Abstract
Purpose
To determine the contribution of intestinal PepT1 on the permeability and oral absorption of the β-lactam antibiotic drug cefadroxil.
Methods
The effective permeability (Peff) of cefadroxil was evaluated in wild-type and PepT1 knockout mice following in situ single-pass intestinal perfusions. The plasma concentration-time profiles of cefadroxil were also examined after oral gavage.
Results
The Peff (cm/s) of cefadroxil in wild-type mice was 0.49×10-4 in duodenum, 0.80×10-4 in jejunum, 0.88×10-4 in ileum and 0.064x10-4 in colon. The Peff (cm/s) in PepT1 knockout mice was significantly reduced in small intestine, but not in colon, as shown by values of 0.003×10-4, 0.090×10-4, 0.042×10-4 and 0.032×10-4, respectively. Jejunal uptake of cefadroxil was saturable (Km=2-4 mM) and significantly attenuated by the sodium-proton exchange inhibitor 5-(N,N-dimethyl)amiloride. Jejunal permeability of cefadroxil was not affected by L-histidine, glycine, cephalothin, p-aminohippurate or N-methylnicotinamide. In contrast, cefadroxil permeability was significantly reduced by glycylproline, glycylsarcosine, or cephalexin. Finally, PepT1 ablation resulted in 23-fold reductions in peak plasma concentrations and 14-fold reductions in systemic exposure of cefadroxil after oral dosing.
Conclusions
The findings are definitive in demonstrating that PepT1 is the major transporter responsible for the small intestinal permeability of cefadroxil as well as its enhanced oral drug performance.
Keywords: cefadroxil, intestinal permeability, knockout mice, oral absorption, PepT1
INTRODUCTION
Proton-coupled oligopeptide transporters (POTs) are a family of membrane-bound proteins that are present in a broad range of species, from bacteria to mammals (1). Physiologically, the POTs transfer di- and tripeptides, themselves products of protein degradation, and other peptide-like molecules across biological membranes into the cell cytosol. Although the process occurs against a concentration gradient, it is energized by the inwardly directed symport of protons down their concentration gradient and negative membrane potential. At present, four mammalian peptide transporters have been cloned, that is PepT1 (SLC15A1), PepT2 (SLC15A2), PhT1 (SLC15A4) and PhT2 (SLC15A3), details of which are available in these excellent reviews (2-4).
PepT1, cloned first from a rabbit intestinal cDNA library (5), is a high-capacity low-affinity transporter that moves small peptides with different sequences and charges, but not single amino acids or peptides longer than 3 amino acid residues. It is expressed predominantly in the small intestine, and to a lesser extent in the kidney, pancreas and liver (2-4). Previous studies in rodents have shown that PepT1 was expressed at the apical membrane of enterocytes in the duodenum, jejunum and ileum of mice, but not in the colon (6,7). It was also demonstrated that the effective permeability of glycylsarcosine (GlySar) was reduced 10-fold in the small intestine of PepT1 knockout mice as compared to wild-type animals (7). Moreover, the jejunal permeability of GlySar was significantly reduced by co-perfusions of cefadroxil at saturating concentrations in wild-type mice.
Cefadroxil, a first generation cephalosporin, is used to treat a diverse range of bacterial infections, such as urinary tract infections caused by E. coli and P. mirabilis, and pneumonia caused by S. pyogenes (8). This broad-spectrum aminocephalosporin drug has a high bioavailability despite its anionic charge in the intestine and poor lipophilicity. Cefadroxil is very stable in vivo and is neither hydrolyzed in the acidic environment of the stomach nor degraded by intra- or extracellular enzymes. The oral availability of cefadroxil is not affected by the presence of food and > 90% of the drug is recovered unchanged in the urine over 24 hr. The drug also has a relatively long half-life (~ 95 min) and exhibits only about 20% binding to plasma proteins (8,9).
Due to the resemblance of its chemical structure to physiological occurring peptides, such as the presence of an α-amino group, carboxylic end and peptide bond (10), cefadroxil was verified as a substrate of PepT1 (11). Consistent with this finding, the intestinal transport of cefadroxil was nonlinear and shown to obey Michaelis-Menten kinetics (12-14). However, other transporters have also been implicated in the transport of cefadroxil. For example, renal PepT2 was responsible for most of the tubular reabsorption of cefadroxil, thereby, increasing the half-life of the drug and increasing its exposure in different tissues (15). Given its location at the apical membrane of choroid plexus, PepT2 also acts to transport cefadroxil from CSF into this tissue, decreasing the concentration of drug in brain (16,17). Cefadroxil has a net negative charge at physiological pH and is transported by the organic anion transporters (OATs) (18,19). These transporters are present at the basolateral membrane of epithelial cells in renal proximal tubules, and are thought to be responsible for the active secretion of negatively charged endogenous and exogenous compounds, including cefadroxil. Although OAT1 (SLC22A6) and OAT3 (SLC22A8) are present in human kidney, OAT3 plays a stronger role in the active secretion of cephalosporins (20). And finally, experiments in Xenopus oocytes expressing the rat organic anion transporting polypeptide 2 Oatp2 (Slco1a4) showed that cefadroxil was also a substrate for this transporter (21).
