Abstract
Self-assembled monolayers are a unique class of nanostructured materials, with properties determined by their molecular lattice structures, as well as the interfaces with their substrates and environments. As with other nanostructured materials, defects and dimensionality play important roles in the physical, chemical, and biological properties of the monolayers. In this review, we discuss monolayer structures ranging from surfaces (two-dimensional) down to single molecules (zero-dimensional), with a focus on applications of each type of structure, and on techniques that enable characterization of monolayer physical properties down to the single-molecule scale.
1. Introduction
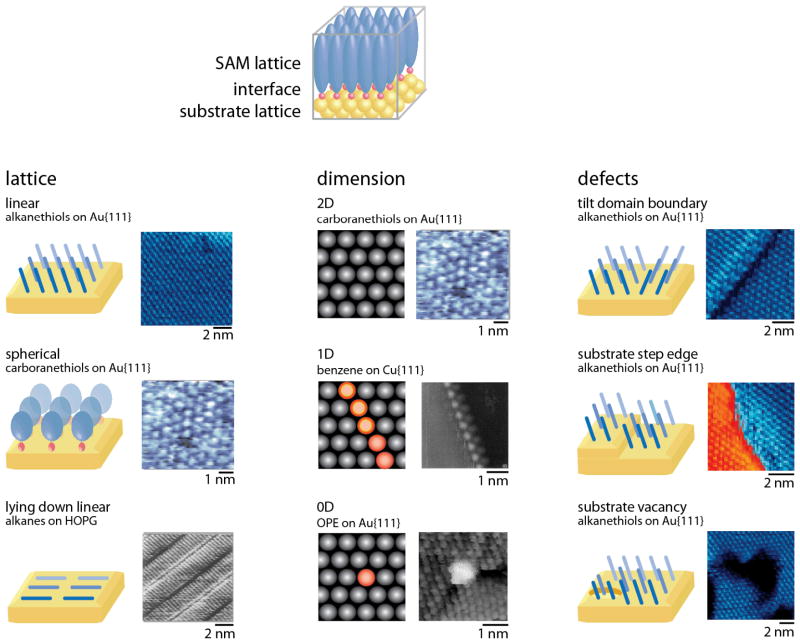
The unique chemistry of nanostructured materials evolves due to balance between the lattice and bonding structures of the materials, the chemistries of their interfaces, the dimensionalities of their structures, and the types and distributions of defects (Figure 1). In comparison with other nanoscale materials, the chemistries and structures of self-assembled monolayers (SAMs) are largely determined by their interfaces, which account for a relatively high proportion of the atoms (typical monolayer thickness ranges from 1–3 nm1), and also frequently dominate the energetics of structure formation. For instance, in the most common synthetic SAMs, alkanethiols on gold, the enthalpy of the gold–sulfur bond formation (~50 kcal/mol)2 is several times larger than the combined interactions of the alkyl tails with surrounding molecules (1–2 kcal/mol·CH2).3 Importantly, if the enthalpy of assembly at one interface provides a strong driving force for monolayer formation, it is possible to tune many other material properties and still achieve ordered lattices.
Figure 1.
Self-assembled monolayers have molecular lattices that optimize interactions both with substrate lattices and between molecules in the monolayers, leading to a variety of lattice structures. Molecular structures in monolayers exhibit restricted dimensionality, similar to other nanostructured materials, which changes molecular behavior through directional coupling and other effects. Adapted with permission from refs. 19, 127, and 163. Defects in monolayer structure arise from substrate structure or molecular interactions, and create reactive sites in materials that can be used to control and to characterize molecular properties. Adapted with permission from ref. 12.
Monolayer structures optimize both interactions with the substrate and intermolecular interactions.1,4–6 Lattice structures in monolayers are determined by both the substrate lattice and the chemistries and structures of the molecules forming the monolayers. In alkanethiol monolayers, linear molecules are attached to gold surfaces via Au–S bonds; both the organization of the sulfur headgroups on the gold lattice and the packing of the alkyl tails influence molecular lattice formation. For other molecules, such as adamantanethiols and carboranethiols, the tails are bulkier than the headgroups, and can play larger roles in lattice formation.6 In still other monolayers, such as those formed based on noncovalent interactions with graphite, molecules lie down, maximizing their interactions with the substrate.4
Although monolayers are typically considered to be two-dimensional (2D) structures, it is equally possible to assemble one-dimensional (1D) and zero-dimensional (0D) molecular structures on surfaces. As with nanocrystals, the definitions of dimensionality arise from the physical properties of interest in the structure – that is, anisotropic molecules that assemble across a 2D surface, but display strong directional coupling may be considered as 1D structures.7,8 Similarly, individual functional molecules deposited on surfaces can act as 0D structures, with properties that can be controlled and measured individually.9,10 Dimensionality at the molecular scale can also be combined with large-scale patterning processes such as soft lithography.1,11
Defects are important in understanding and predicting the behavior of monolayers.12 Since monolayers are often tightly coupled to solid substrates, irregularities in the substrate lattices (such as atomic step edges) can create offsets in the monolayers.5 Defects can also arise from the molecular lattices. For instance, many classes of molecules tilt relative to the surface normal,5 creating areas of heterogeneous structure between domains of molecules oriented in different azimuthal directions. Still other defects can be created as monolayers are formed. When alkanethiols are assembled on gold from solution, thiols can extract gold atoms from the surface, resulting in one-atom-deep ‘etch pits’ in the Au{111} substrate surface that disrupt monolayer structures.5 These and other defects can be selectively removed by subsequent monolayer and substrate dynamics and processing.13
Importantly, as with other nanostructured materials, defects are often the most reactive sites in the materials, and dominate both access of additional molecules to the substrates and the dynamics of the systems. In the context of monolayers, this reactivity can be exploited to design 1D and 0D structures within 2D monolayers, or to nucleate processes such as molecular exchange.14–20
Characterization of lattice structures and defects in SAMs and related structures relies on surface-sensitive tools that provide chemical, electronic, and/or topographic information.12,21 Typical 2D techniques providing chemical information about surfaces and adsorbates include grazing angle Fourier transform infrared (FTIR) spectroscopy, Raman spectroscopy, electron diffraction (transmission electron microscopy, TEM, and low energy electron diffraction, LEED), near-edge X-ray absorption fine structure (NEXAFS), and neutron scattering.1 Scanning probe techniques – atomic force microscopy (AFM) and scanning tunneling microscopy (STM) – provide more localized information, down to the single-molecule scale.21 In this review, we discuss the physical properties and applications of self-assembled monolayers as nanostructured materials, with a focus on assembled structures ranging from 2D (full monolayers) down to 0D (single molecules), including methods for top-down patterning of monolayer structures. Throughout, we select examples highlighting the breadth of molecular functions available through these materials, and specialized characterization techniques and methods that enable quantitative measurements of these properties down to the single-molecule scale.
2. Two-dimensional structure and function
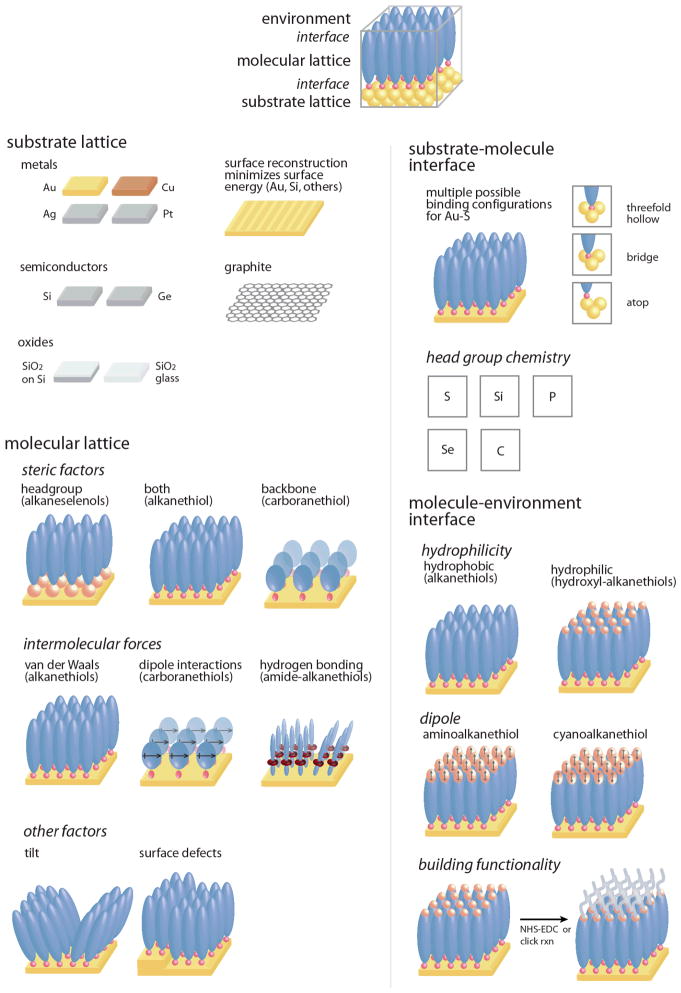
Studies of 2D monolayers on solid surfaces began as early as the 1940s,22 and now encompass systems ranging from alkanes and aromatic molecules to much larger macromolecules, and substrates from glass to single-crystalline metal surfaces.1,4,23–25 Self-assembled monolayer structure is influenced by the substrate lattice, the interface between the molecular head group functionality and the substrate, a molecular lattice formed based on head group and backbone chemistries, and an exposed interface dominated by the chemical functionality of the tail group (Figure 2). Monolayers of alkanethiols on Au{111} are the most common and most studied;1 thus, we discuss many aspects of monolayer formation and structure in relation to these systems. The strategies developed for controlling monolayers of thiols on Au{111} will, over time, be translated to other molecule–substrate systems.6,26
Figure 2.
Two-dimensional molecular structures on surfaces form via the interplay between substrates and molecular lattices, and interactions at interfaces.
2.1. Substrate lattices
Monolayers have been formed on metals including Au,1,12,27 Ag,27–29 Cu,27,30,31 Pd,30,32 Pt,33,34 Ni,30,35 Fe,30 Ti,36 Te,37 Hg,38 and GaIn.39,40 Since bonding with the substrate is often a critical driving force for monolayer formation, the substrate atomic lattice and electronic structure are key. The Au{111} crystal face is often used in monolayer formation for a number of reasons–gold readily forms bonds with thiols, and such surfaces are easily formed, commercially available, and stable under normal atmospheric conditions. Other metal surfaces (such as Ag, Cu, Pt, and Pd) are more easily oxidized; these other substrates are often used in studies of monolayers under vacuum, in which molecules are evaporated onto the surface rather than deposited from solution.
Semiconductors such as Si, Ge, GaAs, and InP can also be used as substrates for monolayer formation.41–46 Silicon is particularly widely used due to its electronic applications; however, silicon forms a native oxide under normal atmospheric conditions. Thus, chemistry has developed to interface with both silicon and its oxide.47–49 Other semiconductors have also been explored as monolayer substrates, including GaN50 and ZnSe.51
Highly oriented pyrolytic graphite (HOPG) is a semimetal and forms atomically flat surfaces over relatively large areas (>1000 nm on an edge). Although the surface does not readily form covalent bonds to common functional groups such as thiols or amines, it can be used as a substrate for monolayers in which aromatic groups in the monolayers form π-stacking interactions with graphite (or graphene) surfaces.4,52–55
2.2. Substrate-head-group interfaces
The substrate–molecule interface influences both monolayer structures and serves as the electronic connection to the substrate. Thus, understanding these interfaces is important in predicting structures, stabilities, and physical properties (such as conductance) of SAMs, as well as of molecules matrix-isolated within them.
Although there are many possible pairings of substrate and molecular head group chemistries, the substrate–molecule interface is more complex than would be predicted based solely on bonding between substrates and the functional head groups. The substrate lattice presents multiple chemically non-equivalent binding sites, involving one or more atoms. Further, substrate surfaces often reconstruct to minimize energy; these changes create lower symmetry surface structures that also influence the interfaces. Binding of the functional head group can lead to additional surface structural changes, and restrictions on orbital hybridization can influence the angle between molecular backbones and surfaces.
Surface reconstruction is widely observed for metal and semiconductor surfaces.56,57 Here, we briefly discuss the relationship between surface reconstruction and alkanethiol SAM formation on Au{111} as an illustrative example of the complexities of these rearrangements.
Substrate atoms on bare Au{111} surfaces compact slightly, forming a reconstruction with both face-centered-cubic- and hexagonally close-packed-stacked zones and a (22×√3) unit cell relative to the Au{111} lattice, referred to as the herringbone reconstruction. When sulfur-containing molecules (including thiols, disulfides, and thioethers) bind to Au{111}, the formation of Au–S bonds removes the reconstruction, a phenomenon that is possible to visualize by STM. The nominal strength of Au–S bonds (~50 kcal/mol) is greater than that of Au–Au bonds,28,58 and the ability to remove the reconstruction can be used as a qualitative descriptor of molecule–substrate bond strengths. For instance, thioethers have weaker molecule–substrate bonds than thiols; however, SAMs of thioethers nonetheless lift the Au{111} herringbone reconstruction.59–62 In contrast, adsorbed benzene does not lift the native Au{111} reconstruction.63
The growing consensus is that thiols lift selected atoms to sit at a subset of sites on the restored Au{111} lattice; these are referred to as Au adatoms.64 It has been known for some time that thiolates move as a Au-thiolate complex at defect sites such as step edges.65 Recent calculations show that there are multiple stable surface reconstructions (similar to within 0.2 eV): one sulfur bound to a three-fold hollow site, one sulfur bound to a bridge site, one sulfur bound to one Au adatom, and two sulfur atoms bound to one Au adatom.66–71 Thiols on Ag{111} exhibit different binding properties than those on Au{111},72 typically binding at bridge and three-fold hollow sites, and are tilted at angles closer to the surface normal.29 Selenols on Au{111} have more promiscuous binding, and as a result Moiré patterns are observed in STM images.73
The multiplicity of possible binding structures highlights the importance of techniques for characterizing the buried interfaces. Some chemical properties can be characterized at large (micron to millimeter) scales using techniques such as FTIR spectroscopy and X-ray photoelectron spectroscopy (XPS), or at intermediate scales by measuring reductive desorption electrochemically.20 However, characterization at the sub-nanometer scale with STM-based techniques provides complementary information about heterogeneous structure important for understanding reconstructions. Weiss and coworkers found that the largest buried dipole in a SAM can be mapped by varying the tunneling gap distance in STM imaging. For alkanethiols on Au{111}, this locates the Au–S bond; thus, correlating buried dipoles (head groups) with topography (tail groups) enabled the first single-molecule tilt angle measurement.74 A similar spectroscopy technique applied to cyclohexanethiol on Au{111} distinguishes between binding at bridge and atop sites.2,24,27
In addition to the molecules with head groups based on sulfur, discussed above,1,28,25 many other head groups are available, including isocyanides,75 Si,76–78 P,50,79–81 Se,29,73,82,83 carboxylic acids,84,85 and head groups that allow noncovalent π-stacking interactions on highly oriented graphite, such as cyclodextrins,86–89 pentacene,90 and peptides.91–93 While these surface connectors are relatively less explored, comparisons between molecule-substrate systems provide insight into how to translate surface attachment and patterning strategies from one system to another, and will hopefully lead to the identification of new strategies.
Selenols provide a useful comparison with thiols since they come from the same group in the periodic table.83,94–96 In contrast to the hexagonal lattices formed by alkanethiols, alkaneselenols form either densely packed distorted hexagonal lattices incommensurate with the underlying Au{111} substrate, or commensurate linear missing-row structures.73 These differences in bonding likely contribute to the differences in conductance observed between alkaneselenol and alkanethiol SAMs.97 Short (n = 2–6) n-alkaneselenols follow the odd/even rule for stability (even chain lengths are more stable), as do alkanethiols. However, selenols are more stable toward exchange than alkanethiols of equivalent length,98 which has been attributed to differences in bonding configurations (sp3 and sp).29 Polyfunctional head groups increase binding strength; for instance, diselenides and dithiols displace monothiolate monolayers on Au, similar to chelating effects found for other molecules.94
Silanes and silanols are typically used to form monolayers on silica (SiO2) surfaces.25,99,100 Head groups based on phosphorus (including phosphonates, phosphines, and phosphonic acids) are also common. Phosphorus headgroup binding on metals, such as Au, is generally weaker than thiol binding, but strong enough (in the case of trimethylphosphine) to lift the Au reconstruction.79 Phosphonic acids form stable monolayers on Ti and Au substrates,36 and phosphonates and alkylphosphonic acids assemble on GaN and nitinol (NiTi), respectively.50,101 Alkylphosphonates assemble on Al2O3, TiO2, ZrO2, planar mica, TiO2 and SiO2;80 however, monolayer structures are typically found to be less regular, and largely based on backbone and tailgroup interactions.
