Abstract
Ultrasonography (US) and the new applications US elastography (USE) and contrast-enhanced US (CEUS) are used in the screening of thyroid nodules, for which fine-needle aspiration biopsy (FNAB) is the best single diagnostic test. The aim of the study was to compare the sensitivity, specificity, positive predictive value (PPV), and accuracy of the four examinations in nodules with cytological and histological diagnoses. The study used data from US, FNAB, USE (elasticity (ELX 2/1) index), and CEUS (Peak index and time to peak (TTP) index) evaluated in 73 thyroid nodules in 63 consecutive patients likely to undergo surgery. Cytological-histological correlation was available for 38 nodules. No correlation emerged between nodule size and cytological results. A significant (P=0.03) positive correlation between cumulative US findings and cytological results was found. In addition, significant correlations between cumulative US findings and cytology (P=0.02) and between cumulative US findings and histology (P<0.0001) were found. US showed the best specificity and PPV, and FNAB the best sensitivity. There was no significant difference in the ELX 2/1 index, Peak index, or TTP index among nodules subdivided according to cytological scores. No significant correlation was found between ELX 2/1 index, Peak index, and TTP index, on the one hand, and nodule size, US cumulative findings, cytology, and histology on the other hand. The sensitivity of the ELX 2/1 index was high, but its specificity was very low. The accuracy and PPV of USE were lower than those of the other procedures. Only the correlation between Peak index and cumulative US findings reached a value close to significance. Our ultimate aim is to minimise unnecessary thyroidectomy. US and FNAB continue to play a central diagnostic role. The use of a US score showed high specificity and PPV. The specificity of FNAB was low in this selected series because of the numbers of indeterminate cytological responses. USE and CEUS are innovative techniques that need to be standardized. The ELX 2/1 index, Peak index, and TTP index seem to be unrelated to histology. The best statistical data on USE and CEUS concerned their sensitivity and PPV, respectively. At present, USE and CEUS are too time-consuming and of limited utility in selecting patients for surgery.
Keywords: Thyroid nodules, Cytology, Histology, Ultrasonography, Elastosonography, Contrast-enhanced ultrasonography
1. Introduction
Thyroid nodules are a common clinical problem, with prevalence varying according to the method of examination used. Ultrasonography (US) is the most sensitive diagnostic tool for detecting the presence of thyroid nodule(s) (Henrichsen and Reading, 2011). Thyroid nodules are more common in elderly patients, females, and patients with an iodine deficiency. When a nodule is found, the most important clinical problem is to exclude malignancy, which accounts for approximately 5% of all thyroid nodules, irrespective of their size (Gharib et al., 2006). In differentiating malignant from benign thyroid nodules, cytological investigation by means of fine-needle aspiration biopsy (FNAB) is the best single test (Castro and Gharib, 2003; Nam-Goong et al., 2004; Sidoti et al., 2006). The ultimate aim of FNAB is to reassure the patient and to avoid recourse to surgery when this is not otherwise indicated (Sidoti et al., 2006). Literature data indicate that FNAB yields useful cytological results in about 80% of cases (Burch, 1995; Sidoti et al., 2006; Gharib and Papini, 2007). Nevertheless, non-diagnostic examinations in US-guided FNAB range from 3% to 16% (Danese et al., 1998; Baloch et al., 2003; Redman et al., 2006; Sidoti et al., 2006; Gharib and Papini, 2007) and indeterminate (follicular) lesions range from 6% to 20% (Baloch et al., 2003; Foppiani et al., 2003; Sidoti et al., 2006; Gharib and Papini, 2007; Rago et al., 2007). When non-diagnostic FNABs are referred for surgery, the likelihood of malignancy is reported to vary widely, from 5% to 37%, probably depending on patient selection (Chow et al., 2001; Yang et al., 2007; Lewis et al., 2009; Garcia-Pascual et al., 2011). Moreover, in about one quarter of FNAB-indeterminate lesions, thyroid cancer has been found after surgery (Mihai et al., 2009).
Molecular biology techniques, such as searching for genetic alterations (Nikiforova et al., 2003; Gomez Saez, 2010) or protein expression (Bartolazzi et al., 2008; Gomez Saez, 2010), have not yet led to a significant improvement in distinguishing cases of thyroid malignancy from those of inadequate or indeterminate cytology. On the other hand, the role of US in this effort is complex, being both operator- and machine-dependent and linked to an adequate standardized terminology. In a multicentre retrospective study, the US features of malignant nodules were: taller than wide in shape, irregular margin, marked hypo-echogenicity, microcalcification and macrocalcification; however, the sensitivity of each individual parameter was low, ranging from 40% to 48% (Kim et al., 2002). Spot microcalcifications are reported to be significantly associated to thyroid cancer, but their lower frequency in microcarcinomas reduces sensitivity. This suggests that this single finding is not a major predictor of malignancy in nodules smaller than 1 cm (Moon et al., 2011).
US elastography (USE) is a relatively new technique for measuring the elasticity of tissues. Carcinoma tissue is harder and firmer than the thyroid parenchyma or benign thyroid nodules. USE quantifies the firmness of the tissue and displays this as a colour map, on which a hard consistency appears in blue and a soft constancy in red, with yellow to green indicating intermediate values from soft to hard. The strain index (SI) has been suggested as a good predictive factor for malignancy (Lyshchik et al., 2005). Recently, some (Rago et al., 2010) but not all (Lippolis et al., 2011) authors have reported that USE may be an important tool in the diagnosis of thyroid malignancy in nodules in which FNAB is non-diagnostic or indeterminate between benign and malignant. USE has also been claimed to be useful in selecting patients who are candidates for surgery (Rago et al., 2010; Cakir et al., 2011). Finally, in the last 10 years, some contrast agents have been introduced in order to better characterize solitary thyroid nodules by focusing on time-intensity curves; contrast is observed significantly sooner in thyroid carcinomas than in benign hyperplastic nodules and adenomas (Spiezia et al., 2001). Moreover, regular and monophasic washout curves are more frequent in benign lesions, while they are mainly irregular and polyphasic in malignant lesions (Argalia et al., 2002). The new availability of a second generation of micro-bubble-based contrast agents enables continuous imaging at low acoustic power. Contrast-enhanced US (CEUS) is a new technique, and its potential in diagnosing thyroid carcinomas is not yet known (Bartolotta et al., 2006; Zhang et al., 2010).
