Abstract
Gastroduodenal Crohn’s disease (CD) is rare and the response to standard medical therapy is often poor. Anti-tumor necrosis factor therapy has revolutionised the treatment of CD. We present a patient with pyloric stenosis associated with CD which improved with Adalimumab therapy. We recommend considering anti-tumor necrosis factor therapy in symptomatic gastroduodenal CD.
Keywords: Pyloric stenosis, Crohn’s disease, Anti-tumor necrosis factor therapy, Adalimumab
INTRODUCTION
CD is a chronic inflammatory condition and can involve the gut from the lips to the anus. Gastroduodenal involvement is seen in 0.5%-4% of all patients with CD[1-4]. While most patients with gastroduodenal disease are asymptomatic[5], some of them do develop features of gastric outlet obstruction[2]. Historically, stricturing gastroduodenal CD has been treated with resection[6,7], gastrojejunostomy bypass[7] and strictureplasty[8]. These have been associated with recurrence and morbidity. Corticosteroids and parenteral nutrition have been reported to be effective in acute gastric outlet obstruction secondary to CD while both thiopurines and methotrexate have been effective in maintaining remission[9-11]. Endoscopic balloon dilatation as adjunct to aggressive medical intervention has been described but has high recurrence rates[12,13]. There have been case reports describing the efficacy of anti-TNF therapy in stricturing gastroduodenal CD[14,15]. The patients in these case reports went through the combined treatment with mesalazine, corticosteroids, proton pump inhibitors and total parenteral nutrition before being considered for infliximab infusions.
CASE REPORT
A 23-year-old woman presented to the Gastroenterology Clinic in September 2010 with a 11 mo history of worsening bloating, malodorous belching, vomiting, reflux symptoms, 3 kg weight loss and borborygmi. She was diagnosed to have Crohn’s disease (CD) in 2005 and had undergone a subtotal colectomy with ileorectal anastomosis in 2006. The pathology of the resected specimen was in keeping with CD. She later developed perianal fistulating disease in 2007 which was treated with initial Seton insertion and later marsupialisation. She was intolerant of azathioprine, developing abnormal liver function tests and was on methotrexate 20 mg once a week orally since June 2008. She had also been treated for suspected small intestinal bacterial overgrowth with intermittent antibiotics between October 2008 and August 2010, with no symptomatic improvement. During this time, her lower gastrointestinal and perianal disease was well controlled on methotrexate.
In August 2010, her C reactive protein started rising and reached 78 mg/L (normal range: 0-5 mg/L). Her faecal calprotectin was high at 1840 ìg/g of faeces (normal: < 60 ìg/g) in September 2010. However, a flexible sigmoidoscopy in September 2010 showed a normal looking mucosa up to 35 cm from the anal verge. Random biopsies from the rectum and remaining colon showed minimally active, mild colitis with granuloma formation consistent with CD. A gastroscopy done at the same time was inconclusive due to food residue suggesting delayed gastric emptying.
She had an magnetic resonance imaging (MRI) of her small bowel in October 2010 which showed gross gastric dilatation with possible duodenal stricture (Figure 1A). The majority of the oral contrast had not passed into her small intestine. The rest of the small bowel was slightly dilated in keeping with post colectomy appearance. A repeat gastroscopy, after a prolonged fast, in December 2010 showed reflux oesophagitis, a normal stomach, and significant pyloric stenosis. The pylorus was negotiated and the first and second part of duodenum appeared normal. The biopsies from the pylorus showed focally erosive active chronic antral gastritis with intestinal metaplasia with no evidence of helicobacter infection. The pyloric inflammation was likely due to her CD. She was discussed at a Multi Disciplinary Team meeting. The surgical consensus was that she would likely require a subtotal gastrectomy. She was offered a trial of an anti-tumor necrosis factor (TNF) alpha antagonist therapy and she opted for Adalimumab. In January 2011, she was started on adalimumab with induction dose of 160 mg and 80 mg with maintenance dose of 80 mg subcutaneously every fortnight in addition to methotrexate 20 mg orally once a week. Over the next 11 mo, her symptoms gradually improved. By November 2011, she had gained 17 kg since starting Adalimumab and reported no further vomiting, belching or borborygmi.
