Abstract
The microtubule-associated protein tau is expressed throughout the nervous system, most highly in neurons but also in glial cells. Its functions in adult and aging mammals remain to be defined. Previous studies in mouse models found either protective or detrimental effects of genetic tau ablation. While tau ablation prevented synaptic, network and cognitive dysfunctions in several models of Alzheimer’s disease and made mice more resistant to epileptic seizures, a recent study described a parkinsonian phenotype in aging Tau knockout mice. Here we tested cognition and motor functions in Tau+/+, Tau+/− and Tau−/− mice around 1 and 2 years of age. Tau ablation did not impair cognition and caused only minor motor deficits that were much more subtle than those associated with the aging process. Tau ablation caused a mild increase in body weight, which correlated with and may have contributed to some of the motor deficits. However, tau ablation did not cause significant dopaminergic impairments, and dopamine treatment did not improve the motor deficits, suggesting that they do not reflect extrapyramidal dysfunction.
Keywords: Tau knockout, parkinsonian, aging
1. Introduction
Reduction of the microtubule-associated protein tau prevents cognitive impairments in transgenic models designed to assess the pathogenic effects of amyloid-β (Aβ) or apolipoprotein E4 (Andrews-Zwilling, et al., 2010; Ittner, et al., 2010; Roberson, et al., 2007; Roberson, et al., 2011), proteins that – like tau itself – are thought to promote the development of Alzheimer’s disease (AD) (Huang and Mucke, 2012). Alzheimer’s disease is the most common neurodegenerative disease. It causes prolonged suffering and disability in the elderly and takes an enormous toll on patient families and society in general (Alzheimer's Association, 2012). Its prevalence is expected to increase ≥3-fold by 2050 (Alzheimer's Association, 2012), threatening health care systems worldwide and underscoring the need to develop better strategies to prevent and halt this devastating illness (Golde, et al., 2011; Huang and Mucke, 2012).
Because tau reduction might be beneficial in AD and other “tauopathies” (Morris, et al., 2011b), it is interesting to study the consequences of this intervention in experimental models, especially in regards to cognitive and motor functions. Up to 8 months of age, Tau−/− mice had no impairments in the Morris water maze and other tests of learning, memory and exploratory behaviors (Ittner, et al., 2010; Lei, et al., 2012; Roberson, et al., 2011; Roberson, et al., 2007). At 10–12 months, Tau−/− mice also performed like wildtype Tau+/+ controls in the Morris water maze and radial arm water maze (Dawson, et al., 2010). However, a recent study identified Y-maze deficits in Tau−/− mice at 12 months of age (Lei, et al., 2012). We were unable to find information in the literature on the cognitive performance of older Tau−/− mice.
Tau−/− mice have also been reported to have motor deficits. One line of Tau−/− mice showed deficits in rod walking and wire hang tests at 10–11 weeks of age (Ikegami, et al., 2000). However, no additional behavioral analyses of this line appear to have been published since this original report. In a second, more widely used line of Tau−/− mice (Dawson, et al., 2001), subtle motor deficits were detected at 3.0–4.5-months, when the Tau−/− mice showed an increased latency to cross a balance beam and an increased number of slipped steps, but no impairments in several other motor tests (Morris, et al., 2011a). The effect of tau ablation on motor behavior in older Tau−/− mice from this line is controversial. One group found no impairment on the Rota rod at 10–12 months (Dawson, et al., 2010), whereas another found deficits in the Rota rod, pole test and open field test at 12 months (Lei, et al., 2012). Lei, et al. (2012) attributed the deficits they observed to a loss of dopaminergic neurons in the substantia nigra caused by iron accumulation in the brain (Lei, et al., 2012).
To address these discrepancies and fill the knowledge gaps identified above, we assessed middle-aged and old Tau+/+, Tau+/− and Tau−/− mice in a battery of behavioral tests and evaluated motor components of their central nervous system histopathologically and pharmacologically.
2. Methods
2.1 Mice
Tau+/+, Tau+/− and Tau−/− mice (Dawson, et al., 2001) on a C57BL/6 background were used at various ages, as specified in the text. An all-female cohort was used for the initial characterization of motor deficits at 12–15 months of age (Fig. 2); all other cohorts included males and females (Table S1). Mice had ad libitum access to food (Picolab Rodent Diet 20, Labdiet) and water. At the end of experiments, mice were deeply anesthetized with Avertin and killed by transcardial perfusion with saline. One hemibrain was frozen on dry ice. The other hemibrain was post-fixed for 24–48 hours in 4% paraformaldehyde in PBS at 4°C. All experiments were approved by the Committee on Animal Research of the University of California, San Francisco.