Limitations to USE could be: the selection of nodules, difficulty in achieving standardized compression of the neck region, subjectivity in attributing an elasticity score and intra- and inter-observer variabilities. Here, we report our experience of USE, in which the elasticity index (ELX 2/1) was adopted. Provided directly by the software of the equipment, this index is, in our opinion, less operator-dependent than the colour score developed by Ueno and Ito (2004) for breast imaging which has been employed in previous papers (Rago et al., 2010; Cakir et al., 2011; Lippolis et al., 2011). In CEUS, too, the mathematical approach is crucial, owing to the complexity of data analysis (Molinari et al., 2010); we report our experience of using a contrast software showing both colour maps and time-intensity curves. The final aim was to compare the results of US-guided FNAB, USE, and CEUS with the final histological report. It has not yet been established whether USE and CEUS are interesting, but expensive and time-consuming, new techniques to be confined to research centres, or whether they have the potential for broad application in the endocrinological setting.
2. Materials and methods
2.1. Patients
From January 2010 to June 2011, this prospective study enrolled 63 consecutive patients who were deemed candidates for surgery on the basis of the size of their nodular goitre or cytological results. Thirty-seven patients had uninodular goitre, while in 26 the goitre was multinodular. Laboratory data were compatible with Hashimoto’s thyroiditis in three patients. Fifty-one patients were females and 12 were males; their age range was 20–81 years [(55.9±14.7) years; mean±standard deviation (SD)]. After US-guided FNAB performed on 73 nodules, all patients were invited to undergo further evaluation by means of USE and CEUS. Selection criteria were: age over 18 years, nodules suitable for USE (i.e., devoid of prevalent fluid content or coarse calcifications), and indication for thyroidectomy. The primary aim of the study was to compare the impacts of US-FNAB, USE and CEUS in the preoperative detection of thyroid carcinoma. All patients gave informed consent to participate in the study. The three patients with Hashimoto’s thyroiditis were on levothyroxine therapy, and another eight patients were undergoing thyroid stimulating hormone (TSH)-reductive therapy. Only one patient was on methimazole therapy for pre-toxic goitre. All patients underwent laboratory evaluations. TSH and free-T4 (f-T4) were measured by means of ultra-sensitive chemiluminescence immunoassay (Roche Diagnostics, Mannheim, Germany). Normal ranges are: 0.3–4.2 mIU/L for TSH, and 12.0–22.0 pmol/L for f-T4. Auto-antibodies against thyroperoxidase (TPOAb) were evaluated by means of the Dia Sorin assay (Saluggia, Italy); concentrations <100 mIU/L were regarded as negative. Serum calcitonin (CT) was assayed by chemiluminescence immunoassay (Dia Sorin); in our laboratory, the upper limit of the normal CT range is <10 ng/L; our institutional cut-off for CT evaluation in needle washing after FNAB is 30 ng/L (Massaro et al., 2009).
2.2. FNAB cytology and histology
The three dimensions of the nodules were measured by means of US, the largest diameter being recorded as the nodule size. FNAB was performed under US assistance in the solitary or dominant nodules, as already reported (Sidoti et al., 2006), and following the current guidelines (Castro and Gharib, 2003; Gharib and Papini, 2007). The adequacy of an aspirate was defined in accordance with previously reported criteria (Layfield et al., 2009). Cytomorphological results were subdivided into five diagnostic categories (Thy 1–5) according to the BTA criteria (British Thyroid Association, 2007). When thyroid surgery was performed, the histological diagnosis was made in accordance with the World Health Organization guidelines (Heidinger and Sobin, 1974). Except for USE and CEUS data, all available clinical data were given to the pathologist.
2.3. Thyroid US and USE
All thyroid glands were examined by means of high-resolution US with a colour-Doppler module (AU 5 Idea, Esaote Biomedica, Genoa) equipped with a 7.5-MHz linear probe; US-FNAB was performed with the aid of this same equipment. In accordance with US guidelines (Gharib and Papini, 2007; Moon et al., 2011), the following parameters were investigated: echogenicity vs. non-nodular tissue, presence or absence of halo sign, presence or absence of microcalcifications, and flow pattern of the nodule. All USE examinations were performed by the same operator (Dr. Gianni TURTULICI) by means of a MyLab 70 XvG US scanner (Esaote Biomedica) equipped with an LA-522 linear probe working in the range of 7–12 MHz and the software for the quantification of the USE features of the tissue. Static and moving images were recorded in accordance with Lyshchik et al. (2005) at least three times to obtain mean values. The elasticity score (ELX 2/1) index was calculated as the ratio between the elasticity features of the selected region-of-interest (ROI) located on US-normal thyroid tissue and the ROI of the nodule under investigation. An ELX 2/1 index less than 1 indicates a soft nodule, while an ELX 2/1 index higher than 2 indicates a hard nodule. ELX 2/1 indexes between 1 and 2 are regarded as indicating nodules of intermediate hardness. We considered the ELX 2/1 index directly reported on the screen of the equipment, as this is a less operator-dependent variable than the elasticity colour-scale extrapolated from breast tissue (Ueno and Ito, 2004) to the thyroid gland by some authors (Rago et al., 2010; Cakir et al., 2011; Lippolis et al., 2011).
2.4. Thyroid CEUS
Once written informed consent had been obtained, CEUS images were acquired by the MyLab 70 US scanner while the patient was in the fasting condition. CEUS was performed by using a non-destructive US mode after bolus injection of SonoVue (4.8 ml; Bracco, Milan). CEUS video-clips were digitally recorded and analysed by means of Q-Contrast software V.4.0. (Bracco). Time-intensity curves within selected ROI and colour maps were acquired. Nodule and healthy thyroid tissue values of peak contrast enhancement (Peak) and time to peak (TTP) were calculated. Peak and TTP are reported as indexes (Peak index, TTP index) derived from the ratio between the values from the ROI of the nodule and the ROI of normal thyroid tissue. In our preliminary experiences, colour maps and time-intensity curves showed a variable contrast peak and an inhomogeneous TTP pattern in nodules with malignant cytology, while in nodules with benign cytology, a contrast peak higher than that of the surrounding healthy thyroid and homogeneous TTP colour maps were obtained.
