Abstract
In a recent article, Gorissen et al report on 795 patients with primary colorectal anastomosis operated on during the period 2008-2010 for different colorectal conditions at two centres. The leakage rate was significantly higher among patients who were administered non-steroidal anti-inflammatory drugs (NSAIDs) in the perioperative course. A dose-response relationship could also be traced, where longer NSAID use yielded a higher risk of anastomotic breakdown. However, as this study is observational in design, confounding by indication may be present and there is also a risk of residual confounding from unmeasured covariates. Moreover, the question whether different affinity for the cyclooxygenase enzyme is important in different NSAIDs seems to be largely unanswered. The results, conclusions and clinical relevance of the aforementioned study, including the possible effects of different types of NSAIDs, are discussed. While acknowledging that this study represents the best attempt so far in establishing the causal relationship between perioperative NSAID use and anastomotic leakage, the need for further research in this important area is underlined.
Keywords: Cancer, Colon, Non-steroidal anti-inflammatory drugs, Leakage, Rectum, Anastomotic leak
COMMENTARY ON HOT ARTICLES
We read with interest the recent article by Gorissen et al[1] from the Netherlands reporting data from two teaching hospitals concerning use of non-steroidal anti-inflammatory drugs (NSAIDs) and anastomotic leakage in colorectal surgery.
Anastomotic leakage is a feared complication that increases morbidity, mortality and healthcare costs[2,3]. Identified risk factors include malnutrition, smoking, comorbidity and, in rectal cancer surgery, male gender, lack of a protective stoma, and anastomotic level[4-7]. Whether or not the level of vessel ligation is a risk factor is still under debate[8].
NSAIDs are currently widely used in perioperative analgesia as a means of providing effective pain control while decreasing the need for opioid drugs and, subsequently, reducing the risk of nausea, constipation and ileus[9,10]. Since NSAIDs interfere with the normal inflammation present in wound healing, it is not illogical to infer a risk of impaired anastomotic healing. This possible negative factor must be thoroughly scrutinized, especially since any detrimental effects on anastomotic integrity may overshadow the positive effects of opioid-sparing NSAIDs.
NSAIDs are a heterogeneous group of drugs, traditionally subdivided by their effects on the enzymes of the cyclooxygenase (COX) class. The COX enzymes are responsible for the production of prostaglandin H2, the first step in the prostanoid biosynthesis. With the revelation that most of the adverse effects of NSAID used seemed to be linked to decreased COX-1 activity, and that the beneficial anti-inflammatory effects seemed to be associated with inhibition of COX-2, the development of selective COX-2 inhibitors was initiated. Several of the classical NSAIDs have been reappraised and turned out to be mostly COX-1 selective, which thus reflected their tendency to cause gastrointestinal side effects. However, the positive effects of selective COX-2 inhibition have subsequently been questioned, as any lesser rate of gastrointestinal adverse effects may have been outweighed by negative effects such as increased risk of cardiovascular events[11].
As regards to colorectal surgery, recent retrospective clinical studies have also indicated a negative effect on anastomotic healing[12-14], as well as some, but not all, experimental investigations[15-17]. In these studies, COX-2 inhibitors in particular have been implicated, as these have been compared unfavourably with mostly COX-1 selective compounds; more specifically, the use of celecoxib and diclofenac compared to ibuprofen seemed to be associated with high leakage rates[12-14]. This is of special note, as the classical NSAID diclofenac usually is classified as “weakly COX-2 selective” along with celecoxib, while ibuprofen is classified as “weakly COX-1 selective”[18]. Thus, some of the debate regarding potential effects on anastomotic healing has revolved around not only NSAID use in general, but also the different affinity for the two main COX enzymes.
In the present article, Gorissen et al[1] analyzed retrospectively 795 patients who underwent primary colorectal anastomosis during a three-year period from 2008 to 2010 at two Dutch teaching hospitals. Indications for surgery included both benign disease and colorectal malignancy, and elective as well as emergency surgery was performed. All patients were treated according to a fast-track surgery protocol including epidural anaesthesia, while the use of NSAIDs was not standardized, and was thus administered as per physician preference. The patients were divided into four groups according to NSAID use, where the terms “non-selective” and “selective” NSAIDs were used to differentiate older and newer classes of NSAIDs, roughly differentiated by the later being ostensibly more selective towards COX-2. The largest group was non-users (n = 471). Of the NSAID users, 201 patients used non-selective NSAIDs, 79 used selective COX-2 inhibitors and 44 used both non-selective and selective drugs. It should be noted that the authors defined diclofenac as a non-selective NSAID while meloxicam and celecoxib were categorized as selective NSAIDs.