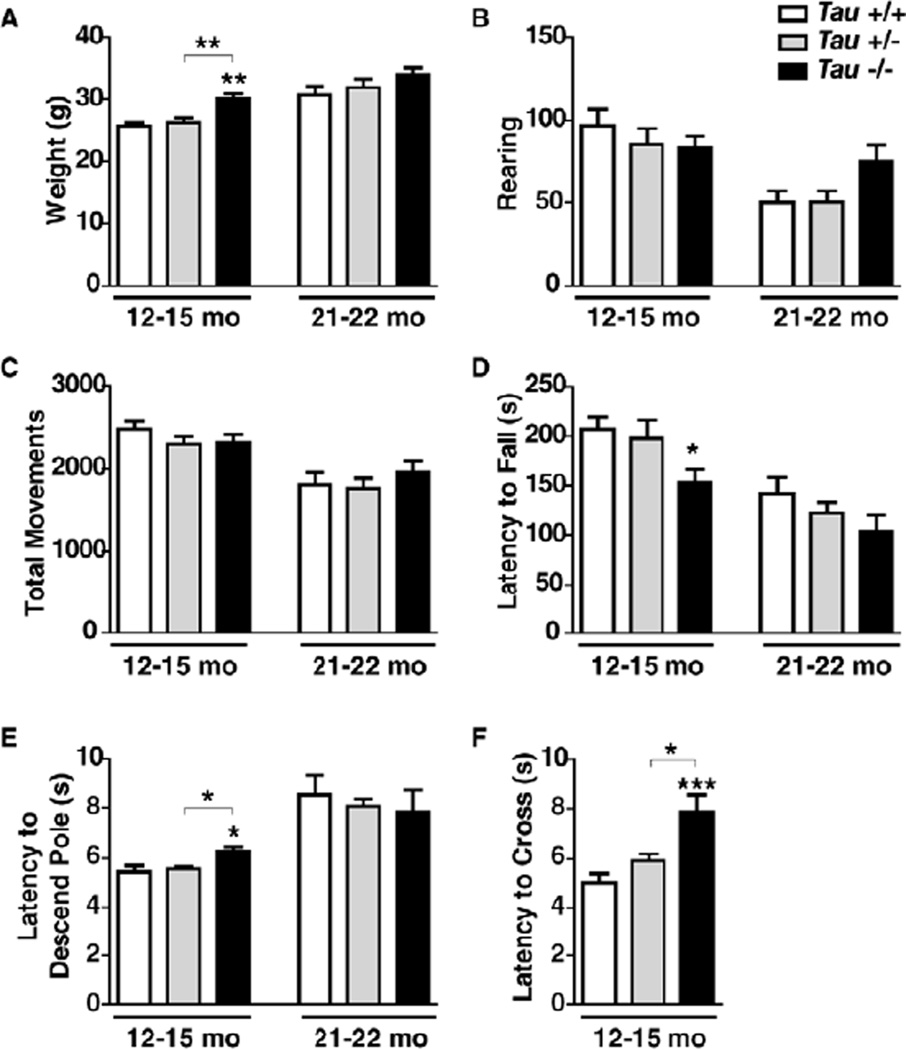
Figure 2.
Complete, but not partial, tau reduction and aging are associated with weight gain and subtle motor deficits. Mice of the indicated genotypes were weighed and analyzed in different motor tests at 12–15 or 21–22 months of age (n=11–14 mice per age and genotype). (A) Body weight. Two-way ANOVA: genotype (p=0.002) and age (p<0.0001) effects but no interaction (p=0.69). (B, C) Rearing (B) and total movements (C) were measured in the open field test. Two-way ANOVA: age effect (B, p<0.0001; C, p<0.0001) but no genotype effect (B, p=0.42; C, p=0.55) or interaction (B, p=0.09; C, p=0.44). (D) Rota rod test. Two-way ANOVA: genotype (p=0.01) and age (p<0.0001) effects but no interaction (p=0.67). (E) Pole test. Two-way ANOVA: age effect (p<0.0001) but no genotype effect (p=0.88) or interaction (p=0.31). (F) Balance beam. *p<0.05, **p<0.01, ***p<0.001 vs. age-matched wildtype mice or as indicated by brackets (Tukey test). Data are means ± SEM.
2.2 Behavioral testing
Unless specified otherwise, mice were acclimated to the testing room for at least one hour before testing. All testing apparatuses were cleaned with 70% ethanol between mice, unless otherwise indicated, and with 10% bleach after each testing day. Open field, pole and balance beam tests were carried out as described (Morris, et al., 2011a). Experimenters were blinded to the genotype of mice.
2.2.1 Morris Water Maze
Spatial learning and memory was assessed as described (Morris, et al., 1982) in a Morris water maze consisting of a pool (120 cm in diameter) of water made opaque with white, non-toxic tempura paint. Water temperature was held at 20±1°C. Extra maze cues were posted on the walls of the testing room. Data were acquired with Ethovision XT (Noldus Information Technology, the Netherlands).
Pretraining
Before hidden platform training, mice were exposed to the water maze by having them swim down a rectangular channel for 4 trials. The hidden escape platform was placed in the middle of the channel. If a mouse was unable to find and mount the platform for 60 s, it was guided to it and allowed to sit on it for up to 10 s.
