Abstract
Objective
To evaluate the changes in Tp-e interval (an interval from the peak to the end of the T wave), QT interval and Tp-e/QT ratio of the body surface ECG in patients with left ventricular hypertrophy (LVH).
Methods
The Tp-e interval and QT interval were measured on body surface ECGs in 42 patients without either hypertension or LVH (control group), 41 patients having hypertension but not LVH (non-LVH group), and 38 patients with both hypertension and LVH (LVH group).
Results
The mean corrected QT (QTc) interval, and mean corrected Tp-e[T(p-e)c] interval were significantly longer in the LVH group (0.430±0.021s vs. 0.409±0.019s, p < 0.01; 0.098±0.013s vs. 0.088±0.011s, respectively) than those in the control group. The Tp-e/QT ratio was also amplified in LVH group (0.232±0.028 vs.0.218±0.027) (p < 0.05).
Conclusion
LVH increased the QT interval, Tp-e interval and Tp-e/QT ratio of the body surface ECG.
Keywords: hypertension, left ventricular hypertrophy, QT interval, Tp-e interval, arrhythmia
INTRODUTION
Left ventricular hypertrophy (LVH) is a well-recognized risk factor for cardiovascular disease, and is associated with malignant ventricular arrhythmias, leading to sudden cardiac death (SCD)[1],[2]. Several animal studies have demonstrated that LVH can lead to alterations of ion channel density and expression, prolonged action potential duration and transmural dispersion of repolarization (TDR)[3],[4].
Amplification of TDR has long been known as a substrate for ventricular arrhythmias[5]. The Tp-e interval correlates well with TDR, whereas the QT interval is closely approximated by the midmyocardial cell (M cell) action potential duration[6]. Thus the purpose of this study is to investigate the changes in the QT and Tp-e intervals and Tp-e/QT ratio in the left chest leads of the body surface ECG in patients with LVH.
MATERIALS AND METHODS:
Clinical materials
One hundred and twenty-one inpatients from March 2008 to September 2008 in the First Affiliated Hospital of Medical College of Xi'an Jiaotong University were enrolled into the study. All patients underwent echocardiography to determine LVH. Hypertension was diagnosed according to the JNC7 criteria[7], and LVH was defined by any of the following three ECG criteria[8]: ① LVMI > 125 g/m2 (male), > 110 g/m2 (female); ② LVPWT ≥ 12 mm; ③ IVST ≥ 12 mm, where LVMI, LVPWT and IVST denote left ventricular mass index[9], left ventricular posterior wall thickness, and interventricular septal thickness, respectively. According to the criteria of hypertension and LVH, patients were divided into 3 groups: ① control group (n = 42), patients without LVH and with no history of hypertension; ② non-LVH group (n = 41), patients without LVH but with a history of hypertension, ③ LVH group (n = 38), patients with LVH and a history of hypertension (Table 1).
Table 1. Clinical characteristics of patients in the three groups.
| Control group | non-LVH group | LVH group | |
| Number of patients | 42 | 41 | 38 |
| Age (years) | 57.70 ± 11.40 | 56.90 ± 12.70 | 58.30 ± 14.20 |
| Number of male | 23 | 23 | 20 |
| Serum potassium (mEq/L) | 4.24 ± 0.22 | 4.20 ± 0.31 | 4.22 ± 0.38 |
| Heart rate (time/min) | 68.20 ± 10.40 | 66.40 ± 14.80 | 65.90 ± 12.30 |
There were no significant differences between groups.
None of the study subjects were treated with agents that could affect the QT interval. Patients with secondary hypertension, electrolyte disorders, atrial fibrillation, bundle branch block, atrioventricular block, Wolff-Parkinson-White syndrome, myocardial infarction, or who had pacemakers were excluded from the study.
Electrocardiographic measurements
A standard 12-lead ECG was recorded at 25 mm/s, 1 mV/cm calibration. All subjects were in sinus rhythm. Electrocardiographic intervals were measured manually by an experienced physician using the lead V4-V6. The QT interval was defined as the time from the onset of the QRS complex to the end of the T wave at which the isoelectric line intersected a tangential line drawn at the maximal down slope of a positive T wave. The QTpeak interval was measured as the time from the onset of QRS complex to the point at the peak of a positive T wave. The difference between the QT and QTpeak intervals was taken as the Tp-e interval. Three consecutive QT and QTpeak intervals were measured and averaged. All the intervals were corrected for heart rate by using Bazett's formula[10]. The low-amplitude T wave (amplitude < 0.1 mV), inverted T wave, biphasic and bifurcated T wave (interval between two peaks > 0.15 s) were excluded.
