Abstract
Integrin α5β1 is an important therapeutic target that can be inhibited using an aldolase antibody (Ab)-derived chemical-Ab (chem-Ab) for the treatment of multiple human diseases, including cancers. A fairly optimized anti-integrin α5β1 chem-Ab 38C2-4e was obtained using an in situ convergent chemical programming (CP) approach, which minimized the time and efforts needed to develop a chem-Ab. Multiple Ab-programming agents (PAs) 4a-e could be prepared rapidly using the Cu-catalyzed alkyne-azide coupling (Cu-AAC) reaction of an α5β1 inhibitor 2 with multiple linkers 3a-e, either before or after conjugating the linkers into Ab 38C2 binding sites. In these two-steps processes, the products after step 1 can be used in next step without performing an extensive purification or analysis of the Ab-PAs or Ab-linker conjugates affording chem-Abs 38C2-(4a-e). Flow cytometry assay was used to determine binding of the chem-Abs to U87 human glioblastoma cells expressing α5β1 integrin, and identify 38C2-3e as the strongest binder. Further studies revealed that 38C2-3e strongly inhibited proliferation of U87 cells and tube formation of HUVEC in matrigel assay, as well as tumor growth and metastasis of 4T1 cells in vivo.
Keywords: Integrin alpha(5)beta(1), chemical programming, antibody 38C2, Aldolase antibody, cancer, chemical-antibody (chem-Ab), in-situ convergent strategy
Integrins are noncovalently bound heterodimeric glycoproteins that interact with extracellular receptors, and transmit signals across the cell membrane in both directions.1,2 Their interactions are critical for the cell functions, maintaining the tissue homoeostasis, and repairing tissue injuries.3,4 Integrins are present on a wide variety of cells under both normal as well as pathological conditions, but their expressions are often perturbed in latter situations. Many integrins, including α5β1, overexpress on tumor cells and/or on endothelial cells in angiogenic tumor vasculature, play an important role in tumor angiogenesis, growth and metastasis.5,6,7,8 It has been shown that integrin α5β1 and its main ligand – fibronectin – are highly relevant targets for the treatment of human cancers.9,10,11,12,13 A disruption in integrin α5β1-fibronectin binding using selective inhibitors of the integrin, including small molecules as well as antibodies (Abs), induces apoptosis of the activated endothelial cells, and inhibits tumor angiogenesis, growth and metastases. Indeed, several inhibitors of α5β1 integrin, including small molecules and monoclonal Ab, are undergoing clinical trials for the treatment of human cancers and age-related macular degeneration (AMD) (www.clinicaltrials.gov). We are also developing anti-α5β1 chemical-Abs (chem-Abs) using a chemical programming (CP) strategy14,15 that may prove therapeutically useful for the treatment of human cancers and AMD. In this communication, we describe synthesis and optimization of an anti-α5β1 chem-Ab using an in situ convergent CP approach, and results of the in vitro and in vivo studies with a fairly optimized anti-α5β1 chem-Ab.
We have developed several chem-Abs by programming Ab 38C216 and related aldolase Abs17 with low molecular weight synthetic inhibitors that targeted integrins αvβ3, αvβ5, and αvβ6.14,15,18,19,20,21 There are additional chem-Abs that targeted endothelin receptor,22 or bound two different targets.23,24 In vitro and in vivo studies have revealed that the chem-Abs possessed long serum half-life like a classical Ab, and they are therapeutically more effective than the low molecular weight inhibitors.15,25 Construction of such chem-Abs is achieved by modifying synthetic inhibitors with a proprietary linker that selectively react into Ab binding sites through the reactive lysine residues. We anticipated that an anti-α5β1 chemical-Ab could be prepared similarly using Ab 38C2, and a synthetic inhibitor of integrin α5β1 as the Ab-programming agent (PA). However, to further facilitate the discovery and optimization of a chem-Ab, we have developed an in situ convergent CP approach that affords multiple chem-Abs using aldolase Abs and immediate precursors of the Ab-PAs, i.e., functionalized inhibitors and linkers, in parallel. In this approach, multiple bifunctional linkers react with a functionalized inhibitor (Method 1) or into Ab 38C2 binding sites (Method 2) first, and then the intermediates react with the Ab or inhibitor, respectively, as shown in Scheme 1. For the sake of convenience, both inhibitors and linkers are functionalized with alkyne and azide functions that undergo Cu-catalyzed alkyne-azide coupling (Cu-AAC or Click reaction)26 affording the coupled products. The intermediates from step 1 can be used in step 2 without undergoing an extensive purification and/or analysis of the products, and the resulting chem-Abs after step 2 are dialyzed before analyzing their bindings to cells.
Scheme 1.