The overall leakage rate was 9.9% (10.0% for right colonic, 8.7 for left colonic and 12.4 for rectal anastomoses). Among the patients who were not administered NSAIDs, the leakage rate was 7.6%, while users of any NSAID sustained a leakage rate of 13.2%; the multivariable odds ratio (OR) with 95%CIs was 1.84, 1.13 to 2.98. The leakage rates for non-selective NSAID, selective NSAID and combination users amounted to 14.5, 9.0, and 16%, respectively, with corresponding ORs (and 95%CIs) of 2.13 (1.24 to 3.65), 1.16 (0.49 to 2.75) and 1.86 (0.75 to 4.61), respectively (Figure 1). Interestingly, an analysis of the duration of NSAID use yielded significant differences between use of any NSAID for three days or more compared with use for only one or two days, with longer use being associated with a higher rate of anastomotic leakage (16.6% vs 10.0%).
Figure 1.
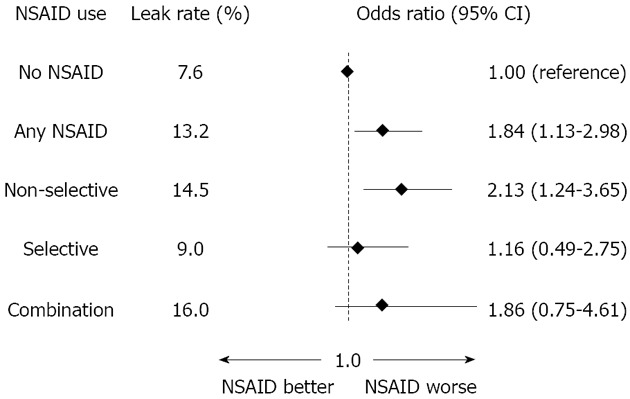
Multivariable logistic regression with non-steroidal anti-inflammatory drugs as exposure and anastomotic leakage as outcome in 795 patients operated on with a primary colorectal anastomosis[1]. NSAIDs:Non-steroidal anti-inflammatory drugs.
In the current study, the use of NSAIDs as compared to non-use seemed to be associated with an increased risk of leakage from colorectal anastomoses. This in our opinion is, to date, the best designed and most adequately powered study to establish this relationship. However, the question of whether COX-1 and/or COX-2 inhibitors are responsible for this increase in risk is still debatable. The stratification into “non-selective”, “selective” and “combination” users dilutes the statistical power, which may in itself make any conclusions hard to justify; moreover, the decision to classify diclofenac as a non-selective NSAID may have confounded these results, as diclofenac is comparable to both celecoxib and meloxicam regarding COX-2 affinity[11]. Taking this into consideration, the current study may not quite disprove that truly selective NSAIDs are a risk factor for leakage. The aforementioned retrospective studies, in which the use of diclofenac and celecoxib has been compared to ibuprofen, are therefore difficult to relate to the current study, as ibuprofen did not seem to be used.
Furthermore, the patient population in this study is quite heterogeneous, as patients undergoing both elective and emergency surgery and with malign and benign disease were included. Nevertheless, the authors have adjusted for known confounders including e.g. resection type, but residual confounding is hard to rule out due to the observational nature of the study. This study design is also subject to confounding by indication, duly acknowledged by the authors, as NSAIDs might have been administered because of increasing pain due to leakage or factors leading to leakage, e.g. anastomotic tension. Surgical technique might also be of concern, as there is a surprisingly high rate of leaks from the colonic anastomoses, amounting to about 10%. This may explain the postoperative mortality of 4.2% for all patients, which does not compare well to even population-based figures[19].
In conclusion, the authors have produced the best attempt so far in establishing the causal relationship between postoperative NSAID exposure and colorectal anastomotic leakage. However, this result must be interpreted with caution and might not yet justify the discontinuance of established practices of administering NSAID in this setting. Although this study corroborates the general perception that NSAID use may increase the risk of anastomotic leakage, there is still considerable uncertainty regarding the effects of inhibiting the COX subtypes. Further research is certainly warranted and could include large register studies as well as prospective studies with meticulous collection of data concerning NSAID exposure, including timing of administration postoperatively, type of substance, duration and dosage. Ideally, a multi-centre randomized controlled trial would be suitable in order to ultimately answer the question of whether any NSAID use causes anastomotic leakage; however, one might question the ethics of performing such a study, considering the mounting observational evidence against NSAID use.