Understanding the contribution of PepT1 toward the absorption and disposition of drugs has been a goal of several research groups for the past couple of decades. This transporter can influence the pharmacokinetics, especially the biopharmaceutical properties, of important therapeutic drugs including some β-lactam antibiotics, angiotensin-converting enzyme inhibitors and antiviral prodrugs, and the anticancer agent bestatin (2-4). However, no studies have provided definitive evidence on the quantitative contribution and relevance of PepT1 in the intestinal permeability and oral absorption of pharmacologically active agents including cefadroxil. Therefore, we proposed to study the in situ intestinal permeability of cefadroxil in wild-type and PepT1 knockout mice as a function of drug concentration, perfusate pH, regional permeability, and specificity. The in vivo absorption and disposition of cefadroxil were also examined in both genotypes after oral dosing of drug.
MATERIALS AND METHODS
Chemicals
[3H]Cefadroxil (0.8 Ci/mmol) was obtained from Moravek Biochemicals and Radiochemicals (Brea, CA). All others chemicals, including unlabeled cefadroxil, were purchased from Sigma-Aldrich (St. Louis, MO).
Animals
All studies were performed in 8-10 week old gender-matched wild-type (PepT1+/+) and PepT1 knockout (PepT1-/-) mice (22). The animals were kept in a temperature controlled room with 12-hr light and dark cycles, and access to a standard diet and water ad libitum. All of the procedures were approved by the University of Michigan Committee on Use and Care of Animals (UCUCA), and adhered to the Principles of Laboratory Animal Care (NIH publication #85-23, revised in 1985).
In Situ Single-Pass Jejunal Perfusions
Wild-type and PepT1 knockout mice were fasted overnight prior to experimentation. Following sodium pentobarbital (40 mg/kg ip) anesthesia, the mice were placed on a heated pad to maintain body temperature and isopropyl alcohol was used to sterilize the abdomen. The abdomen was opened through a midline incision to expose the abdominal cavity and the small intestine. An 8 cm segment of the proximal jejunum was isolated, 2 cm distal from the ligament of Treitz, after which the intestinal segment was rinsed and cleaned with isotonic saline solution. Two glass cannulas (1.9 mm in diameter) were then inserted at the proximal and distal ends of this segment and fixed firmly in place with silk suture. Subsequently, animals were transferred to a temperature-controlled chamber (31°C) to maintain body temperature and the inlet cannula was connected to a 10 mL syringe containing 10 μM cefadroxil in perfusate buffer (pH 6.5). This buffer contained 10 mM 2-(N-morpholino)ethanesulfonic acid (MES), 135 mM sodium chloride, and 5 mM of potassium chloride. The intestinal segment was perfused at a rate of 0.1 mL/min for 90 min using a syringe pump (Harvard Apparatus, South Natick, MA). Water flux was measured using a gravimetric method and the animals were sacrificed at the end of experimentation.
For concentration-dependent uptake studies, the perfusate contained cefadroxil over a wide range of values (0.01 – 25 mM). For pH-dependent studies (5.5 to 7.5), different ratios of 10 mM (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (HEPES)-tris(hydroxymethyl)aminomethane (Tris) and 10 mM MES – Tris were mixed, keeping the osmolarity of the buffer constant. Luminal pH was also altered by co-perfusing 10 μM cefadroxil with 0.1 mM dimethyl-amiloride (DMA). Specificity studies were performed by coperfusing 10 μM cefadroxil with 25 mM of the following potential inhibitors: L-histidine (LHis), glycine (Gly), glycylproline (GlyPro), glycylsarcosine (GlySar), cephalexin, cephalothin, p-aminohippurate (PAH), or N-methylnicotinamide (NMN).
In Situ Single-Pass Regional Perfusions in Intestines
Different regions of the small and large intestines were perfused simultaneously in wild-type and PepT1 knockout mice using a similar procedure to that described above for jejunal perfusions. However, for these experiments, a 2 cm length of duodenum was isolated (starting at the end of the stomach and ending at the ligament of Treitz), along with an 8 cm segment of jejunum (starting 2 cm distal to the ligament of Treitz), a 6 cm segment of ileum (starting 1 cm proximal to the cecum, and a 4 cm segment of colon (starting 1 cm distal to the cecum). All experiments were performed in pH 6.5 perfusate and 10 μM cefadroxil.
Analytical Method
Samples from the in-situ perfusion experiments were assayed for cefadroxil by high performance liquid chromatography (HPLC) using a Waters 616 pump, a Waters 717 autosampler, and a Waters 2487 dual λ absorbance detector set at 265 nm (Milford, MA). Separation was achieved on a Waters ODS-3 column (250 × 4.6 mm) using an isocratic mobile phase of 10% acetonitrile containing 0.01% trifluoroacetic acid (TFA) and 90% water containing 0.01% TFA, ambient temperature, at a flow rate of 1.0 mL/min. Prior to analysis, the perfusate samples were centrifuged at 11000 g for 10 min and 20 μL of supernatant was injected into the HPLC system. The calibration curves of cefadroxil standards were linear over the concentration range of 0.001 to 25 mM, and the retention time for cefadroxil was 11 min.