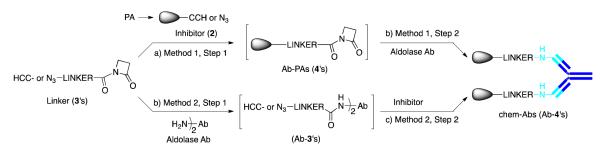
In situ convergent chemical programming (CP) approach for synthesis of the aldolase Ab-derived chemical-antibodies (chem-Abs), Key: (a) Cu wire, Aq. CuSO4, CH3CN, 24 h, then CupriSorbTM, 3 h, filtration using nanopore filter; (b) Ab 38C2 and compound 3’s or 4’s in DMSO, PBS, pH 6.5, 16 h, then dialysis using Amicon; (c) 2 Aq. CuSO4, THPTA ligand,34 Na-ascorbate, aminoguanidine.HCl, DMSO.
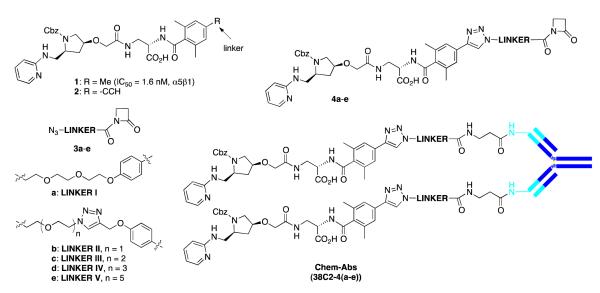
There are numerous potent anti-α5β1 integrin inhibitors27,28,29,30 that could be modified with a linker and conjugated to Ab 38C2 giving anti-α5β1 chem-Abs. Initially, we focused on compound 127 (Figure 1), and synthesized an analogous compound 2 that possessed an alkyne function for introducing a linker enroute the Ab-PAs, 4’s, and chem-Abs 38C2-4’s. The linker site in compound 2 was established based upon the structure activity relationship data around compound 1, and our prior studies with the anti-αvβ3 and αvβ5 chem-Abs.14,15,18-21 Conjugation of compound 2 into Ab 38C2 binding sites could be mediated through a series of bifunctional linkers 3’s, different from each other only in length, possessing an azide group. As described above in Scheme 1, compound 2 could react with linkers 3’s, and the resulting Ab PAs 4’s conjugate with Ab 38C2 (method 1); or, linkers 3’s could conjugate with Ab 38C2, and then react with compound 2 (method 2), giving chem-Abs 38C2-4’s. Syntheses and partial analysis of intermediate 2, linkers 3’s, and Ab-PAs 4’s, as well as their precursors, are described in supporting information (SI).
Figure 1.
Structure of integrin α5β1 inhibitors, antibody programming agents (Ab-PAs), and chem-Abs.
First, we examined a feasibility of the in situ convergent methods by constructing chem-Ab 38C2-4a using Ab 38C2, compound 2, and linker 3a, as described in Scheme 1, and also by classical way, and examining bindings of the resulting samples to U87 cells overexpressing integrin α5β1.31 Thus, in in situ method 1, azide-linker 3a was treated with an excess (3 equivalents) of alkyne-inhibitor 2 (Step 1) using Cu-ACC condition.32 After a complete consumption of linker 3a was confirmed using LC-MS and excess Cu was removed using CupriSorbTM,33 the resulting mixture containing the Ab-PA 4a was reacted with Ab 38C2 (Step 2) giving 38C2-4a. In method 2, Ab 38C2 was first programmed using linker 3a (3 equivalents), and the resulting 38C2-3a (Step 1) was subsequently treated with an excess (10 equivalents) of compound 2 under Finn’s Cu-AAC condition34 (Step 2) to afford 38C2-4a. In our classical method, 38C2-4a was prepared using PA 4a that was purified and authenticated spectroscopically before reacting with Ab 38C2 (method 3). Formation of the chem-Ab 38C2-4a samples by all three methods was confirmed using the Methodol Assay.35 Chem-Ab 38C2-4a samples were dialyzed using 10K MW cut Amicon Ultra Centrifugal Filter and filtered before use, which also removed any unreacted small molecules and Cu reagents. An examination using FACS revealed that both samples of 38C2-4a’s bound to human U87 astrocytoma cells possessed a nearly identical binding profile (SI Figure S-1).