2.5. Statistical analysis
Owing to the small samples of subjects, non-parametric tests were used for statistical evaluation. The correlation coefficient r was calculated by means of Spearman correlation (Sr). Data are reported as mean±standard error of mean (SEM), if not otherwise reported. Significance was set at P≤0.05. A US score (from 0 to 5) was arbitrarily calculated for the nodule under evaluation, with one point being assigned for the presence of each of the following radiological findings: solid, hypo-echoic, microcalcification, internal vascularisation, and irregular shape. For the purpose of correlation, the histology was scored as follows: 1=benign, 2=minimally invasive with indeterminate potential of malignancy, and 3=malignant.
Sensitivity, specificity, positive predictive value (PPV), and accuracy were evaluated in 38 nodules with available histological data. After US, we arbitrarily considered nodules with scores 3–5 and malignant histology to be true positives (TP) and nodules with scores 1–2 and benign histology to be true negatives (TN). Nodules with scores 3–5 and benign histology were regarded as false positives (FP), while nodules with scores 1–2 and malignant histology were regarded as false negatives (FN). In accordance with Lewis et al. (2009), after FNAB, those nodules in which cytology was either indeterminate or malignant (Thy 3–5) and histology was malignant were considered TP, while those which had both benign cytology (Thy 2) and histology were considered TN. Thy 3–5 nodules with benign histology were classified as FP, while Thy 2 nodules with malignant histology were classified as FN. After USE, nodules with an ELX 2/1 index >1 (hard nodules and nodules of intermediate hardness) and malignant histology were considered to be TP, while those with an ELX 2/1 index ≤1 and benign histology were considered to be TN. Nodules with an ELX 2/1 index >1 and benign histology were classified as FP, and those with an ELX 2/1 index ≤1 and malignant histology FN. After CEUS, nodules with a Peak index ≤1 and/or TTP index ≥1 and malignant histology were deemed TP, while those with a Peak index >1 and/or TTP index <1 and benign histology were deemed TN. Nodules with a Peak index ≤1 and/or TTP index ≥1 and benign histology were classified as FP, and those with a Peak index >1 and/or TTP index <1 and malignant histology as FN. The following formulae were employed: sensitivity [TP/(TP+FN)], specificity [TN/(TN+FP)], PPV [TP/(TP+FP)], and accuracy [(TP+TN)/(TP+TN+FN+FP)].
3. Results
3.1. Clinical data and US
At the time of FNAB evaluation, the results of thyroid function tests, irrespective of any therapy under way, were in the normal range (f-T4 12.8–21.7 pmol/L; TSH 0.3–4.2 mIU/L) in all subjects. Only one subject displayed slightly elevated CT levels (12 ng/L), but suspicion of medullary-hormone hypersecretion was not confirmed by CT evaluation on the needle-washing after FNAB. The FNAB procedure was well tolerated, causing only occasional local pain.
Nodules ranged in size from 4 to 50 mm [(17.9±1.1) mm; median 15.5 mm]. There was no correlation between nodular sizes and the results of cytology according to BTA criteria. The percentages of US parameters observed in the nodules were: 75% hypoechogenic, 73% solid, 55% with intra-nodular vascularisation, 19% with spot micro-calcifications, and 18% with irregular margins. There was a significant (n=67, Sr 0.26; P=0.03) positive correlation between the combined set of these five US findings and the results of FNAB expressed according to BTA criteria.
Histology was available in 33 of the 44 patients in whom surgery was performed. Benign histology (6 follicular adenomas; 8 hyperplastic nodules; 2 Hurtle adenomas) was found in 16 nodules, while a final diagnosis of malignancy (19 papillary thyroid carcinomas, 2 follicular variant of papillary thyroid carcinomas) or potential malignancy (1 minimally invasive follicular tumour) was reported in 22 nodules. In 13 patients with non-diagnostic (n=1), benign (n=10), and indeterminate (n=2) cytology, a further follow-up period was decided upon. Six patients (n=4 Thy 2 and n=2 Thy 3) were lost to follow up. In the 33 patients for whom histology was available, a significant correlation was seen between cumulative US findings (n=38 nodules) and both cytology (n=38, Sr 0.37; P=0.02) and histology (n=38, Sr 0.65; P<0.0001). Sensitivity, specificity, accuracy, and PPV calculated for US scores and Thy scores from nodules with available histology are reported in Table 1. US showed the best specificity and PPV values, and FNAB the best sensitivity.
Table 1.
Sensitivity, specificity, accuracy, and positive predictive value (PPV) calculated according to US, FNAB, USE, and CEUS data from nodules with available histology
| Method | Sensitivity (%) | Specificity (%) | Accuracy (%) | PPV (%) |
| US | 75 | 94 | 84 | 94 |
| FNAB | 95 | 28 | 63 | 59 |
| USE | 94 | 11 | 55 | 56 |
| CEUS | 68 | 67 | 64 | 76 |
3.2. Histology, USE, and CEUS
There was 100% concordance between malignant histology and a Thy 5 result on cytology, and 100% concordance between benign histology and a Thy 2 result on cytology. Malignant histology was found in 85% of nodules with Thy 4 cytology, but in only 25% of nodules with Thy 3 cytology.
An ELX 2/1 index was available for 82% of nodules. There were no significant differences in ELX 2/1 indexes among nodules subdivided according to Thy scores 2–5 (Table 2). For illustrative purposes, USE images are shown in Figs. 1 and 2; the ELX 2/1 index calculated by the software are shown. The ELX 2/1 index was successfully recorded in 34 (89%) of the 38 nodules with available histology. In the other 4 nodules, the ELX 2/1 index was unobtainable owing to the prevalent retro-jugular right extension of an estimated 35-mm nodule, the contiguity with the trachea of a 10-mm isthmus nodule and the loss of data. The sensitivity of the ELX 2/1 index calculated from USE was high, but specificity was very low; accuracy and PPV were lower than those of the other procedures (Table 1). No significant correlation was found between ELX 2/1 index and nodule size, cumulative US findings (score), cytology (Thy) or histology.
Table 2.