Oral Administration of Cefadroxil
Wild-type and PepT1 mice were fasted overnight (~ 14 hr) prior to each experiment. Unlabeled cefadroxil was dissolved in 200 μL of water along with 0.5 μCi/g (3H)cefadroxil for a total dose of 44.5 nmol/g. The solution was then administered by oral gavage to the mice using a 20G needle. Blood samples (15 - 20 μL) were collected by tail transections at 5, 10, 15, 20, 30, 45, 60, 90 and 120 min after oral dosing. The blood was collected in PCR tubes containing 1 μl of 7.5% EDTA and centrifuged at 3000 g for 3 min, room temperature. An aliquot of plasma (5 μL) was then placed in a scintillation vial containing 6 mL of CytoScint scintillation fluid (MP Biomedicals, Solon, OH). Radioactivity of the sample was measured using a dual channel liquid scintillation counter (LS 6000 SC; Beckman Coulter Inc., Fullerton, CA). Mice remained awake during the serial blood sampling and had free access to water throughout the experiment.
Data Analysis
The effective permeability (Peff) of cefadroxil was calculated according to the complete radial mixing model (parallel tube) as described previously (23,24):
| (1) |
where Q is the perfusion rate, Cin is the inlet concentration of cefadroxil, Cout is the outlet concentration of cefadroxil (corrected for water flux), and R and L are the radius and length of the intestinal segment, respectively. The steady-state flux (J) through the intestinal membrane was calculated as:
| (2) |
and the apparent Michaelis-Menten parameters V′max and K′m were estimated according to a single saturable term as follows:
| (3) |
The flux equation above, referenced to inlet (or bulk fluid) concentrations of drug, was also modified to account for drug concentrations at the intestinal wall (12,25). Accordingly, the Peff was separated into its component parts, the aqueous permeability (Paq) and the membrane permeability (Pw) such that:
| (4) |
where Paq was estimated by:
| (5) |
In this equation, A is a unitless constant (A = 2.50 Gz + 1.125), R is the radius, and D is the diffusion coefficient of cefadroxil in water. Gz is the Graetz number, which is the ratio of the mean residence time of a fluid in the intestine to the mean radial diffusion time of a solute, and is calculated according to:
| (6) |
Once the aqueous and wall permeabilities are known, concentrations of drug at the surface of the epithelial cell membrane (Cw) can be calculated as:
| (7) |
and the intrinsic Michaelis-Menten parameters Vmax and Km estimated as:
| (8) |
With respect to the oral dosing of cefadroxil, the plasma concentration-time profiles were analyzed using a noncompartmental approach (WinNonlin v5.3; Pharsight, Inc., Mountain View, CA). Pharmacokinetic parameters included the peak plasma concentration (Cmax), the time to reach a peak concentration (Tmax), and the area under the curve from time zero to 120 min (AUC0-120).
Statistical Analysis
The data are presented as mean ± standard error (SE). In order to test for significant differences between multiple groups, a one-way analysis of variance was used followed by either Tukey's or Dunnett's test. A p value ≤ 0.05 was considered statistically significant. Prism v4.0 software (GraphPad, La Jolla, CA) was used to perform nonlinear regression analyses of the flux data and to estimate the Michaelis-Menten parameters. Goodness of fit was determined by evaluating the standard error of parameter estimates, by the coefficient of determination (r2), and by visual inspection of the residual plots.
RESULTS
Concentration-Dependent Uptake Studies
To probe the saturable kinetics of cefadroxil transport in jejunum, in situ perfusions were performed over the concentration range of 0.01 – 25 mM for drug. As shown in Fig. 1 (panel A), the uptake of cefadroxil was nonlinear in which the V′max and K′m values were 4.8 ± 0.4 nmol/cm2/sec and 3.8 ± 1.2 mM, respectively (r2 = 0.894). By subtracting out the aqueous resistance from the total membrane resistance, and referencing the steady-state flux of cefadroxil to membrane surface concentrations (Fig. 1, panel B), the intrinsic transport parameters were estimated as Vmax = 4.5 ± 0.3 nmol/cm2/sec and Km = 2.1 ± 0.6 mM (r2 = 0.898). Thus, the uptake of cefadroxil was mediated by a low-affinity transporter (i.e., mM Michaelis constants) and by a single transport system, as corroborated by the linear slope observed in the Woolf-Augustinsson-Hofstee transformation of the data (Fig. 1, panel C).
Fig. 1.
Concentration-dependent uptake of cefadroxil (0.01-25 mM) in the jejunum of wild-type mice where Cin is the inlet concentration of cefadroxil in perfusate (A) and Cw is the estimated concentration of cefadroxil at the membrane wall (B), and as represented by a Woolf-Augustinnson-Hofstee transformation (C). All studies were performed in pH 6.5 buffer. Data are reported as mean ± SE (n=4-8).
Regional Permeability Studies
To determine if differences existed in the regional activity of PepT1, the effective permeability of 10 μM cefadroxil was measured in four regions of the intestines in mice. As shown in Fig. 2, the permeability of cefadroxil was substantially lower in the duodenum (4%), jejunum (12%) and ileum (5%) of PepT1 knockout mice as compared to that in wild-type mice. In contrast, there was no significant difference between genotypes in the permeability of cefadroxil in colon, which was < 10% of values in the small intestine of wild-type animals. In addition, there were no statistically significant differences in cefadroxil permeability between all four intestinal segments of PepT1 knockout mice.
Fig. 2.
Effective permeability of 10 μM cefadroxil in different intestinal regions of wild-type and PepT1 knockout mice. All studies were performed in pH 6.5 buffer. Data are reported as mean ± SE (n=4-8). Treatment groups with the same capital letter (A or B) are not statistically different.