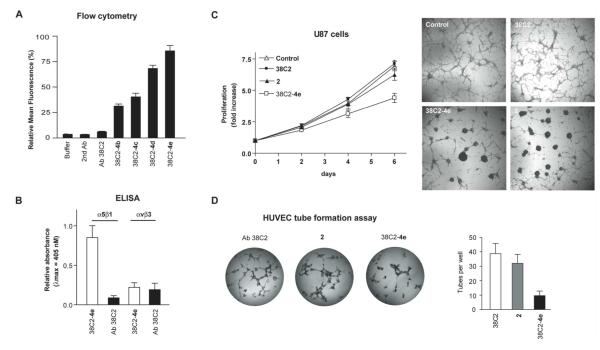
Next, we prepared chem-Abs 38C2-4(b-e) using linkers 3b-e, compound 2 and Ab 38C2 via the Ab-PAs 4b-e, and determined their bindings to U87 cells using flow cytometry. The results, shown in Figure 2A, indicated that all four chem-Abs, 38C2-4(b-e), bound to U87 cells. No binding was recorded with the –ve controls, including Ab 38C2, buffer or the secondary Ab alone, as expected. In this manner, we have identified 38C2-4e as a fairly optimized chem-Ab for subsequent studies, which included binding studies with the 4T1 murine breast cancer cells, ELISA experiments, in vitro cell proliferation and angiogenesis assay, and the efficacy evaluation using tumor and metastases models of 4T1 cells. Again, binding of the chem-Ab 38C2-4e to 4T1 cells was examined, as described above with U87 cells. The results (SI Figure S-2) confirmed a positive binding of the chem-Ab 38C2-4e to 4T1 cells, albeit weakly, which correlated with the weak expression level of the integrin on this cell line. Using ELISA assay (Figure 2B), we found that 38C2-4e possessed high affinity for α5β1 integrin, but not for αvβ3 integrin, suggesting that the inhibitor 2 retained its affinity for the α5β1 integrin even after modification with the linker and conjugation to Ab 38C2. The latter finding is significant as both α5β1 and αvβ3 integrins bind RGD motif in proteins and the RGD mimetics, and selectivity is often an issue for the inhibitors of these integrins.36
Figure 2.
In vitro evaluation of the chem-Abs. (A) Bar graph showing the binding affinities of chem-Abs 38C2-4(b-e) to U87 human glioblastoma cells as determined using FACS. Cells (1.5 × 105 cells/ml) were incubated with chem-Abs (15 μg/ml) in PBS buffer containing (1% FCS and 100 nm MnCl2) for 2 hr at 4 °C, and with FITC labeled anti mouse goat Ab (1 μg/ml) for 1 hour at 4 °C. Secondary Ab and Ab 38C2 (15 Jg/ml) were used as –ve controls. The y axis gives the relative mean fluorescence in linear scale, and the x axis describes sample names. (B) Bar graph showing relative binding of chem-Ab 38C2-4e to integrin α5β1 and αvβ3 using ELISA. Chem-Ab 38C2-4e and Ab 38C2 were added into wells of Immulon 2HB well plates (DYNE Technologies) that have been coated with purified integrin α5β1 or αvβ3 protein and blocked with 10% BSA in PBS. After plates were incubated and washed, binding of Ab 38C2 and chem-Ab 38C2-4e was determined using biotinylated anti-mouse Ab (Vector Lab, 1:500 dilution in binding buffer, 2 Jg/ ml, 100 Jl), avidin-horseradish peroxidase reagent, and AEC (3-amino-9-ethylcarbazole). Results were obtained by measuring the relative absorptions at 405 nM using the UV spectrophotometer. (C) Inhibitory effects of 38C2-4e on U87 cell proliferation. Cells were treated with compound 2 (1 μM), chem-Ab 38C2-4e (1 JM), buffer, and Ab 38C2 (1 JM) alone at 37 °C. After 0, 48, 96, and 144 hrs incubation periods, cell proliferation was determined using the MTT (3-(4,5-dimethyl-thiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfo-phenyl)-2H-tetrazolium) assay (Left). Image from each well scanned using a microscope on day 6 (Right). (D) Inhibitory effects of 38C2-4e on HUVEC tube formation. HUVEC cells layered on top of the gels in the wells of a 96-well microtiter plate were incubated with the test compound 2 (1 μM), chem-Ab 38C2-4e (1 μM), and Ab 38C2 (1 JM) at 37°C for 72 hrs, and the image from each well was scanned using a microscope (Left) and analyzed by counting fragments of tubes (Right). All experiments were carried out in triplicate.