Values of the ELX 2/1 index obtained from USE, and Peak index and TTP index observed after CEUS in thyroid nodules subdivided according to Thy score on FNAB
| Thy score | ELX 2/1 index |
Peak index |
TTP index |
||||||
| Mean±SEM | Range | n | Mean±SEM | Range | n | Mean±SEM | Range | n | |
| Thy 2 | 1.45±0.18 | 0.12–2.60 | 16 | 1.26±0.11 | 0.79–2.07 | 12 | 1.06±0.11 | 0.58–2.25 | 12 |
| Thy 3 | 1.40±0.10 | 0.60–3.30 | 27 | 0.92±0.09 | 0.33–1.67 | 17 | 0.93±0.03 | 0.70–1.20 | 17 |
| Thy 4 | 1.63±0.14 | 0.80–2.50 | 11 | 1.29±0.23 | 0.43–2.61 | 10 | 0.88±0.11 | 0.22–1.30 | 10 |
| Thy 5 | 1.65±0.16 | 1.20–2.30 | 6 | 0.90±0.22 | 0.28–1.78 | 6 | 1.60±0.42 | 0.46–3.00 | 6 |
n: number of nodules evaluated
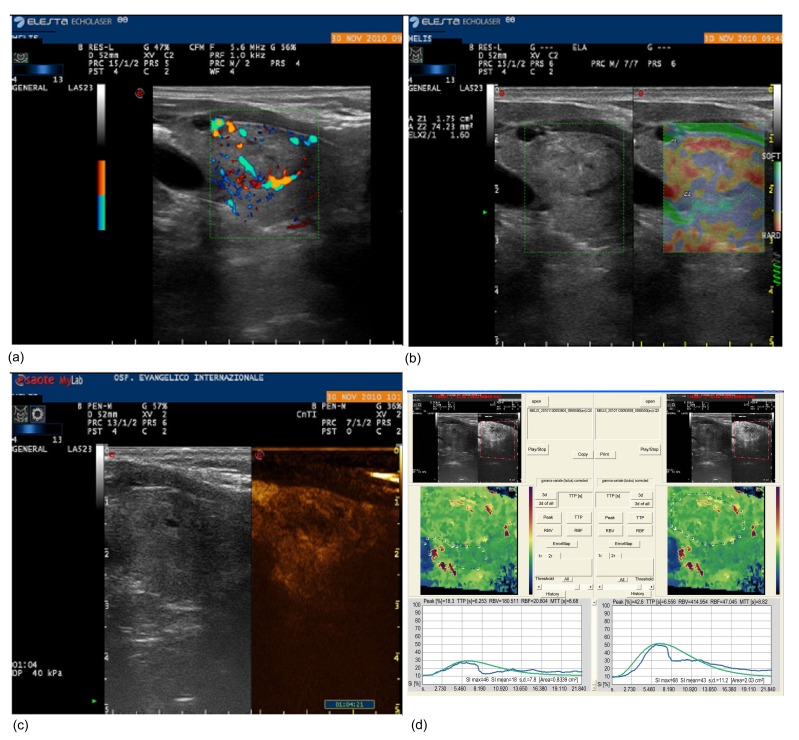
Fig. 1.
Colour-Doppler US (a), USE (b), and CEUS (c, d) evaluations of a 20-mm right nodule (US score 3) with suspicious cytology (Thy 4) and benign histology
In (b), the first ROI (Z1) is located on the nodule and the second (Z2) on the surrounding healthy thyroid. The ELX 2/1 index calculated by the software was 1.60. As shown in (d), the contrast peak colour maps and time-intensity curves are needed to calculate the Peak index (0.43) and TTP index (0.95)
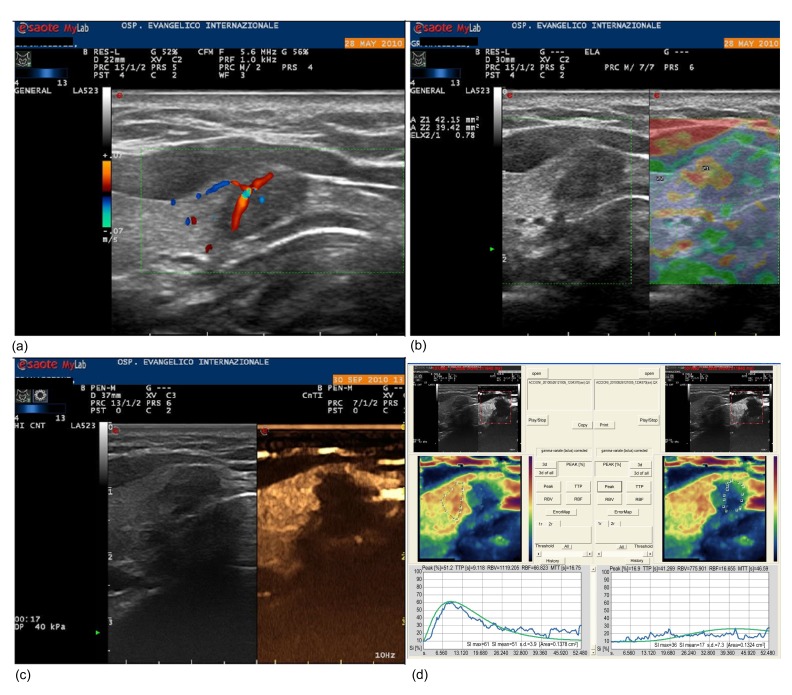
Fig. 2.
Colour-Doppler US (a), USE (b), and CEUS (c, d) evaluations of a 10-mm right paraisthmic nodule (US score 3) with suspicious cytology (Thy 4) and malignant histology
In (b), the first ROI (Z1) is located on the nodule and the second (Z2) on the surrounding healthy thyroid. The ELX 2/1 index calculated by the software is 0.78. As shown in (d), the contrast peak colour maps and time-intensity curves are needed to calculate the Peak index (2.61) and TTP index (0.22)
CEUS was performed on 45 nodules (62%); no adverse events or side effects were recorded during or immediately after the injection of the contrast agent. There was no significant difference in Peak index or TTP index among nodules subdivided according to Thy scores 2–5 (Table 2). Illustrative CEUS images are reported in Figs. 1 and 2; the intensity curves of the ROI obtained from the nodule under investigation and from surrounding normal thyroid tissue are shown. CEUS was performed on 28 of the 38 nodules for which histology was available. CEUS could not be performed in 10 nodules owing to refusal of the intravenous injection of contrast (n=4), the impossibility of studying bilateral nodules in the same scheduled CEUS (n=3), and the technical impossibility of recording adequate video-clips (n=3). No significant correlations were found between Peak index or TTP index after CEUS and nodule size, cumulative US findings (score), cytology (Thy), or histology. Only the correlation between Peak index after CEUS and cumulative US findings reached a value close to significance (P=0.09). PPV was the best statistical result for CEUS (Table 1).