Monolayers can also form based on noncovalent interactions with substrates. Noncovalently bound molecular monolayers have been studied by STM, in many cases at low temperature, to stabilize the self-assembled structures sufficiently for imaging.102,103 Many conjugated molecules can also form 2D assemblies on surfaces, including styrene,104 pentacene,90 pyrene,25 and larger graphitic molecules.105,106 Peptides can also assemble noncovalently on graphite surfaces, typically forming linear arrangements due to hydrogen-bonding; such structures are discussed more extensively in the 1D section.92,93,107,108
2.3. Molecular lattices
Molecular lattice structures are influenced by both molecule–substrate interfaces and the packing of the molecular backbones. The relative influence of these contributions can vary due to factors including interfacial bond strengths and sizes of the molecular backbones. Molecular interactions within lattices include van der Waals, dipole–dipole, and hydrogen-bonding interactions. van der Waals interactions between molecules in lattices contribute to stability. In alkanethiol–Au SAMs, this contribution increases with alkyl chain length, as each methylene (-CH2-) unit contributes approximately 1 kcal/mol.30 Alkanethiolates on Au{111} tilt roughly 30° relative to the surface normal, to maintain nominally all-trans configurations that maximize van der Waals interactions.25,74 As chain lengths increase in alkanethiols, the range of accessible backbone tilt angles is constrained due to steric effects.109 Monolayers on other substrates have different tilt angles, again determined by the substrate lattice and maximizing intermolecular interactions.5,110
Chemical functional groups incorporated into the molecular backbone can influence molecular lattice structures. For instance, 3-mecapto-N-nonylpropionamide (1ATC9) is chemically similar to the more commonly used decanethiol, with an amide functional group replacing a methylene unit near the thiol headgroup. Two phases have been observed, one normal to the surface, the other tilted 18° relative to the surface normal.111 The amide groups enable hydrogen bonding between molecules in the monolayer in the tilted conformation, increasing intermolecular interaction strength by ~6 kcal/mol, and increasing the electronic polarizability of the monolayer.91,111,112 Such interactions can cause phase segregation, creating monolayers with two distinct types of domains: 1ATC9 monolayers phase separate from n-alkanethiols of equivalent length based on their hydrogen-bonding networks.113 Amide-bonding networks in SAMs have also been used for charge transport across Ag/SAM//Ga2O3/EGaIn junctions.114
Even functional groups that introduce relatively strong interactions such as hydrogen bonding can disrupt monolayer formation if they interfere with molecular packing. The di-amide and tri-amide counterparts of the system described above, 3-mercapto-N-(N′-n-hexylacetamido)propionamide (2ATC9) and 3-mercapto-N-(N′-(N′ ′-n-propylacetamido)acetamido)propionamide, do not form ordered monolayers, at least in the outermost alkyl layers measured with the STM.113,115
Other chemical functional groups can be added within the molecular backbones to impact monolayer formation, including alkenes, alkynes, diacetylene, aryl groups, oligo(phenylene ethynylene), oligo(ethylene glycol), sulfones, and azobenzenes.14,15,25,116,117 Functional groups can be used to create a wide variety of properties in monolayers, including switchable conductivity,118 targeted capture of biological molecules,119 or the ability to crosslink under an electron beam120 or restructure under an ion beam121 to act as a molecular resist.
Cage molecules such as adamantanes and carboranes are classes of SAM substituents in which the cage serves as a short, sterically bulky backbone.6,82,122,123 Thiolated cage molecules thus have large molecular lattice constants relative to alkanethiols (~0.7 nm vs. 0.5 nm), and weaker intermolecular interactions,124 meaning they are easily displaced from Au{111} surfaces by alkanethiols.6,123 Adamantane cages can be engineered to orient normal or tilted relative to the surface, by thiolating at either a primary (2-adamantanethiol) or tertiary (1-adamantanethiol) carbon, making it possible to create or to eliminate tilt defects based on small changes in molecular structure.123 Larger diamondoid structures also form monolayers with a variety of interesting structures and properties.6,125,126
As with alkanethiols, carboranethiols have been designed to have molecular dipoles that contribute to molecular lattice interactions;6,127 carboranethiols with strong lateral dipole–dipole interactions dominate surface coverage when codeposited with carboranethiols that lack such interactions.127 Dipole effects can also be important in noncovalently bound monolayers. For instance, styrene, under vacuum and low temperature, forms dipole-organized assemblies on Au{111}.104
2.4 Molecule-environment interfaces
Terminal functional groups determine the physical properties at environmental interfaces. Terminal group chemistries can vary widely, including methyl groups, amines, nitriles, carboxylic acids, sulfides, alcohols, ferrocenes, pyrroles, fullerenes, and biomolecules.1,119,128–137
Interface physical properties can be changed dramatically based on relatively straightforward chemical changes to terminal groups. The conventionally used methyl terminal group for alkanethiolates produces hydrophobic SAMs, whereas hydroxyl-terminated alkanethiols assemble into hydrophilic SAMs, even though the bulk of the backbone is hydrophobic. Similarly, SAMs can present large numbers of aligned dipoles at the interface, which can be reversed based on terminal substituents, for instance electron-rich amines vs. electron-poor nitriles.130
Specialized terminal groups create additional possibilities for monolayer function. Azobenzene terminal groups isomerize on exposure to UV (365 nm) and blue (450 nm) light, respectively.14,15,138 Pyrrole-terminated SAMs polymerize upon plasma exposure, forming conductive and mechanically active polymers.134 Hydroxyl-terminated SAMs have also been used to immobilize Au nanoparticles on surfaces.139
Simple functional groups displayed at SAM–environment interfaces can be used for further reactions to display more complex molecules on surfaces. Common functional groups incorporated into SAMs for reactions to create biological interfaces include carboxylic acids, amines, and azides.119,140–145 Care is needed in further functionalization of SAMs as the reaction exothermicity of vigorous surface reactions can disrupt the assemblies and nanostructures previously formed.11,13,142,146
Carboxylic acids can be used in standard synthetic reactions (such as N-hydroxy-succinimide–ethylenediaminecarbodiimide, NHS-EDC, coupling) to create amide linkages – such reactions have been used to attach benzensulfonamide ligands for enzymes,147 as well as for attachment of small-molecule neurotransmitters, their precursors, proteins, and other molecules.119,136,137 Interactions with large molecules such as receptor proteins are in some cases more specific when ligands are distributed several nanometers apart on the surface; this is discussed further in the section on 0D assemblies. Amines, similarly, can be used for coupling reactions, which have been used to display functional groups including nitrophenyl phosphonates80,101 and neurotransmitters148 on surfaces, to interact with molecules in solution.
Azides displayed on surfaces can be used for a copper(I)-catalyzed azide–alkyne cycloaddition known as the ‘click’ reaction due to its high reaction efficiency.149 This approach has been used, for instance, to attach peptides to surfaces to study cell adhesion.150,151 This reaction has also been carried out using a copper AFM tip to create localized molecular patterns.152
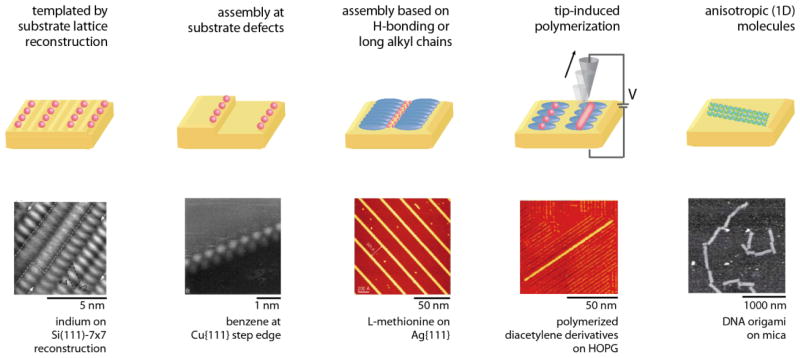
3. One-dimensional assemblies
One-dimensional structures on surfaces can be templated by anisotropy in the surface, created by depositing anisotropic (inherently 1D) molecules, or can arise from interactions such as hydrogen bonding between molecules (Figure 3).52 Once assemblies are formed, some types of molecules (such as those containing diacetylene functionalities) can polymerize to create covalently bound structures.8,153,154 Linear structures may be aligned in a number of ways, including external electric or magnetic fields, or capillary interactions with a receding solvent.155 One-dimensional assemblies have been used to create directionally conductive monolayers, for linear transport of nanoscale objects, and to induce macroscale motion by causing directional strain in a substrate.156–158
Figure 3.
Strategies for 1D self-assembly. One-dimensional structures can be templated by substrates, either through anisotropic substrate lattices formed when substrates reconstruct to minimize surface energy, or at atomic step edges. One-dimensional structures can also be self-assembled from anisotropic molecules to form ordered assemblies due to interactions such as directional hydrogen-bonding or interactions between long alkyl chains. Such assemblies can then be polymerized to form covalently bound 1D structures. Finally, intrinsically 1D molecules such as DNA may be patterned on substrates directly. Adapted with permission from refs. 163, 168, 173, 181, and 335.
3.1. Substrate surface reconstruction
Solid crystalline surfaces typically have unsatisfied bonds, in some cases leading the top layer of the surface to reconstruct to minimize surface energy, either on the pristine surface,41,159 or as adsorbates bind.160 For instance, pristine Si(100) surfaces prepared in ultrahigh vacuum (<10−10 torr) and thermally annealed, reconstruct to form Si dimers with π-bond character (the Si(100)–2×1 phase).41 As noted above, such features can template assembly.
3.2. Surface defects and electronic standing waves
Surface defects such as atomic step edges and vacancies cause perturbations in the local electronic density of states,105,161,162 that modulate the binding of adsorbates and create linear arrays, as for benzene adsorbed to Cu{111} step edges.163 Linear arrays of magnetic atoms105 (such as Co on Pt{111}164) have been of particular interest due to theoretical predictions of unique 1D magnetic properties.165,166 Adsorbates can also perturb surface electronic structure and template the binding of further adsorbates,163,167 as found for L-methionine on Ag{111}, which forms linear arrays168 that can be used to template further adsorption of species such as Fe atoms.169
3.3 Anisotropic molecules
Adsorption of anisotropic molecules also leads to 1D surface structures. Both synthetic and natural linear molecules have been used for this purpose. Synthetic examples include bistable rotaxanes,170 dumbbell-shaped molecules with controlled numbers of electron-accepting cyclobis(paraquat-p-phenylene) rings threaded on the shafts; each ring has a pair of available docking stations with which it associates via electron donor–acceptor interactions. Such molecules deposited on surfaces have been both characterized at the single-molecule level,171 and used to do macroscale work (cantilever tip deflections up to 2 μm) based on electrochemically controlled motion of the rings between their two stable positions on the shaft.157,172
Natural 1D structures may also be studied on surfaces – DNA has been deposited on surfaces both in its natural linear state and as components of DNA origami structures with controlled morphology.173 Linear origami structures have been used to perform assembly-line tasks, loading three different gold nanoparticles cargoes onto a DNA walker transiting the line (each step ~2 h), in a programmable fashion.174,175 Peptides forming regular secondary structures, such as β sheets, have been assembled on surfaces to illuminate the process of amyloid plaque formation.176 Biologically important protein assemblies (such as muscular actin–myosin filaments) are also deposited on surfaces. Small (1–4 μm) metallized actin filaments have been used as nanoscale transporters on myosin-coated surfaces, with speeds of ~200 nm/s (vs. ~4 μm/s for unmetallized actin), controlled by the addition of ATP.156
3.4 Noncovalent interactions
Noncovalent interactions between molecules, such as hydrogen-bonding or substrate-mediated interactions, are used to template 1D assembly. In addition to the hydrogen-bonded peptide structures mentioned above, organic molecules can form 1D arrays.52,105 Typical structures include a rigid framework (often benzene, anthracene, or a porphyrin), and substituents that facilitate directional intermolecular interactions.4,177
For instance, dicarboxylic acids with long alkyl substitutents (such as isophthalic and terephthalic acid derivatives) form linear arrays based on both hydrogen bonding between carboxylic acids and interdigitation of the long alkyl tails.4,7 In this context, the interdigitated alkyl tails form 1D lamellar structures, with the carboxylic acids organizing at the periphery of each lamella. Lamellar structures can also be formed from other functionalized alkanes (including alcohols, amines, and bromides), or even long alkanes such as tricontane (30 carbons) based on van der Waals forces.178 Tilt angles of the molecules relative to the lamellar axis vary based on interactions between the functional head groups.179
3.5 Polymerization
Polymerization of molecules self-assembled on surfaces can produce covalently bound 1D structures.8,153,154,180 For example, diacetylene derivatives such as nonacosa-10,12-diynoic acids have been assembled on HOPG, using long alkyl chains154 or hydrogen-bonding functional groups such as isophthalic acids153 to align the alkyne moieties. Polymerization is either initiated across the entire surface, using UV light,154 or at a single point, using a STM probe tip.181 Polymerization creates linear polydiacetylene chains that terminate at domain edges or defects, with HOMO-LUMO gaps that decrease with increasing chain length,154 and conductivities that increase substantially (up to 1000×) with iodine doping.180 Films of polymerized pentacosa-10,12-diynoic ethanolamide exhibit source–drain modulation when a gate bias is applied, in contrast to less-ordered polymerized films,182 suggesting the importance of aligning 1D features for applications in devices.
4. Zero-dimensional assemblies
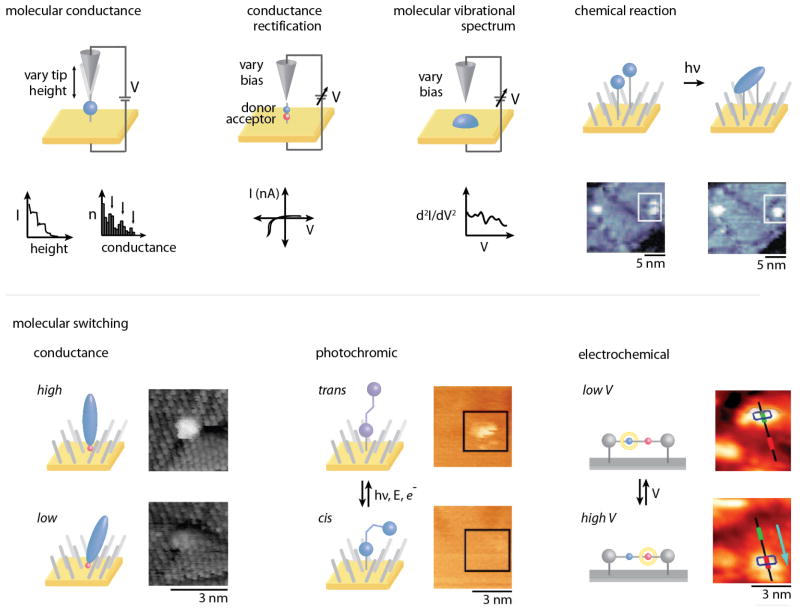
Zero-dimensional molecular features consisting of individual molecules or small molecular clusters have been studied for their electronic, mechanical, magnetic, optical, biological, and chemical properties.21 Studies at this level illuminate unique properties that otherwise are lost through averaging in ensemble measurements. Here, we select illustrative examples, classified based on molecular function (Figure 4).
Figure 4.
Molecular conductance is measured in a STM break junction by monitoring current as the STM probe tip is moved away from the surface (top left). A donor-bridge-acceptor molecular rectifier exhibits an asymmetric I–V curve, also measured with STM (top center). Photodimerization of paired anthracene phenylene ethynylene derivatives isolated in a SAM is visualized with STM (top right). Adapted with permission from ref. 230. An isolated oligo(phenylene ethynylene) molecule inserted in a dodecanethiolate SAM on Au{111} undergoes reversible conductance switching (bottom left). Adapted with permission from ref. 19. Azobenzene inserted in a dodecanethiolate SAM undergoes reversible photoisomerization (bottom middle). Adapted with permission from ref. 15. The ring of a surface-bound rotaxane shuttles between two stations as the electrochemical potential in the cell is cycled from 0.1 V to 0.5 V (bottom right). Adapted with permission from ref. 171.