Statistical analysis
Data are presented as mean±SD. Data analyses were performed by using SPSS11.5 software. One-way analysis of variance was used to determine statistical significance of the differences in QT, QTpeak, Tp-e interval and Tp-e/QT ratio among the three groups. A value of P < 0.05 was considered statistically significant.
RESULTS
Patient characteristics
There were no significant differences in age, gender, serum potassium concentration or heart rate among the three groups (Table 1).
Comparison of the corrected QT peak, QT and T(p-e)c interval among the three groups
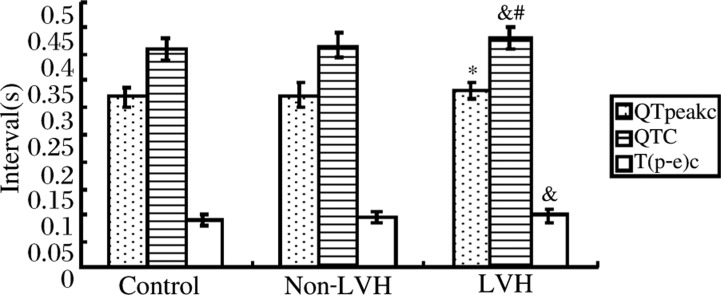
The mean corrected QTpeak (QTpeakc) interval, and QT(QTc) interval were significantly longer in the LVH group than these in the control group, especially in the case of the QTc interval. Compared with the control group, the non-LVH group exhibited no QTpeakc interval or QTc interval prolongation. When comparing the two groups with hypertension, there was a significant increase in the QTc interval in LVH group (Table 2,Fig. 1).
Table 2. The QTpeakc, QTc and T(p-e)c intervals of the three patient groups.
| Control group | Non-LVH group | LVH group | |
| QTpeakc interval(s) | 0.321 ± 0.019 | 0.324 ± 0.023 | 0.332 ± 0.015* |
| QTc interval(s) | 0.409 ± 0.019 | 0.417 ± 0.023 | 0.430 ± 0.021&# |
| T(p-e)c interval(s) | 0.088 ± 0.011 | 0.093 ± 0.010 | 0.098 ± 0.013& |
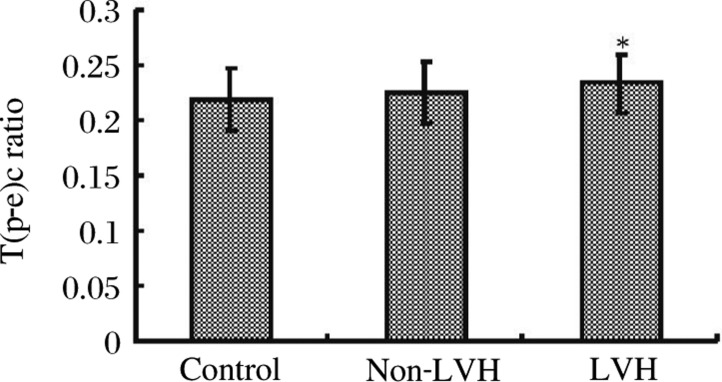
| T(p-e)c/QTc ratio | 0.218 ± 0.027 | 0.225 ± 0.028 | 0.232 ± 0.028* |
Compared with control group, *P < 0.05, &P < 0.01; comparison of non-LVH group with LVH group, #P < 0.05.
Fig. 1. The QTpeakc, QTc and T(p-e)c intervals compared among the three groups. Compared with control group, *P < 0.05, &P < 0.01; comparison of non-LVH group with LVH group, #P < 0.05.
The mean corrected Tp-e [T(p-e)c] interval was significantly increased in the LVH group when compared to the control group, because of preferential prolongation of the QT (QTc) interval. There was no statistically significant difference in T(p-e)c interval between the control group and non-LVH group, or between the non-LVH group and LVH group (p = 0.052) (Table 2, Fig. 1).
Comparison of the T(p-e)c/QTc ratio among the three groups
The mean T(p-e)c/QTc ratios in the control group, non-LVH group and LVH group were 0.218±0.027, 0.225±0.028, and 0.232±0.028, respectively. This upward trend in the T(p-e)c/QTc ratio was only statistically significant when the LVH group value was compared with the control group (p < 0.05). There was no significant difference between the non-LVH and LVH groups (Table 2, Fig. 2).
Fig. 2. The T(p-e)c/QTc ratio compared among the three groups. Compared with control group, *P < 0.05.
DISCUSSION
The chief findings of the present study are that ① the LVH group had longer QTpeak, QT and Tp-e intervals than the control group, which reflect the repolarization in the left ventricles, and ② the Tp-e/QT ratio is amplified in the LVH group.
