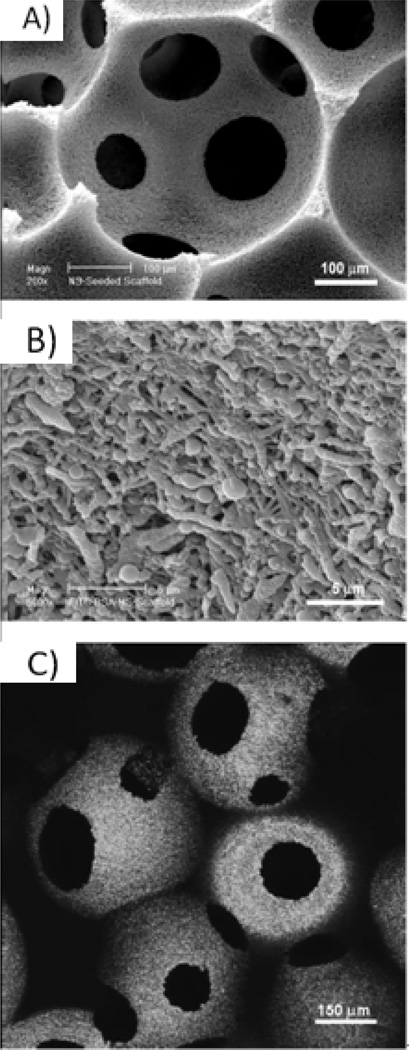
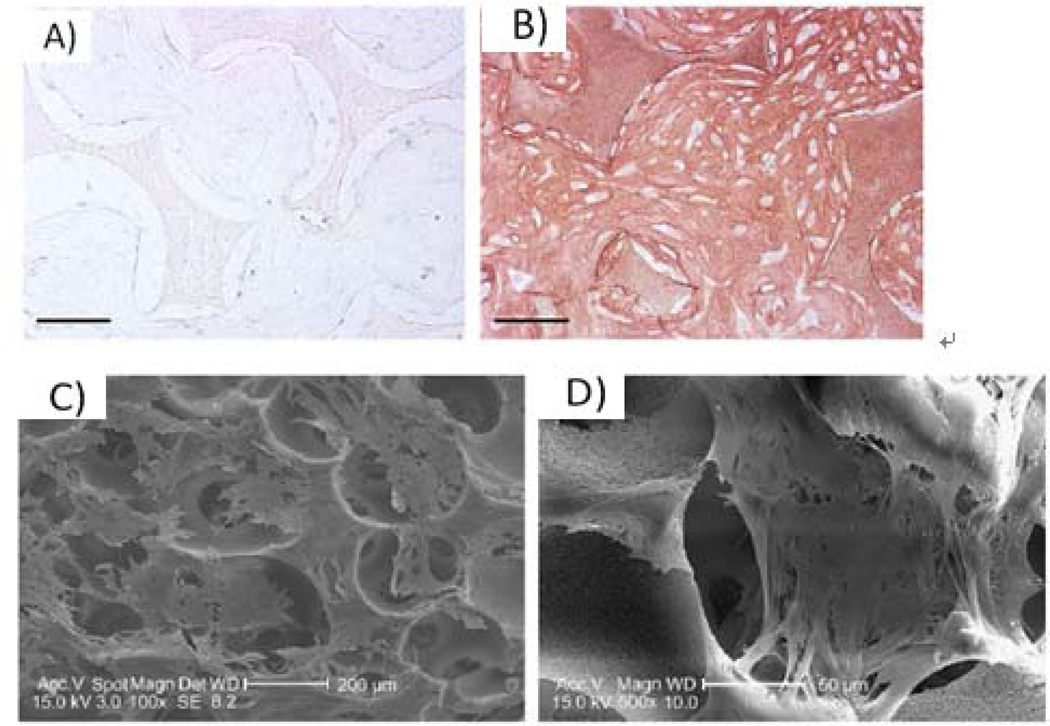
The knowledge gained from bone tissue formation has been applied to generate cartilage. By determining the ideal environment for both tissues, the entire osteochondral interface can be regenerated, which is especially important in osteoarthritic joint restoration. One stem cell type used for cartilage formation is human MSCs (hMSCs), which also have potential to differentiate into bone and fat tissues94. Similar to osteogenesis, chondrogenesis was improved by growth factor supplementation and designed architectural features. The addition of transforming growth factor (TGF)-β1 improved aggrecan, collagen type II, and Sox-9 gene expression of hMSCs. GAG accumulation and collagen type II deposition (Figure 5A and 5B) also increased at 6 weeks of culture with TGF-β1. In addition to important NF architecture, the designed, interconnected macropore network favorably allowed hMSC aggregation within the 250–425µm pores (Figure 5C and 5D)87. This aggregation likely induced differentiation by facilitating the condensation process that is integral in chondrogenic differentiation87. Once again, the NF macroporous scaffold with necessary growth factors provided an advantageous environment for tissue regeneration.
Figure 5.
hMSCs culture on 3D NF PLLA scaffold. Immunohistochemical type II collagen stain after constructs cultured for 6 weeks (A) without TGF-β1 or (B) with TGF-β1, showing much more type II collagen deposition with TGF- β1, scale bar=100um; (C,D) SEM micrographs of hMSCs after 24 hours of culture showing aggregation within macropores. From Hu et al.87, Copyright©2009 by Elsevier. Reprinted with permission of Elsevier.