Single-molecule electronic properties in particular have been extensively investigated with the goal of producing single-molecule devices performing the functions of macroscale circuit components.183,184 Such studies began in 1974 with a theoretical proposal of a molecular rectifier by Aviram and Ratner,185 and now encompass both theoretical and experimental work on molecular resistors, rectifiers, electronic and photochromic switches, and other devices.186–188
Just as decreasing the size of macroscale circuit components increases the importance of interface issues such as line edge roughness, the interface between a molecular device and its environment (including electrodes and surrounding molecules) is critical. Electrode–molecule coupling, determined by band alignment, contact bonding, and contact geometry, plays a critical role in electron transport properties. Thus, with proper control over electrode interfaces, electronic properties of contacts can be tuned.97,184,187,189
4.1 Characterization
Scanning probe microscopes are widely used for molecular electronic characterization, since they can both image target molecules in their environments and position one electrode (the scanning probe tip) with sub-nanometer precision in three dimensions.190–192 In addition to interfaces with the substrate, nearby conductive molecules can influence the electronic properties of target molecules. To reduce the effects of such interactions, active molecules can be distributed in less conducting monolayer matrices.9,21 One method for doing this is solution deposition of conductive molecules inserted into existing ordered alkanethiolate (or other) SAMs (Figure 4).9,10 Target molecules have high probabilities of deposition at domain boundaries and other defects in SAMs, typically resulting in few-nm spacings between molecules, adequate for electrical isolation. This method was used by Weiss and co-workers to test the conductance switching mechanism of isolated oligo(phenylene ethynyl) (OPE) thiolate molecules inserted into self-assembled dodecanthiolate monolayers.9,19,193
Active molecules can also be co-deposited at low concentration with alkanethiolates, dispersing them within the alkanethiolate domains; however, high-temperature (~80 °C) annealing after deposition can cause the active molecules to phase segregate.194
Single molecules can be also suspended between contacts in break junctions.192,195,196 In this configuration, one electrode is initially in contact with or in close proximity to the other, and is slowly drawn away to break contact. If bifunctional (for instance dithiolated) conductive molecules are present in the junction, quantized conductance decreases are observed as the number of molecules bridging the junction decreases. Junction lengths can be controlled to ±1 Å, and the junction is typically broken and reformed repeatedly to test reproducibility. Alternatively, controlled junction lengths can be achieved in static conformations using techniques such as on-wire lithography.197
Further molecular measurements can be made using scanning tunneling spectroscopy.191 Inelastic electron tunneling spectroscopy (IETS) is performed using STM by holding the tip stationary over a molecule (i.e., without varying the tip–sample separation or the lateral position), and varying the voltage while monitoring the current at low temperature (4 K). This yields a current–voltage curve dependent on the vibrational spectrum of the molecule, providing both structural and electronic information about the single-molecule junction.198,199 Comparable measurements have been made in break junctions.200,201
4.2 Molecular function
4.2.1 Resistors
Single-molecule conductance is sensitive to several factors, in addition to the chemical structures of molecules. These include the chemistry, geometry, and electronic properties of the contacts with the electrode (thiol, amine, etc.), as well as interactions with surrounding molecules.97,202–204 Molecular energy levels (particularly the highest occupied molecular orbital, HOMO, and lowest unoccupied molecular orbital, LUMO) shift on binding to the electrodes. This shift determines the difference between molecular energy levels and the Fermi levels of the electrodes; alignment of either the HOMO or LUMO with the Fermi level typically increases conductivity.203,204
Conductance measurements based on repeated break-junction formation can generate histograms of values for thousands of Au–molecule–Au junctions, which are important due to variations in conductance with electrode geometry.205 Conductance is often reported in relation to the quantum point contact value G0 = 77.4 μS, which is the ideal conductance value for two metal electrodes bridged by a single atom of the same metal.202 Conjugated molecules with extended π systems have higher conductances than unconjugated molecules such as alkanethiols.206 Conductance also varies with the chemistry of the contacts, with reported conductance orders of R–P > R–S > R–NH2 > R–COOH for saturated alkanes,207,208 and differences between thiolates and selenolates.97 Again, values and relative order depend on the specific backbones and electrodes, as band alignment effects dominate.209
4.2.2 Rectifiers and diodes
Certain molecules with two conjugated groups having donor–bridge–acceptor structures exhibit asymmetric conduction, or rectification.210 Several factors contribute to rectification. One is the magnitude of the Schottky barrier at each interface, which depends on the mismatch between the molecular HOMO or LUMO and the substrate Fermi level.211,212 Another is the placement of the donor–bridge–acceptor structure relative to the two electrodes. Asymmetric placement (often achieved using alkyl spacers with different lengths) increases rectification.121,213 Rectification also occurs due to differences in energy levels between the donor and acceptor groups.213 Distinguishing this contribution from the formation of a Schottky barrier requires the use of the same metal for both electrodes, first achieved by Metzger and coworkers.214 Rectification ratios (ratio of conductances at positive and negative voltages of the same magnitude) of up to 3000 at ±1 V have been measured in a cationic donor–bridge–acceptor dye (Fig. 4B).215 Experimentally, the same challenges described above for resistors apply here; for instance, rectification depends on the structure of the junction between the electrodes. In addition, the rectification ratio has been observed to decrease with successive measurements, complicating quantification.
4.2.3 Switches
Molecular switches transition between two (or more) electronic, structural, or optical states in response to external stimuli. The behavior of most such molecules is initially characterized in solution. Surface-bound switching is of interest because it creates the possibility of using switch states in devices; however, surface attachment often impacts switching, meaning careful characterization is important.
Certain classes of molecules undergo switching between high and low conductance states. Oligo(phenylene ethynylene)s are widely studied examples, in which switching occurs due to hybridization changes at the metal–molecule contact.16,19 As with the molecules discussed above, switching behavior depends on the local environment. For instance, placing OPE molecules in well-ordered alkanethiol SAM matrices increases stability and reduces stochastic switching frequency. Hydrogen-bonding interactions with appropriately designed amide-containing alkanethiol SAMs have been used to stabilize one or both switch states.18,216
Photochromic molecules switch between conformational states upon exposure to light of characteristic wavelengths, with switching behavior that is often different on surfaces vs. in solution. For instance, azobenzene molecules, containing two benzene rings linked through an N=N double bond, undergo photoisomerization from a lower-energy trans conformation to a higher-energy cis conformation when irradiated at ~365 nm.217 Isomerization on surfaces introduces additional considerations. If molecules lie flat on bare metal surfaces, isomerization is typically quenched due to electronic coupling with the surface.217,218 If molecules are elevated off the surface on bulky legs to decouple them from the substrate, switching is again observed;218 however, the photon absorption cross-section is reduced relative to that in solution,219 and the reaction can proceed via a different mechanism.220 Switching can also be achieved by depositing the molecules at defects in background SAM matrices, linked to the surface through tethers long enough to place the azo functionality protruding beyond the matrix.15 In this case, the structure of the tether plays a key role in mediating switching.209,218,221
Placing functional molecules on surfaces also opens new possibilities for controlling switching. For instance, azobenzene switching on surfaces can be initiated using electric fields or tunneling electrons from a STM probe tip.209
Mechanically interlocked molecules such as catenanes and rotaxanes operate based on the motion of two or more noncovalently linked molecules relative to each other.222–225 Rotaxanes are comprised of dumbbell-shaped molecules with a ring-shaped molecule threaded on the shaft. The ring can move between two stations on the shaft based on the oxidation state of the preferred tetrathiafulvalene (TTF) station. Tethering these molecules to solid surfaces enables them to perform work,157 but also raises the possibility that interactions with the surface or neighboring molecules may affect their function.
The motion of individual rings can be tracked using electrochemical STM (with solution covering the surface), as the potential is raised and lowered (from ~0.1 to ~0.5 V), reversibly changing the oxidation state of the TTF station. Since the shafts are much less conductive than the rings, only the rings are visible in STM images. Observing the motions of ~100 rings as the potential is cycled reveals a distribution of ring displacements around the ~3 nm distance between stations of the fully extended molecule, with an average displacement of ~2 nm.171 This suggests that the shaft binds to the surface without the ends fully extended. Direct measurements such as those described inform strategies for optimizing molecular function for devices, such as development of rotaxane switches with more rigid shafts. Rigid shafts would have the further advantage of being able to transmit greater force in assembled, cooperative devices.157,158
4.2.4 Single-molecule reactions
Isolating individual molecules or pairs of molecules on surfaces as 0D structures enables chemical reactions to be initiated and monitored at the single-molecule level. Again, STM is a useful tool, since electronic excitation can trigger chemical reactions, which can then be observed with the microscope; many STM measurements are performed at low temperature (4 K) under ultrahigh vacuum conditions (pressures <10−10 torr), both reducing the likelihood of unwanted side reactions with gas-phase molecules, and slowing reaction kinetics.226
Such single-molecule chemistry was pioneered by Ho,227 and Meyer and Rieder.228 Ho and coworkers performed single-molecule dissociation of pyridine and benzene molecules on Cu(001).227 Meyer, Rieder, and coworkers induced Ullmann coupling (formation of biphenyl from two iodobenzene molecules) on Cu{111}.228 Weiss and coworkers later extended single-molecule reaction studies, inducing Ullman coupling with a scanning tunneling microscope and differentiating reactive intermediates from products based on IETS measurements.229 Reaction mechanisms for catalytic chemistry on metal surfaces are technologically important, and often difficult to monitor using standard characterization techniques under reaction conditions.
In addition to observing important and normally short-lived reaction intermediates, 0D molecular assemblies on surfaces can be used to control reaction pathways to select for paths that would be unfavorable in solution. Pairs of thiolated anthracene phenylene ethynylene derivatives deposited at defects in alkanethiolate SAMs are confined in head-to-head arrangements.230 When photoexcited, the molecules dimerize via cycloaddition, since they cannot access the head-to-tail conformation that would otherwise be sterically favored in solution.
4.2.5 Biological applications of 0D assembly
Isolating individual biomolecules on surfaces provides unique opportunities for understanding their structure and function,21,231–233 although many systems benefit from careful selection of passivating monolayers to control interactions between the target molecules and the substrates.1 Proteins are relatively large (~4 nm diameter for a 50 kD globular protein234) and can interact with other nearby proteins on surfaces, meaning that if proteins are targeted to surfaces by ligands, it is useful to have the ligands distributed several nanometers apart on surfaces. This is possible using the defect-based insertion strategy discussed previously.137,235 For instance, 5-hydroxytryptophan (5-HTP), a serotonin precursor, has been covalently bound to tethers via its additional carboxyl group. The use of insertion-directed self-assembly distributes 5-HTP at defects in SAM matrices, effectively spacing these molecules far apart. The use of the 5-HTP precursor, instead of the neurotransmitter serotonin (5-hydroxytryptamine) itself, leaves all epitopes of serotonin accessible for molecular recognition. These surfaces have been used to capture native serotonin receptor proteins.119,148 Substrates capable of biomolecule-specific recognition can ultimately be used in tandem with other methods, such as surface mass spectrometry, to identify new target molecules.145
5. Molecular patterning and its applications
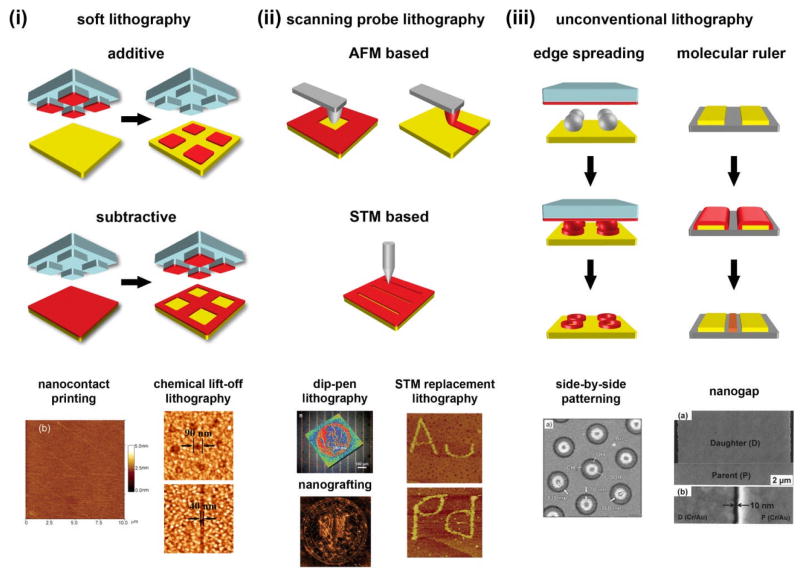
In the previous sections, we have described how molecular interactions of alkanethiols within SAMs and with the surrounding environment can be controlled and characterized to provide structural and functional information with sub-nanometer resolution. Integrating such functions into electronic and biosensing devices requires registry with macroscale features, often created by larger scale molecular patterning. Molecular patterning has been carried out on a variety of solid surfaces (including Au, Ag, Cu, Ge, Pd, and SiO2), utilizing an array of techniques in surface chemistry to generate patterns ranging in scale from microns to nanometers.44,47,49,236–240 Terminal functional groups displayed at interfaces define both physical and chemical surface properties, making it possible to tailor surfaces down to the nanoscale through chemical patterning.14,15,241–247 In this section, we briefly discuss molecular patterning of SAMs through soft lithography, scanning probe lithography, and related techniques. Detailed descriptions of these techniques have been provided elsewhere and in previous reviews.1,11,25,248–253
5.1 Patterning strategies
5.1.1 Soft lithography
In soft lithography, a pliable elastomeric stamp is molded from polydimethylsiloxane (PDMS) or another polymeric material; molecular inks are then applied to stamps, which are used to transfer inks to surfaces, reproducing the stamp features.254 Stamps are typically produced by casting liquid PDMS onto master molds with predefined features fabricated by photolithography or EBL. Features in masters are negatively replicated in PDMS stamps. For example, trenches and protruding features on the master molds will create protruding and depressed features on PDMS stamps, respectively.
Although a variety of molecular inks are currently available, alkanethiols are the original (and still the most commonly used) inks for conventional soft lithography.254 The soft PDMS stamp conforms to the surface topography of substrates, making it possible to pattern curved substrates.255
Molecular ink features can be used in several ways. Inked patterns act as chemical etch resists, protecting patterned areas while material in the exposed areas is removed.256,257 As a result, stamp features are transferred into the underlying solid substrates. Unpatterned areas can also be backfilled, to self-assemble different molecules to create surfaces displaying chemically distinct functional groups in patterned vs. unpatterned areas. Such surfaces have been investigated in the context of preventing biofouling258–260 and guiding cell growth.261,262
Two key factors relating to inks limit the fidelity of feature reproduction in soft lithography. Alkanethiol inks can diffuse laterally on the surfaces of substrates beyond the contact areas (surface diffusion) during printing, creating halos around patterned features.235 Additionally, low molecular weight inks are volatile, enabling ink vapor deposition in noncontacted areas.263 Both phenomena decrease pattern fidelity, becoming more important as feature sizes are reduced to the nanoscale. Several modifications to soft lithography have been developed to address these challenges.235,244,254,264,265
5.1.2 High-molecular-weight inks
Using higher molecular weight inks lowers ink vapor pressure, reducing vapor deposition to noncontacted areas during ink transfer.263,266 High-molecular-weight inks also minimize lateral diffusion around patterned features. For instance, Delamarche et al. demonstrated that by controlling both ink concentration and stamp-substrate contact time, microcontact printing of eicosanethiol (a 20-carbon alkanethiol) can be used to create features ~80 nm in size.263 Even smaller feature sizes (~40 nm) were achieved using dendrimeric polymers, which are more massive than commercially available alkanethiols.267 Although high-molecular-weight inks minimize lateral diffusion and vapor deposition, their sizes can cause inks to precipitate on stamp surfaces prior to contact, causing post-printing patterning problems on substrates.268 In addition, polymeric inks do not provide robust etch resist properties for device fabrication.269
5.1.3 Microcontact displacement printing (μDP)
Another method for retaining line edge sharpness by preventing lateral diffusion of alkanethiol inks is referred to as microcontact displacement printing.142,265,270 Here, labile adamantanethiols are first self-assembled on substrates. Because adamantanethiols have weak intermolecular interactions, lower molecule–substrate bond densities, and mismatched lattices relative to the displacing molecules, they are readily displaced by alkanethiol inks in the stamped areas. Adamantanethiol molecules remaining in the unpatterned regions around patterned features prevent ink molecules from diffusing outside the contact areas, improving line-edge roughness.265 An important consideration in this method is that the molecular ink must form a sufficiently robust monolayer to displace the existing weakly bound adamantanethiol SAM.