Integrin inhibitors inhibit tumor growth mainly by interfering with the tumor angiogenesis processes, as well as by inhibiting the proliferation of tumor cells expressing target integrins.37,38,39,40,41 We determined the effects of 38C2-4e and compound 2 on cell proliferation using U87 cells. For this, cells were treated with compound 2 (1 μM) or 38C2-4e (1 μM), and number of live cells determined on day 2, 4, and 6. Image from each well was scanned using a microscope on day 6. Results are shown in Figure 2C. Evidently, chem-Ab 38C2-4e decreased proliferation of U87 cells significantly (Figure 2C-Left), which amounted to approximately 40% inhibition after 6 days. Compound 2 also showed approximately 15% inhibition. After 72 hours of exposure to 38C2-4e or compound 2, U87 cells rounded up and detached to form clusters, i.e. spheroids (Figure 2C-Right). In contrast, fewer U87 cells formed spheroids in control and 38C2 treatment. Similar results were reported with α5β1 inhibitor SJ749, which inhibited proliferation of U87 cells at 10 JM concentrations by 20% and formed cell spheroids.36
To determine and analyze the anti-angiogenic effects of anti-α5β1 chem-Ab 38C2-4e on tumor vascularization, human endothelial cell function was assessed in vitro in matrigel using the endothelial cell tube formation assay, as described in the literature.42 HUVEC tube formation was observed as early as 5 hrs after assay initiation versus under normal conditions, and the vascular tubes were formed within 24 hrs. The results are shown in Figure 2D. Evidently, 38C2-4e inhibited the angiogenesis at 1 μM concentration significantly than using the compound 2 alone. As expected, the tube formation wasn’t apparent in the control experiment using Ab 38C2 alone. These data indicated that the 38C2-4e is most likely a high affinity integrin α5β1 blocking Ab that can suppress tumor angiogenesis and tumor growth effectively.
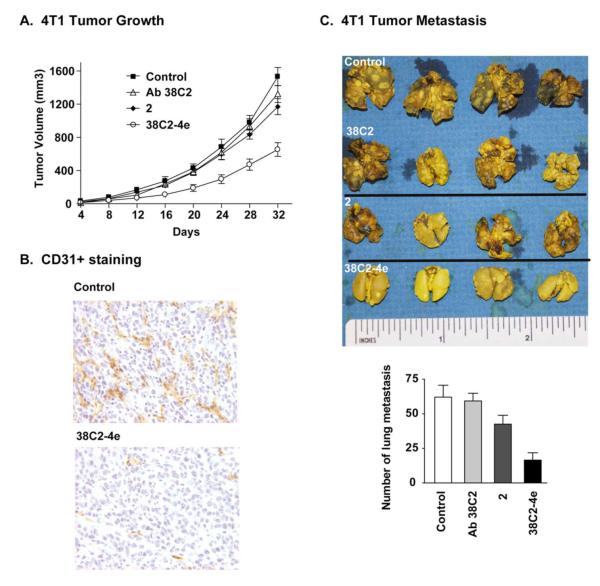
Results of the above-described studies with 38C2-4e, as well as prior in vitro and/or in vivo studies with the anti-α5β1 integrin antibody and small molecule inhibitor SJ749, clearly suggested that the former could also inhibit tumor growth and/or metastasis without causing any general toxicity to normal cells.36-40 Because both the small molecule integrin inhibitors and anti-integrin Abs are usually less toxic than most anti-cancer cytotoxins, such as doxorubicin and paclitaxel, currently used in clinic, they can be administered at a high dosage. Uses of the integrin inhibitors at a high dosage may also be necessary as they are often pro-angiogenic at a low concentration.43 Thus, we determined in vivo efficacy of 38C2-4e using 4T1 murine mammary carcinoma model. Compound 2 and Ab 38C2 alone and buffer were used as the control groups. All treatment regimens were administered every fifth day starting on day 4 after tumor induction. As shown in Figures 3A and 3B, the results confirmed that there were significant tumor growth and metastasis inhibition in experimental groups treated with 38C2-4e. There are no weight loss and any other apparent signs of toxicity. On day 32, tumor volumes of 38C2-4e treated group animals were significantly smaller than the control groups (Figure 3A) (p<0.01, n=6). The vascular density (CD31 positive in Figure 3B) was reduced in tumor sections of treated Mice. This data is in consistent with the in vitro assay of the tube formation, indicating that the 38C2-4e is a high affinity-blocking anti-α5β1 integrin Ab that suppresses angiogenesis under pathologic conditions, such as tumor growth. Treatment with 38C2-4e has also shown significant inhibitory effects on spontaneous metastasis of 4T1 murine mammary carcinoma, as compared to the control groups, including those treated with buffer, Ab 38C2, or compound 2 alone (Figure 3C). These results are significant because 4T1 spontaneous metastasis is a highly aggressive model and has great clinical relevance.44
Figure 3.