6.3 Dentin
NF scaffolds have also been used in dental applications for tooth defect repair with the differentiation of human dental pulp stem cells (hDPSCs) into odontoblasts, which form dentin. Dentin is the mineralized tissue below the enamel layer of the tooth that encases the dental pulp within the tooth. It has similar components to bone, namely hydroxyapatite and type I collagen95, making NF scaffolds an ideal candidate for dentin tissue engineering. The nanofibrous scaffolds advantageously enhance the odontogenic differentiation of hDPSCs, compared with control scaffolds96. When seeded on NF scaffolds, hDPSCs differentiated into odontoblasts in both ‘Dex’ media (containing Dex, ascorbic acid, and β-glycophosphate) and in ‘BMP-7+Dex’ media (containing the Dex media with BMP-7)97. However, the ‘BMP-7+Dex’ media had higher quality dentin formation in vitro and in vivo. In vitro there was higher ALP activity, gene expression of odontogenic markers, and calcium content. When hDPSC-seeded scaffolds were implanted in vivo in nude mice, more hard tissue formation and ECM deposition, as well as higher dentin sialo protein staining97. Therefore, the NF scaffold in combination with odontogenic soluble factors propelled hDPSC differentiation for dentinogenesis.
6.4 Bladder and vascular tissues
NF scaffolds have also been applied to deliver stem cells for vascular formation and urological tissue engineering. In one example, BMSCs were first induced to differentiate into smooth muscle cells (SMC) using a combination of myogenic growth factors, bladder ECM and dynamic culture. The myogenically differentiated cells were then seeded onto NF 3D PLLA scaffolds with porous structure, which were found to facilitate the cell-matrix penetration, maintain myogenic differentiation of these cells, and promote tissue remolding with rich capillary formation, showing potential for bladder tissue engineering98. Similarly, the porous NF 3D PLLA scaffolds were also found to support vascular tissue formation from SMCs derived from ESCs99. In a different study, electrospun nanofibers made of polyhydroxybutyrate (PHB) and its copolymer poly-3-hydroxybutyrate-co-3-hydroxyvalerate (PHB-HV), combined with endothelial differentiation medium, were found to promote the differentiation of human adipose tissue-derived stem cells (hASCs) into the endothelial lineage100. Electrospun hydrogel nanofiber was also found to encourage MSC differentiation into smooth muscle- or endothelial- like cells depending on the elasticity of the hydrogel101. In this specific nanofiber hydrogel system, an elasticity of 3kPa encouraged MSC differentiation into endothelial cells, while higher elasticity (of >8kPa) favored MSC differentiation into smooth muscle cells101.
6.4 Overall biological effects
In summary, several types of stem cells have been successfully utilized in various tissue engineering applications using a novel three-dimensional NF macroporous scaffold. The scaffold has many positive biological effects on stem cell differentiation by closely mimicking natural ECM with synthetic nanofibers and permitting cell/nutrient migration through the interconnected porous network. The scaffold can also incorporate controlled release nanospheres containing growth factors84, which greatly enhanced the regeneration of bone, cartilage, and dentin as exemplified previously. The synthetic scaffolds with multi-level architecture have been integral in tissue engineering applications and will be applied to a wider array of tissue regeneration needs, such as neural regeneration in future work.
7. Conclusion
Research on stem cell biology and modern biomaterials has created exciting opportunities for tissue regeneration and cell-based therapy. Biomaterials can now be rationally designed to provide a defined local biochemical and mechanical microenvironment with dynamic regulations on stem cell development for desired tissue formation. Phase separation technique has produced ECM-mimicking 3D NF scaffolds from various synthetic and naturally-derived materials, while diverse micro/nano-technologies were introduced to engineer complex biophysical micro-environment. Drug delivery strategies and surface modification further brought desired properties to regulate cell-matrix interactions. These technologies provide us with valuable tools for different tissue engineering solutions. By logically selecting cell source, scaffolds, and bioactive stimuli, various tissues including bone, dentin, cartilage, bladder and vascular tissues have been developed. As the field of tissue engineering continuous to evolve, more complex strategies will be developed for potential therapeutic applications.
8. Expert opinion
8.1 Current state of tissue engineering field
Investigational models in tissue engineering have evolved rapidly, which are derived from interdisciplinary and multidisciplinary research by cell biologists, chemists, biomaterial engineers, computer scientists, and medical doctors. Stem cells showed great potential in tissue engineering ever since they were first brought into the field in the 1990’s, when researchers used MSCs with site-specific delivery vehicles to repair cartilage, bone, tendon, marrow stroma, muscle, and other connective tissues102. Biomaterial effects, deriving from material physical structure, local chemistry and mechanical properties, have been utilized to control cell proliferation and differentiation for several stem cell types and tissue types16,38,87,89,92,103. Discovery and increased understanding of growth factors, as well as drug delivery strategies, have offered researchers control over cellular proliferation, differentiation, migration, adhesion and gene expression84,104,105 in a temporal and spatial manner. The progress in tissue engineering has changed the conventional tissue repair protocols, with several tissue engineering therapies tested in clinical trials. For example, Stratagraft employing collagen matrix as supporting materials for both a dermis and a fully-stratified, biologically-functional epidermis has been tested clinically as human skin substitute106. In another example, tubular scaffolds made of degradable PLLA/PCL reinforced by woven PGA have been used to deliver bone marrow derived mononuclear cells in a cardiovascular surgery as tissue engineered vascular grafts107. Recently, successful human tracheobronchial replacement to restore lung function in a patient was reported using a decellularized human airway construct repopulated with epithelial cells and chondrocytes of MSC origin108. There is also a limited number of clinical trials combining MSCs with biomaterials. Hydroxyapatite porous scaffolds, for example, have been employed to deliver MSCs for bone defect repair in patients109–111. Stem cells and biomaterials have also been used together with growth factors to increase the healing potential in a clinical treatment of a mandibular defect112.