Proton-Dependent Permeability Studies
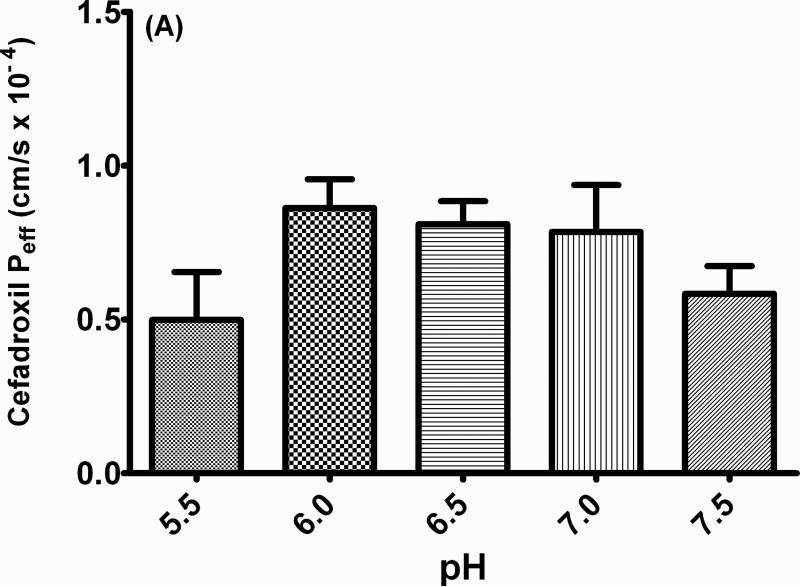
The effective permeability of 10 μM cefadroxil was initially evaluated over a range of physiological pH values in perfusate (i.e., from 5.5 to 7.5). As observed in Fig. 3 (panel A), cefadroxil permeability was somewhat higher at pH 6.0 - 7.0, although no significant differences were noted over the entire pH range. To further probe this relationship, DMA (an inhibitor of proton/sodium exchange at the apical membrane) was co-perfused with cefadroxil (Fig. 3, panel B). As observed in this drug interaction study, DMA inhibited the PepT1-mediated permeability of cefadroxil, presumably by reducing the driving force for proton:substrate co-transport across the intestinal membrane.
Fig. 3.
Proton-dependent permeability of 10 μM cefadroxil in the jejunum of wild-type mice as a function of pH 5.5 to 7.5 (A) and 0.1 mM of the sodium-proton inhibitor dimethyl-amiloride (DMA), at pH 6.5 (B). Data are reported as mean ± SE (n=4-8). ** p < 0.01, as compared to the control value.
Substrate Specificity Studies
The specificity of jejunal permeability for 10 μM cefadroxil was evaluated by co-perfusing this drug with a variety of potential transport inhibitors (25 mM). In particular, several transport mechanisms were probed with the use of L-His (i.e., substrate for peptide/histidine transporters) and Gly (i.e., substrate for amino acid transporters), GlyPro, GlySar and cephalexin (i.e., substrates for dipeptide transporters), PAH (i.e., substrate for organic acid transporters) and NMN (i.e., substrate for organic cation transporters). As shown in Fig. 4, the effective permeability of cefadroxil was significantly decreased by the addition of GlyPro, GlySar and cephalexin, indicating that cefadroxil transport in the jejunum was specific for apically located PepT1. On the other hand, no significant effect was observed when L-His, Gly, cephalothin, PAH or NMN was added to the perfusate solution.
Fig. 4.
Permeability of 10 μM cefadroxil in the jejunum of wild-type mice when co-perfused with potential inhibitors (25 mM). All studies were performed in pH 6.5 buffer. Data are reported as mean ± SE (n=4-8). **p < 0.01 and ***p < 0.001, as compared to the control value.
Oral Administration Studies
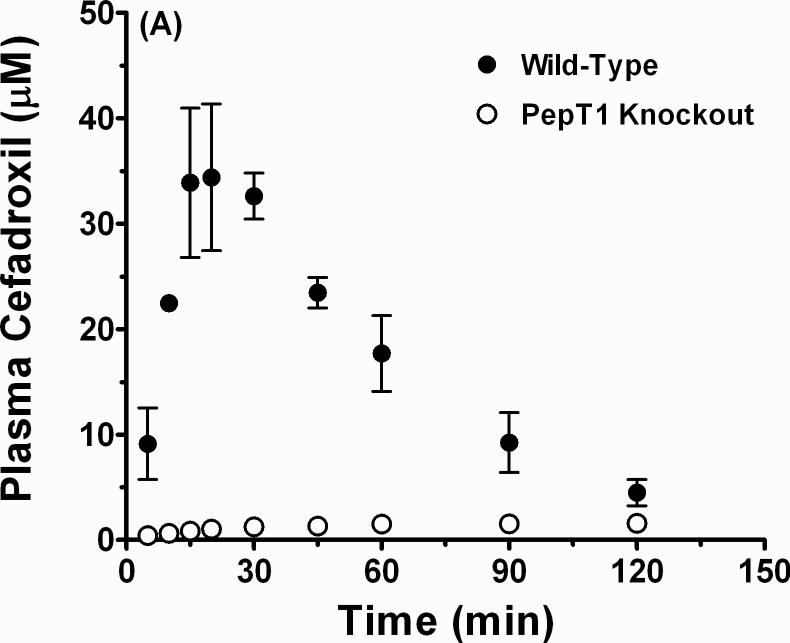
Fig. 5 shows that the plasma concentration-time profiles of cefadroxil after oral dosing were substantially lower in PepT1 knockout mice than in wild-type animals. Moreover, absorption appears to be the rate-limiting process in mice lacking intestinal PepT1 as the peak value(s) are rather flat and prolonged. With respect to our preliminary pharmacokinetic analysis (n=3), the Tmax was almost 4-fold higher (100 ± 20 min in PepT1 knockout vs. 27 ± 3 min in wild-type animals; p < 0.05), the Cmax 20- to 25-fold lower (1.6 ± 0.3 μM in PepT1 knockout vs. 36.4 ± 5.9 μM in wild-type animals; p < 0.01), and the AUC0-120 10- to 15-fold lower (156 ± 29 min•μM in PepT1 knockout vs. 2088 ± 124 min•μM in wild-type animals; p < 0.001) during PepT1 ablation. These results demonstrate that the oral availability of cefadroxil is significantly improved by the presence of intestinal PepT1.