5.1.4 Microcontact insertion printing (μCIP)
If surfaces are instead passivated with more strongly bound alkanethiol SAMs prior to patterning, ink molecules are inserted at defects in the existing SAMs (as opposed to displacement).271,272 This produces dilute patterns of ink molecules in the stamped areas. For work with biomolecules, this capability is particularly useful. If small-molecule probe molecules are distributed across surfaces at defects, relatively large target proteins and antibodies can be captured specifically based on function.119,137,140,141,148,273,274 In contrast, full monolayers of small-molecule targets result in non-specific binding, because of the prevalence of exposed interacting functional groups.137,141,275
5.1.5 Reactive spreading lithography
If ink vapor formation is restricted, lateral diffusion of inks can be used to advantage to create nanoscale gaps between patterned areas by carefully controlling both ink concentration and stamp-substrate contact time. Xia et al. performed microcontact printing under water, regulating lateral ink diffusion to create alkanethiol SAM features separated by ~100 nm gaps.276 Because stamps and substrates were immersed in water and a relatively high-molecular-weight, water-insoluble ink was used, ink vapor was not formed. Controlled lateral spreading has also been exploited in a technique called edge spreading lithography, discussed below.277
5.1.6 Catalytic microcontact printing
In contrast to the techniques discussed above, catalytic microcontact printing (CμCP) patterns substrates without requiring ink transfer, avoiding altogether problems associated with lateral and vapor diffusion. In this technique, polymeric stamp surfaces are functionalized or inked with chemical species that catalyze reactions or react with functional groups on substrates in stamped areas.278 A notable feature of CμCP is the interfacial reaction that takes place between the chemically functionalized surfaces of polymeric stamps and the reactive surfaces of SAM-modified substrates. The mechanism of interfacial reactions has been widely discussed, since tethering both reaction partners to solid surfaces would be expected to hinder their reaction.279,280
Patterning with CμCP has produced feature sizes as small as ~20 nm and found diverse applications in surface chemistry, biology, and catalysis.144,281–283 Key concerns for CμCP include optimizing chemical conjugation for the polymeric stamps and eliminating competing conjugating pathways that reduce reaction yields.284 Extension to smaller feature sizes (<20 nm) would enable single-molecule biological studies.
5.1.7 Chemical lift-off lithography
Chemical lift-off lithography (CLL)58 extends the concept of CμCP by allowing reactions to take place between stamp surfaces and substrate-bound SAMs, which is followed by lift-off of reacted molecules from monolayers as the stamp is removed. In CLL, PDMS stamps with molded relief patterns are activated by oxygen plasma treatment and brought into conformal contact with SAM-modified Au substrates. The activated patterns react with certain classes of chemical functional groups on the exposed surfaces (e.g., alcohols but not unmodified alkanes). Contact-induced reactions form covalent bonds, allowing reacted molecules to be lifted off surfaces in areas where the stamps make contact.58 Exposed gold areas can either be chemically etched or backfilled with other alkanethiols to create multicomponent patterned SAMs. For example, biotin-terminated alkanethiols were self-assembled on post-lift-off hydroxyl tri(ethylene glycol) SAM-modified Au substrates, to create small-molecule patterned substrates for biorecognition. Patterning by CLL can produce sharp 40-nm gaps with a single patterning step using nanopatterned masters;58 alternatively, micropatterned masters can be shifted in registry through multiple lift-off steps to produce features narrower than 50 nm.
Investigation of post-lift-off PDMS stamps by X-ray photoelectron spectroscopy indicated that Au was removed from the underlying substrates, suggesting that the stamp-SAM interactions are at least as strong as the Au–Au bonds at the substrate surface. Thus, CLL serves as a potential route to investigate the widely discussed nature of SAM-substrate interactions.
5.2 Scanning probe lithography
As described in previous sections, scanning probe microscopy can be used to characterize and provide structures and function of alkanethiol SAMs on solid substrates with Ångström resolution. In this section, we discuss the use of scanning probe microscopy in lithography to achieve high-resolution molecular nanoscale patterning, including AFM-based techniques such as dip-pen nanolithography (DPN), nanografting, nanoshaving, and STM-based techniques.
5.2.1 Techniques based on atomic force microscopy: Dip-pen nanolithography and nanografting
Dip-pen nanolithography, developed and commercialized by Mirkin and coworkers, transfers molecular ink from an AFM tip to substrates via a water meniscus.251 Because ink transfer is mediated by the meniscus, feature size and resolution are controlled not only by the probe tip sharpness (the probe tip radius of curvature is typically ~10 nm), but also by tip–surface contact duration, ink diffusion dynamics, chemical structures of molecular inks, temperature, and relative humidity.285–287 Thiols, proteins, nucleotides, DNA, and polymers have all been used as molecular inks for DPN.251,288–296 Relative to microcontact printing, DPN typically produces smaller features but at slower speeds since it is a serial process. However, patterning speeds are increased by utilizing multiple tips simultaneously.275,297–300
While DPN uses an AFM tip to add molecules to substrates, molecules in SAMs can also be removed by dragging the tips through SAMs with scanning forces higher than those used for imaging, a process referred to as nanoshaving.301,302 Performing this process with the surface immersed in a solution of different SAM-forming molecule is called nanografting, and allows the exposed areas to be backfilled by other molecules, creating multi-component surfaces.302 These techniques retain the properties of high spatial resolution and can be performed under many different chemical environments.303 They have been widely used for fabricating molecular patterns with sub-100-nm resolution.301,304–307 Nanografting has been used to regulate and to study reaction mechanisms,308 and to fabricate multicomponent nanostructures for molecular electronic and biological applications.274,301,309–311
Similar to DPN, the main limitations of nanoshaving and nanografting are relatively slow patterning speeds and small patterning areas. However, in addition to creating arbitrary 2D patterns, 3D nanostructures can also be fabricated through simple chemical reactions on features created by nanografting.312
5.2.2 Techniques based on scanning tunneling microscopy
Similar to AFM-based patterning techniques, STM tips can also be used to create high-resolution (sub-20 nm) patterns by applying high biases (3–4 V, in contrast to typical imaging voltages of magnitude ≤ 1 V) between the STM probe tip and conducting or semiconducting samples, causing desorption of molecules from surfaces.313,314 If desorption is performed under a liquid, exposed areas can be backfilled with other molecules, a process known as STM-replacement lithography (STM-RL).315,316 Although this technique produces precise features, patterns are formed slowly and only over small areas, even relative to AFM-based techniques. Additionally, high applied biases can chemically modify the STM probe tip itself, decreasing feature precision.11
5.3 Other techniques
In addition to conventional soft-lithography and scanning probe lithography techniques described above, other strategies have also produced high-quality molecular-scale patterns. In this section, we will discuss two examples of unconventional patterning strategies: edge spreading lithography and molecular rulers.
5.3.1 Edge spreading lithography
Lateral diffusion of alkanethiol inks can be controlled to fabricate patterns on Au substrates.276 Edge spreading lithography (ESL) leverages diffusion in a multi-step process to create narrow features only in areas to which the ink diffuses, rather than in both stamped and diffusion-coated areas. Elastomeric stamps first transfer alkanethiols to substrates with existing template features. The ink diffuses along the template features to the surface, creating narrow molecular features around their edges.277,317 Subsequent removal of the template leaves narrow molecular features on substrates. The template features can either be patterned through conventional lithography or by techniques such as colloidal lithography; the use of conventional lithography enables patterning over large areas, while colloidal lithography enables creation of relatively small features.317
Multiple rounds of ESL can be performed on the same surface with different molecules, creating concentric patterns around the preformed features.318 This procedure provides precise 2D control of chemical patterns on surfaces for applications in metal nanostructure fabrication and biology.318,319
5.3.2 Molecular ruler lithography
Molecular ruler lithography combines a top-down patterning approach (EBL) with a bottom-up approach (SAM formation) to fabricate high-resolution nanostructures.320 Parent features are first fabricated through EBL or photolithography. Subsequently, multiple SAMs with well-defined thicknesses are deposited on the parent features through electrostatic interactions, by alternating layers of end-functionalized α,ω-mercaptoalkanoic acid with layers of a polycation. This process creates small, precisely controlled gaps (4–100 nm) between two parent features. Importantly, the chemistry of the parent features, substrate, and SAM constituents must be chosen such that the SAMs wet only the parent features and not the substrate. Finally, daughter features are created through controlled deposition of metals across the entire surface; removing the SAM layers reveals the original parent features, as well as daughter features formed in the gaps. The lengths of the molecules and the numbers of alternating layers used to create the multilayer resists control feature thickness, acting as a ruler to vary both the spacing between the parent and daughter features and the daughter feature sizes.320–334 This process expands chemical patterning control of molecules from 2D to 3D.
6. Perspectives
Molecular monolayers represent one of the limits of nanostructured materials, and, in some cases, enable control of structure and environment down to the single-molecule level. In 2D monolayers, the molecular lattice structures and physical properties can be tuned based on substrate structures, and the steric and chemical functionalities of the molecules making up the monolayers. Combining monolayer strategies with top-down patterning enables 2D monolayer structures to be created with features with below 100 nm to the wafer scale.11 The self- and directed-assembly strategies described above enable control down to the sub-nanometer scale.
To date, the properties of 2D monolayers have been explored extensively; however, restricting additional dimensions can provide even greater control over function and provide insight into molecular processes. For instance, 1D structures can direct coupling between atoms and molecules or enable motion of nanoscale objects across surfaces, while 0D structures allow the course of individual molecular reactions to be controlled and monitored.
Studying molecular structures and processes at the sub-nanometer scale in real space makes it uniquely possible to understand how nanoscale properties change based on interactions with the environment, particularly defects and neighboring molecules. Since interfaces, defects, and heterogeneous structures are critical in applications from device performance to biological function, characterization tools that provide additional information about interfaces will open new fields of study and new means of control.
Figure 5.
Overview of molecular patterning strategies. (i) Soft lithography. Left: 42-nm lines of dendrimers on Si surfaces fabricated by additive nanocontact printing. Adapted with permission from ref. 267. Right: Biotin-streptavidin recognition patterns on Au substrates fabricated by subtractive chemical lift-off lithography. Adapted with permission from ref. 58. (ii) Scanning probe lithography. Left: Large-area patterning of 1-octadecanethiol with dip-pen nanolithography; and nanografting aldehyde-terminated thiols into decanethiol SAMs. Adapted with permission from refs. 299 and 336. Right: Replacement of dodecanethiol SAMs with ferrocenyl undecyl thioacetate molecules on Au and Pd substrates assisted by high-bias (>2 V) scanning with STM probes. Adapted with permission from ref. 337. (iii) Unconventional lithography. Left: Side-by-side patterning for carboxy- (bright), hydroxy- (gray), and methyl-terminated (dark) thiolate monolayers on Au substrates. Adapted with permission from ref. 318. Right: A 10-nm gap fabricated by molecular ruler technique. Adapted with permission from ref. 322.
Acknowledgments
We thank the Department of Energy (Grants #DE-FG02-08ER46546 and #DE-FG02-07ER15877), the National Science Foundation (Grants #CHE-1013042 and #CHE-1124984), and the Kavli Foundation for support of the work described here. S.A.C. acknowledges support from an NIH Postdoctoral Fellowship (7F32GM0892202).
Biographies
Shelley A. Claridge is a NIH Postdoctoral Fellow with Paul Weiss at UCLA. She completed her PhD in Chemistry at UC Berkeley in 2008 with Paul Alivisatos and Jean M. J. Fréchet, working on synthesis and bioconjugation of inorganic nanocrystals. From 1997 to 2003 she worked as a software engineer. She received a BS in Mathematics, Biochemistry, and Genetics from Texas A&M University in 1997. Her research interests are in single-molecule biological structure and biological-inorganic interfaces.

Wei-Ssu Liao received his BS degree from National Cheng Kung University, Taiwan, in 2000. In 2002, he received his MS from National Taiwan University, Taiwan. He received his PhD from Texas A&M University in 2009, working under the direction of Prof. Paul Cremer. He is currently a postdoctoral scholar working with Profs. Paul Weiss and Anne Andrews at UCLA. His research experience and interests lie in bioanalytical chemistry, surface chemistry, and plasmonic nanomaterials.
John C. Thomas obtained his BS at the University of Texas at San Antonio in 2008, while performing research in the field of palladium coordination chemistry in the lab of Prof. Judith A. Walmsley. He is currently working towards his PhD with Prof. Paul Weiss at UCLA. His graduate research focuses on the scanning tunneling microscopy and spectroscopy of cage molecules for self-assembly, probing buried functionality of self-assembled systems, and extending the capabilities of the scanning tunneling microscope to make novel measurements at the molecular and atomic scales. This research is performed at low temperature (4 K), in extreme high vacuum, and also in catalytically relevant environments.
Yuxi Zhao obtained her BS in Macromolecular Science from Fudan University (China) in 2009 and wrote her diploma thesis in the field of inorganic-organic hybrid solar cells. She is currently pursuing her PhD in the Department of Chemistry at UCLA with Prof. Paul Weiss. Her research focuses on coupling photons to single molecules to guide the design of molecules for organic photovoltaics.
Huan H. Cao received his BS in Chemistry from Marshall University, Huntington, WV in 2008. His undergraduate research in the Norton Nanolaboratories focused on fabricating DNA constructs attached to Au nanodots on Si/SiO2 surfaces, which can be used as templates for building bioelectronic devices. He is presently working toward his PhD degree with Profs. Paul Weiss and Anne Andrews. His research spans the physical and neural sciences, which include exploring unconventional molecular patterning techniques on solid substrates, investigating reaction mechanism at interfaces between molecular self-assembled monolayers and polymeric surfaces, and fabricating bioselective substrates capable of capturing and sorting biomolecules from complex media.
Sarawut Cheunkar graduated in 2004 with a BS in Chemistry from Mahidol University, Thailand. In 2005, he was awarded a full scholarship from the Ministry of Education, Royal Thai Government, to pursue his PhD working with Profs. Anne Andrews and Paul Weiss, first at the Pennsylvania State University, and currently at the California NanoSystems Institute at UCLA. He employs self-assembled monolayer techniques to develop neurotransmitter-functionalized surface chemistries. After completing his PhD, Mr. Cheunkar will join the faculty at the School of Bioresources and Technology, King Mongkut’s University of Technology.
Andrew Serino received his BS from Penn State in 2010. His senior thesis was on characterizing intermolecular interactions in various self-assembled monolayers. He is currently pursuing a PhD in Materials Science and Engineering at the UCLA under the supervision of Prof. Paul Weiss. His research focus is on characterization of self-assembled materials and their utilization in devices.
Anne Milasincic Andrews earned her PhD in Chemistry at the National Institute of Mental Health. At UCLA, she is a Professor of Psychiatry and Chemistry & Biochemistry, and a member of the Semel Institute for Neuroscience & Human Behavior, Hatos Center for Neuropharmacology, and California NanoSystems Institute. Among her awards are an Eli Lilly Outstanding Young Analytical Chemist Award, an American Parkinson’s Disease Association Research Award, and a NARSAD Independent Investigator Award. She is an Associate Editor of ACS Chemical Neuroscience. Her basic and translational research interests focus on anxiety and depression, and at the nexus of nanoscience and neuroscience.
Paul S. Weiss received his PhD in Chemistry in 1986 from UC Berkeley. He was a postdoctoral fellow at AT&T Bell Laboratories and IBM Almaden Research Center. He began his academic career at Penn State, becoming Distinguished Professor of Chemistry and Physics before moving to UCLA in 2009. At UCLA, he is the Fred Kavli Chair in NanoSystems Sciences, the Director of the California NanoSystems Institute, and Distinguished Professor of Chemistry & Biochemistry and Materials Science & Engineering. He is the founding Editor-in-Chief of ACS Nano. His research interests are in single-molecule/assembly function, chemical patterning, self-assembly, and nanoscale analyses.