8.2 Current challenges
The limited success of tissue engineering in clinics using MSC and biomaterials is attributed to several reasons. Besides regulatory issues as well as economic concerns, there are significant scientific and technological limitations. There is much to learn regarding the mechanisms underlying stem cell development. Intercellular and intracellular signaling pathways are often difficult to determine due to issues such as functional redundancy, autoregulation, and lack of availability of knockout animal models. Nano/micro-technologies also need to be further advanced to provide more precise control over the biophysics of 3D scaffolds at multiple scales, in order to better systematically study the cell/matrix interactions. Also, suitable dosing and duration of growth factors used in tissue engineering, understanding of the induced complex cascade that define the appropriate expression sequence, as well as the synergic effects of multiple growth factors need to be further addressed113. In addition, more in vivo studies are needed despite cost barriers, especially in large animal models, to provide better perspectives for human clinical trials. Successful overcoming these current challenges would allow for better tissue formation with more physiological properties.
8.3 Future of the field
The next decade would present us more challenges and exciting research topics in the rapidly developing field of tissue engineering. Hybrid approach would be applied to produce customer designed bio-mimetic/bio-inspired artificial extracellular matrices, incorporating optimal physical structures and local chemistry to improve stem cell support. Stem cells delivered by these designed scaffolds can then be guided and stimulated to reconstitute a functional tissue in vivo. Multiple cell types may also be employed with spatial control, e.g. in the form of printed 3D cells/organs, to generate complex tissues ex vivo, with the needed vascular architectures and tissue hierarchy.
Along with the advancement of biomaterials, the fate of stem cells will be more precisely controlled. The resulting higher quality tissues will lead to more clinical trials and applications of the stem cell beyond tissue engineering therapies.
Article highlights box.
Synthetic polymer scaffolds with nanofibrous structure mimic the collagen physical structure to enhance cell/matrix interactions.
Thermally-induced phase separation allows the construction of nanofibrous scaffolds from natural and synthetic polymers in a 3D fashion with control over micro-structure and overall 3D shapes.
The scaffolds with designed features of nanofibers and macropores positively affect stem cell adhesion, proliferation, differentiation, and tissue formation.
In bone tissue regeneration, polymer/ceramic nanocomposites are advantageous to mimic both the structure and composition of natural bone.
Growth factors are a key component in tissue engineering and are delivered by nanospheres on scaffolds to spatiotemporally regulate stem cell fate and tissue regeneration.
Several types of stem cells were differentiated on nanofibrous scaffolds to form bone, cartilage, dentin, and smooth muscle tissues.
Acknowledgements
The authors gratefully acknowledge the research grant support from the NIH (NIDCR DE015384, DE017689 and DE022327), NSF (DMR-1206575) and DOD (W81XWH-12-2-0008). MJG was partially supported by the NIH/NIDCR Training Grant (T32 DE 007057) at the University of Michigan School of Dentistry.
Abbreviations
- 3D
three dimensional
- ALP
alkaline phosphatase
- BMP-7
bone morphogeneic protein-7
- BSP
bone sialo protein
- CAD
computer assisted design
- CAM
computer assisted manufacture
- CATE
computer-aided tissue engineering
- Dex
dexamethasone
- DOXY
doxycycline
- ECM
extracellular matrix
- ES cell
embryonic stem cell
- FDA
Food and Drug Administration
- hAFSCs
human amniotic fluid-derived stem cells
- HAP
Hydroxyapatite
- hDPSCs
human dental pulp stem cells
- hESC-MSC
human embryonic stem cell derived mesenchymal stem cell
- hMSCs
human embryonic stem cell
- MSCs
mesenchymal stem cells
- NF
nanofibrous
- OCN
bone markers osteocalcein
- PCL
poly(ε-caprolactone)
- PDAC
poly(diallyldimethylammonium chloride)
- PDGF
platelet-derived growth factor
- PDLLA
poly(DL-lactide)
- PGA
poly(glycolic acid)
- PHAA-g-PLLA
poly(hydroxyalkyl (meth)acrylate)-graft-poly(L-lactic acid)
- PHB
poly(hydroxyl butyrate)
- PLA
poly(lactic acid)
- PLGA
poly(lactic-co-glycolic acid)
- PLLA
poly(L-lactic acid)
- PU
poly(urethane)
- rhPDGF-BB
recombinant human platelet-derived growth factor BB
- SBF
simulated body fluid
- SFF
solid free form
- TGF
transforming growth factor
- TIPS
thermally induced phase separation
Footnotes
Declaration of interest:
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
References
- 1.Langer R, Vacanti JP. Tissue Engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2. De Coppi P, Bartsch G, Jr, Siddiqui MM, Xu T, Santos CC, Perin L, et al. Isolation of amniotic stem cell lines with potential for therapy. Nature Biotechnology. 2007;25(1):100–106. doi: 10.1038/nbt1274.. Human and rodent amniotic fluid–derived stem (AFS) cells provide an attractive alternative cell source for tissue regeneration.
- 3.Saltzman WM, Olbricht WL. Building drug delivery into tissue engineering. Nature Reviews Drug Discovery. 2002;1(3):177–186. doi: 10.1038/nrd744. [DOI] [PubMed] [Google Scholar]
- 4. Ma PX. Biomimetic materials for tissue engineering. Advanced Drug Delivery Reviews. 2008;60(2):184–198. doi: 10.1016/j.addr.2007.08.041.. This article reviews biomimetic approaches in biomaterials for cell and biomolecule delivery in tissue engineering.