Inhibition of primary tumor growth and metastasis by the chem-Ab 38C2-4e. (A) Tumor induction was performed by s.c. injection of 5 × 105 4T1 cells in the right flank of six-week-old BALB/c mice. Four different groups of mice were treated with PBS (200 Jl), Ab 38C2 (150 Jg in 200 Jl buffer), chem-Ab 38C2-4e (150 Jg in 200 Jl PBS), or equimolar concentration of compound 2, starting on day 4 after the tumor induction. Each mouse was given i.p. injection on day 4, 8, 12, 16, 20, 24, and 32 (total 7 injections per mouse), and tumor volumes were measured using a microcaliper and calculated by the formula V = 1/2(LXW2), where L is Length (longest dimension) and W is Width (shortest dimension).45 The difference in tumor growth between 38C2-4e and 2 was significant (n = 6 mice per group, P <0.01). (B) Shown are the representative tumor sections of the vascular density CD31 staining. (C) Representative lung specimens and statistical analysis of lung metastasis of control, Ab 38C2, 2 and chem-Ab 38C2-4e. (n=6, p<0.01). Lung metastases were determined by examining the lung sections using H & E staining. All animal experiments were performed using procedures that have been reviewed and approved by the Institutional Animal Care and Use Committee at The Scripps Research Institute. The Scripps Research Institute maintains an assurance with the Public Health Service and is registered with the Department of Agriculture and is in compliance with all regulations relating to animal care and welfare.
In general, affinity of a chem-Ab to cellular target strongly depends upon the length and type of linker that connects a targeting moiety to Ab 38C2 through the lactam or DK function.18 The above-described anti-α5β1 integrin chem-Abs 38C2-4’s also behaved similarly, as their binding to U87 cells was stronger as linker increased from 4b to 4e, though with an exception. Chem-Ab 38C2-4a possessed stronger binding than 38C2-4b and 38C2-4c, but the latter had longer linker than the former. The exact cause of this observation remains to be ascertained. Second, linker length of the fairly optimized chem-Ab 38C2-4e is already quite long, but it remains to be seen whether further elongation will have any effects on the affinity of the resulting anti-α5β1 integrin chem-Abs. It is likely that the recently described crystal structure of 33F12 Fab-JW hapten (5-((4-(3,5-dioxohexyl)phenyl)amino)-5-oxo-pentanoic acid) complex46 may shed some light on the development of new linkers in 3’s and 4’s, necessary to maximize binding of chem-Abs 38C2-4’s to their target. However, all these studies remain the subject of our future investigations.
In conclusion, an aldolase Ab-derived anti-α5β1 integrin chem-Ab was prepared and optimized using an in situ convergent CP approach, facilitated by the Cu-catalyzed Alkyne-azide coupling reaction. Binding of the resulting five homologous chem-Abs to U87 human glioblastoma cells expressing α5β1 integrin using flow cytometry assay revealed that chem-Ab 38C2-4e was a fairly optimized anti-α5β1 integrin chem-Ab. In vitro and in vivo studies confirmed that chem-Ab 38C2-4e inhibit tumor growth, angiogenesis and metastasis of murine 4T1 cells. We anticipate that the chem-Ab 38C2-4e can be further optimized using analogous compounds and/or linkers, and developed as highly efficient diagnostic tool and possibly as therapeutic applications.
Supplementary Material
ACKNOWLEDGMENT
Authors thank to the US National Cancer Institute (CA120289 to SCS, and CA127535 to CL) and the US Department of Defense (W81XWH-09-1-0690 to SCS, and W81XWH-07-1-0389 to CL) for the funding support.
SUPPORTING INFORMATION. Synthesis, analyses, and examination of the chem-Abs 38C2-4’s, and their precursors. This information is available free of charge via the Internet at http://pubs.acs.org/
Footnotes
Conflict of Interests.Rajib K. Goswami, Yuan Liu, None, and Cheng Liu: None. Richard A. Lerner and Subhash C. Sinha: Inventor on a patent related to chemical programming technology.