Fig. 5.
Plasma concentration-time profiles of cefadroxil in wild-type and PepT1 knockout mice following a 44.5 nmol/g oral dose of drug. Data are reported as mean ± SE (n=3) in which the y-axis is displayed on a linear scale (A) and on a logarithmic scale (B).
DISCUSSION
The findings in this paper are novel in quantifying the contribution of intestinal PepT1, relative to other active and/or passive transport processes, in the absorption of a peptide-like drug cefadroxil. Using a combination of in situ perfusions and in vivo oral dosing studies in genetically modified mice, we found that: 1) jejunal uptake of cefadroxil was saturable with a Km on the order of 2 to 4 mM; 2) the permeability of cefadroxil in different regions of small intestine was about 10-fold lower in PepT1 knockout compared to wild-type mice; 3) no difference was observed between genotypes in the colonic permeability of cefadroxil, which was less than 10% of values in the small intestine of wild-type mice; 4) the jejunal permeability of cefadroxil was dependent on the proton-motive force; 5) cefadroxil permeability in the jejunum was specific for PepT1; and 6) the oral availability of cefadroxil after oral administration was substantially reduced in mice deficient in intestinal PepT1.
For passively absorbed drugs, rat and human permeability estimates based on jejunal perfusions are highly correlated and both values can be used with precision to predict in vivo absorption in human (26). However, the permeability estimates are 3.6 times higher in human than in rat regardless of the drugs’ physical-chemical properties. More importantly, significant deviations from linearity (Peff,human vs. Peff,rat) are observed for compounds that are actively transported. Although human permeability studies are not available for cefadroxil, such estimates are available for cephalexin, which is also an aminocephalosporin drug that serves as a substrate for PepT1. Thus, the Peff,human of 1.56 × 10-4 cm/s for cephalexin (27) is similar to our permeability estimate of 0.80 × 10-4 cm/s for cefadroxil in wild-type mice during in situ jejunal perfusions. Mice appear to be a good surrogate of human for studies investigating the transport of peptides/mimetics by PepT1, as described previously (7,28). In particular, these two species are similar with respect to driving forces (i.e., transporter stimulated by proton gradient and inside-negative membrane potential), substrate specificity and affinity, PepT1 expression in the intestine and kidney, and its apical localization in epithelial cells. In contrast, the rat exhibits greater gene expression profiles of intestinal PepT1 as compared to mice and human.
Several studies have demonstrated that cefadroxil can inhibit the uptake of GlySar in different experimental systems such as Caco-2 cell monolayers (29,30), LLC-PK1 cells stably transfected with PepT1 cDNA (31), and during in situ jejunal perfusions in mice (7). Other studies have demonstrated the direct uptake of [3H]cefadroxil in Xenopus oocytes expressing the cloned intestinal transporter PepT1 (11,14). However, fewer studies have described the transport properties of cefadroxil in animal models of small intestine (12,13,32,33) and no studies have quantitated the PepT1-mediated contribution in the intestinal absorption of this drug. PepT1 is a low-affinity transporter with Km values in the lower mM range for its substrates (2-4). In our in situ jejunal perfusion studies in wild-type mice, the estimated Michaelis constants for cefadroxil (K′m = 3.8 mM based on bulk fluid concentrations and Km = 2.1 mM based on intestinal wall concentrations) were consistent with its low-affinity transport by PepT1. These values were also consistent with the Michaelis constants of 5.9 mM (12) and 6.6 mM (13) for cefadroxil during in situ perfusions in proximal small intestine, and with the Michaelis constant of 1.1 mM for cefadroxil in PepT1 cRNA-injected Xenopus oocytes (11).
The pH-dependent transport of cefadroxil was demonstrated in Caco-2 cells in which the drug exhibited a “bell-shaped” curve with a maximum uptake at apical pH values of 6.0 to 6.5 (14). Whereas drug uptake increased by about 4-fold when shifting the pH from 7.4 to 6.0, the uptake of cefadroxil was decreased by 50% when further reducing the pH to 5.5. In contrast, our in situ perfusion studies in wild-type mice showed that the jejunal permeability of cefadroxil was not significantly affected by buffer pH (Fig. 3, panel A). This finding is similar to our previous study with GlySar, using the same animal model, where the permeability of dipeptide was increased by only 36% at pH 5.5 but no differences were observed at pH values of 5.0, 6.0, 6.5, 7.0 and 7.4 (7). The acidic microclimate layer that is present at the apical membrane of intestinal epithelial cells containing an intact blood supply can explain the “apparent” discrepancy between these in vitro and in situ studies. This layer, formed by mucus and sodium/proton exchange at the brush border membrane, creates an environment that is highly resistant to changes by bulk fluid pH in the intestine (34,35). As a result, the proton dependence of cefadroxil permeability was further probed by adding DMA to the buffer during our in situ jejunal perfusions. As observed in Fig. 3 (panel B), the permeability of cefadroxil was reduced by about 60% during these co-perfusion experiments, indicating an indirect effect of sodium/proton inhibition on the PepT1-mediated uptake of substrate at the apical membrane of enterocytes.