Hidden platform training
Following pre-training, mice were trained for 5 days to locate a hidden platform (10×10 cm) submerged 1.5 cm below the water surface. The platform location was fixed, while the mouse drop locations were changed between trials. Mice were trained in four sessions per day with 3 hours of rest between sessions. Each session consisted of two trials with inter-trial intervals of approximately 15 min. Trials were terminated after 60 s. If a mouse did not find the platform within this time period, it was gently guided to the platform and allowed to sit on it for 15 s. Latency to locate the platform, as well as distance to the platform and swim speeds were recorded for the subsequent data analysis.
Probe trials
Twenty four hours after the last hidden platform training, the platform was removed and mice were allowed to swim in the pool for 60 s. The drop location was opposite to where the platform used to be during hidden platform training. The percentage of time mice spent in the target quadrant and the number of times they crossed the original platform location were recorded.
2.2.2 Novel Object Recognition (NOR)
On two consecutive days, mice were placed into a plexiglass chamber (20×20 cm) containing two identical objects and allowed to explore for 10 min. Forty-eight hours after training, mice were tested in the same chamber for 10 min after one of the objects was replaced with a novel object. Time spent exploring each object was recorded. The novel object position alternated between mice to eliminate any possible right-left bias. Objects were cleaned with 70% ethanol between each mouse.
2.2.3 Open field
Spontaneous movements in the open field were assessed using a Flex-Field/Open Field Photobeam Activity System (San Diego Instruments, CA). Each mouse was placed in the center of a clear plastic chamber (41×41×30 cm) containing two arrays of 16×16 photobeams and allowed to explore for 15 min. Movements were recorded through beam breaks.
2.2.4 Rota rod
Five mice were placed on the Rota Rod (Med Associates Inc, VT) for simultaneous testing and the computer recorded photobeam interruptions when mice fell off the rotating rod. Photobeams were interrupted by the tester if the mouse held onto the rod without walking for three full rotations. Each mouse was given three trials with a maximum time on the Rota Rod of 300 s per trial and a 10-min rest period between trials. Mice were first trained at a constant speed of 16 rpm for three trials. The next two days, they were trained for three trials in the morning and three trials in the afternoon at rotation speeds accelerating from 4 to 40 rpm over 300 s. The average latency to fall off the Rota Rod on the afternoon of the last day was analyzed.
2.2.5 Balance beam
The balance beam consisted of two platforms connected by a removable plastic beam leading to an opaque box on top of one platform. On the first day, mice were trained on a thick, round beam by placing them first a few inches from the box and leading them into the box, and then by placing them halfway across the beam and leading them into the box, if leading was required. The mice were then trained three times across the whole length of the beam with approximately 10 min rest periods between trials. On the second day, mice were again trained three times on the thick beam. On the third day, the thick beam was replaced with a thin, square beam and the mice were tested three times. The average latency to cross the thin beam and the average number of times a foot slipped while crossing the beam during the testing sessions were analyzed. A trial was excluded from analysis if the mouse dragged its hind limbs across the beam for >50% of the distance. At 21–22 months of age, mice of all genotypes were unable to walk across the balance beam on the third day.
2.2.6 Pole test
The pole consisted of a thin wooden dowel and a cross-shaped wooden base placed in a clean cage. Rubber bands were wrapped around the dowel at intervals of approximately 1.5 inches to increase traction. Mice were placed at the top of the pole facing downwards and latency to descend the pole was measured. Trials were excluded if the mouse jumped or slid down the pole rather than climbed down. On the first day, mice were trained in two trials with approximately 10 min of rest between trials. On the second day, they were given five trials and the shortest latency to descend the pole was analyzed. The pole was cleaned with 70% ethanol between testing groups of mice from separate home cages.
2.2.7 Pole test with 3,4-dihydroxyphenylalanine (L-DOPA)/benserazide treatments
Acute treatment with L-DOPA/benserazide was designed as a cross-over trial. On day 1, mice were trained twice on the pole without injection. On day 2, mice were given an intraperitoneal (i.p.) injection of saline or of L-DOPA (20 mg/kg) and benserazide HCl (5 mg/kg) (Sigma) in saline. The mice were then transferred to the testing room and, 30 min later, given five pole test trials. On day 3, treatments were reversed, so that mice injected with saline on day 2 now received L-DOPA/benserazide and vice versa. Mice were then transferred to the testing room and tested as on day 2.
The same cohort of mice was used to assess the effect of chronic L-DOPA/benserazide treatment. Three days after finishing the acute treatments, mice were given five trials on the pole test to assess their performance before chronic drug treatment. They were then given water bottles containing L-DOPA (200mg/L) and benserazide (50mg/L) in 0.2% ascorbic acid (Sigma) as described (Lei, et al., 2012). The bottles were changed daily and fluid/drug intake per cage of group-housed mice was monitored daily. The average weight of each mouse was calculated from measurements obtained at the beginning and end of the treatment. For each cage, the average daily drug intake was divided by the total weight of mice in the cage to estimate their approximate daily dose in mg of drug per kg of body weight. On the seventh day of chronic treatment, mice were given five trials in the pole test. Half of the mice were sacrificed the day after the last pole test. The remaining mice were placed on normal drinking water to allow for drug washout and sacrificed six days later.