- 5.Odorico JS, Kaufman DS, Thomson JA. Multilineage differentiation from human embryonic stem cell lines. Stem Cells. 2001;19(3):193–204. doi: 10.1634/stemcells.19-3-193. [DOI] [PubMed] [Google Scholar]
- 6.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 7. Varghese S, Elisseeff J, Hwang NS. Controlled differentiation of stem cells. Advanced Drug Delivery Reviews. 2008;60(2):199–214. doi: 10.1016/j.addr.2007.08.036.. This review presents recent findings and differentiation models of human embryonic stem cells.
- 8. Mitragotri S, Lahann J. Physical approaches to biomaterial design. Nature Materials. 2009;8(1):15–23. doi: 10.1038/nmat2344.. This review summarizes the biological effects of physical properties of materials including size, shape, mechanical properties, surface texture and compartmentalization, and their related synthesis methods as well as applications.
- 9.Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, et al. Silk-based biomaterials. Biomaterials. 2003;24(3):401–416. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 10. Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324(5923):59–63. doi: 10.1126/science.1169494.. A novel system of photodegradable gels that allow real-time remote control of material properties or chemistry is present in this article.
- 11. Luo Y, Shoichet MS. A photolabile hydrogel for guided three-dimensional cell growth and migration. Nature Materials. 2004;3(4):249–254. doi: 10.1038/nmat1092.. This work provides a means to construct programmed spatial features in transparent systems for tissue engineering applications.
- 12.Salinas CN, Anseth KS. The enhancement of chondrogenic differentiation of human mesenchymal stem cells by enzymatically regulated RGD functionalities. Biomaterials. 2008;29(15):2370–2377. doi: 10.1016/j.biomaterials.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benoit DSW, Schwartz MP, Durney AR, Anseth KS. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nature Materials. 2008;7(10):816–823. doi: 10.1038/nmat2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma PX. Scaffolds for tissue fabrication. Materials Today. 2004;7(5):30–40. [Google Scholar]
- 15. Engler A, Sen S, Sweeney H, Discher D. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044.. Naive mesenchymal stem cells (MSCs) are shown here to specify lineage and commit to phenotypes with extreme sensitivity to tissue-level elasticity.
- 16.Mizuno M, Shindo M, Kobayashi D, Tsuruga E, Amemiya A, Kuboki Y. Osteogenesis by bone marrow stromal cells maintained on type I collagen matrix gels in vivo. Bone. 1997;20(2):101. doi: 10.1016/s8756-3282(96)00349-3. [DOI] [PubMed] [Google Scholar]
- 17.Ma PX. Tissue Engineering. In: Kroschvitz JI, editor. Encyclopedia of Polymer Science and Technology. 3rd ed. John Wiley & Sons; 2004. [Google Scholar]
- 18.Zhang R, Ma PX. Processing of polymer scaffolds: Phase separation. In: Atala A, Lanza R, editors. Methods of Tissue Engineering. San Diego: Academic Press; 2001. [Google Scholar]
- 19.Pachence JM, Kohn J. Chapter 22 - Biodegradable Polymers (Second Edition) In: Robert PL, Robert L, Josepph VacantiA2, Robert P, Lanza RL, Joseph V, editors. Principles of Tissue Engineering (Second Edition) San Diego: Academic Press; 2000. pp. 263–277. [Google Scholar]
- 20.Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR. J. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27(18):3413–3431. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 21.Ma PX, Langer R. Degradation, structure and properties of fibrous poly(glycolic acid) scaffolds for tissue engineering. In: Mikos AG, editor. Polymers in Medicine and Pharmacy. Materials Research Society; 1995. pp. 99–104. [Google Scholar]
- 22.Reed AM, Gilding DK. Biodegradable polymers for use in surgery - poly(glycolic)/poly(lactic acid) homo and copolymers: 2. In vitro degradation. Polymer. 1981;22(4):494–498. [Google Scholar]
- 23.Zhang R, Ma PX. Degradation behavior of porous poly (α-hydroxy acids)/hydroxyapatite composite scaffolds. Polymer Preprints. 2000;41(2):1618–1619. [Google Scholar]
- 24.Eling B, Gogolewski S, Pennings AJ. Biodegradable materials of poly(l-lactic acid): 1. Melt-spun and solution-spun fibres. Polymer. 1982;23(11):1587–1593. [Google Scholar]
- 25.Pitt CG, Gratzl MM, Kimmel GL. Aliphatic polyesters II. The degradation of poly (DL-lactide), poly (ε-caprolactone), and their copolymers in vivo. Biomaterials. 1981;2(4):215–220. doi: 10.1016/0142-9612(81)90060-0. [DOI] [PubMed] [Google Scholar]
- 26.Miller ND, Williams DF. On the biodegradation of poly-β-hydroxybutyrate (PHB) homopolymer and poly-β-hydroxybutyrate-hydroxyvalerate copolymers. Biomaterials. 1987;8(2):129–137. doi: 10.1016/0142-9612(87)90102-5. [DOI] [PubMed] [Google Scholar]
- 27.Sokolsky-Papkov M, Agashi K, Shakesheff K, Domb AJ. Polymer carriers for drug delivery in tissue engineering. Advanced Drug Delivery Reviews. 2007;59(4–5):187–206. doi: 10.1016/j.addr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Bonzani IC, Adhikari R, Houshyar S, Mayadunne R, Gunatillake P, Stevens MM, et al. Synthesis of two-component injectable polyurethanes for bone tissue engineering. Biomaterials. 2007;28(3):423–433. doi: 10.1016/j.biomaterials.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 29.Guelcher SA, Srinivasan A, Dumas JE, Didier JE, McBride S, Hollinger JO. Synthesis, mechanical properties, biocompatibility, and biodegradation of polyurethane networks from lysine polyisocyanates. Biomaterials. 2008;29(12):1762–1775. doi: 10.1016/j.biomaterials.2007.12.046. [DOI] [PubMed] [Google Scholar]