4. Discussion
Among modern imaging modalities, high-resolution US is the most sensitive diagnostic modality for the detection of thyroid nodules, and it is necessary to perform US on all nodules found on palpation (Gharib et al., 2006; Gharib and Papini, 2007; Henrichsen and Reading, 2011; Moon et al., 2011). In addition, US can evaluate the size and characteristics of non-palpable nodules, guide FNAB, and reveal lymph-node metastases. In clinical practice, US is increasingly being used as an adjunct to physical thyroid examination. It is conceivable that the number of referrals to endocrinologists for newly (incidentally) discovered thyroid nodules will also dramatically increase, thus raising concerns as to how to manage this ‘epidemic’ (Gharib et al., 2006; Gharib and Papini, 2007). No single US pattern should be used alone as a definite criterion of malignancy, since considerable overlapping of US features has been found in benign and malignant lesions (Kim et al., 2002; Moon et al., 2011). On the other hand, some US characteristics, such as irregular or blurred margins, micro-calcifications, marked hypo-echogenicity, and taller-than-wide shape, are reported to be more predictive of thyroid malignancy (Horvath et al., 2009; Moon et al., 2011).
In our study, after investigating five US parameters (hypoechogenic, solid, intra-nodular vascularisation, micro-calcifications, irregular margins) in the nodules under evaluation, we observed a significant positive correlation between the combined set of these findings and both cytology and histology. In our selected population of nodules, the diagnostic power (both sensitivity and PPV) of standard, relatively inexpensive and rapid colour-Doppler US proved to be high. That analysing several US parameters reveals a higher incidence of malignancy was also reported by Horvath et al. (2009), who used the so-called thyroid imaging reporting and data system (TIRADS). In their study, malignancy was found in over 80% of nodules classified as probably malignant (TIRADS 5) on US, while in our nodules histology revealed malignancy in 100% of those in which four or five parameters proved positive. However, a limitation of both this and Horvath et al. (2009)’s studies is that not all US-evaluated nodules were subsequently operated on at once. A further limitation of US scores is the rate of proven malignancy in nodules showing low US risk. In our population, 14% of nodules with a 1–2 score proved malignant on histology, and in nodules classified by TIRADS as probably benign, up to 5% malignancy was found (Horvath et al., 2009).
When a thyroid nodule is found, FNAB remains the best and easiest means of distinguishing between malignant and benign lesions (Burch, 1995; Sidoti et al., 2006; Gharib and Papini, 2007). False-negative rates, between 0.2% and 5.0% (Gharib and Papini, 2007; Nayar and Ivanovic, 2009), are deemed acceptable. Nodular size is crucial to a reliable cytology result, and some authors (Kim et al., 2009), but not all (Nam-Goong et al., 2004), discourage the routine use of US-FNAB to diagnose thyroid nodules of less than 1 cm. In our study, no correlation between nodular size and cytology results was found. In our opinion, as already reported in the literature (Sidoti et al., 2006; Gharib and Papini, 2007; Kim et al., 2009), only specific reasons, such as US features of cancer, history of thyroid irradiation, a family history of thyroid cancer, or local symptoms, justify FNAB in thyroid nodules of less than 1 cm.
Indeterminate lesions are currently reported in FNAB series as placing another limitation on this procedure (Baloch et al., 2003; Foppiani et al., 2003; Sidoti et al., 2006; Gharib and Papini, 2007; Rago et al., 2007; Mihai et al., 2009; Rago et al., 2010). Cytological-histological correlation has demonstrated cancer in 1/4 patients with cytological features of follicular neoplasia (Mihai et al., 2009; Rago et al., 2010). The likelihood of malignancy has been reported to be higher in men and in large (>4 cm) nodules with Thy 3 cytology (Mihai et al., 2009); however, the present data and those of other authors (Kim et al., 2003) do not support this association.
USE has recently been introduced in some centres to overcome the limitations of FNAB. The efficacy of USE in distinguishing benign from malignant thyroid nodules varies widely, ranging from 34% to 100% in different studies (Lyshchik et al., 2005; Rubaltelli et al., 2009; Rago et al., 2010; Vorlander et al., 2010; Cakir et al., 2011; Lippolis et al., 2011). USE may be viewed as a form of palpation by means of US and, as such, the examination may be influenced by operator’s experience. More recently, USE machines have been able to measure tissue hardness both qualitatively (colour score) and quantitatively (defined value of hardness). Vorlander et al. (2010) measured strain values in 309 thyroid nodules and compared the results with histopathological diagnoses; the PPV proved to be 42%. In a recent study by Cakir et al. (2011), in which 391 nodules in 292 patients were evaluated, the SI, calculated on both transverse and longitudinal measurements, was significantly higher in malignant nodules than in benign ones. However, the elevated standard deviation could suggest a non-normal distribution of the SI, and the correlation between the SI value and the final histological diagnosis is not easy to discern in Cakir et al. (2011)’s study. Moreover, cumulative US features seemed to be more predictive of malignancy than the SI value (Cakir et al., 2011).
Our USE equipment and program report the ELX 2/1 index, which is the ratio between the hardness observed in ROI in normal tissue and ROI in the nodular tissue. The ELX 2/1 index in our limited number of nodules did not correlate significantly with the results of either cytology or histology. Moreover, in another study, in which a 2nd generation USE machine was used to examine a significant number of nodules, a high rate of false-negative diagnoses of malignancy was found (Vorlander et al., 2010). Cakir et al. (2011) observed that the ability of the colour score 5 (hard) to predict malignancy was lower than the ability of the score 1 (soft) to predict benignity. The reasons for this discrepancy with regard to Rago et al. (2010)’s data were attributed to the qualitative (operator-dependent) method of USE and study population selection (Thy 1–3). Although ELX 2/1 and SI seem to be less operator-dependent, they have so far added nothing to standard US in terms of the ability to predict malignancy. Nevertheless, it must be borne in mind that these are the first studies to calculate hardness by means of USE, and we cannot exclude the possibility that this non-invasive procedure might, in the future, assist conventional methods of distinguishing between benign and malignant thyroid nodules. At present, we disagree with the proposal by some authors (Vorlander et al., 2010) that USE should substitute FNAB.