Figure 1.
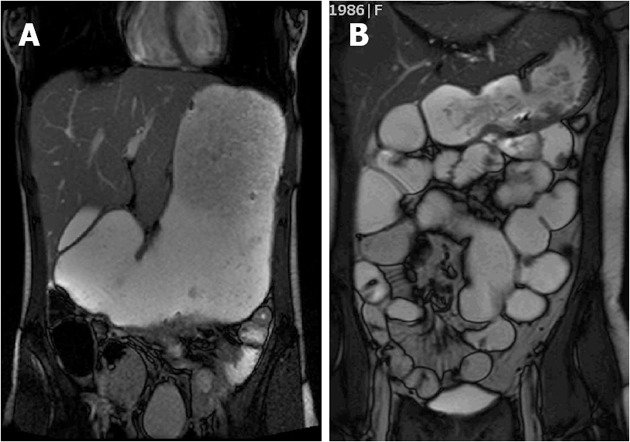
Magnetic resonance imaging. A: Gross gastric dilatation before anti-tumor necrosis factor therapy; B: Significantly reduced stomach size but with some focal small intestinal dilatation.
Her repeat MRI scan showed that the gastric dilatation was significantly less (Figure 1B). The oral contrast was seen to be passing into the small intestine and colon. Though there were focal areas of small bowel dilatation, they were likely to be secondary to adhesions or fibrotic strictures. The patient continues to take Adalimumab fortnightly.
DISCUSSION
There has been one case report of a patient with isolated gastric CD with gastric outlet obstruction initially who went into remission with infliximab infusions[16]. When her symptoms recurred, she was restarted on infliximab. However, the patient developed mild arthralgias and later and acute infusion reaction. This led to the patient being started on Adalimumab in addition to azathioprine and prednisolone. However, there was no significant clinical improvement after 3 mo. Topical corticosteroid therapy in form of fluticasone inhaler was introduced. The patient improved subsequently and went into clinical remission. Duodenal CD has been treated successfully with a combination of azthioprine, adalimumab and pantoprazole[17]. The patient had isolated non-stenotic duodenal CD.
This is the first reported case in which the patient with symptomatic pyloric CD has responded to Adalimumab therapy. This patient was on an apparently adequate doses of oral methotrexate, although drug absorption may have been suboptimal, her lower gastrointestinal and perianal disease were well controlled. However, this was apparently not enough to control disease activity in the upper gut. By opting for Adalimumab directly, we avoided the side effects of long term corticosteroids and the morbidity associated with total parenteral nutrition, frequent endoscopic dilatations, surgery and repeated admissions to hospital for infliximab infusions.
In conclusion, we report a novel and safe way of treating stricturing gastroduodenal CD with Adalimumab. Treatment can be imparted with minimal disturbance to patient’s routine life. Adalimumab merits consideration in symptomatic gastroduodenal CD.