- 30.Gunatillake P, Mayadunne R, Adhikari R. Recent developments in biodegradable synthetic polymers. In: El-Gewely MR, editor. Biotechnology Annual Review. Elsevier; 2006. pp. 301–347. [DOI] [PubMed] [Google Scholar]
- 31.Leong KW, Kost J, Mathiowitz E, Langer R. Polyanhydrides for controlled release of bioactive agents. Biomaterials. 1986;7(5):364–371. doi: 10.1016/0142-9612(86)90007-4. [DOI] [PubMed] [Google Scholar]
- 32.Alza Corporation (Palo Alto, CA), assignee Drug delivery devices manufactured from poly(orthoesters) and poly(orthocarbonates) USA: 1978. [Google Scholar]
- 33.Elisseeff J, McIntosh W, Fu K, Blunk T, Langer R. Controlled-release of IGF-I and TGF-β1 in a photopolymerizing hydrogel for cartilage tissue engineering. Journal of Orthopaedic Research. 2001;19(6):1098–1104. doi: 10.1016/S0736-0266(01)00054-7. [DOI] [PubMed] [Google Scholar]
- 34.Anderson DG, Levenberg S, Langer R. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nature Biotechnology. 2004;22(7):863–866. doi: 10.1038/nbt981. [DOI] [PubMed] [Google Scholar]
- 35.Zhang R, Ma PX. Poly(α-hydroxyl acids)/hydroxyapatite porous composites for bone- tissue engineering. I. Preparation and morphology. Journal of Biomedical Materials Research. 1999;44(4):446–455. doi: 10.1002/(sici)1097-4636(19990315)44:4<446::aid-jbm11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 36.Liu X, Ma PX. Polymeric scaffolds for bone tissue engineering. Annals of Biomedical Engineering. 2004;32(3):477–486. doi: 10.1023/b:abme.0000017544.36001.8e. [DOI] [PubMed] [Google Scholar]
- 37.Woo KM, Chen VJ, Ma PX. Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. Journal of Biomedical Materials Research - Part A. 2003;67(2):531–537. doi: 10.1002/jbm.a.10098. [DOI] [PubMed] [Google Scholar]
- 38.Hu J, Liu X, Ma PX. Induction of osteoblast differentiation phenotype on poly(l-lactic acid) nanofibrous matrix. Biomaterials. 2008;29(28):3815–3821. doi: 10.1016/j.biomaterials.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang R, Ma PX. Synthetic nano-fibrillar extracellular matrices with predesigned macroporous architectures. Journal of Biomedical Materials Research. 2000;52(2):430–438. doi: 10.1002/1097-4636(200011)52:2<430::aid-jbm25>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 40. Chen VJ, Smith LA, Ma PX. Bone regeneration on computer-designed nano-fibrous scaffolds. Biomaterials. 2006;27(21):3973–3979. doi: 10.1016/j.biomaterials.2006.02.043.. Computer designed scaffolding
- 41.Liu X, Ma PX. Phase separation, pore structure, and properties of nanofibrous gelatin scaffolds. Biomaterials. 2009;30(25):4094–4103. doi: 10.1016/j.biomaterials.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma PX, Zhang R. Synthetic nano-scale fibrous extracellular matrix. Journal of Biomedical Materials Research. 1999;46(1):60–72. doi: 10.1002/(sici)1097-4636(199907)46:1<60::aid-jbm7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 43.Chen VJ, Ma PX. Nano-fibrous poly(L-lactic acid) scaffolds with interconnected spherical macropores. Biomaterials. 2004;25(11):2065–2073. doi: 10.1016/j.biomaterials.2003.08.058. [DOI] [PubMed] [Google Scholar]
- 44.Chen VJ, Ma PX. The effect of surface area on the degradation rate of nano-fibrous poly(l-lactic acid) foams. Biomaterials. 2006;27(20):3708–3715. doi: 10.1016/j.biomaterials.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 45.Liu X, Ma PX. The nanofibrous architecture of poly(l-lactic acid)-based functional copolymers. Biomaterials. 2010;31(2):259–269. doi: 10.1016/j.biomaterials.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wei G, Ma PX. Partially nanofibrous architecture of 3D tissue engineering scaffolds. Biomaterials. 2009;30(32):6426–6434. doi: 10.1016/j.biomaterials.2009.08.012.. 3D nanofibrous scaffold processing and application
- 47.Hu J, Sun X, Ma PX. Porous nanofibrous PLLA scaffolds for vascular tissue engineering. Biomaterials. 2010;31(31):7971–7977. doi: 10.1016/j.biomaterials.2010.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma H, Hu J, Ma PX. Polymer scaffolds for small-diameter vascular tissue engineering. Advanced Functional Materials. 2010;20(17):2833–2841. doi: 10.1002/adfm.201000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei G, Ma PX. Macroporous and nanofibrous polymer scaffolds and polymer/bone-like apatite composite scaffolds generated by sugar spheres. Journal of Biomedical Materials Research - Part A. 2006;78(2):306–315. doi: 10.1002/jbm.a.30704. [DOI] [PubMed] [Google Scholar]
- 50.Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC, et al. Engineering vascularized skeletal muscle tissue. Nature Biotechnology. 2005;23(7):879–884. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 51.Zhang S, Huang Y, Yang X, Mei F, Ma Q, Chen G, et al. Gelatin nanofibrous membrane fabricated by electrospinning of aqueous gelatin solution for guided tissue regeneration. Journal of Biomedical Materials Research Part A. 2009;90(3):671–679. doi: 10.1002/jbm.a.32136. [DOI] [PubMed] [Google Scholar]