The latest technique involving US equipment for the study of thyroid vascularisation uses micro-bubble contrast agents. It is well known that the thyroid gland has an abundant microvasculature, and that the parenchyma of normal thyroid shows rapid uniform enhancement after intravenous injection of contrast agents. Nodules differ from the normal vascular structure, and hence their enhancement differs from that of the normal parenchyma (Argalia et al., 2002; Bartolotta et al., 2006). Argalia et al. (2002) have demonstrated that CEUS time-intensity curves can provide an indirect description of intra-nodular vascularisation, which seems to be “anarchic” in malignant nodules.
Some studies carried out with the first-generation air-based contrast agent analyzed time-intensity curves in the characterization of solitary thyroid nodules, but did not provide conclusive data (Spiezia et al., 2001). SonoVue is a new, second-generation, stabilized micro-bubble preparation containing sulphur hexafluoride. SonoVue has low solubility, is isotonic, and does not contain potentially antigenic gas. An early report showed that SonoVue can enable some specific contrast-enhancement patterns to be identified in various focal liver lesions (Bartolotta et al., 2005). In a small series of patients, Bartolotta et al. (2006) reported a CEUS pattern specific for malignancy; malignant nodules displayed mainly absent or faintly dotted vascularisation, whereas benign lesions showed only diffuse vascularisation, whether homogeneous or inhomogeneous. Nevertheless, in their study, CEUS findings seemed to be more closely related to nodule size than to histology (Bartolotta et al., 2006). In a study by Zhang et al. (2010), four enhancement patterns were observed: homogeneous, heterogeneous, ring, and absent enhancements. The authors reported as the most meaningful findings of their study that ring enhancement can identify benign thyroid nodules, whereas heterogeneous enhancement can identify malignant thyroid nodules with high sensitivity and specificity. In this selected cohort of patients, 24% of mixed nodules and 66% of solid nodules were malignant. Nodules showing enhancement were more likely to be benign, whereas nodules showing heterogeneous enhancement were more suspicious and FNAB should therefore be recommended (Zhang et al., 2010). Spiezia et al. (2001) showed that carcinomas displayed a shorter time to the peak of the perfusion curve, but that peak intensity and washout were not statistically different from those seen in benign nodules. Conversely, Friedrich-Rust et al. (2010) recently reported that the time-intensity CEUS curves did not prove useful in distinguishing between benign and malignant nodules, and in our experience the Peak index and TTP index were not related to cytological and histological results. CEUS presents some limitations: the examination is expensive, only one nodule can be evaluated for each injection of the micro-bubble agent, there is no established standard for the patterns of enhancement, and calcification in a nodule may affect CEUS imaging. So far, it is difficult to compare the results of the different studies.
The final aim of the endocrinologist’s effort is to minimize unnecessary thyroidectomy in the current setting of epidemic thyroid nodule pathology. A properly performed colour-Doppler US examination and a satisfactory cytological result from FNAB continue to play a central diagnostic role. USE and CEUS are innovative techniques, which need to be standardized. At present, they seem to be too time-consuming and of limited utility in selecting patients for surgery. Further studies based on totally blinded evaluation are needed in order to provide more conclusive evidence of the role of USE and CEUS in the management of thyroid nodules.
References
- 1.Argalia G, de Bernardis S, Mariani D, Abbattista T, Taccaliti A, Ricciarelli L, Faragona S, Gusella PM, Giuseppetti GM. Ultrasonographic contrast agent: evaluation of time intensity curve in the characterisation of solitary thyroid nodules. Radiol Med. 2002;103(4):407–413. [PubMed] [Google Scholar]
- 2.Baloch Z, LiVolsi VA, Jain P, Jain R, Aljada I, Mandel S, Langer JE, Gupta PK. Role of repeat fine-needle aspiration biopsy (FNAB) in the management of thyroid nodules. Diagn Cytopathol. 2003;29(4):203–206. doi: 10.1002/dc.10361. [DOI] [PubMed] [Google Scholar]
- 3.Bartolazzi A, Orlandi F, Maggiorato E, Volante M, Arecco F, Rossetto R, Palestrini N, Ghigo E, Capotti M, Bussolanti G, et al. Galectin-3-expression analysis in the surgical selection of follicular thyroid nodules with indeterminate fine-needle aspiration cytology: a prospective multicentre study. Lancet Oncol. 2008;9(6):543–549. doi: 10.1016/S1470-2045(08)70132-3. [DOI] [PubMed] [Google Scholar]
- 4.Bartolotta TV, Midiri M, Quaia E, Bertolotto M, Galia M, Cademartiri F, Legalla R, Cardinale AE. Benign focal liver lesions: spectrum of findings on SonoVue-enhanced pulse-inversion ultrasonography. Eur Radiol. 2005;15(8):1643–1649. doi: 10.1007/s00330-005-2668-2. [DOI] [PubMed] [Google Scholar]
- 5.Bartolotta TV, Midiri M, Galia M, Runza G, Attard M, Savoia G, Lagalla R, Cardinale AE. Qualitative and quantitative evaluation of solitary thyroid nodules with contrast-enhanced ultrasound; initial results. Eur Radiol. 2006;16(10):2234–2241. doi: 10.1007/s00330-006-0229-y. [DOI] [PubMed] [Google Scholar]