Footnotes
Part of the chemistry of functional nanomaterials themed issue.
References
- 1.Love JC, Estroff LA, Kriebel JK, Nuzzo RG, Whitesides GM. Chem Rev. 2005;105:1103–1169. doi: 10.1021/cr0300789. [DOI] [PubMed] [Google Scholar]
- 2.Nuzzo RG, Zegarski BR, Dubois LH. J Am Chem Soc. 1987;109:733–740. [Google Scholar]
- 3.Dubois LH, Nuzzo RG. Annu Rev Phys Chem. 1992;43:437–463. [Google Scholar]
- 4.De Feyter S, De Schryver FC. Chem Soc Rev. 2003;32:139–150. doi: 10.1039/b206566p. [DOI] [PubMed] [Google Scholar]
- 5.Poirier GE. Chem Rev. 1997;97:1117–1127. doi: 10.1021/cr960074m. [DOI] [PubMed] [Google Scholar]
- 6.Hohman JN, Claridge SA, Kim M, Weiss PS. Mat Sci Eng R. 2010;70:188–208. [Google Scholar]
- 7.De Feyter S, Gesquiere A, Abdel-Mottaleb MM, Grim PCM, De Schryver FC, Meiners C, Sieffert M, Valiyaveettil S, Mullen K. Acc Chem Res. 2000;33:520–531. doi: 10.1021/ar970040g. [DOI] [PubMed] [Google Scholar]
- 8.Okawa Y, Aono M. Nature. 2001;409:683–684. doi: 10.1038/35055625. [DOI] [PubMed] [Google Scholar]
- 9.Bumm LA, Arnold JJ, Cygan MT, Dunbar TD, Burgin TP, Jones L, Allara DL, Tour JM, Weiss PS. Science. 1996;271:1705–1707. [Google Scholar]
- 10.Cygan MT, Dunbar TD, Arnold JJ, Bumm LA, Shedlock NF, Burgin TP, Jones L, Allara DL, Tour JM, Weiss PS. J Am Chem Soc. 1998;120:2721–2732. [Google Scholar]
- 11.Saavedra HM, Mullen TJ, Zhang PP, Dewey DC, Claridge SA, Weiss PS. Rep Prog Phys. 2010;73:036501. [Google Scholar]
- 12.Weiss PS. Acc Chem Res. 2008;41:1772–1781. doi: 10.1021/ar8001443. [DOI] [PubMed] [Google Scholar]
- 13.Bumm LA, Arnold JJ, Charles LF, Dunbar TD, Allara DL, Weiss PS. J Am Chem Soc. 1999;121:8017–8021. [Google Scholar]
- 14.Zheng YB, Payton JL, Chung CH, Liu R, Cheunkar S, Pathem BK, Yang Y, Jensen L, Weiss PS. Nano Lett. 2011;11:3447–3452. doi: 10.1021/nl2019195. [DOI] [PubMed] [Google Scholar]
- 15.Kumar AS, Ye T, Takami T, Yu BC, Flatt AK, Tour JM, Weiss PS. Nano Lett. 2008;8:1644–1648. doi: 10.1021/nl080323+. [DOI] [PubMed] [Google Scholar]
- 16.Moore AM, Dameron AA, Mantooth BA, Smith RK, Fuchs DJ, Ciszek JW, Maya F, Yao Y, Tour JM, Weiss PS. J Am Chem Soc. 2006;128:1959–1967. doi: 10.1021/ja055761m. [DOI] [PubMed] [Google Scholar]
- 17.Lewis PA, Inman CE, Yao Y, Tour JM, Hutchison JE, Weiss PS. J Am Chem Soc. 2004;126:12214–12215. doi: 10.1021/ja038622i. [DOI] [PubMed] [Google Scholar]
- 18.Lewis PA, Inman CE, Maya F, Tour JM, Hutchison JE, Weiss PS. J Am Chem Soc. 2005;127:17421–17426. doi: 10.1021/ja055787d. [DOI] [PubMed] [Google Scholar]
- 19.Donhauser ZJ, Mantooth BA, Kelly KF, Bumm LA, Monnell JD, Stapleton JJ, Price DW, Jr, Rawlett AM, Allara DL, Tour JM, Weiss PS. Science. 2001;292:2303–2307. doi: 10.1126/science.1060294. [DOI] [PubMed] [Google Scholar]
- 20.Saavedra HM, Barbu CM, Dameron AA, Mullen TJ, Crespi VH, Weiss PS. J Am Chem Soc. 2007;129:10741–10746. doi: 10.1021/ja071116z. [DOI] [PubMed] [Google Scholar]
- 21.Claridge SA, Schwartz JJ, Weiss PS. ACS Nano. 2011;5:693–729. doi: 10.1021/nn103298x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bigelow WC, Pickett DL, Zisman WA. Journal of Colloid Science. 1946:1. doi: 10.1016/0095-8522(47)90058-5. [DOI] [PubMed] [Google Scholar]
- 23.Sagiv J. J Am Chem Soc. 1980:102. [Google Scholar]
- 24.Nuzzo RG, Allara DL. J Am Chem Soc. 1983;105:4481–4483. [Google Scholar]
- 25.Smith RK, Lewis PA, Weiss PS. Prog Surf Sci. 2004;75:1–68. [Google Scholar]
- 26.Bent SF. ACS Nano. 2007;1:10–12. doi: 10.1021/nn700118k. [DOI] [PubMed] [Google Scholar]
- 27.Laibinis PE, Whitesides GM, Allara DL, Tao YT, Parikh AN, Nuzzo RG. J Am Chem Soc. 1991;113:7152–7167. [Google Scholar]
- 28.Ulman A. Chem Rev. 1996;96:1533–1554. doi: 10.1021/cr9502357. [DOI] [PubMed] [Google Scholar]
- 29.Shaporenko A, Ulman A, Terfort A, Zharnikov M. J Phys Chem B. 2005;109:3898–3906. doi: 10.1021/jp045052f. [DOI] [PubMed] [Google Scholar]
- 30.Vericat C, Vela ME, Benitez G, Carro P, Salvarezza RC. Chem Soc Rev. 2010;39:1805–1834. doi: 10.1039/b907301a. [DOI] [PubMed] [Google Scholar]
- 31.Azzaroni O, Vela ME, Fonticelli M, Benítez G, Carro P, Blum B, Salvarezza RC. J Phys Chem B. 2003;107:13446–13454. [Google Scholar]
- 32.Love JC, Wolfe DB, Haasch R, Chabinyc ML, Paul KE, Whitesides GM, Nuzzo RG. J Am Chem Soc. 2003;125:2597–2609. doi: 10.1021/ja028692+. [DOI] [PubMed] [Google Scholar]
- 33.Floridia Addato MaA, Rubert AA, Benítez GA, Fonticelli MH, Carrasco J, Carro P, Salvarezza RC. J Phys Chem C. 2011;115:17788–17798. [Google Scholar]
- 34.Zangmeister CD, Picraux LB, van Zee RD, Yao Y, Tour JM. Chem Phys Lett. 2007;442:390–393. [Google Scholar]
- 35.Bengio S, Fonticelli M, Benitez G, Creus AH, Carro P, Ascolani H, Zampieri G, Blum B, Salvarezza RC. J Phys Chem B. 2005;109:23450–23460. doi: 10.1021/jp052915b. [DOI] [PubMed] [Google Scholar]
- 36.Mani G, Johnson DM, Marton D, Dougherty VL, Feldman MD, Patel D, Ayon AA, Agrawal CM. Langmuir. 2008;24:6774–6784. doi: 10.1021/la8003646. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura T, Miyamae T, Nakai I, Kondoh H, Kawamoto T, Kobayashi N, Yoshimura YSD, Ohta T, Nozoye H, Matsumoto M. Langmuir. 2005;21:3344–3353. doi: 10.1021/la047620o. [DOI] [PubMed] [Google Scholar]
- 38.Rampi MA, Schueller OJA, Whitesides GM. Appl Phys Lett. 1998;72:1781–1783. [Google Scholar]
- 39.Chiechi RC, Weiss EA, Dickey MD, Whitesides GM. Angew Chem-Int Edit. 2008;47:142–144. doi: 10.1002/anie.200703642. [DOI] [PubMed] [Google Scholar]
- 40.Hohman JN, Kim M, Wadsworth GA, Bednar HR, Jiang J, LeThai MA, Weiss PS. Nano Lett. 2011;11:5104–5110. doi: 10.1021/nl202728j. [DOI] [PubMed] [Google Scholar]
- 41.Buriak JM. Chem Rev. 2002;102:1271–1308. doi: 10.1021/cr000064s. [DOI] [PubMed] [Google Scholar]
- 42.Bent SF. Surf Sci. 2002;500:879–903. [Google Scholar]
- 43.Kachian JS, Wong KT, Bent SF. Acc Chem Res. 2010;43:346–355. doi: 10.1021/ar900251s. [DOI] [PubMed] [Google Scholar]
- 44.Hohman JN, Kim M, Bednar HR, Lawrence JA, McClanahan PD, Weiss PS. Chem Sci. 2011;2:1334–1343. [Google Scholar]
- 45.McGuiness CL, Shaporenko A, Mars CK, Uppili S, Zharnikov M, Allara DL. J Am Chem Soc. 2006;128:5231–5243. doi: 10.1021/ja058657d. [DOI] [PubMed] [Google Scholar]
- 46.Lim H, Carraro C, Maboudian R, Pruessner MW, Ghodssi R. Langmuir. 2004;20:743–747. doi: 10.1021/la035404u. [DOI] [PubMed] [Google Scholar]
- 47.Wasserman SR, Tao YT, Whitesides GM. Langmuir. 1989;5:1074–1087. [Google Scholar]
- 48.Schreiber F. Prog Surf Sci. 2000;65:151–256. [Google Scholar]
- 49.Maury P, Peter M, Mahalingam V, Reinhoudt DN, Huskens J. Adv Funct Mater. 2005;15:451–457. [Google Scholar]
- 50.Ito T, Forman SM, Cao C, Li F, Eddy CR, Mastro MA, Jr, Holm RT, Henry RL, Hohn KL, Edgar JH. Langmuir. 2008;24:6630–6635. doi: 10.1021/la800716r. [DOI] [PubMed] [Google Scholar]
- 51.Noble-Luginbuhl AR, Nuzzo RG. Langmuir. 2001;17:3937–3944. [Google Scholar]
- 52.Elemans JAAW, Lei S, De Feyter S. Angew Chem-Int Edit. 2009;48:7298–7332. doi: 10.1002/anie.200806339. [DOI] [PubMed] [Google Scholar]
- 53.De Feyter S, De Schryver FC. J Phys Chem B. 2005;109:4290–4302. doi: 10.1021/jp045298k. [DOI] [PubMed] [Google Scholar]
- 54.Xu L, Miao X, Ying X, Deng W. J Phys Chem C. 2012;116:1061–1069. [Google Scholar]
- 55.Xue Y, Zimmt MB. J Am Chem Soc. 2012;134:4513–4516. doi: 10.1021/ja2115019. [DOI] [PubMed] [Google Scholar]
- 56.Somorjai GA, Li Y. Introduction to Surface Chemistry and Catalysis. John Wiley and Sons, Inc; Hoboken, New Jersey: 2010. [Google Scholar]
- 57.Duke CB. Chem Rev. 1996;96:1237–1259. doi: 10.1021/cr950212s. [DOI] [PubMed] [Google Scholar]
- 58.Liao WS, Cheunkar S, Cao HH, Bednar HR, Andrews AM, Weiss PS. Science. 2012 doi: 10.1126/science.1221774. in press. [DOI] [PubMed] [Google Scholar]
- 59.Tierney HL, Han JW, Jewell AD, Iski EV, Baber AE, Sholl DS, Sykes ECH. J Phys Chem C. 2011;115:897–901. [Google Scholar]
- 60.Bellisario DO, Jewell AD, Tierney HL, Baber AE, Sykes ECH. J Phys Chem C. 2010;114:14583–14589. [Google Scholar]
- 61.Tierney HL, Baber AE, Sykes ECH, Akimov A, Kolomeisky AB. J Phys Chem C. 2009;113:10913–10920. [Google Scholar]
- 62.Jensen SC, Baber AE, Tierney HL, Sykes EC. ACS Nano. 2007;1:22–29. doi: 10.1021/nn700042b. [DOI] [PubMed] [Google Scholar]
- 63.Han P, Mantooth BA, Sykes ECH, Donhauser ZJ, Weiss PS. J Am Chem Soc. 2004;126:10787–10788. doi: 10.1021/ja049113z. [DOI] [PubMed] [Google Scholar]
- 64.Yu M, Bovet N, Satterley C, Bengió S, Lovelock K, Milligan P, Jones R, Woodruff D, Dhanak V. Phys Rev Lett. 2006;97:166102. doi: 10.1103/PhysRevLett.97.166102. [DOI] [PubMed] [Google Scholar]
- 65.Stranick SJ, Parikh AN, Allara DL, Weiss PS. J Phys Chem. 1994;98:11136–11142. [Google Scholar]
- 66.Hakkinen H. Nat Chem. 2012;4:443–455. doi: 10.1038/nchem.1352. [DOI] [PubMed] [Google Scholar]
- 67.Maksymovych P, Voznyy O, Dougherty DB, Sorescu DC, Yates JT. Prog Surf Sci. 2010;85:206–240. [Google Scholar]
- 68.Cossaro A, Mazzarello R, Rousseau R, Casalis L, Verdini A, Kohlmeyer A, Floreano L, Scandolo S, Morgante A, Klein ML, Scoles G. Science. 2008;321:943–946. doi: 10.1126/science.1158532. [DOI] [PubMed] [Google Scholar]
- 69.Chaudhuri A, Lerotholi TJ, Jackson DC, Woodruff DP, Dhanak VR. Surf Sci. 2010;604:227–234. [Google Scholar]
- 70.Mazzarello R, Cossaro A, Verdini A, Rousseau R, Casalis L, Danisman M, Floreano L, Scandolo S, Morgante A, Scoles G. Phys Rev Lett. 2007;98:016102. doi: 10.1103/PhysRevLett.98.016102. [DOI] [PubMed] [Google Scholar]
- 71.Otálvaro D, Veening T, Brocks G. J Phys Chem C. 2012;116:7826–7837. [Google Scholar]
- 72.Iski EV, El-Kouedi M, Calderon C, Wang F, Bellisario DO, Ye T, Sykes ECH. Electrochim Acta. 2011;56:1652–1661. [Google Scholar]
- 73.Monnell JD, Stapleton JJ, Jackiw JJ, Dunbar T, Reinerth WA, Dirk SM, Tour JM, Allara DL, Weiss PS. J Phys Chem B. 2004;108:9834–9841. doi: 10.1021/jp044186q. [DOI] [PubMed] [Google Scholar]
- 74.Han P, Kurland AR, Giordano AN, Nanayakkara SU, Blake MM, Pochas CM, Weiss PS. ACS Nano. 2009;3:3115–3121. doi: 10.1021/nn901030x. [DOI] [PubMed] [Google Scholar]
- 75.Murphy KL, Tysoe WT, Bennett DW. Langmuir. 2004;20:1732–1738. [Google Scholar]
- 76.Lio A, Charych DH, Salmeron M. J Phys Chem B. 1997;101:3800–3805. [Google Scholar]
- 77.Allara DL, Parikh AN, Rondelez F. Langmuir. 1995;11:2357–2360. [Google Scholar]
- 78.Bourgeat-Lami E. J Nanosci Nanotech. 2002;2:1–24. doi: 10.1166/jnn.2002.075. [DOI] [PubMed] [Google Scholar]
- 79.Jewell A, Tierney H, Sykes E. Phys Rev B. 2010;82:205401. [Google Scholar]
- 80.Gouzman I, Dubey M, Carolus MD, Schwartz J, Bernasek SL. Surf Sci. 2006;600:773–781. [Google Scholar]
- 81.Jewell AD, Kyran SJ, Rabinovich D, Sykes EC. Chem Eur J. 2012;18:7169–7178. doi: 10.1002/chem.201102956. [DOI] [PubMed] [Google Scholar]
- 82.Hohman JN, Kim M, Schupbach B, Kind M, Thomas JC, Terfort A, Weiss PS. J Am Chem Soc. 2011;133:19422–19431. doi: 10.1021/ja2063988. [DOI] [PubMed] [Google Scholar]
- 83.Huang FK, Horton RC, Myles DC, Garrell RL. Langmuir. 1998;14:4802–4808. [Google Scholar]
- 84.Taylor CE, Schwartz DK. Langmuir. 2003;19:2665–2672. [Google Scholar]