Footnotes
Peer reviewers: Leif A Israelsson, MD, PhD, Associate Professor, Department of Surgery, Sundsvall County Hospital, 85186 Sundsvall, Sweden; Zohar Levi, MD, Department of Gastroenterology, Rabin Medical Center, Beilinson Campus, Petah Tiqwa 49100, Israel; Dr. Alexander Lyall, Aberdeen Royal Infirmary, Department of Surgery, Ashgrove House, Foresterhill Road, Aberdeen AB25 2ZN, Scotland, United Kingdom
S- Editor Jiang L L- Editor A E- Editor Xiong L
References
- 1.Gorissen KJ, Benning D, Berghmans T, Snoeijs MG, Sosef MN, Hulsewe KW, Luyer MD. Risk of anastomotic leakage with non-steroidal anti-inflammatory drugs in colorectal surgery. Br J Surg. 2012;99:721–727. doi: 10.1002/bjs.8691. [DOI] [PubMed] [Google Scholar]
- 2.Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg. 2011;253:890–899. doi: 10.1097/SLA.0b013e3182128929. [DOI] [PubMed] [Google Scholar]
- 3.Frye J, Bokey EL, Chapuis PH, Sinclair G, Dent OF. Anastomotic leakage after resection of colorectal cancer generates prodigious use of hospital resources. Colorectal Dis. 2009;11:917–920. doi: 10.1111/j.1463-1318.2008.01728.x. [DOI] [PubMed] [Google Scholar]
- 4.Kingham TP, Pachter HL. Colonic anastomotic leak: risk factors, diagnosis, and treatment. J Am Coll Surg. 2009;208:269–278. doi: 10.1016/j.jamcollsurg.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 5.Law WL, Chu KW. Anterior resection for rectal cancer with mesorectal excision: a prospective evaluation of 622 patients. Ann Surg. 2004;240:260–268. doi: 10.1097/01.sla.0000133185.23514.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matthiessen P, Hallböök O, Andersson M, Rutegård J, Sjödahl R. Risk factors for anastomotic leakage after anterior resection of the rectum. Colorectal Dis. 2004;6:462–469. doi: 10.1111/j.1463-1318.2004.00657.x. [DOI] [PubMed] [Google Scholar]
- 7.Richards CH, Campbell V, Ho C, Hayes J, Elliott T, Thompson-Fawcett M. Smoking is a major risk factor for anastomotic leak in patients undergoing low anterior resection. Colorectal Dis. 2012;14:628–633. doi: 10.1111/j.1463-1318.2011.02718.x. [DOI] [PubMed] [Google Scholar]
- 8.Rutegård M, Hemmingsson O, Matthiessen P, Rutegård J. High tie in anterior resection for rectal cancer confers no increased risk of anastomotic leakage. Br J Surg. 2012;99:127–132. doi: 10.1002/bjs.7712. [DOI] [PubMed] [Google Scholar]
- 9.Marret E, Kurdi O, Zufferey P, Bonnet F. Effects of nonsteroidal antiinflammatory drugs on patient-controlled analgesia morphine side effects: meta-analysis of randomized controlled trials. Anesthesiology. 2005;102:1249–1260. doi: 10.1097/00000542-200506000-00027. [DOI] [PubMed] [Google Scholar]
- 10.Kehlet H. Postoperative opioid sparing to hasten recovery: what are the issues? Anesthesiology. 2005;102:1083–1085. doi: 10.1097/00000542-200506000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Warner TD, Mitchell JA. Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic. FASEB J. 2004;18:790–804. doi: 10.1096/fj.03-0645rev. [DOI] [PubMed] [Google Scholar]
- 12.Holte K, Andersen J, Jakobsen DH, Kehlet H. Cyclo-oxygenase 2 inhibitors and the risk of anastomotic leakage after fast-track colonic surgery. Br J Surg. 2009;96:650–654. doi: 10.1002/bjs.6598. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg J, Harvald T. Severe complications with diclofenac after colonic resection. Dis Colon Rectum. 2007;50:685. doi: 10.1007/s10350-006-0860-3. [DOI] [PubMed] [Google Scholar]
- 14.Klein M, Andersen LP, Harvald T, Rosenberg J, Gogenur I. Increased risk of anastomotic leakage with diclofenac treatment after laparoscopic colorectal surgery. Dig Surg. 2009;26:27–30. doi: 10.1159/000193329. [DOI] [PubMed] [Google Scholar]
- 15.Klein M, Krarup PM, Burcharth J, Ågren MS, Gögenur I, Jorgensen LN, Rosenberg J. Effect of diclofenac on cyclooxygenase-2 levels and early breaking strength of experimental colonic anastomoses and skin incisions. Eur Surg Res. 2011;46:26–31. doi: 10.1159/000321706. [DOI] [PubMed] [Google Scholar]
- 16.Cahill RA, Sheehan KM, Scanlon RW, Murray FE, Kay EW, Redmond HP. Effects of a selective cyclo-oxygenase 2 inhibitor on colonic anastomotic and skin wound integrity. Br J Surg. 2004;91:1613–1618. doi: 10.1002/bjs.4722. [DOI] [PubMed] [Google Scholar]
- 17.de Hingh IH, van Goor H, de Man BM, Lomme RM, Bleichrodt RP, Hendriks T. Selective cyclo-oxygenase 2 inhibition affects ileal but not colonic anastomotic healing in the early postoperative period. Br J Surg. 2006;93:489–497. doi: 10.1002/bjs.5288. [DOI] [PubMed] [Google Scholar]
- 18.Rang H, Dale M, Ritter J. Rang and Dale’s Pharmacology, 6th edition. London, England: Churchill Livingstone; 2007. [Google Scholar]
- 19.Borowski DW, Bradburn DM, Mills SJ, Bharathan B, Wilson RG, Ratcliffe AA, Kelly SB. Volume-outcome analysis of colorectal cancer-related outcomes. Br J Surg. 2010;97:1416–1430. doi: 10.1002/bjs.7111. [DOI] [PubMed] [Google Scholar]