- 6.British Thyroid Association. London: Royal College of Physicians of London and the British Thyroid Association; 2007. Guidelines for the Management of Thyroid Cancer in Adults. Available from http://www.british-thyroid-association.org. [Google Scholar]
- 7.Burch HB. Evaluation and management of the solid nodule. Endocrinol Metab Clin North Am. 1995;24(4):663–710. [PubMed] [Google Scholar]
- 8.Cakir B, Aydin C, Koruklouglu B, Ozdemir D, Sisman LC, Tuzun D, Oguz A, Guler G, Guney G, Kusdemir A, et al. Diagnostic value of elastosonographically determined strain index in the differential diagnosis of benignant and malignant thyroid nodules. Endocrine. 2011;39(1):89–98. doi: 10.1007/s12020-010-9416-3. [DOI] [PubMed] [Google Scholar]
- 9.Castro MR, Gharib H. Thyroid fine-needle aspiration biopsy: progress, practice, and pitfalls. Endocr Pract. 2003;9(2):128–136. doi: 10.4158/EP.9.2.128. [DOI] [PubMed] [Google Scholar]
- 10.Chow LS, Gharib H, Goellner JR, van Heerden JA. Nondiagnostic thyroid fine-needle aspiration biopsy aspiration cytology: management dilemmas. Thyroid. 2001;11(12):1147–1151. doi: 10.1089/10507250152740993. [DOI] [PubMed] [Google Scholar]
- 11.Danese D, Schiaccitano S, Farsetti A, Andreoli M, Pontecorvi A. Diagnostic accuracy of conventional versus sonography-guided fine-needle aspiration biopsy of thyroid nodules. Thyroid. 1998;8(1):15–21. doi: 10.1089/thy.1998.8.15. [DOI] [PubMed] [Google Scholar]
- 12.Foppiani L, Tancredi M, Ansaldo GL, Ceppa P, Aurati L, Torre GC, Minuto F, Giusti M. Absence of histological malignancy in a patient cohort with follicular lesion on fine-needle aspiration. J Endocrinol Invest. 2003;26(1):29–34. doi: 10.1007/BF03345119. [DOI] [PubMed] [Google Scholar]
- 13.Friedrich-Rust M, Sperber A, Holzer K, Diener J, Grunwald F, Badenhoop K, Weber S, Kriener S, Hermann E, Bechstein WO, et al. Real-time elastography and contrast-enhanced ultrasound for the assessment of thyroid nodules. Exp Clin Endocrinol Diabetes. 2010;118(9):602–609. doi: 10.1055/s-0029-1237701. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Pascual L, Barahona MJ, Balsells M, del Pozo C, Anglada-Barcelo J, Casalots-Casado J, Veloso E, Torres J. Complex thyroid nodules with nondiagnostic fine needle aspiration cytology: histopathologic outcomes and comparison of the cytological variants (cystic vs. acellular) Endocrine. 2011;39(1):33–40. doi: 10.1007/s12020-010-9409-2. [DOI] [PubMed] [Google Scholar]
- 15.Gharib H, Papini E. Thyroid nodules: clinical importance, assessment, and treatment. Endocrinol Metab Clin North Am. 2007;36(3):707–735. doi: 10.1016/j.ecl.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Gharib H, Papini E, Valcalvi R, Baskin HJ, Crescenzi A, Dottorini ME, Duick DS, Guglielmi R, Hamilton CR, Jr, Zeiger MA, et al. American Association of Clinical Endocrinologists and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocr Pract. 2006;12(1):63–102. doi: 10.4158/EP.12.1.63. [DOI] [PubMed] [Google Scholar]
- 17.Gomez Saez JM. Diagnostic usefulness of tumor markers in the thyroid cytological samples extracted by fine-needle aspiration biopsy. Endocr Metab Immune Disord Drug Targets. 2010;10(1):47–56. doi: 10.2174/187153010790828000. [DOI] [PubMed] [Google Scholar]
- 18.Heidinger CHR, Sobin LH. Histological Typing of Thyroid Tumors. In: Roto Satag AS, editor. International Histological Classification of Tumors. Vol. 11. Geneva: World Health Organization; 1974. pp. 17–27. [Google Scholar]
- 19.Henrichsen TL, Reading CC. Thyroid ultrasonography. Part 2: nodules. Radiol Clin North Am. 2011;49(3):417–424. doi: 10.1016/j.rcl.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Horvath E, Majlis S, Rossi R, Franco C, Niedmann JP, Castro A, Dominguez M. An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J Clin Endocrinol Metab. 2009;94(5):1748–1751. doi: 10.1210/jc.2008-1724. [DOI] [PubMed] [Google Scholar]
- 21.Kim DW, Lee EJ, Kim SH, Kim TH, Lee SH, Kim DH, Rho MH. Ultrasound-guided fine-needle aspiration biopsy of thyroid nodules: comparison in efficacy according to nodule size. Thyroid. 2009;19(1):27–31. doi: 10.1089/thy.2008.0106. [DOI] [PubMed] [Google Scholar]
- 22.Kim EK, Park CS, Chung WY, Oh KK, Kim DI, Lee JT, Yoo HS. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol. 2002;178(3):687–691. doi: 10.2214/ajr.178.3.1780687. [DOI] [PubMed] [Google Scholar]
- 23.Kim ES, Nam-Goong IS, Gong G, Hong SJ, Kim WB, Shong YK. Postoperative findings and risk for malignancy in thyroid nodules with cytological diagnosis of so-called “follicular neoplasm”. Korean J Intern Med. 2003;18(2):94–97. doi: 10.3904/kjim.2003.18.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Layfield LJ, Cibas ES, Gharib H, Mandel SJ. Thyroid aspiration cytology: current status. CA Cancer J Clin. 2009;59(2):99–110. doi: 10.3322/caac.20014. [DOI] [PubMed] [Google Scholar]
- 25.Lewis CM, Chang KP, Pitman M, Faquin WC, Randolph GW. Thyroid fine-needle aspiration biopsy: variability in reporting. Thyroid. 2009;19(7):717–723. doi: 10.1089/thy.2008.0425. [DOI] [PubMed] [Google Scholar]