- 85.Lim MS, Feng K, Chen XX, Wu N, Raman A, Nightingale J, Gawalt ES, Korakakis D, Hornak LA, Timperman AT. Langmuir. 2007;23:2444–2452. doi: 10.1021/la061914n. [DOI] [PubMed] [Google Scholar]
- 86.Li J, Li X, Ni X, Wang X, Li H, Leong KW. Biomater. 2006;27:4132–4140. doi: 10.1016/j.biomaterials.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 87.Uemura S, Tanoue R, Yilmaz N, Ohira A, Kunitake M. Materials. 2010;3:4252–4276. doi: 10.3390/ma3084252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dorokhin D, Hsu SH, Tomczak N, Reinhoudt DN, Huskens J, Velders AH, Vancso GJ. ACS Nano. 2010;4:137–142. doi: 10.1021/nn901109x. [DOI] [PubMed] [Google Scholar]
- 89.Gonzalez-Campo A, Hsu SH, Puig L, Huskens J, Reinhoudt DN, Velders AH. J Am Chem Soc. 2010;132:11434–11436. doi: 10.1021/ja1048658. [DOI] [PubMed] [Google Scholar]
- 90.Hu WS, Tao YT, Hsu YJ, Wei DH, Wu YS. Langmuir. 2005;21:2260–2266. doi: 10.1021/la047634u. [DOI] [PubMed] [Google Scholar]
- 91.Clegg RS, Reed SM, Hutchison JE. J Am Chem Soc. 1998;120:2486–2487. [Google Scholar]
- 92.Mao X, Wang Y, Liu L, Niu L, Yang Y, Wang C. Langmuir. 2009;25:8849–8853. doi: 10.1021/la901342r. [DOI] [PubMed] [Google Scholar]
- 93.Nowinski AK, Sun F, White AD, Keefe AJ, Jiang S. J Am Chem Soc. 2012;134:6000–6005. doi: 10.1021/ja3006868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Prato M, Toccafondi C, Maidecchi G, Chaudhari V, Harish MNK, Sampath S, Parodi R, Esaulov VA, Canepa M. J Phys Chem C. 2012;116:2431–2437. [Google Scholar]
- 95.Chaudhari V, Kotresh HMN, Srinivasan S, Esaulov VA. J Phys Chem C. 2011;115:16518–16523. [Google Scholar]
- 96.Choi J-S, Kang H-G, Ito E, Hara M, Noh J-G. Bull Kor Chem Soc. 2011;32:2623–2627. [Google Scholar]
- 97.Monnell JD, Stapleton JJ, Dirk SM, Reinerth WA, Tour JM, Allara DL, Weiss PS. J Phys Chem B. 2005;109:20343–20349. doi: 10.1021/jp044186q. [DOI] [PubMed] [Google Scholar]
- 98.Szelagowska-Kunstman K, Cyganik P, Schupbach B, Terfort A. Phys Chem Chem Phys. 2010;12:4400–4406. doi: 10.1039/b923274p. [DOI] [PubMed] [Google Scholar]
- 99.Livage J. J Solid State Chem. 1986;64:322–330. [Google Scholar]
- 100.Sanchez C, Boissière C, Grosso D, Laberty C, Nicole L. Chem Mater. 2008;20:682–737. [Google Scholar]
- 101.Petrović Ze, Katić J, Metikoš-Huković M, Dadafarin H, Omanovic S. J Electrochem Soc. 2011;158:F159–F165. [Google Scholar]
- 102.Baber AE, Lawton TJ, Sykes ECH. J Phys Chem C. 2011;115:9157–9163. [Google Scholar]
- 103.Otero R, Rosei F, Besenbacher F. Annu Rev Phys Chem. 2006;57:497–525. doi: 10.1146/annurev.physchem.57.032905.104634. [DOI] [PubMed] [Google Scholar]
- 104.Baber AE, Jensen SC, Sykes EC. J Am Chem Soc. 2007;129:6368–6369. doi: 10.1021/ja0709526. [DOI] [PubMed] [Google Scholar]
- 105.Han P, Weiss PS. Surf Sci Rep. 2012;67:19–81. [Google Scholar]
- 106.Yan L, Zheng YB, Zhao F, Li S, Gao X, Xu B, Weiss PS, Zhao Y. Chem Soc Rev. 2012;41:97–114. doi: 10.1039/c1cs15193b. [DOI] [PubMed] [Google Scholar]
- 107.Arakawa H, Umemura K, Ikai A. Nature. 1992;358:171–173. doi: 10.1038/358171a0. [DOI] [PubMed] [Google Scholar]
- 108.Mao X, Wang C, Ma X, Zhang M, Liu L, Zhang L, Niu L, Zeng Q, Yang Y. Nanoscale. 2011;3:1592–1599. doi: 10.1039/c0nr00782j. [DOI] [PubMed] [Google Scholar]
- 109.Torres E, Blumenau AT, Biedermann PU. Chemphyschem. 2011;12:999–1009. doi: 10.1002/cphc.201000803. [DOI] [PubMed] [Google Scholar]
- 110.Poirier GE. J Vac Sci Technol B. 1996;14:1453–1460. [Google Scholar]
- 111.Moore AM, Yeganeh S, Yao Y, Claridge SA, Tour JM, Ratner MA, Weiss PS. ACS Nano. 2010;4:7630–7636. doi: 10.1021/nn102371z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Clegg RS, Hutchison JE. J Am Chem Soc. 1999;121:5319–5327. [Google Scholar]
- 113.Lewis PA, Smith RK, Kelly KF, Bumm LA, Reed SM, Clegg RS, Gunderson JD, Hutchison JE, Weiss PS. J Phys Chem B. 2001;105:10630–10636. [Google Scholar]
- 114.Yoon HJ, Shapiro ND, Park KM, Thuo MM, Soh S, Whitesides GM. Angew Chem Int Ed Engl. 2012;51:4658–4661. doi: 10.1002/anie.201201448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Smith RK, Reed SM, Lewis PA, Monnell JD, Clegg RS, Kelly KF, Bumm LA, Hutchison JE, Weiss PS. J Phys Chem B. 2001;105:1119–1122. [Google Scholar]
- 116.Kim M, Hohman JN, Serino AC, Weiss PS. J Phys Chem C. 2010;114:19744–19751. [Google Scholar]
- 117.Grave C, Risko C, Shaporenko A, Wang Y, Nuckolls C, Ratner MA, Rampi MA, Zharnikov M. Adv Funct Mater. 2007;17:3816–3828. [Google Scholar]
- 118.Donhauser ZJ, Mantooth BA, Kelly KF, Bumm LA, Monnell JD, Stapleton JJ, Price DW, Rawlett AM, Allara DL, Tour JM, Weiss PS. Science. 2001;292:2303–2307. doi: 10.1126/science.1060294. [DOI] [PubMed] [Google Scholar]
- 119.Vaish A, Shuster MJ, Cheunkar S, Singh YS, Weiss PS, Andrews AM. ACS Chem Neurosci. 2010;1:495–504. doi: 10.1021/cn1000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Geyer W, Stadler V, Eck W, Zharnikov M, Golzhauser A, Grunze M. Appl Phys Lett. 1999;75:2401–2403. [Google Scholar]
- 121.Chabinyc ML, Chen XX, Holmlin RE, Jacobs H, Skulason H, Frisbie CD, Mujica V, Ratner MA, Rampi MA, Whitesides GM. J Am Chem Soc. 2002;124:11730–11736. doi: 10.1021/ja020506c. [DOI] [PubMed] [Google Scholar]
- 122.Bould J, Machacek J, Londesborough MG, Macias R, Kennedy JD, Bastl Z, Rupper P, Base T. Inorg Chem. 2012;51:1685–1694. doi: 10.1021/ic202000b. [DOI] [PubMed] [Google Scholar]
- 123.Kim M, Hohman JN, Morin EI, Daniel TA, Weiss PS. J Phys Chem A. 2009;113:3895–3903. doi: 10.1021/jp810048n. [DOI] [PubMed] [Google Scholar]
- 124.Jobbins MM, Raigoza AF, Kandel SA. J Phys Chem C. 2011;115:25437–25441. [Google Scholar]
- 125.Willey TM, Fabbri JD, Lee JRI, Schreiner PR, Rokin AA, Tkachenko BA, Fokina NA, Dahl JEP, Carlson RMK, Vance AL, Yang Wl, Terminello LJ, van Buuren T, Melosh NA. J Am Chem Soc. 2008;130:10536–10544. doi: 10.1021/ja711131e. [DOI] [PubMed] [Google Scholar]
- 126.Wang YY, Kioupakis E, Lu XH, Wegner D, Yamachika R, Dahl JE, Carlson RMK, Louie SG, Crommie MF. Nat Mater. 2008;7:38–42. doi: 10.1038/nmat2066. [DOI] [PubMed] [Google Scholar]
- 127.Hohman JN, Zhang P, Morin EI, Han P, Kim M, Kurland AR, McClanahan PD, Balema VP, Weiss PS. ACS Nano. 2009;3:527–536. doi: 10.1021/nn800673d. [DOI] [PubMed] [Google Scholar]
- 128.Wang H, He Y, Ratner BD, Jiang S. J Biomed Mater Res A. 2006;77:672–678. doi: 10.1002/jbm.a.30586. [DOI] [PubMed] [Google Scholar]
- 129.Lee SH, Lin WC, Chang CJ, Huang CC, Liu CP, Kuo CH, Chang HY, You YW, Kao WL, Yen GJ, Kuo DY, Kuo YT, Tsai MH, Shyue JJ. Phys Chem Chem Phys. 2011;13:4335–4339. doi: 10.1039/c0cp02437f. [DOI] [PubMed] [Google Scholar]
- 130.Romaner L, Heimel G, Zojer E. Phys Rev B. 2008;77:045113. [Google Scholar]
- 131.Carot ML, Macagno VA, Paredes-Olivera P, Patrito EM. J Phys Chem C. 2007;111:4294–4304. [Google Scholar]
- 132.Watcharinyanon S, Moons E, Johansson LSO. J Phys Chem C. 2009;113:1972–1979. [Google Scholar]
- 133.Chambers RC, Inman CE, Hutchison JE. Langmuir. 2005;21:4615–4621. doi: 10.1021/la050104t. [DOI] [PubMed] [Google Scholar]
- 134.Yagüe JL, Agulló N, Fonder G, Delhalle J, Mekhalif Z, Borrós S. Plasma Proc Pol. 2010;7:601–609. [Google Scholar]
- 135.Patnaik A, Setoyama H, Ueno N. J Chem Phys. 2004;120:6214–6221. doi: 10.1063/1.1651062. [DOI] [PubMed] [Google Scholar]
- 136.Shuster MJ, Vaish A, Szapacs ME, Anderson ME, Weiss PS, Andrews AM. Adv Mater. 2008;20:164–167. [Google Scholar]
- 137.Shuster MJ, Vaish A, Cao HH, Guttentag AI, McManigle JE, Gibb AL, Martinez MM, Nezarati RM, Hinds JM, Liao WS, Weiss PS, Andrews AM. Chem Comm. 2011;47:10641–10643. doi: 10.1039/c1cc13002a. [DOI] [PubMed] [Google Scholar]
- 138.Cho J, Berbil-Bautista L, Levy N, Poulsen D, Frechet JM, Crommie MF. J Chem Phys. 2010;133:234707. doi: 10.1063/1.3519557. [DOI] [PubMed] [Google Scholar]
- 139.Smith RK, Nanayakkara SU, Woehrle GH, Pearl TP, Blake MM, Hutchison JE, Weiss PS. J Am Chem Soc. 2006;128:9266–9267. doi: 10.1021/ja061040r. [DOI] [PubMed] [Google Scholar]
- 140.Vaish A, Liao WS, Shuster MJ, Hinds JM, Weiss PS, Andrews AM. Anal Chem. 2011;83:7451–7456. doi: 10.1021/ac2016536. [DOI] [PubMed] [Google Scholar]
- 141.Shuster MJ, Vaish A, Gilbert ML, Martinez-Rivera M, Nezarati RM, Weiss PS, Andrews AM. J Phys Chem C. 2011;115:24778–24787. [Google Scholar]
- 142.Saavedra HM, Thompson CM, Hohman JN, Crespi VH, Weiss PS. J Am Chem Soc. 2009;131:2252–2259. doi: 10.1021/ja807648g. [DOI] [PubMed] [Google Scholar]
- 143.Perez-Luna VH, O’Brien MJ, Opperman KA, Hampton PD, Lopez GP, Klumb LA, SPS J Am Chem Soc. 1999;121:6469–6478. [Google Scholar]
- 144.Lahiri J, Ostuni E, Whitesides GM. Langmuir. 1999;15:2055–2060. [Google Scholar]
- 145.Mrksich M. ACS Nano. 2008;2:7–18. doi: 10.1021/nn7004156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Weck M, Jackiw JJ, Rossi RR, Weiss PS, Grubbs RH. J Am Chem Soc. 1999:121. [Google Scholar]
- 147.Lahiri J, Isaacs L, Tien J, Whitesides GM. Anal Chem. 1999;71:777–790. doi: 10.1021/ac980959t. [DOI] [PubMed] [Google Scholar]
- 148.Vaish A, Shuster MJ, Cheunkar S, Weiss PS, Andrews AM. Small. 2011;7:1471–1479. doi: 10.1002/smll.201100094. [DOI] [PubMed] [Google Scholar]
- 149.Kolb HC, Finn MG, Sharpless KB. Angew Chem-Int Edit. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 150.Sun XL, Stabler CL, Cazalis CS, Chaikof EL. Bioconj Chem. 2006;17:52–57. doi: 10.1021/bc0502311. [DOI] [PubMed] [Google Scholar]
- 151.Qin GT, Santos C, Zhang W, Li Y, Kumar A, Erasquin UJ, Liu K, Muradov P, Trautner BW, Cai CZ. J Am Chem Soc. 2010;132:16432–16441. doi: 10.1021/ja1025497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Paxton WF, Spruell JM, Stoddart JF. J Am Chem Soc. 2009;131:6692–6694. doi: 10.1021/ja9015974. [DOI] [PubMed] [Google Scholar]
- 153.Grim PCM, De Feyter S, Gesquiere A, Vanoppen P, Rucker M, Valiyaveettil S, Moessner G, Mullen K, De Schryver FC. Angew Chem-Int Edit. 1997;36:2601–2603. [Google Scholar]
- 154.Akai-Kasaya M, Shimizu K, Watanabe Y, Saito A, Aono M, Kuwahara Y. Phys Rev Lett. 2003;91:255501. doi: 10.1103/PhysRevLett.91.255501. [DOI] [PubMed] [Google Scholar]
- 155.Bensimon A, Simon A, Chiffaudel A, Croquette V, Heslot F, Bendimon D. Science. 1994;265:2096–2098. doi: 10.1126/science.7522347. [DOI] [PubMed] [Google Scholar]
- 156.Patolsky F, Weizmann Y, Willner I. Nat Mater. 2004;3:692–695. doi: 10.1038/nmat1205. [DOI] [PubMed] [Google Scholar]
- 157.Juluri BK, Kumar AS, Liu Y, Ye T, Yang YW, Flood AH, Fang L, Stoddart JF, Weiss PS, Huang TJ. ACS Nano. 2009;3:291–300. doi: 10.1021/nn8002373. [DOI] [PubMed] [Google Scholar]
- 158.Li DB, Paxton WF, Baughman RH, Huang TJ, Stoddart JF, Weiss PS. MRS Bull. 2009;34:671–681. [Google Scholar]
- 159.Kumpf C, Marks LD, Ellis D, Smilgies D, Landemark E, Nielsen M, Feidenhans R, Zegenhagen J, Bunk O, Zeysing JH, Su Y, Johnson RL. Phys Rev Lett. 2001;86:3586–3589. doi: 10.1103/PhysRevLett.86.3586. [DOI] [PubMed] [Google Scholar]
- 160.Besenbacher F. Rep Prog Phys. 1996;59:1737–1802. [Google Scholar]
- 161.Eigler DM, Schweizer EK. Nat Chem. 1990;344:524–526. [Google Scholar]