2.3 Striatal tissues
Frozen hemibrains were covered in 2% agarose and chopped into 450-µm slices (McIlwain Tissue Chopper, Mickle Laboratory Engineering Co. Ltd., UK). Slices were microdissected in sterile PBS on ice. The striatum was isolated from 4–5 slices per mouse. Striatal tissues were immediately frozen on dry ice and stored at −80°C. For each mouse, half of the striatal tissue was used for western blot analysis, while the other half was sent to the Vanderbilt Neurochemistry Core Laboratory for determination of bioamine levels by HPLC.
2.4 Western blot
Striatal samples were homogenized in 100µL buffer (PBS, 1mM DTT, 0.5mM EDTA, 0.5% Triton-X100, 0.1M PMSF, phosphatase inhibitor cocktails 2 and 3 (Sigma) and protease inhibitor tablets (Roche complete mini)) with a hand-held homogenizer. Samples were then sonicated (Episonic mutli-functional bioprocessor 1000, Epigentek Group Inc., NY) at an amplitude of 40% in an ice bath twice for 5 min, incubated for 20–30 min on ice, and spun at 10,000×g at 4°C for 10 min. The supernatant was collected. Protein concentration was measured by BCA assay (Thermo Scientific). Samples were run on a 4–12% bis-tris gel (Bio-rad) and transferred by iBlot onto a nitrocellulose membrane (Invitrogen), which was blocked for 1 hour in 5% milk/PBS. Blots were incubated overnight with primary antibodies (rabbit anti-tyrosine hydroxylase (1:1000 AB152, Millipore) plus anti-alpha tubulin (1:500,000 B512, Sigma)), followed by incubation with secondary antibodies (680LT donkey anti-mouse (1:10,000, LI-COR Biosciences) and 800CW goat anti-rabbit (1:10,000, LI-COR Biosciences)) and quantitation of signals by an Odyssey system (LI-COR Biosciences).
2.5 Perl iron staining
Brain sections were stained as previously described (Perl and Good, 1992). Briefly, sections were stained in a solution of 1% potassium ferrocyanide and 1% hydrochloric acid. They were then rinsed with distilled water and counterstained at 60°C for 15 s in a solution of 0.1% nuclear fast red, 5% aluminum sulfate and trace amounts of thymol (storage preservative). The sections were rinsed well in water and then mounted and analyzed. Focal brain injury, elicited in anesthetized wildtype mice as described (Mucke, et al., 1991), was used as a positive control.
2.6 Statistics
All data was analyzed by GraphPad Prism (GraphPad Software Inc., CA). Power calculations were done using the Statistical Solutions, LLC Power & Sample Size Calculator as a two-tailed t-test comparing Tau+/+ and Tau−/− groups within each age group. Alpha was defined as 0.0167 for each test to correct for multiple comparisons. The lower sample number and the higher standard deviation between the two groups were used in power calculations for striatal dopamine and tyrosine hydroxylase levels. All comparisons made had power higher than 0.80 to detect a 40% decrease from levels in Tau+/+ mice. Null hypotheses were rejected at the p < 0.05 level.
3. Results
3.1 Chronic lack of tau does not impair learning and memory in old age
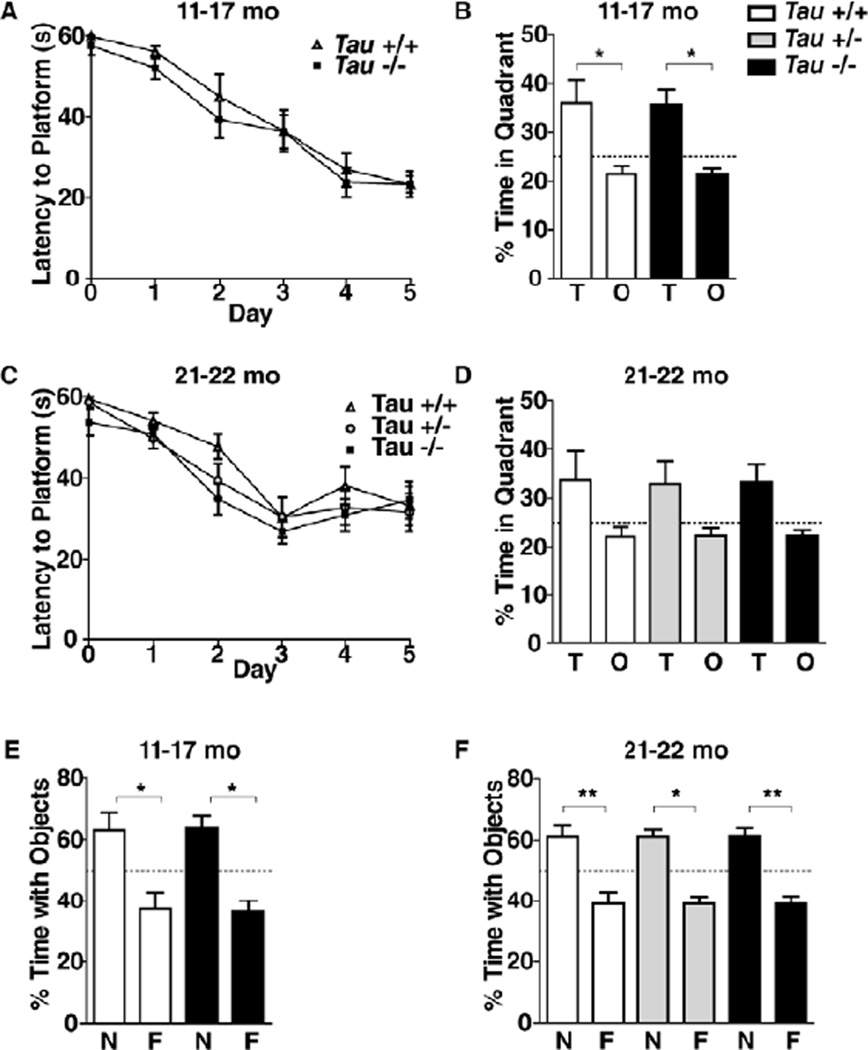