Reference
- 1.Hynes RO. Integrins: Bidirectional, Allosteric Signalling Machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 2.Hu P, Luo BH. Integrin Bidirectional Signaling Across The Plasma Membrane. J. Cell Physiol. 2012 doi: 10.1002/jcp.24154. doi: 10.1002/jcp.24154. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Berrier AL, Yamada KM. Cell-Matrix Adhesion. J. Cell Physiol. 2007;213:565–573. doi: 10.1002/jcp.21237. [DOI] [PubMed] [Google Scholar]
- 4.Danen EH, Sonnenberg A. Integrins In Regulation Of Tissue Development And Function. J. Pathol. 2003;201:632–641. doi: 10.1002/path.1472. [DOI] [PubMed] [Google Scholar]
- 5.Avraamides CJ, Garmy-Susini B, Varner JA. Integrins In Angiogenesis And Lymphangiogenesis. Nat. Rev. Cancer. 2008;8:604–617. doi: 10.1038/nrc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caswell PT, Vadrevu S, Norman JC. Integrins: Masters And Slaves Of Endocytic Transport. Nature Rev. Mol. Cell Biol. 2009;10:843–853. doi: 10.1038/nrm2799. [DOI] [PubMed] [Google Scholar]
- 7.Chiodoni C, Colombo MP, Sangaletti S. Matricellular Proteins: From Homeostasis To Inflammation, Cancer, And Metastasis. Cancer Metastasis Rev. 2010;29:295–307. doi: 10.1007/s10555-010-9221-8. [DOI] [PubMed] [Google Scholar]
- 8.Weis SM, Cheresh DA. Tumor Angiogenesis: Molecular Pathways And Therapeutic Targets. Nat. Med. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 9.Yao ES, Zhang H, Chen Y-Y, Lee B, Chew K, Moore D, Park C. Increased β1 Integrin Is Associated with Decreased Survival in Invasive Breast Cancer. Cancer Res. 2007;67:659–664. doi: 10.1158/0008-5472.CAN-06-2768. [DOI] [PubMed] [Google Scholar]
- 10.Han SW, Roman J. Extracellular Matrix And Aberrant Signaling In Lung Carcinoma: Role Of Fibronectin In The Control Of Human Lung Carcinoma Cell Growth, Apoptosis And Resistance To Therapy. Curr. Signal Transduct. Ther. 2007;2:1–10. [Google Scholar]
- 11.Nam J-M, Onodera Y, Bissell MJ, Park CC. Breast Cancer Cells In Three-Dimensional Culture Display An Enhanced Radioresponse After Coordinate Targeting Of Integrin A5β1 And Fibronectin. Cancer Res. 2010;70:5238–5248. doi: 10.1158/0008-5472.CAN-09-2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desgrosellier JS, Cheresh DA. Integrins In Cancer: Biological Implications And Therapeutic Opportunities. Nature Rev. Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cox D, Brennan M, Moran N. Integrins As Therapeutic Targets: Lessons And Opportunities. Nat. Rev. Drug Discov. 2010;9:804–820. doi: 10.1038/nrd3266. [DOI] [PubMed] [Google Scholar]
- 14.Rader C, Sinha SC, Popkov M, Lerner RA, Barbas CF., III A Chemically Programmed Monoclonal Antibody For Cancer Therapy. Proc. Natl. Acad. Sci. USA. 2003;100:5396–5400. doi: 10.1073/pnas.0931308100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo F, Das S, Mueller BM, Barbas CF, III, Lerner RA, Sinha SC. Breaking The One Antibody-One Target Axiom. Proc. Natl. Acad. Sci. USA. 2006;103:11009–11014. doi: 10.1073/pnas.0603822103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner J, Lerner RA, Barbas CF., III Efficient Aldolase Catalytic Antibodies That Use The Enamine Mechanism Of Natural Enzymes. Science. 1995;270:1797–1800. doi: 10.1126/science.270.5243.1797. [DOI] [PubMed] [Google Scholar]
- 17.Zhong G, Lerner RA, Barbas CF., III Broadening The Aldolase Catalytic Antibody Repertoire By Combining Reactive Immunization And Transition State Theory: New Enantio- And Diastereoselectivities. Angew. Chem. Int. Ed. 1999;38:3738–3741. doi: 10.1002/(sici)1521-3773(19991216)38:24<3738::aid-anie3738>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 18.Li L-S, Rader C, Matsushita M, Das S, Barbas CF, III, Lerner RA, Sinha SC. Chemical-Immunotherapy: Design, Synthesis And Evaluation Of Novel Integrin-Targeting Devices. J. Med. Chem. 2004;47:5630–5640. doi: 10.1021/jm049666k. [DOI] [PubMed] [Google Scholar]
- 19.Rader C, Turner JM, Heine A, Shabat D, Sinha SC, Wilson IA, Lerner RA, Barbas CF., III A Humanized Aldolase Antibody For Selective Chemotherapy And Adaptor Immunotherapy. J. Mol. Biol. 2003;332:889–899. doi: 10.1016/s0022-2836(03)00992-6. [DOI] [PubMed] [Google Scholar]