To confirm that PepT1 was the only transporter responsible for the intestinal absorption of cefadroxil, high concentrations (25 mM) of known PepT1 substrates were added to buffer during the in situ perfusion studies (Fig. 4). Thus, the dipeptides GlyPro and GlySar, and the aminocephalosporin drug cephalexin, significantly reduced the intestinal permeability of cefadroxil. On the other hand, the lack of effect by high concentrations (25 mM) of the amino acids L-histidine and glycine, the organic acid PAH, the organic base TEA, and the nonaminocephalosporin drug cephalothin, indicated that other potential transporters of cefadroxil were not a factor in its jejunal permeability. The minor (to negligible) permeability of cefadroxil in the small and large intestines of PepT1 knockout mice would also argue against other transporters as being important in drug absorption (Fig. 2).
Our in situ perfusion experiments in mice were designed to reflect the intestinal concentrations one would observe after oral administration of cefadroxil in human over a dose range of 250 – 1000 mg. Assuming a stomach fluid volume of 250 mL (36,37), the concentration of cefadroxil entering the small intestine would be estimated at 2.8 to 11.0 mM over this range, values that were covered by our concentration-dependent study in which cefadroxil was studied from 0.005 to 25 mM. Likewise, cefadroxil was dosed at 44.5 nmol/g in our oral absorption studies where the Cmax observed in wild-type mice was 36.4 μM (Fig. 5). This concentration reflected clinically relevant concentrations in human given that Cmax values of 25 to 50 μM were reported after oral cefadroxil doses of 250 to 500 mg (8,38,39).
It is noteworthy that the 10-fold reduction in cefadroxil permeability in small intestine during the in situ studies is reflected by similar changes (10- to 15-fold) in the systemic exposure of cefadroxil after oral dosing. This finding differs from the approximate 2-fold reductions that were observed for GlySar in PepT1 knockout vs. wild-type mice after oral dipeptide administration (22,28,40). The reasons for this difference may be two-fold: 1) given the larger size of cefadroxil (MW 363) over GlySar (MW 146), passive absorption plays a much reduced role as drug transits through the gastrointestinal tract, and 2) the 10- to 15-fold reduction in cefadroxil systemic exposure may reflect, to some extent, the fact that blood levels were only obtained for two hours. It is possible that, given the divergent terminal slopes (i.e., decreasing for wild-type mice and flat for PepT1 knockout animals), the differences in oral availability between genotypes would lessen over time. In this second scenario, the intestine's residual length and long residence times would allow passive processes (e.g., diffusion, paracellular flux) to play a bigger role in the absence of PepT1. This latter scenario is intriguing as it could also explain why no obvious phenotypic abnormalities were observed between wild-type and PepT1 knockout mice (22).
CONCLUSIONS
In conclusion, the results from these studies are unique in characterizing, for the first time, the in situ and in vivo transport properties of cefadroxil in wild-type and PepT1 knockout mice. The findings are definitive in demonstrating that intestinal PepT1 plays a pivotal role in improving both the rate and extent of absorption for a peptide-like drug. Moreover, the findings strongly support the increasing effort to exploit intestinal PepT1 as a targeting strategy for prodrugs and new chemical entities.
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant R01-GM035498] (to D.E.S.).
REFERENCES
- 1.Daniel H, Spanier B, Kottra G, Weitz D. From bacteria to man: archaic proton-dependent peptide transporters at work. Physiol. 2006;21:93–102. doi: 10.1152/physiol.00054.2005. [DOI] [PubMed] [Google Scholar]
- 2.Rubio-Aliaga I, Daniel H. Peptide transporters and their roles in physiological processes and drug disposition. Xenobiotica. 2008;38:1022–1042. doi: 10.1080/00498250701875254. [DOI] [PubMed] [Google Scholar]
- 3.Brandsch M, Knutter I, Bosse-Doenecke E. Pharmaceutical and pharmacological importance of peptide transporters. J Pharm Pharmacol. 2008;60:543–585. doi: 10.1211/jpp.60.5.0002. [DOI] [PubMed] [Google Scholar]
- 4.Smith DE, Clémençon B, Hediger MA. Proton-coupled oligopeptide transporter family SLC15: physiological, pharmacological and pathological implications. Mol Aspects Med. doi: 10.1016/j.mam.2012.11.003. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fei YJ, Kanai Y, Nussberger S, Ganapathy V, Leibach FH, Romero MF, Singh SK, Boron WF, Hediger MA. Expression cloning of a mammalian proton-coupled oligopeptide transporter. Nature. 1994;368:563–566. doi: 10.1038/368563a0. [DOI] [PubMed] [Google Scholar]