- 52.Matthews JA, Wnek GE, Simpson DG, Bowlin GL. Electrospinning of Collagen Nanofibers. Biomacromolecules. 2002;3(2):232–238. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 53.Yang F, Murugan R, Wang S, Ramakrishna S. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26(15):2603–2610. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 54.Yoshimoto H, Shin YM, Terai H, Vacanti JP. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003;24(12):2077–2082. doi: 10.1016/s0142-9612(02)00635-x. [DOI] [PubMed] [Google Scholar]
- 55.Zong X, Ran S, Kim K-S, Fang D, Hsiao BS, Chu B. Structure and Morphology Changes during in Vitro Degradation of Electrospun Poly(glycolide-co-lactide) Nanofiber Membrane. Biomacromolecules. 2003;4(2):416–423. doi: 10.1021/bm025717o. [DOI] [PubMed] [Google Scholar]
- 56.Reneker DH, Chun I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology. 1996;7(3):216–223. [Google Scholar]
- 57.Jose MV, Thomas V, Xu Y, Bellis S, Nyairo E, Dean D. Aligned Bioactive Multi-Component Nanofibrous Nanocomposite Scaffolds for Bone Tissue Engineering. Macromolecular Bioscience. 2010;10(4):433–444. doi: 10.1002/mabi.200900287. [DOI] [PubMed] [Google Scholar]
- 58.Nishida K. Corneal Reconstruction with Tissue-Engineered Cell Sheets Composed of Autologous Oral Mucosal Epithelium. New England Journal of Medicine. 2004;351(12):1187. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- 59.Wang P, Hu J, Ma PX. The engineering of patient-specific, anatomically shaped, digits. Biomaterials. 2009;30(14):2735–2740. doi: 10.1016/j.biomaterials.2009.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nerem RM, Seliktar D. Vascular tissue engineering. Annual Review of Biomedical Engineering. 2001;3:225–243. doi: 10.1146/annurev.bioeng.3.1.225. [DOI] [PubMed] [Google Scholar]
- 61.Germain L, Remy-Zolghadri M, Auger F. Tissue engineering of the vascular system: from capillaries to larger blood vessels. Medical & Biological Engineering & Computing. 2000 Mar;38(2):232–240. [PubMed] [Google Scholar]
- 62.Teebken OE, Haverich A. Tissue engineering of small diameter vascular grafts. European Journal of Vascular and Endovascular Surgery. 2002 Jun;23(6):475–485. doi: 10.1053/ejvs.2002.1654. [DOI] [PubMed] [Google Scholar]
- 63.Akao M, Sakatsume M, Aoki H, Takagi T, Sasaki S. In vitro mineralization in bovine tooth germ cell cultured with sintered hydroxyapatite. Journal of Materials Science: Materials in Medicine. 1993;4(6):569–574. [Google Scholar]
- 64.Puleo DA, Holleran LA, Doremus RH, Bizios R. Osteoblast responses to orthopedic implant materials in vitro. Journal of Biomedical Materials Research. 1991;25(6):711–723. doi: 10.1002/jbm.820250603. [DOI] [PubMed] [Google Scholar]
- 65.Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. Journal of Biomedical Materials Research. 2000;51(3):475–483. doi: 10.1002/1097-4636(20000905)51:3<475::aid-jbm23>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 66.Ma PX, Zhang R. Microtubular architecture of biodegradable polymer scaffolds. Journal of Biomedical Materials Research. 2001;56(4):469–477. doi: 10.1002/1097-4636(20010915)56:4<469::aid-jbm1118>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 67.Ma PX, Zhang R, Xiao G, Franceschi R. Engineering new bone tissue in vitro on highly porous poly(α-hydroxyl acids)/hydroxyapatite composite scaffolds. Journal of Biomedical Materials Research. 2001;54(2):284–293. doi: 10.1002/1097-4636(200102)54:2<284::aid-jbm16>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 68.Wei G, Ma PX. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials. 2004;25(19):4749–4757. doi: 10.1016/j.biomaterials.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 69.The Regents of the University of Michigan, assignee. Porous composite materials. USA: 2001. [Google Scholar]
- 70.The Regents of the University of Michigan, assignee. Porous composite materials. USA: 2005. [Google Scholar]
- 71.Zhang R, Ma PX. Biomimetic polymer/apatite composite scaffolds for mineralized tissue engineering. Macromolecular Bioscience. 2004;4(2):100–111. doi: 10.1002/mabi.200300017. [DOI] [PubMed] [Google Scholar]
- 72.Liu X, Smith LA, Hu J, Ma PX. Biomimetic nanofibrous gelatin/apatite composite scaffolds for bone tissue engineering. Biomaterials. 2009;30(12):2252–2258. doi: 10.1016/j.biomaterials.2008.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.He C, Xiao G, Jin X, Sun C, Ma PX. Electrodeposition on Nanofibrous Polymer Scaffolds: Rapid Mineralization, Tunable Calcium Phosphate Composition and Topography. Advanced Functional Materials. 2010;20:3568–3576. doi: 10.1002/adfm.201000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric Control of Cell Life and Death. Science. 1997;276(5317):1425–1428. doi: 10.1126/science.276.5317.1425.. This work points out that local geometric properties can control cell growth and viability, which represents a fundamental mechanism for developmental regulation within the tissue microenvironment.