A few studies have demonstrated that LVH resulted in a marked increase in ventricular repolarization time[11],[12]. Because the end of repolarization of the M cells coincides with the end of the T wave, and the end of repolarization of the epicardial cells coincides with the peak of the T wave, it is not surprising that the QTpeak and QT intervals in the LVH group are longer than those in the control group. Although the underlying mechanism is unknown, it is probably associated with the increased wall thickness. This is further supported by pulmonary hypertensive patients who have longer QTpeak and QT intervals in the right chest leads of the body surface ECG[13].
Another important finding of this study is that Tp-e and Tp-e/QT ratio is amplified in the LVH group. This is likely because of the disproportional prolongation of the Tp-e interval relative to QT interval in LVH patients. This indicates that the Tp-e interval, like the QT interval, is also positively correlated to ventricular wall thickness.
The Tp-e interval has been accepted as an easy, assessable measure of TDR, which is related to arrhythmogenesis. This suggests that in LVH there is a high transmural heterogeneity of repolarization, and hence has high arrhythmic risk.
We provide the first evidence that LVH leads to a notable increase in the Tp-e/QT ratio. Several studies have demonstrated that the ratio may serve as a good clinical predictor of SCD in long QT (LQT) syndrome and other ion channel diseases [14],[15]. As LVH is similar to acquired LQT syndrome in some aspects[16], it is easy to understand the high incidence of SCD in LVH patients.
The Tp-e and QT intervals vary greatly with heart rate and across species. However, the Tp-e/QT ratio remains relatively constant in a narrow range of values[14]. Drugs blocking IKr or enhancing late INa or ICa-L cause an increase in the Tp-e/QT ratio, and vice versa[17]. By using the arterially perfused rabbit ventricular wedge preparation, Liu et al[18] found that azithromycin significantly prolonged both the QT and Tp-e intervals without changing the Tp-e/QT ratio, and at higher doses carried a much smaller torsade de pointes (TdP) risk than those agents amplifying the Tp-e/QT ratio. More interestingly, Yamaguchi et al[19]demonstrated that a Tp-e/QT ratio exceeding 0.28 was tightly correlated with the risk of developing TdP in patients with acquired LQT syndrome.
Some researchers also observed the effect of LVH on QT and T(p-e) intervals in left chest leads[20]. However, they did not investigate the change in Tp-e/QT ratio or measure the heart rate and serum potassium among the patients. Because age, gender and electrolyte disturbance[21],[22] have obvious effects on the repolarization time, they were matched in our three groups.
Reference
- 1.de Simone G, Gottdiener JS, Chinali M, Maurer MS. Left ventricular mass predicts heart failure not related to previous myocardial infarction: the Cardiovascular Health Study. Eur Heart J. 2008;29:741–7. doi: 10.1093/eurheartj/ehm605. [DOI] [PubMed] [Google Scholar]
- 2.Gerdts E, Okin PM, de Simone G, Cramariuc D, Wachtell K, Boman K, et al. Gender differences in left ventricular structure and function during antihypertensive treatment. The Losartan Intervention for Endpoint Reduction in Hypertension Study. Hypertension. 2008;51:1109–14. doi: 10.1161/HYPERTENSIONAHA.107.107474. [DOI] [PubMed] [Google Scholar]
- 3.Luo X, Lin H, Pan Z, Xiao J, Zhang Y, Lu Y, et al. Down-regulation of miR-1/miR-133 contributes to re-expression of pacemaker channel genes HCN2 and HCN4 in hypertrophic heart. J Biol Chem. 2008;283:20045–52. doi: 10.1074/jbc.M801035200. [DOI] [PubMed] [Google Scholar]
- 4.Marionneau C, Brunet S, Flagg TP, Pilgram TK, Demolombe S, Nerbonne JM. Distinct cellular and molecular mechanisms underlie functional remodeling of repolarizing K+ currents with left ventricular hypertrophy. Circ Res. 2008;102:1406–15. doi: 10.1161/CIRCRESAHA.107.170050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang JF, Shan QJ, Yang B, Chen ML, Zou JG, Xu DJ, et al. Tpeak-Tend interval as a new risk factor for arrhythmic event in patient with Brugada syndrome. JNMU. 2007;21:213–7. [PubMed] [Google Scholar]