- 6.Shen H, Smith DE, Yang T, Huang YG, Schnermann JB, Brosius FC., 3rd Localization of PEPT1 and PEPT2 proton-coupled oligopeptide transporter mRNA and protein in rat kidney. Am J Physiol. 1999;276:F658–F665. doi: 10.1152/ajprenal.1999.276.5.F658. [DOI] [PubMed] [Google Scholar]
- 7.Jappar D, Wu SP, Hu Y, Smith DE. Significance and regional dependency of peptide transporter (PEPT) 1 in the intestinal permeability of glycylsarcosine: in situ single-pass perfusion studies in wild-type and Pept1 knockout mice. Drug Metab Dispos. 2010;38:1740–1746. doi: 10.1124/dmd.110.034025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanrisever B, Santella PJ. Cefadroxil: a review of its antibacterial, pharmacokinetic and therapeutic properties in comparison with cephalexin and cephradine. Drugs. 1986;32(Suppl 3):1–16. doi: 10.2165/00003495-198600323-00003. [DOI] [PubMed] [Google Scholar]
- 9.Buck RE, Price KE. Cefadroxil, a new broad-spectrum cephalosporin. Infection. 1980;8(Suppl 5):S532–S537. doi: 10.1128/aac.11.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsuji A. Intestinal absorption of β-lactam antibiotics. In: Taylor MD, Amidon GL, editors. Peptide-based drug design. American Chemical Society; Washington DC: 1995. pp. 101–134. [Google Scholar]
- 11.Boll M, Markovich D, Weber W-M, Korte H, Daniel H, Murer H. Expression cloning of a cDNA from rabbit small intestine related to proton-coupled transport of peptides, β-lactam antibiotics and ACE-inhibitors. Pflügers Arch – Eur J Physiol. 1994;429:146–149. doi: 10.1007/BF02584043. [DOI] [PubMed] [Google Scholar]
- 12.Sinko PJ, Amidon GL. Characterization of the oral absorption of beta-lactam antibiotics. I. Cephalosporins: determination of intrinsic membrane absorption parameters in the rat intestine in situ. Pharm Res. 1988;5:645–650. doi: 10.1023/a:1015974920682. [DOI] [PubMed] [Google Scholar]
- 13.Sánchez-Picó A, Peris-Ribvera J-E, Toledano C, Torres-Molina F, Casabó V-G, Martín-Villodre A, Plá-Delfina JM. Non-linear intestinal absorption kinetics of cefadroxil in the rat. J Pharm Pharmacol. 1989;41:179–185. doi: 10.1111/j.2042-7158.1989.tb06425.x. [DOI] [PubMed] [Google Scholar]
- 14.Wenzel U, Gebert I, Weintraut H, Weber W-M, Clauß W, Daniel H. Transport characteristics of differently charged cephalosporin antibiotics in oocytes expressing the cloned intestinal peptide transporter PEPT1 and in human intestinal Caco-2 cells. J Pharmacol Exp Ther. 1996;277:831–839. [PubMed] [Google Scholar]
- 15.Shen H, Ocheltree SM, Hu Y, Keep RF, Smith DE. Impact of genetic knockout of PEPT2 on cefadroxil pharmacokinetics, renal tubular reabsorption, and brain penetration in mice. Drug Metab Dispos. 2007;35:1209–1216. doi: 10.1124/dmd.107.015263. [DOI] [PubMed] [Google Scholar]
- 16.Ocheltree SM, Shen H, Hu Y, Xiang J, Keep RF, Smith DE. Mechanisms of cefadroxil uptake in the choroid plexus: studies in wild-type and PEPT2 knockout mice. J Pharmacol Exp Ther. 2004;308:462–467. doi: 10.1124/jpet.103.060400. [DOI] [PubMed] [Google Scholar]
- 17.Shen H, Keep RF, Hu Y, Smith DE. PEPT2 (Slc15a2)-mediated unidirectional transport of cefadroxil from cerebrospinal fluid into choroid plexus. J Pharmacol Exp Ther. 2005;315:1101–1108. doi: 10.1124/jpet.105.090654. [DOI] [PubMed] [Google Scholar]
- 18.Takeda M, Babu E, Narikawa S, Endou H. Interaction of human organic anion transporters with various cephalosporin antibiotics. Eur J Pharmacol. 2002;438:137–142. doi: 10.1016/s0014-2999(02)01306-7. [DOI] [PubMed] [Google Scholar]
- 19.Khamdang S, Takeda M, Babu E, Noshiro R, Onozato ML, Tojo A, Enomoto A, Huang XL, Narikawa S, Anzai N, Piyachaturawat P, Endou H. Interaction of human and rat organic anion transporter 2 with various cephalosporin antibiotics. Eur J Pharmacol. 2003;465:1–7. doi: 10.1016/s0014-2999(03)01381-5. [DOI] [PubMed] [Google Scholar]
- 20.Ueo H, Motohashi H, Katsura T, Inui K-I. Human organic anion transporter hOAT3 is a potent transporter of cephalosporin antibiotics, in comparison with hOAT1. Biochem Pharmacol. 2005;70:1104–1113. doi: 10.1016/j.bcp.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 21.Nakakariya M, Shimada T, Irokawa M, Koibuchi H, Iwanaga T, Yabuuchi H, Maeda T, Tamai I. Predominant contribution of rat organic anion transporting polypeptide-2 (Oatp2) to hepatic uptake of beta-lactam antibiotics. Pharm Res. 2008;25:578–585. doi: 10.1007/s11095-007-9427-9. [DOI] [PubMed] [Google Scholar]