Tau+/+, Tau+/−, and Tau−/− littermates were obtained from breedings between Tau+/− mice (C57Bl/6J). To determine whether chronic reduction (Tau+/−) or ablation (Tau−/−) of tau impairs learning and memory in middle-aged or old mice, we behaviorally tested two independent cohorts of mice at 11–17 or 21–22 months of age. The Morris water maze and a novel object recognition test were used to assess spatial versus non-spatial learning and memory, respectively. In the Morris water maze, tau ablation had no effect on learning and memory in 11–17-month-old mice (Fig.1 A,B). Reduction and ablation of tau did not significantly affect learning and memory in 21–22-month-old mice either (Fig. 1C,D). Tau ablation did not affect learning and memory in the novel object recognition test in 11–17-month-old mice (Fig. 1E) and neither did tau reduction or ablation in 21–22-month-old mice (Fig. 1F).
Figure 1.
Lack of tau since early development does not impair learning and memory in middle-aged and old mice. (A–D) Independent cohorts of mice comprising the indicated genotypes were tested in the Morris water maze at 11–17 months (A, B; n=8 mice per genotype) or 21–22 months (C, D; n=11–13 mice per genotype) of age. mo, months (A, C) Learning curves during hidden-platform training. Repeated-measures ANOVA: time effect (A, p<0.0001; C, p<0.0001), but no genotype effect (A, p=0.37; C, p=0.28) or interaction (A, p=0.95; C, p=0.70). (B, D) Memory retention in the probe trial (platform removed) 24 h after the end of the last training trial. Repeated measures ANOVA: quadrant effect (B, p=0.002; D, p=0.006), but no genotype effect (B, p=0.98; D, p=1.0) or interaction (B, p=0.98; D, p=1.0). T, target quadrant; O, other quadrant. Dotted line indicates chance. (E, F) The same groups of mice were trained in a novel object recognition paradigm and tested 48h later. Repeated measures ANOVA: object effect (E, p=0.002; F, p<0.0001), but no genotype effect (E, p=0.47; F, p=0.42) or interaction (E, p=0.91; F, p=1.0). N, novel object; F, familiar object. *p<0.05, **p<0.01 (Bonferroni post hoc test). Data are means ± SEM.
3.2 Ablation of tau and aging cause weight gain and subtle motor deficits
To determine whether chronic reduction or ablation of tau impairs motor functions in middle-aged or old mice, we assessed two cohorts of Tau+/+, Tau+/−, and Tau−/− mice in several motor tests at 12–15 or 21–22 months of age. Mice of all genotypes gained weight as they aged, but Tau−/− mice weighed more than Tau+/+ and Tau+/− mice at 12–15 months and showed a trend in this direction at 21–22 months (Fig. 2A). Tau genotype did not significantly alter rearing or overall activity in the open field, although both were reduced by aging (Fig. 2B,C) and Tau−/− mice showed a trend towards increased rearing at the older age (Fig. 2B). Aging also reduced the latency to fall off the Rota rod, as did tau ablation (Fig. 2D). Because fall latency in 12–15-month-old Tau−/− mice strongly correlated with body weight at 12–15 months of age (R2=0.64, p=0.001; Fig. S1A), this deficit may be caused primarily by their increased weight.