- 26.Lippolis PV, Tognini S, Materazzi G, Polini A, Mancini R, Ambrosini CE, Dardano A, Basolo F, Seccia M, Miccoli P, et al. Is elastography actually useful in the presurgical selection of thyroid nodules with indeterminate cytology? J Clin Endocrinol Metab. 2011;96(11):E1826–E1830. doi: 10.1210/jc.2011-1021. [DOI] [PubMed] [Google Scholar]
- 27.Lyshchik A, Higashi T, Asato R, Tenaka S, Ito J, Mai JJ, Pellot-Barakat C, Insana MF, Brill AB, Saga T, et al. Thyroid gland tumor diagnosis at US elastography. Radiology. 2005;237(1):202–211. doi: 10.1148/radiol.2363041248. [DOI] [PubMed] [Google Scholar]
- 28.Massaro F, Dolcino M, Degrandi R, Ferone D, Mussap M, Minuto F, Giusti M. Calcitonin assay in wash-out fluid after fine-needle aspiration biopsy in patients with a thyroid nodule and border-line value of the hormone. J Endocrinol Invest. 2009;32(4):308–312. doi: 10.1007/BF03345717. [DOI] [PubMed] [Google Scholar]
- 29.Mihai R, Parker AJ, Roskell D, Sadler GP. One in four patients with follicular thyroid cytology (THY 3) has a thyroid carcinoma. Thyroid. 2009;19(1):33–37. doi: 10.1089/thy.2008.0200. [DOI] [PubMed] [Google Scholar]
- 30.Molinari F, Mantovani A, Deandrea M, Limone P, Garberoglio R, Suri JS. Characterization of a single thyroid nodule by contrast-enhanced 3-D ultrasound. Ultrasound Med Biol. 2010;36(10):1616–1625. doi: 10.1016/j.ultrasmedbio.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Moon WJ, Baek JH, Jung SL, Kim DW, Kim EK, Kim JY, Kwak JY, Lee JH, Lee JH, Lee YH, et al. Ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations. Korean J Radiol. 2011;12(1):1–14. doi: 10.3348/kjr.2011.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nam-Goong IS, Kim HY, Gong G, Lee HK, Hong SJ, Kim WB, Shong YK. Ultrasonography-guided fine-needle aspiration of thyroid incidentaloma: correlation with pathological findings. Clin Endocrinol (Oxf) 2004;60(1):21–28. doi: 10.1046/j.1365-2265.2003.01912.x. [DOI] [PubMed] [Google Scholar]
- 33.Nayar R, Ivanovic M. The indeterminate thyroid fine-needle aspiration. Cancer Cytopathol. 2009;117(3):195–202. doi: 10.1002/cncy.20029. [DOI] [PubMed] [Google Scholar]
- 34.Nikiforova MN, Kimura ET, Gandhi M, Biddinger PW, Knauf JA, Basolo F, Zhu Z, Giannini R, Salvatore G, Fusco A, et al. BRAF mutations in thyroid tumors are restricted to papillary carcinoma and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab. 2003;88(11):5399–5404. doi: 10.1210/jc.2003-030838. [DOI] [PubMed] [Google Scholar]
- 35.Rago T, di Coscio G, Basolo F, Scutari M, Elisei R, Berti P, Miccoli R, Romani R, Faviana P, Pinchera A, et al. Combined clinical, thyroid ulltrasound and cytological features help to predict thyroid malignancy in follicular and Hurthle cell thyroid lesions: results from a series of 505 consecutive patients. Clin Endocrinol (Oxf) 2007;66(1):13–20. doi: 10.1111/j.1365-2265.2006.02677.x. [DOI] [PubMed] [Google Scholar]
- 36.Rago T, Scutari M, Santini F, Loiacono V, Piaggi P, di Coscio G, Basolo F, Berti P, Pinchera A, Vitti P. Real-time elastosonography: useful tool for refining the presurgical diagnosis in thyroid nodules with indeterminate or nondiagnostic cytology. J Clin Endocrinol Metab. 2010;95(12):5274–5280. doi: 10.1210/jc.2010-0901. [DOI] [PubMed] [Google Scholar]
- 37.Redman R, Zalaznick H, Mazzaferri EL, Massoll NA. The impact of assessing specimen adequacy and number of needle passes for fine-needle aspiration biopsy of thyroid nodules. Thyroid. 2006;16(1):55–60. doi: 10.1089/thy.2006.16.55. [DOI] [PubMed] [Google Scholar]
- 38.Rubaltelli L, Corradin S, Dorigo A, Stabilito M, Tregnaghi A, Borsato S, Stramare R. Differential diagnosis of benignant and malignant thyroid nodules at elastosonography. Ultraschall Med. 2009;30(2):175–179. doi: 10.1055/s-2008-1027442. [DOI] [PubMed] [Google Scholar]
- 39.Sidoti M, Marino G, Resmini E, Augeri C, Cappi C, Cavallero D, Lagasio C, Ceppa P, Minuto F, Giusti M. The rationale use of fine needle aspiration biopsy (FNAB) in diagnosing thyroid nodules. Minerva Endocrinol. 2006;31(2):159–172. [PubMed] [Google Scholar]
- 40.Spiezia S, Farina R, Cerbone G, Assanti AP, Iovino V, Siciliani M, Lombardi G, Colao A. Analysis of color Doppler signal intensity variation after levovist injection: a new approach to the diagnosis of thyroid nodules. J Ultrasound Med. 2001;20(3):223–231. doi: 10.7863/jum.2001.20.3.223. [DOI] [PubMed] [Google Scholar]
- 41.Ueno E, Ito A. Diagnosis of breast cancer by elasticity imaging. Eizo Joho Med. 2004;36(12):2–6. [Google Scholar]
- 42.Vorlander C, Wolf J, Saalablan S, Lienenluke RH, Wahl RA. Real-time ultrasound elastography—a non-invasive diagnostic procedure for evaluating dominant thyroid nodules. Langenbeck’s Arch Surg. 2010;395(7):865–871. doi: 10.1007/s00423-010-0685-3. [DOI] [PubMed] [Google Scholar]
- 43.Yang J, Schnadig V, Logrono R, Wasserman PG. Fine-needle aspiration for thyroid nodules: a study of 4703 patients with histological and clinical correlations. Cancer. 2007;111(5):306–315. doi: 10.1002/cncr.22955. [DOI] [PubMed] [Google Scholar]
- 44.Zhang B, Jiang YX, Liu JB, Yang M, Dai Q, Zhu QL, Gao P. Utility of contrast-enhanced ultrasound for evaluation of thyroid nodules. Thyroid. 2010;20(1):51–57. doi: 10.1089/thy.2009.0045. [DOI] [PubMed] [Google Scholar]