- 6.Yan GX, Antzelevitch C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long-QT syndrome. Circulation. 1998;98:1928–36. doi: 10.1161/01.cir.98.18.1928. [DOI] [PubMed] [Google Scholar]
- 7.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 8.Sun NL. Hypertension and heart failure. Chin J Cardiol(in chinese) 2004;32:382–4. [Google Scholar]
- 9.Edwards NC, Steeds RP, Stewart PM, Ferro CJ, Townend JN. Effect of spironolactone on left ventricular mass and aortic stiffness in early-stage chronic kidney disease: a randomized controlled trial. J Am Coll Cardiol. 2009;54:505–12. doi: 10.1016/j.jacc.2009.03.066. [DOI] [PubMed] [Google Scholar]
- 10.Bazett HC. An analysis of the time-relations of electrocardiograms. Ann Noninvasive Electrocardiol. 2006;2:177–94. [Google Scholar]
- 11.Guo D, Young L, Patel C, Jiao Z, Wu Y, Liu T, et al. Calcium-activated chloride current contributes to action potential alternations in left ventricular hypertrophy rabbit. Am J Physiol Heart Circ Physiol. 2008;295:H97–104. doi: 10.1152/ajpheart.01032.2007. [DOI] [PubMed] [Google Scholar]
- 12.Salles GF, Cardoso CR, Leocadio SM, Muxfeldt ES. Recent ventricular repolarization markers in resistant hypertension: are they different from the traditional QT interval? Am J Hypertens. 2008;21:47–53. doi: 10.1038/ajh.2007.4. [DOI] [PubMed] [Google Scholar]
- 13.Hlaing T, Guo D, Zhao X, DiMino T, Greenspon L, Kowey PR, et al. The QT and Tp-e intervals in left and right chest leads: comparison between patients with systemic and pulmonary hypertension. J Electrocardiol. 2005;38(Suppl 4):S154–8. doi: 10.1016/j.jelectrocard.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 14.Gupta P, Patel C, Patel H, Narayanaswamy S, Malhotra B, Green JT, et al. Tp-e/QT ratio as an index of arrhythmogenesis. J Electrocardiol. 2008;41:567–74. doi: 10.1016/j.jelectrocard.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 15.Billman GE, Kukielka M. Novel transient outward and ultra-rapid delayed rectifier current antagonist, AVE0118, protects against ventricular fibrillation induced by myocardial ischemia. J Cardiovasc Pharmacol. 2008;514:352–8. doi: 10.1097/FJC.0b013e31816586bd. [DOI] [PubMed] [Google Scholar]
- 16.Saenen JB, Vrints CJ. Molecular aspects of the congenital and acquired Long QT Syndrome: clinical implications. J Mol Cell Cardiol. 2008;44:633–46. doi: 10.1016/j.yjmcc.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Wang D, Patel C, Cui C, Yan GX. Preclinical assessment of drug-induced proarrhythmias: role of the arterially perfused rabbit left ventricular wedge preparation. Pharmacol Ther. 2008;119:141–51. doi: 10.1016/j.pharmthera.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Liu T, Brown BS, Wu Y, Antzelevitch C, Kowey PR, Yan GX. Blinded validation of the isolated arterially perfused rabbit ventricular wedge in preclinical assessment of drug-induced proarrhythmias. Heart Rhythm. 2006;3:948–56. doi: 10.1016/j.hrthm.2006.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaguchi M, Shimizu M, Ino H, Terai H, Uchiyama K, Oe K, et al. T wave peak-to-end interval and QT dispersion in acquired long QT syndrome: a new index for arrhythmogenicity. Clin Sci (Lond) 2003;105:671–6. doi: 10.1042/CS20030010. [DOI] [PubMed] [Google Scholar]
- 20.Zhao X, Qu L, Peng Z, Li L, Cui C. The difference between QT and T(p-e) intervals in left and right chest leads in patients with hypertension and chronic obstructive pulmonary diseases. Chin J Cardiac Pacing Electrophysiol(in chinese) 2009;23:116–8. [Google Scholar]
- 21.Letsas KP, Efremidis M, Kounas SP, Pappas LK, Gavrielatos G, Alexanian IP, et al. Clinical characteristics of patients with drug-induced QT interval prolongation and torsade de pointes: identification of risk factors. Clin Res Cardiol. 2009;98:208–12. doi: 10.1007/s00392-008-0741-y. [DOI] [PubMed] [Google Scholar]
- 22.Sabir IN, Killeen MJ, Goddard CA, Thomas G, Gray S, Grace AA, et al. Transient alterations in transmural repolarization gradients and arrhythmogenicity in hypokalaemic Langendorff-perfused murine hearts. J Physiol. 2007;581:277–89. doi: 10.1113/jphysiol.2007.128637. [DOI] [PMC free article] [PubMed] [Google Scholar]