- 75.Nitschke M, Schmack G, Janke A, Simon F, Pleul D, Werner C. Low pressure plasma treatment of poly(3-hydroxybutyrate): Toward tailored polymer surfaces for tissue engineering scaffolds. Journal of Biomedical Materials Research. 2002;59(4):632–638. doi: 10.1002/jbm.1274. [DOI] [PubMed] [Google Scholar]
- 76.Liu X, Won Y, Ma PX. Surface modification of interconnected porous scaffolds. Journal of Biomedical Materials Research - Part A. 2005;74(1):84–91. doi: 10.1002/jbm.a.30367. [DOI] [PubMed] [Google Scholar]
- 77.Liu X, Won Y, Ma PX. Porogen-induced surface modification of nano-fibrous poly(l-lactic acid) scaffolds for tissue engineering. Biomaterials. 2006;27(21):3980–3987. doi: 10.1016/j.biomaterials.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 78.Liu X, Won Y, Ma PX. Surface engineering of nano-fibrous biodegradable poly(L-lactic acid) scaffolds for tissue engineering. 2004;2004:243–248. [Google Scholar]
- 79.Schuldiner M, Yanuka O, Itskovitz-Eldor J, Melton DA, Benvenisty N. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(21):11307–11312. doi: 10.1073/pnas.97.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Langer R. Drug delivery and targeting. Nature. 1998;392(6679 SUPPL.):5–10. [PubMed] [Google Scholar]
- 81.Wei G, Pettway GJ, McCauley LK, Ma PX. The release profiles and bioactivity of parathyroid hormone from poly(lactic-co-glycolic acid) microspheres. Biomaterials. 2004;25(2):345–352. doi: 10.1016/s0142-9612(03)00528-3. [DOI] [PubMed] [Google Scholar]
- 82.Jeong B, Bae YH, Lee DS, Kim SW. Biodegradable block copolymers as injectable drug-delivery systems. Nature. 1997;388(6645):860–862. doi: 10.1038/42218. [DOI] [PubMed] [Google Scholar]
- 83. Wei G, Jin Q, Giannobile WV, Ma PX. Nano-fibrous scaffold for controlled delivery of recombinant human PDGF-BB. Journal of Controlled Release. 2006;112(1):103–110. doi: 10.1016/j.jconrel.2006.01.011.. Microspheres for drug delivery embedded on scaffold.
- 84.Wei G, Jin Q, Giannobile WV, Ma PX. The enhancement of osteogenesis by nano-fibrous scaffolds incorporating rhBMP-7 nanospheres. Biomaterials. 2007;28(12):2087–2096. doi: 10.1016/j.biomaterials.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jin Q, Wei G, Lin Z, Sugai JV, Lynch SE, Ma PX, et al. Nanofibrous scaffolds incorporating PDGF-BB microspheres induce chemokine expression and tissue neogenesis in vivo. PLoS ONE. 2008;3(3) doi: 10.1371/journal.pone.0001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Feng K, Sun H, Bradley MA, Dupler EJ, Giannobile WV, Ma PX. Novel antibacterial nanofibrous PLLA scaffolds. Journal of Controlled Release. 2010;146(3):363–369. doi: 10.1016/j.jconrel.2010.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hu J, Feng K, Liu XH, Ma PX. Chondrogenic and osteogenic differentiations of human bone marrow-derived mesenchymal stem cells on a nanofibrous scaffold with designed pore network. Biomaterials. 2009;30(28):5061–5067. doi: 10.1016/j.biomaterials.2009.06.013.. Nanofibrous scaffold for cartilage formation.
- 88.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proceedings of the National Academy of Sciences of the United States of America. 78(12):7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Smith LA, Liu X, Hu J, Wang P, Ma PX. Enhancing osteogenic differentiation of mouse embryonic stem cells by nanofibers. Tissue Engineering - Part A. 2009;15(7):1855–1864. doi: 10.1089/ten.tea.2008.0227.. Embryonic stem cell differentiation for bone tissue engineering using nanofibrous scaffold.
- 90.Hwang NS, Varghese S, Zhang Z, Elisseeff J. Chondrogenic differentiation of human embryonic stem cell-derived cells in arginine-glycine-aspartate-modified hydrogels. Tissue Engineering. 2006;12(9):2695–2706. doi: 10.1089/ten.2006.12.2695. [DOI] [PubMed] [Google Scholar]
- 91.Brown SE, Tong W, Krebsbach PH. The Derivation of Mesenchymal Stem Cells from Human Embryonic Stem Cells. Cells Tissues Organs. 2009;189(1–4):256–260. doi: 10.1159/000151746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hu J, Smith LA, Feng K, Liu X, Sun H, Ma PX. Response of human embryonic stem cell-derived mesenchymal stem cells to osteogenic factors and architectures of materials during in vitro osteogenesis. Tissue Engineering - Part A. 2010;16(11):3507–3514. doi: 10.1089/ten.tea.2010.0097.. Use of human embryonic stem cell-derived mesenchymal stem cells.