At 12–15 months, Tau−/− mice took longer to descend the pole than Tau+/+ and Tau+/− mice, whereas at 21–22 months all groups showed comparable latencies (Fig. 2E). In contrast to the Rota rod results, pole test performance in 12–15-month-old Tau−/− mice did not correlate with body weight (Fig. S1B). Tau ablation also increased the latency to cross a balance beam at 12–15 months of age (Fig. 2F); correlation of latencies with body weight (R2=0.34, p=0.04; Fig. S1C) again suggested that this may not represent a genuine motor deficit. Independent of genotype, the oldest age group could not complete the balance beam test. These findings suggest that complete tau ablation causes subtle motor deficits, in part, by increasing body weight. In contrast, partial reduction of tau had no adverse effects on motor functions even at 21–22 months of age.
3.3 Motor deficits in Tau−/− mice are probably not caused by dopaminergic dysfunction
Motor deficits in aging Tau−/− mice have been attributed to impairments of dopaminergic neurons projecting to the striatum (Lei, et al., 2012). We therefore assessed striatal dopamine and tyrosine hydroxylase (TH) levels of Tau+/+, Tau+/−, and Tau−/− mice at 9–11, 14–20 or 25–26 months of age. We found a gene dose-dependent trend for tau reduction to lower striatal dopamine levels as mice aged (11–16% reduction, Fig. 3A), but this trend did not reach statistical significance. Western blot analysis revealed a statistically significant decrease in TH levels only in Tau+/− mice (16% reduction, Fig. 3B,C) at 25–26 months. Notably, these were the same Tau+/− mice that, compared with age-matched controls, failed to show any behavioral deficits at 21–22 months of age, suggesting that this subtle TH loss may not have a significant impact on motor function. Because others recently reported a much greater (40%) loss of striatal dopamine and TH in 12-month-old Tau−/− mice from the same strain (Lei, et al., 2012), we confirmed by post hoc power calculations (see Methods for details) that our analysis of striatal dopamine and TH levels was powered to detect such a change in all of the groups, if it were present. In contrast to Lei and colleagues, we did not find any iron accumulation in the hippocampus, striatum or substantia nigra of 14-month-old Tau−/− mice (Fig. S2).
Figure 3.
Mild dopaminergic deficits do not contribute to motor deficits in Tau−/−-mice. (A–C) Levels of dopamine (A) and tyrosine hydroxylase (TH) in the striatum (B, C) were measured in mice of the indicated genotypes at 9–11 months (n=5 per genotype), 14–20 months (n=6–7 per genotype) or 25–26 months (n=7–10 per genotype) of age. RU, relative units; TH, tyrosine hydroxylase. (A) Striatal dopamine levels determined by HPLC. Two-way ANOVA: no effects of genotype (p=0.10) or age (p=0.14) and no interaction (p=0.40). (B) Representative western blot showing tyrosine hydroxylase (TH) levels in the striatum of two mice per genotype (age 25–26 months). Alpha tubulin served as a loading control. (C) Quantitation of striatal tyrosine hydroxylase by western blot analysis (n=4–10 mice per genotype and age as specified above). Two-way ANOVA: interaction (p=0.011), but no effects of genotype (p=0.72) or age (p=0.26). Average signals in two 25–26 month wildtype mice on each blot were arbitrarily defined as 1.0 and used to normalize signals across western blots. (D) Pole test deficits in middle-aged Tau knockout mice are not significantly improved by chronic treatment with L-DOPA/benserazide. Mice (n=11–14 per genotype, age: 12–15 months) were assessed in the pole test before and on the 7th day of treatment with L-DOPA (200mg/L) and benserazide (50mg/L) in 0.2% ascorbic acid in their drinking water. Repeated measures two-way ANOVA: genotype effect (p=0.014) but no treatment effect (p=0.97) or interaction (p=0.71). *p<0.05 vs. age-matched wildtype mice (Bonferroni post hoc test of selected columns). Data are means ± SEM.
To further test the hypothesis that motor deficits in Tau−/− mice are caused by dopaminergic deficits, we treated an independent cohort of 12–15-month-old Tau+/+, Tau+/−, and Tau−/− mice with a combination of L-DOPA and benserazide. To determine the efficacy of the treatment, we used the pole test, because it was the only test in which 12–15-month-old Tau−/− mice showed weight-independent motor deficits (Fig S1B). In this second cohort, pole test latency also did not correlate with body weight in Tau−/− mice, and acute i.p. injections of L-DOPA (20mg/kg) and benserazide (5mg/kg) did not improve latency to descend in the pole test in any of the groups (data not shown). We then treated the same mice with L-DOPA/benserazide dissolved in their drinking water for 7 days. On average, the mice received roughly 24 mg/kg/day of L-DOPA and 6 mg/kg/day of benserazide, which is close to the doses (20mg/kg/day L-DOPA and 5mg/kg/day benserazide) Lei et al. reported to significantly improve motor functions in 12-month-old Tau−/− mice (Lei, et al., 2012). On the 7th day of treatment, the mice were again tested in the pole test. Compared to Tau+/+ and Tau+/− mice, Tau−/− mice again took longer to descend the pole, but L-DOPA/benserazide treatment did not significantly improve their performance (Fig. 3D). Thus, compared with age-matched Tau+/+ controls, Tau−/− mice have only subtle dopaminergic deficits and it is unlikely that these deficits contribute significantly to the pole test deficits seen in 12–15-month-old Tau−/− mice.