- 162.Hasegawa S, Avouris P. Phys Rev Lett. 1993;71:1071–1074. doi: 10.1103/PhysRevLett.71.1071. [DOI] [PubMed] [Google Scholar]
- 163.Stranick SJ, Kamna MM, Weiss PS. Science. 1994;266:99–102. doi: 10.1126/science.266.5182.99. [DOI] [PubMed] [Google Scholar]
- 164.Gambardella P, Dallmeyer A, Maiti K, Malagoli MC, Eberhardt W, Kern K, Carbone C. Nature. 2002;416:301–304. doi: 10.1038/416301a. [DOI] [PubMed] [Google Scholar]
- 165.Ising E. Z Phys. 1925;31:253–258. [Google Scholar]
- 166.Bethe H. Z Phys. 1931;71:205–226. [Google Scholar]
- 167.Kamna MM, Stranick SJ, Weiss PS. Isr J Chem. 1996;36:59–62. [Google Scholar]
- 168.Schiffrin A, Riemann A, Auwarter W, Pennec Y, Weber-Bargioni A, Cvetko D, Cossaro A, Morgante A, Barth JV. Proc Natl Acad Sci U S A. 2007;104:5279–5284. doi: 10.1073/pnas.0607867104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pennec Y, Auwarter W, Schiffrin A, Weber-Bargioni A, Riemann A, Barth JV. Nat Nano. 2007;2:99–103. doi: 10.1038/nnano.2006.212. [DOI] [PubMed] [Google Scholar]
- 170.Coskun A, Banaszak M, Astumian RD, Stoddart JF, Grzybowski BA. Chem Soc Rev. 2012;41:19–30. doi: 10.1039/c1cs15262a. [DOI] [PubMed] [Google Scholar]
- 171.Ye T, Kumar AS, Saha S, Takami T, Huang TJ, Stoddart JF, Weiss PS. ACS Nano. 2010;4:3697–3701. doi: 10.1021/nn100545r. [DOI] [PubMed] [Google Scholar]
- 172.Huang TJ, Brough B, Ho CM, Liu Y, Flood AH, Bonvallet PA, Tseng HR, Stoddart JF, Baller M, Magonov S. Appl Phys Lett. 2004;85:5391–5393. [Google Scholar]
- 173.Rothemund PWK. Nature. 2006;440:297–302. doi: 10.1038/nature04586. [DOI] [PubMed] [Google Scholar]
- 174.Gu HZ, Chao J, Xiao SJ, Seeman NC. Nature. 2010;465:202–205. doi: 10.1038/nature09026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wilner OI, Willner I. Chem Rev. 2012;112:2528–2556. doi: 10.1021/cr200104q. [DOI] [PubMed] [Google Scholar]
- 176.Mao X-B, Wang C-X, Wu X-K, Ma X-J, Liu L, Zhang L, Niu L, Guo Y-Y, Li D-H, Yang Y-L, Wang C. Proc Natl Acad Sci U S A. 2011;108:19605–19610. doi: 10.1073/pnas.1102971108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wei YH, Tong WJ, Zimmt MB. J Am Chem Soc. 2008;130:3399–3405. doi: 10.1021/ja075170r. [DOI] [PubMed] [Google Scholar]
- 178.Cyr DM, Venkataraman B, Flynn GW. Chem Mater. 1996;8:1600–1615. [Google Scholar]
- 179.Cyr DM, Venkataraman B, Flynn GW, Black A, Whitesides GM. J Phys Chem. 1996;100:13747–13759. [Google Scholar]
- 180.Takami K, Kuwahara Y, Ishii T, Akai-Kasaya M, Saito A, Aono M. Surf Sci. 2005;591:L273–L279. [Google Scholar]
- 181.Okawa Y, Aono M. J Chem Phys. 2001;115:2317–2322. [Google Scholar]
- 182.Scott JC, Samuel JDJ, Hou JH, Rettner CT, Miller RD. Nano Lett. 2006;6:2916–2919. doi: 10.1021/nl0622449. [DOI] [PubMed] [Google Scholar]
- 183.Joachim C, Gimzewski JK, Aviram A. Nature. 2000;408:541–548. doi: 10.1038/35046000. [DOI] [PubMed] [Google Scholar]
- 184.Moth-Poulsen K, Bjornholm T. Nat Nano. 2009;4:551–556. doi: 10.1038/nnano.2009.176. [DOI] [PubMed] [Google Scholar]
- 185.Aviram A, Ratner MA. Chem Phys Lett. 1974;29:277–283. [Google Scholar]
- 186.Metzger RM. J Mater Chem. 2008;18:4364–4396. [Google Scholar]
- 187.McCreery RL, Bergren AJ. Adv Mater. 2009;21:4303–4322. doi: 10.1002/adma.200802850. [DOI] [PubMed] [Google Scholar]
- 188.Heath JR, Ratner MA. Phys Today. 2003;56:43–49. [Google Scholar]
- 189.Joachim C, Magoga M. Chem Phys. 2002;281:347–352. [Google Scholar]
- 190.Gimzewski JK, Joachim C. Science. 1999;283:1683–1688. doi: 10.1126/science.283.5408.1683. [DOI] [PubMed] [Google Scholar]
- 191.Moore AM, Weiss PS. Annu Rev Anal Chem. 2008;1:857–882. doi: 10.1146/annurev.anchem.1.031207.112932. [DOI] [PubMed] [Google Scholar]
- 192.Chen F, Tao NJ. Acc Chem Res. 2009;42:429–438. doi: 10.1021/ar800199a. [DOI] [PubMed] [Google Scholar]
- 193.Lewis PA, Inman CE, Yao YX, Tour JM, Hutchison JE, Weiss PS. J Am Chem Soc. 2004;126:12214–12215. doi: 10.1021/ja038622i. [DOI] [PubMed] [Google Scholar]
- 194.Donhauser ZJ, Price DW, Tour JM, Weiss PS. J Am Chem Soc. 2003;125:11462–11463. doi: 10.1021/ja035036g. [DOI] [PubMed] [Google Scholar]
- 195.Krans JM, Vanruitenbeek JM, Fisun VV, Yanson IK, Dejongh LJ. Nature. 1995;375:767–769. [Google Scholar]
- 196.Nielsen SK, Noat Y, Brandbyge M, Smit RHM, Hansen K, Chen LY, Yanson AI, Besenbacher F, van Ruitenbeek JM. Phys Rev B. 2003;67:245411. [Google Scholar]
- 197.Qin L, Park S, Huang L, Mirkin CA. Science. 2005;308:113–115. doi: 10.1126/science.1112666. [DOI] [PubMed] [Google Scholar]
- 198.Lorente N, Persson M, Lauhon LJ, Ho W. Phys Rev Lett. 2001;86:2593–2596. doi: 10.1103/PhysRevLett.86.2593. [DOI] [PubMed] [Google Scholar]
- 199.Lauhon LJ, Ho W. Phys Rev B. 1999;60:R8525–R8528. [Google Scholar]
- 200.Reed MA, Zhou C, Muller CJ, Burgin TP, Tour JM. Science. 1997;278:252–254. [Google Scholar]
- 201.Selzer Y, Cabassi MA, Mayer TS, Allara DL. J Am Chem Soc. 2004;126:4052–4053. doi: 10.1021/ja039015y. [DOI] [PubMed] [Google Scholar]
- 202.Chen F, Hihath J, Huang ZF, Li XL, Tao NJ. Annu Rev Phys Chem. 2007;58:535–564. doi: 10.1146/annurev.physchem.58.032806.104523. [DOI] [PubMed] [Google Scholar]
- 203.Xue YQ, Datta S, Ratner MA. J Chem Phys. 2001;115:4292–4299. [Google Scholar]
- 204.Xue Y, Ratner MA. Phys Rev B. 2003;68:115406. [Google Scholar]
- 205.Xu BQ, Tao NJJ. Science. 2003;301:1221–1223. doi: 10.1126/science.1087481. [DOI] [PubMed] [Google Scholar]
- 206.Lindsay SM, Ratner MA. Adv Mater. 2007;19:23–31. [Google Scholar]
- 207.Park YS, Whalley AC, Kamenetska M, Steigerwald ML, Hybertsen MS, Nuckolls C, Venkataraman L. J Am Chem Soc. 2007;129:15768–15769. doi: 10.1021/ja0773857. [DOI] [PubMed] [Google Scholar]
- 208.Chen F, Li XL, Hihath J, Huang ZF, Tao NJ. J Am Chem Soc. 2006;128:15874–15881. doi: 10.1021/ja065864k. [DOI] [PubMed] [Google Scholar]
- 209.Pathem BK, Claridge SA, Zheng YB, Weiss PS. Annu Rev Phys Chem. 2013;64 doi: 10.1146/ANNUREV-PHYSCHEM-040412-110045. in press. [DOI] [PubMed] [Google Scholar]
- 210.Metzger RM. Chem Rev. 2003;103:3803–3834. doi: 10.1021/cr020413d. [DOI] [PubMed] [Google Scholar]
- 211.Geddes NJ, Sambles JR, Jarvis DJ, Parker WG, Sandman DJ. Appl Phys Lett. 1990;56:1916–1918. [Google Scholar]
- 212.Geddes NJ, Sambles JR, Jarvis DJ, Parker WG, Sandman DJ. J Appl Phys. 1992;71:756–768. [Google Scholar]
- 213.Krzeminski C, Delerue C, Allan G, Vuillaume D, Metzger RM. Phys Rev B. 2001;64:085405. [Google Scholar]
- 214.Metzger RM, Xu T, Peterson IR. J Phys Chem B. 2001;105:7280–7290. [Google Scholar]
- 215.Ashwell GJ, Urasinska B, Tyrrell WD. Phys Chem Chem Phys. 2006;8:3314–3319. doi: 10.1039/b604092f. [DOI] [PubMed] [Google Scholar]
- 216.Donhauser ZJ, Mantooth BA, Pearl TP, Kelly KF, Nanayakkara SU, Weiss PS. Japan J Appl Phys. 2002;41:4871–4877. [Google Scholar]
- 217.Zhou XL, Zhu XY, White JM. Surf Sci Rep. 1991;13:73–220. [Google Scholar]
- 218.Comstock MJ, Levy N, Kirakosian A, Cho JW, Lauterwasser F, Harvey JH, Strubbe DA, Frechet JMJ, Trauner D, Louie SG, Crommie MF. Phys Rev Lett. 2007;99:038301. doi: 10.1103/PhysRevLett.99.038301. [DOI] [PubMed] [Google Scholar]
- 219.Comstock MJ, Levy N, Cho J, Berbil-Bautista L, Crommie MF, Poulsen DA, Frechet JMJ. Appl Phys Lett. 2008;92:123107. [Google Scholar]
- 220.Hagen S, Kale P, Leyssner F, Nandi D, Wolf M, Tegeder P. J Chem Phys. 2008;129:164102. doi: 10.1063/1.2997343. [DOI] [PubMed] [Google Scholar]
- 221.Pathem BK, Zheng YB, Payton JL, Song T-B, Yu B-C, Tour JM, Jensen YYL, Weiss PS. J Phys Chem Lett. 2012;3:2388–2394. doi: 10.1021/jz300968m. [DOI] [PubMed] [Google Scholar]
- 222.Davis JJ, Orlowski GA, Rahman H, Beer PD. Chem Comm. 2010;46:54–63. doi: 10.1039/b915122b. [DOI] [PubMed] [Google Scholar]
- 223.Collier CP, Mattersteig G, Wong EW, Luo Y, Beverly K, Sampaio J, Raymo FM, Stoddart JF, Heath JR. Science. 2000;289:1172–1175. doi: 10.1126/science.289.5482.1172. [DOI] [PubMed] [Google Scholar]
- 224.Stoddart JF. Chem Soc Rev. 2009;38:1802–1820. doi: 10.1039/b819333a. [DOI] [PubMed] [Google Scholar]
- 225.Coskun A, Spruell JM, Barin G, Dichtel WR, Flood AH, Botros YY, Stoddart JF. Chem Soc Rev. 2012;41:4827–4859. doi: 10.1039/c2cs35053j. [DOI] [PubMed] [Google Scholar]
- 226.Mayne AJ, Dujardin G, Comtet G, Riedel D. Chem Rev. 2006;106:4355–4378. doi: 10.1021/cr050177h. [DOI] [PubMed] [Google Scholar]
- 227.Lauhon LJ, Ho W. J Phys Chem A. 2000;104:2463–2467. [Google Scholar]
- 228.Hla SW, Bartels L, Meyer G, Rieder KH. Phys Rev Lett. 2000;85:2777–2780. doi: 10.1103/PhysRevLett.85.2777. [DOI] [PubMed] [Google Scholar]
- 229.Blake MM, Nanayakkara SU, Claridge SA, Fernandez-Torres LC, Sykes ECH, Weiss PS. J Phys Chem A. 2009;113:13167–13172. doi: 10.1021/jp903590c. [DOI] [PubMed] [Google Scholar]
- 230.Kim M, Hohman JN, Cao Y, Houk KN, Ma H, Jen AK, Weiss PS. Science. 2011;331:1312–1315. doi: 10.1126/science.1200830. [DOI] [PubMed] [Google Scholar]
- 231.Bustamante C, Macosko JC, Wuite GJL. Nat Rev Mol Cell Biol. 2000;1:130–136. doi: 10.1038/35040072. [DOI] [PubMed] [Google Scholar]
- 232.Kelley AM, Michalet X, Weiss S. Science. 2001;292:1671–1672. doi: 10.1126/science.1060096. [DOI] [PubMed] [Google Scholar]
- 233.Greenleaf WJ, Woodside MT, Block SM. Annu Rev Biophys Biomol Struct. 2007;36:171–190. doi: 10.1146/annurev.biophys.36.101106.101451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Harpaz Y, Gerstein M, Chothia C. Structure. 1994;2:641–649. doi: 10.1016/s0969-2126(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 235.Srinivasan C, Mullen TJ, Hohman JN, Anderson ME, Dameron AA, Andrews AM, Dickey EC, Horn MW, Weiss PS. ACS Nano. 2007;1:191–201. doi: 10.1021/nn7000799. [DOI] [PubMed] [Google Scholar]
- 236.Bain CD, Troughton EB, Tao YT, Evall J, Whitesides GM, Nuzzo RG. J Am Chem Soc. 1989;111:321–335. [Google Scholar]
- 237.Xia YN, Kim E, Whitesides GM. J Electrochem Soc. 1996;143:1070–1079. [Google Scholar]
- 238.Xia YN, Venkateswaran N, Qin D, Tien J, Whitesides GM. Langmuir. 1998;14:363–371. [Google Scholar]
- 239.Xia YN, Kim E, Mrksich M, Whitesides GM. Chem Mater. 1996;8:601–603. [Google Scholar]
- 240.Carvalho A, Geissler M, Schmid H, Michel B, Delamarche E. Langmuir. 2002;18:2406–2412. [Google Scholar]
- 241.Heinemann N, Grunau J, Leissner T, Andreyev O, Kuhn S, Jung U, Zargarani D, Herges R, Magnussen O, Bauer M. Chem Phys. 2012;402:22–28. [Google Scholar]
- 242.Pechenezhskiy IV, Cho J, Nguyen GD, Berbil-Bautista L, Giles BL, Poulsen DA, Frechet JMJ, Crommie MF. J Phys Chem C. 2012;116:1052–1055. [Google Scholar]
- 243.Lee TR, Laibinis PE, Folkers JP, Whitesides GM. Pure Appl Chem. 1991;63:821–828. [Google Scholar]
- 244.Solanki PR, Prabhakar N, Pandey MK, Malhotra BD. Biomed Microdevices. 2008;10:757–767. doi: 10.1007/s10544-008-9188-1. [DOI] [PubMed] [Google Scholar]
- 245.Balamurugan S, Obubuafo A, Soper SA, McCarley RL, Spivak DA. Langmuir. 2006;22:6446–6453. doi: 10.1021/la060222w. [DOI] [PubMed] [Google Scholar]
- 246.Jans K, Bonroy K, De Palma R, Reekmans G, Jans H, Laureyn W, Smet M, Borghs G, Maes G. Langmuir. 2008;24:3949–3954. doi: 10.1021/la703718t. [DOI] [PubMed] [Google Scholar]
- 247.Chaki NK, Vijayamohanan K. Biosens Bioelectron. 2002;17:1–12. doi: 10.1016/s0956-5663(01)00277-9. [DOI] [PubMed] [Google Scholar]