- 22.Hu Y, Smith DE, Ma K, Jappar D, Thomas W, Hillgren KM. Targeted disruption of peptide transporter Pept1 gene in mice significantly reduces dipeptide absorption in intestine. Mol Pharm. 2008;5:1122–1130. doi: 10.1021/mp8001655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Komiya I, Park JY, Kamani A, Ho NFH, Higuchi WI. Quantitative mechanistic studies in simultaneous fluid flow and intestinal absorption using steroids as model solutes. Int J Pharm. 1980;4:249–262. [Google Scholar]
- 24.Kou JH, Fleisher D, Amidon GL. Calculation of the aqueous diffusion layer resistance for absorption in a tube: application to intestinal membrane permeability determination. Pharm Res. 1991;8:298–305. doi: 10.1023/a:1015829128646. [DOI] [PubMed] [Google Scholar]
- 25.Johnson DA, Amidon GL. Determination of intrinsic membrane transport parameters from perfused intestine experiments: a boundary layer approach to estimating the aqueous and unbiased membrane permeabilities. J Theor Biol. 1988;131:93–106. doi: 10.1016/s0022-5193(88)80123-1. [DOI] [PubMed] [Google Scholar]
- 26.Fagerholm U, Johansson M, Lennernäs H. Comparison between permeability coefficients in rat and human jejunum. Pharm Res. 1996;13:1336–1342. doi: 10.1023/a:1016065715308. [DOI] [PubMed] [Google Scholar]
- 27.Lennernäs H. Animal data: the contributions of the Ussing chamber and perfusion systems to predicting human oral drug delivery in vivo. Adv Drug Deliv Rev. 2007;59:1103–1120. doi: 10.1016/j.addr.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 28.Jappar D, Hu Y, Smith DE. Effect of dose escalation on the in vivo oral absorption and disposition of glycylsarcosine in wild-type and Pept1 knockout mice. Drug Metab Dispos. 2011;39:2250–2257. doi: 10.1124/dmd.111.041087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bretschneider B, Brandsch M, Neubert R. Intestinal transport of β-lactam antibiotics: analysis of the affinity at the H+/peptide symporter (PEPT1), the uptake into Caco-2 cell monolayers and the transepithelial flux. Pharm Res. 1999;16:55–61. doi: 10.1023/a:1018814627484. [DOI] [PubMed] [Google Scholar]
- 30.Ganapathy ME, Brandsch M, Prasad PD, Ganapathy V, Leibach FH. Differential recognition of β-lactam antibiotics by intestinal and renal peptide transporters, PEPT 1 and PEPT 2. J Biol Chem. 1995;270:25672–25677. doi: 10.1074/jbc.270.43.25672. [DOI] [PubMed] [Google Scholar]
- 31.Terada T, Saito H, Mukai M, Inui K-I. Recognition of β-lactam antibiotics by rat peptide transporters, PEPT1 and PEPT2, in LLC-PK1 cells. Am J Physiol Renal Physiol. 1997;273:F706–F711. doi: 10.1152/ajprenal.1997.273.5.F706. [DOI] [PubMed] [Google Scholar]
- 32.Naruhashi K, Sai Y, Tamai I, Suzuki N, Tsuji A. PepT1 mRNA expression is induced by starvation and its level correlates with absorptive transport of cefadroxil longitudinally in the rat intestine. Pharm Res. 2002;19:1417–1423. doi: 10.1023/a:1020436028194. [DOI] [PubMed] [Google Scholar]
- 33.Oulianova N, Cheng D, Huebert N, Chen Y. Human oral drugs absorption is correlated to their in vitro uptake by brush border membrane vesicles. Int J Pharm. 2007;336:115–121. doi: 10.1016/j.ijpharm.2006.11.045. [DOI] [PubMed] [Google Scholar]
- 34.Lucas M. Determination of acid surface pH in vivo in rat proximal jejunum. Gut. 1983;24:734–739. doi: 10.1136/gut.24.8.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Högerle ML, Winne D. Drug absorption by the rat jejunum perfused in situ. dissociation from the pH-partition theory and role of microclimate-pH and unstirred layer. Naunyn-Schmiedeberg's Arch Pharmacol. 1983;322:249–255. doi: 10.1007/BF00508339. [DOI] [PubMed] [Google Scholar]
- 36.Dressman J, Butler J, Hempenstall J, Reppas C. The BCS: where do we go from here? Pharm Technol. 2001;25:68–76. [Google Scholar]
- 37.Butler JM, Dressman JB. The developability classification system: application of biopharmaceutics concepts to formulation development. J Pharm Sci. 2010;99:4940–4954. doi: 10.1002/jps.22217. [DOI] [PubMed] [Google Scholar]
- 38.La Rosa F, Ripa S, Prenna M, Ghezzi A, Pfeffer M. Pharmacokinetics of cefadroxil after oral administration in humans. Antimicrob Agents Chemother. 1982;21:320–322. doi: 10.1128/aac.21.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garrigues TM, Martin U, Peris-Ribera JE, Prescott LF. Dose-dependent absorption and elimination of cefadroxil in man. Eur J Clin Pharmacol. 1991;41:179–183. doi: 10.1007/BF00265914. [DOI] [PubMed] [Google Scholar]
- 40.Ma K, Hu Y, Smith DE. Influence of fed-fasted state on intestinal PEPT1 expression and in vivo pharmacokinetics of glycylsarcosine in wild-type and Pept1 knockout mice. Pharm Res. 2012;29:535–545. doi: 10.1007/s11095-011-0580-9. [DOI] [PMC free article] [PubMed] [Google Scholar]