4. Discussion
Several new findings and conclusions emerged from this study. Middle-aged and old Tau−/− mice had age-appropriate spatial and nonspatial learning and memory. Motor deficits were subtle in middle-aged Tau−/− mice and undetectable in old Tau−/− mice, most likely because deficits caused by aging became more prominent than those caused by tau ablation. Because middle-aged Tau−/− mice had no significant reductions in striatal levels of dopamine or TH and L-DOPA/benserazide treatment did not improve their motor deficits, it is unlikely that these deficits were caused by dopaminergic impairments. Some of these deficits may be caused by weight gain and others by alternative mechanisms that have yet to be identified.
Reducing tau by half had no adverse effects on any of the outcome measures until 25–26 months of age. At this age, Tau+/− mice showed a slight reduction in striatal TH levels but no behavioral deficits, compared with age-matched Tau+/+ mice, demonstrating that partial reduction of tau is well tolerated even in old mice. These results extend previous studies demonstrating that Tau+/− mice performed at Tau+/+ control levels in a variety of motor tests at 3.0–4.5 months (Morris, et al., 2011a) and showed no deficits in the Morris Water maze at 4–7 months (Roberson, et al., 2007). They also have potential therapeutic implications, as 50% reduction of tau was sufficient to improve neuronal network activity and cognitive functions in human amyloid precursor protein transgenic mice (Roberson, et al., 2011; Roberson, et al., 2007) and to improve axonal transport in primary neuronal cultures exposed to Aβ oligomers (Vossel, et al., 2010).
The findings of the current study differ in several respects from those reported by Lei and colleagues (Lei, et al., 2012). Specifically, we did not find behavioral deficits in the open field test, abnormal iron accumulation in the brain, or significant decreases in striatal levels of dopamine or TH in middle-aged and old Tau−/− mice, compared with age-matched Tau+/+ controls. In addition, other types of subtle motor deficits we detected in 12–15-month-old Tau−/− mice were not significantly improved by treatment with L-DOPA/benserazide. Because both studies assessed the same Tau−/− model (Dawson, et al., 2001) on the same genetic background strain (C57BL/6) at comparable ages (12 and 24 months versus 12–15 and 21–22 months), it is unlikely that any of these variables accounted for the differences in our results. Additional studies, including by independent groups, are needed to resolve the discrepancies, which might relate to a variety of other factors, including the diet and housing conditions of the mice and the methodological approaches used to study their phenotypes.
Because we did observe a trend towards lower striatal dopamine and TH levels in middle-aged and old Tau+/− and Tau−/− mice, it is possible that genetic reduction or ablation of tau slightly increases the susceptibility of dopaminergic neurons to various injuries, including those caused by aging, exposure to 6-hydroxydopamine (Morris, et al., 2011a) and iron accumulation (Lei, et al., 2012). However, we consider it unlikely that the motor impairments we observed in middle-aged Tau−/− mice reflect extrapyramidal motor dysfunction because these mice showed only subtle trends towards dopaminergic deficits and their motor impairments did not improve with dopamine treatment. One potential cause of motor dysfunction is abnormal weight gain, which was evident in Tau−/− mice at 12–15 months of age and correlated with their impairments on the Rota rod and balance beam. However, pole test deficits did not correlate with weight gain and balance beam deficits were observed in young Tau−/− mice prior to abnormal weight gain (Morris, et al., 2011a), suggesting the involvement of additional factors that remain to be defined.
In conclusion, the genetic ablation of tau causes minimal neurological dysfunction in middle-aged, but not in old Tau−/− mice, as compared with age-matched wildtype controls, and there is little evidence that partial reduction of tau is detrimental. To further assess the safety of this potential therapeutic strategy, it would be desirable to reduce tau levels in rodent models at different ages after their nervous system has developed and matured in the presence of wildtype tau levels.
Supplementary Material
Supplemental Figure 1. Correlation between weight and motor performance in 12–15-month-old Tau−/− mice (n=11–14). (A) Rota rod (R2=0.64, p=0.001 by linear regression). (B) Pole test (R2=0.03, p=0.29). (C) Balance beam (R2=0.34, p=0.04).
Supplemental Figure 2. Lack of iron staining in the brains of 14-month-old Tau−/− mice. (A) Tau+/+ mice that did (left) or did not (right) receive a cerebral needle stab injury were used as a positive control for cerebral iron staining. (B) No iron staining was observed in the brains of uninjured Tau+/+ and Tau−/− mice (n=3 mice per genotype). Scale bars: 10µm, magnification: 430X.