- 248.Kumar A, Biebuyck HA, Whitesides GM. Langmuir. 1994;10:1498–1511. [Google Scholar]
- 249.Xia YN, Whitesides GM. Annu Rev Mater Sci. 1998;28:153–184. [Google Scholar]
- 250.Xia YN, Rogers JA, Paul KE, Whitesides GM. Chem Rev. 1999;99:1823–1848. doi: 10.1021/cr980002q. [DOI] [PubMed] [Google Scholar]
- 251.Piner RD, Zhu J, Xu F, Hong SH, Mirkin CA. Science. 1999;283:661–663. doi: 10.1126/science.283.5402.661. [DOI] [PubMed] [Google Scholar]
- 252.Tseng AA, Notargiacomo A, Chen TP. J Vac Sci Technol B. 2005;23:877–894. [Google Scholar]
- 253.Ruiz SA, Chen CS. Soft Matter. 2007;3:168–177. doi: 10.1039/b613349e. [DOI] [PubMed] [Google Scholar]
- 254.Kumar A, Whitesides GM. Appl Phys Lett. 1993;63:2002–2004. [Google Scholar]
- 255.Jackman RJ, Wilbur JL, Whitesides GM. Science. 1995;269:664–666. doi: 10.1126/science.7624795. [DOI] [PubMed] [Google Scholar]
- 256.Xia YN, Zhao XM, Kim E, Whitesides GM. Chem Mater. 1995;7:2332–2337. [Google Scholar]
- 257.Xia YN, Zhao XM, Whitesides GM. Microelectron Eng. 1996;32:255–268. [Google Scholar]
- 258.Chapman RG, Ostuni E, Yan L, Whitesides GM. Langmuir. 2000;16:6927–6936. [Google Scholar]
- 259.Roberts C, Chen CS, Mrksich M, Martichonok V, Ingber DE, Whitesides GM. J Am Chem Soc. 1998;120:6548–6555. [Google Scholar]
- 260.Lokanathan AR, Zhang S, Regina VR, Cole MA, Ogaki R, Dong MD, Besenbacher F, Meyer RL, Kingshott P. Biointerphases. 2011;6:180–188. doi: 10.1116/1.3647506. [DOI] [PubMed] [Google Scholar]
- 261.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 262.Whitesides GM, Ostuni E, Takayama S, Jiang XY, Ingber DE. Annu Rev Biomed Eng. 2001;3:335–373. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- 263.Delamarche E, Schmid H, Bietsch A, Larsen NB, Rothuizen H, Michel B, Biebuyck H. J Phys Chem B. 1998;102:3324–3334. [Google Scholar]
- 264.Mullen TJ, Srinivasan C, Shuster MJ, Horn MW, Andrews AM, Weiss PS. J Nanopart Res. 2008;10:1231–1240. [Google Scholar]
- 265.Dameron AA, Hampton JR, Gillmor SD, Hohman JN, Weiss PS. J Vac Sci Technol B. 2005;23:2929–2932. [Google Scholar]
- 266.Liebau M, Huskens J, Reinhoudt DN. Adv Funct Mater. 2001;11:147–150. [Google Scholar]
- 267.Li HW, Muir BVO, Fichet G, Huck WTS. Langmuir. 2003;19:1963–1965. [Google Scholar]
- 268.Balmer TE, Schmid H, Stutz R, Delamarche E, Michel B, Spencer ND, Wolf H. Langmuir. 2005;21:622–632. doi: 10.1021/la048273l. [DOI] [PubMed] [Google Scholar]
- 269.Liebau M, Janssen HM, Inoue K, Shinkai S, Huskens J, Sijbesma RP, Meijer EW, Reinhoudt DN. Langmuir. 2002;18:674–682. [Google Scholar]
- 270.Dameron AA, Hampton JR, Smith RK, Mullen TJ, Gillmor SD, Weiss PS. Nano Lett. 2005;5:1834–1837. doi: 10.1021/nl050981j. [DOI] [PubMed] [Google Scholar]
- 271.Mullen TJ, Srinivasan C, Hohman JN, Gillmor SD, Shuster MJ, Horn MW, Andrews AM, Weiss PS. Appl Phys Lett. 2007;90:063114. [Google Scholar]
- 272.Vericat C, Vela ME, Salvarezza RC. Phys Chem Chem Phys. 2005;7:3258–3268. doi: 10.1039/b505903h. [DOI] [PubMed] [Google Scholar]
- 273.Shuster MJ, Vaish A, Szapacs ME, Anderson ME, Weiss PS, Andrews AM. Adv Mater. 2008;20:164–165. [Google Scholar]
- 274.Wadu-Mesthrige K, Amro NA, Liu GY. Scanning. 2000;22:380–388. doi: 10.1002/sca.4950220607. [DOI] [PubMed] [Google Scholar]
- 275.Huo FW, Zheng ZJ, Zheng GF, Giam LR, Zhang H, Mirkin CA. Science. 2008;321:1658–1660. doi: 10.1126/science.1162193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Xia YN, Whitesides GM. J Am Chem Soc. 1995;117:3274–3275. [Google Scholar]
- 277.McLellan JM, Geissler M, Xia YN. J Am Chem Soc. 2004;126:10830–10831. doi: 10.1021/ja0470766. [DOI] [PubMed] [Google Scholar]
- 278.Li XM, Peter M, Huskens J, Reinhoudt DN. Nano Lett. 2003;3:1449–1453. [Google Scholar]
- 279.Wendeln C, Ravoo BJ. Langmuir. 2012;28:5527–5538. doi: 10.1021/la204721x. [DOI] [PubMed] [Google Scholar]
- 280.Sullivan TP, van Poll ML, Dankers PYW, Huck WTS. Angew Chem-Int Edit. 2004;43:4190–4193. doi: 10.1002/anie.200460271. [DOI] [PubMed] [Google Scholar]
- 281.Mizuno H, Buriak JM. J Am Chem Soc. 2008;130:17656–17657. doi: 10.1021/ja807708r. [DOI] [PubMed] [Google Scholar]
- 282.Rozkiewicz DI, Janczewski D, Verboom W, Ravoo BJ, Reinhoudt DN. Angew Chem-Int Edit. 2006;45:5292–5296. doi: 10.1002/anie.200601090. [DOI] [PubMed] [Google Scholar]
- 283.Spruell JM, Sheriff BA, Rozkiewicz DI, Dichtel WR, Rohde RD, Reinhoudt DN, Stoddart JF, Heath JR. Angew Chem-Int Edit. 2008;47:9927–9932. doi: 10.1002/anie.200803480. [DOI] [PubMed] [Google Scholar]
- 284.Mizuno H, Buriak JM. ACS Appl Mater Int. 2009;1:2711–2720. doi: 10.1021/am900602m. [DOI] [PubMed] [Google Scholar]
- 285.Jang JY, Hong SH, Schatz GC, Ratner MA. J Chem Phys. 2001;115:2721–2729. [Google Scholar]
- 286.Sheehan PE, Whitman LJ. Phys Rev Lett. 2002;88:156104. doi: 10.1103/PhysRevLett.88.156104. [DOI] [PubMed] [Google Scholar]
- 287.Rozhok S, Piner R, Mirkin CA. J Phys Chem B. 2003;107:751–757. [Google Scholar]
- 288.Lee KB, Park SJ, Mirkin CA, Smith JC, Mrksich M. Science. 2002;295:1702–1705. doi: 10.1126/science.1067172. [DOI] [PubMed] [Google Scholar]
- 289.Demers LM, Ginger DS, Park SJ, Li Z, Chung SW, Mirkin CA. Science. 2002;296:1836–1838. doi: 10.1126/science.1071480. [DOI] [PubMed] [Google Scholar]
- 290.Lee KB, Lim JH, Mirkin CA. J Am Chem Soc. 2003;125:5588–5589. doi: 10.1021/ja034236p. [DOI] [PubMed] [Google Scholar]
- 291.Noy A, Miller AE, Klare JE, Weeks BL, Woods BW, DeYoreo JJ. Nano Lett. 2002;2:109–112. [Google Scholar]
- 292.Lim JH, Ginger DS, Lee KB, Heo J, Nam JM, Mirkin CA. Angew Chem-Int Edit. 2003;42:2309–2312. doi: 10.1002/anie.200351256. [DOI] [PubMed] [Google Scholar]
- 293.Zhang H, Chung SW, Mirkin CA. Nano Lett. 2003;3:43–45. [Google Scholar]
- 294.Hyun J, Ahn SJ, Lee WK, Chilkoti A, Zauscher S. Nano Lett. 2002;2:1203–1207. [Google Scholar]
- 295.Zhang H, Li Z, Mirkin CA. Adv Mater. 2002;14:1472–1473. [Google Scholar]
- 296.Hampton JR, Dameron AA, Weiss PS. J Phys Chem B. 2005;109:23118–23120. doi: 10.1021/jp055264s. [DOI] [PubMed] [Google Scholar]
- 297.Hong SH, Mirkin CA. Science. 2000;288:1808–1811. doi: 10.1126/science.288.5472.1808. [DOI] [PubMed] [Google Scholar]
- 298.Zhang M, Bullen D, Chung SW, Hong S, Ryu KS, Fan ZF, Mirkin CA, Liu C. Nanotechnology. 2002;13:212–217. [Google Scholar]
- 299.Salaita K, Wang YH, Fragala J, Vega RA, Liu C, Mirkin CA. Angew Chem-Int Edit. 2006;45:7220–7223. doi: 10.1002/anie.200603142. [DOI] [PubMed] [Google Scholar]
- 300.Mirkin CA. ACS Nano. 2007;1:79–83. doi: 10.1021/nn700228m. [DOI] [PubMed] [Google Scholar]
- 301.Liu GY, Xu S, Qian YL. Acc Chem Res. 2000;33:457–466. doi: 10.1021/ar980081s. [DOI] [PubMed] [Google Scholar]
- 302.Xu S, Liu GY. Langmuir. 1997;13:127–129. [Google Scholar]
- 303.Amro NA, Xu S, Liu GY. Langmuir. 2000;16:3006–3009. [Google Scholar]
- 304.Xu S, Miller S, Laibinis PE, Liu GY. Langmuir. 1999;15:7244–7251. [Google Scholar]
- 305.Wadu-Mesthrige K, Xu S, Amro NA, Liu GY. Langmuir. 1999;15:8580–8583. [Google Scholar]
- 306.Wadu-Mesthrige K, Amro NA, Garno JC, Xu S, Liu GY. Biophys J. 2001;80:1891–1899. doi: 10.1016/s0006-3495(01)76158-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Tan YH, Liu M, Nolting B, Go JG, Gervay-Hague J, Liu GY. ACS Nano. 2008;2:2374–2384. doi: 10.1021/nn800508f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Xu S, Laibinis PE, Liu GY. J Am Chem Soc. 1998;120:9356–9361. [Google Scholar]
- 309.Garcia R, Martinez RV, Martinez J. Chem Soc Rev. 2006;35:29–38. doi: 10.1039/b501599p. [DOI] [PubMed] [Google Scholar]
- 310.Liu GY, Amro NA. Proc Natl Acad Sci U S A. 2002;99:5165–5170. doi: 10.1073/pnas.072695699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Josephs EA, Ye T. J Am Chem Soc. 2010;132:10236–10238. doi: 10.1021/ja1039677. [DOI] [PubMed] [Google Scholar]
- 312.Liu JF, Cruchon-Dupeyrat S, Garno JC, Frommer J, Liu GY. Nano Lett. 2002;2:937–940. [Google Scholar]
- 313.Corbitt TS, Crooks RM, Ross CB, Hampdensmith MJ, Schoer JK. Adv Mater. 1993;5:935–938. [Google Scholar]
- 314.Schoer JK, Crooks RM. Langmuir. 1997;13:2323–2332. [Google Scholar]
- 315.Chen J, Reed MA, Asplund CL, Cassell AM, Myrick ML, Rawlett AM, Tour JM, Van Patten PG. Appl Phys Lett. 1999;75:624–626. [Google Scholar]
- 316.Gorman CB, Carroll RL, He YF, Tian F, Fuierer R. Langmuir. 2000;16:6312–6316. [Google Scholar]
- 317.Geissler M, McLellan JM, Xia YN. Nano Lett. 2005;5:31–36. doi: 10.1021/nl048497o. [DOI] [PubMed] [Google Scholar]
- 318.Geissler M, McLellan JM, Chen JY, Xia YN. Angew Chem-Int Edit. 2005;44:3596–3600. doi: 10.1002/anie.200500421. [DOI] [PubMed] [Google Scholar]
- 319.Geissler M, Chalsani P, Cameron NS, Veres T. Small. 2006;2:760–765. doi: 10.1002/smll.200600064. [DOI] [PubMed] [Google Scholar]
- 320.Hatzor A, Weiss PS. Science. 2001;291:1019–1020. doi: 10.1126/science.1057553. [DOI] [PubMed] [Google Scholar]
- 321.Anderson ME, Tan LP, Tanaka H, Mihok M, Lee H, Horn MW, Weiss PS. J Vac Sci Technol B. 2003;21:3116–3119. [Google Scholar]
- 322.Srinivasan C, Hohman JN, Anderson ME, Weiss PS, Horn MW. J Vac Sci Technol B. 2007;25:1985–1988. [Google Scholar]
- 323.Subramanian S, Catchmark JM. J Microlithogr Microfabr Microsyst. 2006;5:049701. [Google Scholar]
- 324.Subramanian S, McCarty GS, Catchmark JM. J Microlithogr Microfabr Microsyst. 2005;4:049701. [Google Scholar]
- 325.Li CB, Hasegawa T, Tanaka H, Miyazaki H, Odaka S, Tsukagoshi K, Aono M. Nanotechnology. 2010;21:495304. doi: 10.1088/0957-4484/21/49/495304. [DOI] [PubMed] [Google Scholar]
- 326.McCarty GS. Nano Lett. 2004;4:1391–1394. [Google Scholar]
- 327.Johnson S, Evans D, Davies AG, Linfield EH, Walti C. Nanotechnology. 2009;20:155304. doi: 10.1088/0957-4484/20/15/155304. [DOI] [PubMed] [Google Scholar]
- 328.Hino T, Tanaka H, Ozawa H, Lida Y, Ogawa T. Colloid Surf A-Physicochem Eng Asp. 2008;313:369–372. [Google Scholar]
- 329.Negishi R, Hasegawa T, Terabe K, Aono M, Ebihara T, Tanaka H, Ogawa T. Appl Phys Lett. 2006;88:223111. [Google Scholar]
- 330.Anderson ME, Mihok M, Tanaka H, Tan LP, Horn MW, McCarty GS, Weiss PS. Adv Mater. 2006;18:1020–1021. [Google Scholar]
- 331.Anderson ME, Smith RK, Donhauser ZJ, Hatzor A, Lewis PA, Tan LP, Tanaka H, Horn MW, Weiss PS. J Vac Sci Technol B. 2002;20:2739–2744. [Google Scholar]
- 332.Tanaka H, Anderson ME, Horn MW, Weiss PS. Jpn J Appl Phys Part 2 -Lett Express Lett. 2004;43:L950–L953. [Google Scholar]
- 333.Anderson ME, Srinivasan C, Jayaraman R, Weiss PS, Horn MW. Microelectron Eng. 2005;78–79:248–252. [Google Scholar]
- 334.Srinivasan C, Anderson ME, Jayaraman R, Weiss PS, Horn MW. Microelectron Eng. 2006;83:1517–1520. [Google Scholar]
- 335.Yeom HW, Takeda S, Rotenberg E, Matsuda I, Horikoshi K, Schaefer J, Lee CM, Kevan SD, Ohta T, Nagao T, Hasegawa S. Phys Rev Lett. 1999;82:4898–4901. [Google Scholar]
- 336.Liu M, Amro NA, Liu G-y. Annu Rev Phys Chem. 2008;59:367–386. doi: 10.1146/annurev.physchem.58.032806.104542. [DOI] [PubMed] [Google Scholar]
- 337.Williams JA, Gorman CB. Langmuir. 2007;23:3103–3105. doi: 10.1021/la061472f. [DOI] [PubMed] [Google Scholar]