- 20.Goswami RK, Huang Z-Z, Forsyth JS, Felding-Habermann B, Sinha SC. Multiple Catalytic Aldolase Antibodies Suitable For Chemical Programming. Bioorg. Med. Chem. Lett. 2009;19:3821–3824. doi: 10.1016/j.bmcl.2009.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goswami RK, Bajjuri KM, Forsyth JS, Das S, Hassenpflug W, Huang ZZ, Lerner RA, Felding-Habermann B, Sinha SC. Chemically Programmed Antibodies Targeting Multiple Alpha(V) Integrins And Their Effects On Tumor-Related Functions In Vitro. Bioconjug. Chem. 2011;22:1535–1544. doi: 10.1021/bc2000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doppalapudi VR, Tryder N, Li L, Aja T, Griffith D, Liao F-F, Roxas G, Ramprasad MP, Bradshaw C, Barbas CF., III Chemically Programmed Antibodies: Endothelin Receptor Targeting Covx-Bodies. Bioorg. Med. Chem. Lett. 2007;17:501–506. doi: 10.1016/j.bmcl.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Gavrilyuk JI, Wuellner U, Salahuddin S, Goswami RK, Sinha SC, Barbas CF., III An Efficient Chemical Approach To Bispecific Antibodies And Antibodies Of High Valency. Bioorg. Med. Chem. Lett. 2009;19:3716–3720. doi: 10.1016/j.bmcl.2009.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doppalapudi VR, Huang J, Liu D, Jin P, Liu B, Li L, Desharnais J, Hagen C, Levin NJ, Shields MJ, Parish M, Murphy RE, Rosario JD, Oates BD, Lai J-Y, Matin MJ, Ainekulu Z, Bhat A, Bradshaw CW, Woodnutt G, Lerner RA, Lappe RW. Chemical Generation of Bispecific Antibodies. Proc. Natl. Acad. Sci. USA. 2010;107:22611–22616. doi: 10.1073/pnas.1016478108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Popkov M, Rader C, Gonzalez B, Sinha SC, Barbas CF., III Small Molecule Drug Activity in Melanoma Models may ee Dramatically Enhanced with an Antibody Effector. Int. J. Cancer. 2006;119:1194–1207. doi: 10.1002/ijc.21924. [DOI] [PubMed] [Google Scholar]
- 26.Kolb HC, Finn MG, Sharpless KB. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 27.Stragies R, Osterkamp F, Zischinsky G, Vossmeyer D, Kalkhof H, Reimer U, Zahn G. Design and Synthesis of a New Class of Selective Integrin α5β1 Antagonists. J. Med. Chem. 2007;50:3786–3794. doi: 10.1021/jm070002v. [DOI] [PubMed] [Google Scholar]
- 28.Delouvrié B, Al-Kadhimi K, Arnould JC, Barry ST, Cross DA, Didelot M, Gavine PR, Germain H, Harris CS, Hughes AM, Jude DA, Kendrew J, Lambert-van der Brempt C, Lohmann JJ, Ménard M, Mortlock AA, Pass M, Rooney C, Vautier M, Vincent JL, Warin N. Structure-Activity Relationship of a Series of Non Peptidic RGD Integrin Antagonists Targeting A5β1: Part 1. Bioorg. Med. Chem. Lett. 2012;22:4111–4116. doi: 10.1016/j.bmcl.2012.04.063. [DOI] [PubMed] [Google Scholar]
- 29.Delouvrié B, Al-Kadhimi K, Arnould JC, Barry ST, Cross DA, Didelot M, Gavine PR, Germain H, Harris CS, Hughes AM, Jude DA, Kendrew J, Lambert-van der Brempt C, Lohmann JJ, Ménard M, Mortlock AA, Pass M, Rooney C, Vautier M, Vincent JL, Warin N. Structure-Activity Relationship of a Series of Non Peptidic RGD Integrin Antagonists Targeting A5β1: Part 2. Bioorg. Med. Chem. Lett. 2012;22:4117–4121. doi: 10.1016/j.bmcl.2012.04.061. [DOI] [PubMed] [Google Scholar]
- 30.Tolomelli A, Gentilucci L, Mosconi E, Viola A, Dattoli SD, Baiula M, Spampinato S, Belvisi L, Civera M. Development of Isoxazoline-Containing Peptidomimetics as Dual αvβ3 and α5β1 Integrin Ligands. ChemMedChem. 2011;6:2264–2272. doi: 10.1002/cmdc.201100372. [DOI] [PubMed] [Google Scholar]
- 31.Friedlander DR, Zagzag D, Shiff B, Cohen H, Allen JC, Kelly PJ, Grume M. Migration of Brain Tumor Cells on Extracellular Matrix Proteins in Vitro Correlates With Tumor Type and Grade and Involves αv and β1 Integrins. Cancer Res. 1996;56:1939–1947. [PubMed] [Google Scholar]
- 32.Himo F, Lovell T, Hilgraf R, Rostovtsev VV, Noodleman L, Sharpless KB, Fokin VV. Copper(I)-Catalyzed Synthesis of Azoles. DFT Study Predicts Unprecedented Reactivity and Intermediates. J. Amer. Chem. Soc. 2005;127:210–216. doi: 10.1021/ja0471525. [DOI] [PubMed] [Google Scholar]
- 33.CupriSorbTM is a synthetic adsorbent that removes copper in its all form, and available from Seachem. http://www.seachem.com.