Footnotes
Peer reviewer: Ole Haagen Nielsen, Professor, Chief Physician, Department of Gastroenterology, Medical Section D112M, Herlev Hospital, University of Copenhagen, Herlev Ringvej 75, DK-2730 Herlev, Denmark
S- Editor Zhai HH L- Editor A E- Editor Xiong L
References
- 1.Isaacs KL. Upper gastrointestinal tract endoscopy in inflammatory bowel disease. Gastrointest Endosc Clin N Am. 2002;12:451–462. doi: 10.1016/s1052-5157(02)00006-5. [DOI] [PubMed] [Google Scholar]
- 2.Shivaraj G, Prakash BD, Sonal V, Shruthi K, Vinayak H, Avinash M. Thyroid function tests: a review. Eur Rev Med Pharmacol Sci. 2009;13:341–349. [PubMed] [Google Scholar]
- 3.Banerjee S, Peppercorn MA. Inflammatory bowel disease. Medical therapy of specific clinical presentations. Gastroenterol Clin North Am. 2002;31:185–202. doi: 10.1016/s0889-8553(01)00012-7. [DOI] [PubMed] [Google Scholar]
- 4.Reynolds HL, Stellato TA. Crohn’s disease of the foregut. Surg Clin North Am. 2001;81:117–135. doi: 10.1016/s0039-6109(05)70276-0. [DOI] [PubMed] [Google Scholar]
- 5.Loftus EV Jr. Upper gastrointestinal tract Crohn’s disease. Clin Perspect Gastroenterol. 2002;5:188–191. [Google Scholar]
- 6.Ross TM, Fazio VW, Farmer RG. Long-term results of surgical treatment for Crohn’s disease of the duodenum. Ann Surg. 1983;197:399–406. doi: 10.1097/00000658-198304000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray JJ, Schoetz DJ, Nugent FW, Coller JA, Veidenheimer MC. Surgical management of Crohn’s disease involving the duodenum. Am J Surg. 1984;147:58–65. doi: 10.1016/0002-9610(84)90035-7. [DOI] [PubMed] [Google Scholar]
- 8.Futami K, Arima S. Role of strictureplasty in surgical treatment of Crohn’s disease. J Gastroenterol. 2005;40 Suppl 16:35–39. doi: 10.1007/BF02990577. [DOI] [PubMed] [Google Scholar]
- 9.Priebe WM, Simon JB. Crohn’s disease of the stomach with outlet obstruction: a case report and review of therapy. J Clin Gastroenterol. 1983;5:441–445. doi: 10.1097/00004836-198310000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Tremaine WJ. Gastroduodenal Crohn’s disease: medical management. Inflamm Bowel Dis. 2003;9:127–128; discussion 131. doi: 10.1097/00054725-200303000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Feagan BG, Fedorak RN, Irvine EJ, Wild G, Sutherland L, Steinhart AH, Greenberg GR, Koval J, Wong CJ, Hopkins M, et al. A comparison of methotrexate with placebo for the maintenance of remission in Crohn’s disease. North American Crohn’s Study Group Investigators. N Engl J Med. 2000;342:1627–1632. doi: 10.1056/NEJM200006013422202. [DOI] [PubMed] [Google Scholar]
- 12.Murthy UK. Repeated hydrostatic balloon dilation in obstructive gastroduodenal Crohn’s disease. Gastrointest Endosc. 1991;37:484–485. doi: 10.1016/s0016-5107(91)70789-x. [DOI] [PubMed] [Google Scholar]
- 13.Matsui T, Hatakeyama S, Ikeda K, Yao T, Takenaka K, Sakurai T. Long-term outcome of endoscopic balloon dilation in obstructive gastroduodenal Crohn’s disease. Endoscopy. 1997;29:640–645. doi: 10.1055/s-2007-1004271. [DOI] [PubMed] [Google Scholar]
- 14.Knapp AB, Mirsky FJ, Dillon EH, Korelitz BI. Successful infliximab therapy for a duodenal stricture caused by Crohn’s disease. Inflamm Bowel Dis. 2005;11:1123–1125. doi: 10.1097/01.mib.0000191612.43584.94. [DOI] [PubMed] [Google Scholar]
- 15.Odashima M, Otaka M, Jin M, Horikawa Y, Matsuhashi T, Ohba R, Koizumi S, Kinoshita N, Takahashi T, Watanabe S. Successful treatment of refractory duodenal Crohn’s disease with infliximab. Dig Dis Sci. 2007;52:31–32. doi: 10.1007/s10620-006-9585-3. [DOI] [PubMed] [Google Scholar]
- 16.Ibrahim SH, Smyrk TC, Faubion WA. Treatment of isolated gastric Crohn’s disease with inhaled corticosteroids. Case Rep Gastroenterol. 2008;2:363–368. doi: 10.1159/000158543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tursi A. Duodenal Crohn’s disease successfully treated with adalimumab. Endoscopy. 2011;43 Suppl 2 UCTN:E22. doi: 10.1055/s-0030-1255893. [DOI] [PubMed] [Google Scholar]