- 93.Sun H, Feng K, Hu J, Soker S, Atala A, Ma PX. Osteogenic differentiation of human amniotic fluid-derived stem cells induced by bone morphogenetic protein-7 and enhanced by nanofibrous scaffolds. Biomaterials. 2010;31(6):1133–1139. doi: 10.1016/j.biomaterials.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Caplan AI. Mesenchymal stem cells. Journal of Orthopaedic Research. 1991;9(5):641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 95.Massa L, Aranachavez V. Odontoblasts: the cells forming and maintaining dentine. The International Journal of Biochemistry & Cell Biology. 2004;36(8):1367–1373. doi: 10.1016/j.biocel.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 96.Wang J, Ma H, Jin X, Hu J, Liu X, Ni L, et al. The effect of scaffold architecture on odontogenic differentiation of human dental pulp stem cells. Biomaterials. 2011;32(31):7822–7830. doi: 10.1016/j.biomaterials.2011.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang J, Liu X, Jin X, Ma H, Hu J, Ni L, et al. The odontogenic differentiation of human dental pulp stem cells on nanofibrous poly(l-lactic acid) scaffolds in vitro and in vivo. Acta Biomaterialia. 2010;6(10):3856–3863. doi: 10.1016/j.actbio.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tian H, Bharadwaj S, Liu Y, Ma H, Ma PX, Atala A, et al. Myogenic differentiation of human bone marrow mesenchymal stem cells on a 3D nano fibrous scaffold for bladder tissue engineering. Biomaterials. 2010;31(5):870–877. doi: 10.1016/j.biomaterials.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hu J, Xie C, Ma H, Yang B, Ma PX, Chen YE. Construction of Vascular Tissues with Macro-Porous Nano-Fibrous Scaffolds and Smooth Muscle Cells Enriched from Differentiated Embryonic Stem Cells. PLoS ONE. 2012;7(4):e35580. doi: 10.1371/journal.pone.0035580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zonari A, Novikoff S, Electo NRP, Breyner NM, Gomes DA, Martins A, et al. Endothelial Differentiation of Human Stem Cells Seeded onto Electrospun Polyhydroxybutyrate/Polyhydroxybutyrate-Co-Hydroxyvalerate Fiber Mesh. PLoS ONE. 2012;7(4):e35422. doi: 10.1371/journal.pone.0035422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wingate K, Bonani W, Tan Y, Bryant SJ, Tan W. Compressive elasticity of three-dimensional nanofiber matrix directs mesenchymal stem cell differentiation to vascular cells with endothelial or smooth muscle cell markers. Acta Biomaterialia. 2012;8(4):1440–1449. doi: 10.1016/j.actbio.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Caplan AI. Mesenchymal stem cells: Cell-based reconstructive therapy in orthopedics. TISSUE ENGINEERING. 2005;11(7–8):1198–1211. doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- 103.Zhang Y, Fan W, Ma Z, Wu C, Fang W, Liu G, et al. The effects of pore architecture in silk fibroin scaffolds on the growth and differentiation of mesenchymal stem cells expressing BMP7. Acta Biomaterialia. 2010;6(8):3021–3028. doi: 10.1016/j.actbio.2010.02.030. [DOI] [PubMed] [Google Scholar]
- 104.Sun H, Feng K, Hu J, Soker S, Atala A, Ma PX. Osteogenic differentiation of human amniotic fluid-derived stem cells induced by bone morphogenetic protein-7 and enhanced by nanofibrous scaffolds. Biomaterials. 2010;31(6):1133–1139. doi: 10.1016/j.biomaterials.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Babensee J, McIntire L, Mikos A. Growth Factor Delivery for Tissue Engineering. Pharm Res. 2000 May 01;17(5):497–504. doi: 10.1023/a:1007502828372. 2000. [DOI] [PubMed] [Google Scholar]
- 106.Schurr MJ, Foster KN, Centanni JM, Comer AR, Wicks A, Gibson AL, et al. Phase I/II clinical evaluation of StrataGraft: A consistent, pathogen-free human skin substitute. Journal of Trauma - Injury, Infection and Critical Care. 2009;66(3):866–873. doi: 10.1097/TA.0b013e31819849d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Matsumura G, Hibino N, Ikada Y, Kurosawa H, Shin’oka T. Successful application of tissue engineered vascular autografts: clinical experience. Biomaterials. 2003;24(13):2303–2308. doi: 10.1016/s0142-9612(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 108.Raya-Rivera A, Esquiliano DR, Yoo JJ, Lopez-Bayghen E, Soker S, Atala A. Tissue-engineered autologous urethras for patients who need reconstruction: an observational study. The Lancet. 377(9772):1175–1182. doi: 10.1016/S0140-6736(10)62354-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marcacci M, Kon E, Moukhachev V, Lavroukov A, Kutepov S, Quarto R, et al. Stem cells associated with macroporous bioceramics for long bone repair; 6- To 7-year outcome of a pilot clinical study. Tissue Engineering. 2007;13(5):947–955. doi: 10.1089/ten.2006.0271. [DOI] [PubMed] [Google Scholar]
- 110.Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, et al. Repair of Large Bone Defects with the Use of Autologous Bone Marrow Stromal Cells. New England Journal of Medicine. 2001;344(5):385–386. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 111.Morishita T, Honoki K, Ohgushi H, Kotobuki N, Matsushima A, Takakura Y. Tissue engineering approach to the treatment of bone tumors: Three cases of cultured bone grafts derived from patients' mesenchymal stem cells. Artificial Organs. 2006;30(2):115–118. doi: 10.1111/j.1525-1594.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- 112.Warnke PH, Springer ING, Wiltfang J, Acil Y, Eufinger H, Wehmöller M, et al. Growth and transplantation of a custom vascularised bone graft in a man. The Lancet. 2004;364(9436):766–770. doi: 10.1016/S0140-6736(04)16935-3. [DOI] [PubMed] [Google Scholar]
- 113.Knight MAF, Evans GRD. Tissue engineering: Progress and challenges. Plastic and Reconstructive Surgery. 2004;114(2):26e–37e. [Google Scholar]