Acknowledgements
We thank X. Wang, G. Yu, W. Guo and K. Ho for technical support, R. Johnson at the Vanderbilt Neurochemistry Core for measuring dopamine, and M. Dela Cruz for administrative support. This work was supported by NIH grants AG022074, AG039259, and NS065780 to L.M., AG5131 and AG022074 to E.M., and by a gift from the S.D. Bechtel, Jr. Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement L.M. has received consulting fees and sponsored research support from Bristol-Myers Squibb and is on the Scientific Advisory Board for iPierian.
References
- Alzheimer’s Association. Alzheimer's disease facts and figures. Alzheimers Dement. 2012;8:131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Andrews-Zwilling Y, Bien-Ly N, Xu Q, Li G, Bernardo A, Yoon SY, Zwilling D, Yan TX, Chen L, Huang Y. Apolipoprotein E4 causes age- and Tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J. Neurosci. 2010;30:13707–13717. doi: 10.1523/JNEUROSCI.4040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson HN, Cantillana V, Jansen M, Wang H, Vitek MP, Wilcock DM, Lynch JR, Laskowitz DT. Loss of tau elicits axonal degeneration in a mouse model of Alzheimer's disease. Neuroscience. 2010;169:516–531. doi: 10.1016/j.neuroscience.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson HN, Ferreira A, Eyster MV, Ghoshal N, Binder LI, Vitek MP. Inhibition of neuronal maturation in primary hippocampal neurons from tau deficient mice. J. Cell Sci. 2001;114:1179–1187. doi: 10.1242/jcs.114.6.1179. [DOI] [PubMed] [Google Scholar]
- Golde TE, Schneider LS, Koo EH. Anti-abeta therapeutics in Alzheimer's disease: The need for a paradigm shift. Neuron. 2011;69:203–213. doi: 10.1016/j.neuron.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148:1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami S, Harada A, Hirokawa N. Muscle weakness, hyperactivity, and impairment in fear conditioning in tau-deficient mice. Neurosci. Lett. 2000;279:129–132. doi: 10.1016/s0304-3940(99)00964-7. [DOI] [PubMed] [Google Scholar]
- Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, Wolfing H, Chieng BC, Christie MJ, Napier IA, Eckert A, Staufenbiel M, Hardeman E, Gotz J. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer's disease mouse models. Cell. 2010;142:387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- Lei P, Ayton S, Finkelstein DI, Spoerri L, Ciccotosto GD, Wright DK, Wong BX, Adlard PA, Cherny RA, Lam LQ, Roberts BR, Volitakis I, Egan GF, McLean CA, Cappai R, Duce JA, Bush AI. Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat. Med. 2012;18:291–295. doi: 10.1038/nm.2613. [DOI] [PubMed] [Google Scholar]
- Morris M, Koyama A, Masliah E, Mucke L. Tau reduction does not prevent motor deficits in two mouse models of Parkinson’s disease. PLoS One. 2011a;6:e29257. doi: 10.1371/journal.pone.0029257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M, Maeda S, Vossel K, Mucke L. The many faces of tau. Neuron. 2011b;70:410–426. doi: 10.1016/j.neuron.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Mucke L, Oldstone MBA, Morris JC, Nerenberg MI. Rapid activation of astrocyte-specific expression of GFAP-lacZ transgene by focal injury. New Biol. 1991;3:465–474. [PubMed] [Google Scholar]
- Perl DP, Good PF. Comparative techniques for determining cellular iron distribution in brain tissues. Ann. Neurol. 1992;32(Suppl):S76–S81. doi: 10.1002/ana.410320713. [DOI] [PubMed] [Google Scholar]
- Roberson ED, Halabisky B, Yoo JW, Yao J, Chin J, Yan F, Wu T, Hamto P, Devidze N, Yu G-Q, Palop JJ, Noebels JL, Mucke L. Amyloid-β/Fyn-induced synaptic, network, and cognitive impairments depend on Tau levels in multiple mouse models of Alzheimer's disease. J. Neurosci. 2011;31:700–711. doi: 10.1523/JNEUROSCI.4152-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu G-Q, Mucke L. Reducing endogenous tau ameliorates amyloid ®-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- Vossel KA, Zhang K, Brodbeck J, Daub AC, Sharma P, Finkbeiner S, Cui B, Mucke L. Tau reduction prevents Aβ-induced impairments in axonal transport. Science. 2010;330:198. doi: 10.1126/science.1194653. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Correlation between weight and motor performance in 12–15-month-old Tau−/− mice (n=11–14). (A) Rota rod (R2=0.64, p=0.001 by linear regression). (B) Pole test (R2=0.03, p=0.29). (C) Balance beam (R2=0.34, p=0.04).
Supplemental Figure 2. Lack of iron staining in the brains of 14-month-old Tau−/− mice. (A) Tau+/+ mice that did (left) or did not (right) receive a cerebral needle stab injury were used as a positive control for cerebral iron staining. (B) No iron staining was observed in the brains of uninjured Tau+/+ and Tau−/− mice (n=3 mice per genotype). Scale bars: 10µm, magnification: 430X.