- 34.Hong Vu., Steinmetz NF, Manchester M, Finn MG. Labeling Live Cells by Copper-Catalyzed Alkyne-Azide Click Chemistry. Bioconj. Chem. 2010;21:1912–1916. doi: 10.1021/bc100272z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sinha SC, Das S, Li L-S, Lerner RA, Barbas CF., III Preparation of integrin alpha(v)beta(3)-targeting Ab 38C2 constructs. Nature Prot. 2007;2:449–456. doi: 10.1038/nprot.2007.3. [DOI] [PubMed] [Google Scholar]
- 36.Meyer A, Auernheimer J, Modlinger A, Kessler H. Targeting RGD Recognizing Integrins: Drug Development, Biomaterial Research, Tumor Imaging and Targeting. Curr. Pharm. Des. 2006;12:2723–2747. doi: 10.2174/138161206777947740. [DOI] [PubMed] [Google Scholar]
- 37.Maglott A, Bartik P, Cosgun S, Klotz P, Rondé P, Fuhrmann G, Takeda K, Martin S, Dontenwill M. The Small A5B1 Integrin Antagonist, SJ749, Reduces Proliferation and Clonogenicity of Human Astrocytoma Cells. Cancer Res. 2006;66:6002–6007. doi: 10.1158/0008-5472.CAN-05-4105. [DOI] [PubMed] [Google Scholar]
- 38.Khalili P, Arakelian A, Chen G, Plunkett ML, Beck I, Parry GC, Donate F, Shaw DE, Mazar AP, Rabbani SA. A Non-RGD-Based Integrin Binding Peptide (ATN-161) Blocks Breast Cancer Growth and Metastasis in Vivo. Mol. Cancer Ther. 2006;5:2271–2280. doi: 10.1158/1535-7163.MCT-06-0100. [DOI] [PubMed] [Google Scholar]
- 39.Sudhakar A, Sugimoto H, Yang C, Lively J, Zeisberg M, Kalluri R. Human Tumstatin and Human Endostatin Exhibit Distinct Antiangiogenic Activities Mediated By Alpha V Beta 3 and Alpha 5 Beta 1 Integrins. Proc. Natl. Acad. Sci. U.S.A. 2003;100:4766–4771. doi: 10.1073/pnas.0730882100. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 40.Yi M, Ruoslahti E. A Fibronectin Fragment Inhibits Tumor Growth, Angiogenesis, and Metastasis. Proc. Natl. Acad. Sci. U.S.A. 2001;98:620–624. doi: 10.1073/pnas.98.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhaskar V, Fox M, Breinberg D, Wong MH, Wales PE, Rhodes S, DuBridge RB, Ramakrishnan V. Volociximab, a Chimeric Integrin Alpha5beta1 Antibody, Inhibits the Growth of VX2 Tumors in Rabbits. Invest. New Drugs. 2008;26:7–12. doi: 10.1007/s10637-007-9078-z. [DOI] [PubMed] [Google Scholar]
- 42.Matsumura T, Wolff K, Petzelbauer P. Endothelial Cell Tube Formation Depends on Cadherin 5 and CD31 Interactions with Filamentous Actin. J. Immunology. 1997;158:3408–3416. [PubMed] [Google Scholar]
- 43.Reynolds AR, Hart IR, Watson AR, Welti JC, Silva RG, Robinson SD, Da Violante G, Gourlaouen M, Salih M, Jones MC, Jones DT, Saunders G, Kostourou V, Perron-Sierra F, Norman JC, Tucker GC, Hodivala-Dilke KM. Stimulation of Tumor Growth and Angiogenesis by Low Concentrations of RGD-Mimetic Integrin Inhibitors. Nature Med. 2009;15:392–400. doi: 10.1038/nm.1941. [DOI] [PubMed] [Google Scholar]
- 44.Aslakson CJ, Miller FR. Selective Events in the Metastatic Process Defined by Analysis of the Sequential Dissemination of Subpopulations of a Mouse Mammary Tumor. Cancer Res. 1992;52:1399–1405. [PubMed] [Google Scholar]
- 45.Sua F;, Kozaka KR, Imaizumib S, Gaoa F, Amneusa MW, Grijalvab V, Nga C, Wagnerb A, Houghb G, Farias-Eisnerb G, Anantharamaiahc GM, Van Lentenb BJ, Navabb M, Fogelmanb AM, Reddy ST, Farias-Eisnera R. Apolipoprotein A-I (apoA-I) and apoA-I mimetic peptides inhibit tumor development in a mouse model of ovarian cancer. Proc. Natl. Acad. Sci. U.S.A. 2010;107:19997–20002. doi: 10.1073/pnas.1009010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu X, Tanaka F, Lerner RA, Barbas CF, III, Wilson IA. Direct Observation of an Enamine Intermediate in Amine Catalysis. J. Am. Chem. Soc. 2009;131:18206–18207. doi: 10.1021/ja907271a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.