Abstract
Objective: The present study was designed to use an in vivo rabbit ear scar model to investigate the efficacy of systemic administration of endostatin in inhibiting scar formation. Methods: Eight male New Zealand white rabbits were randomly assigned to two groups. Scar model was established by making six full skin defect wounds in each ear. For the intervention group, intraperitoneal injection of endostatin was performed each day after the wound healed (about 15 d post wounding). For the control group, equal volume of saline was injected. Thickness of scars in each group was measured by sliding caliper and the scar microcirculatory perfusion was assessed by laser Doppler flowmetry on Days 15, 21, 28, and 35 post wounding. Rabbits were euthanatized and their scars were harvested for histological and proteomic analyses on Day 35 post wounding. Results: Macroscopically, scars of the control group were thicker than those of the intervention group. Significant differences between the two groups were observed on Days 21 and 35 (p<0.05). Scar thickness, measured by scar elevation index (SEI) at Day 35 post wounding, was significantly reduced in the intervention group (1.09±0.19) compared with the controls (1.36±0.28). Microvessel density (MVD) observed in the intervention group (1.73±0.94) was significantly lower than that of the control group (5.63±1.78) on Day 35. The distribution of collagen fibers in scars treated with endostatin was relatively regular, while collagen fibers in untreated controls were thicker and showed disordered alignment. Western blot analysis showed that the expressions of type I collagen and Bcl-2 were depressed by injection of endostatin. Conclusions: Our results from the rabbit ear hypertrophic scar model indicate that systemic application of endostatin could inhibit local hypertrophic scar formation, possibly through reducing scar vascularization and angiogenesis. Our results indicated that endostatin may promote the apoptosis of endothelial cells and block their release of platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF), thereby controlling collagen production by fibroblasts. Blood vessel-targeted treatment may be a promising strategy for scar therapy.
Keywords: Endostatin, Hypertrophic scar, Systemic administration
1. Introduction
Hypertrophic scar (HS) formation is a common complication of wound healing, particularly after burn injuries. HSs are raised, red, rigid, and responsible for serious functional and cosmetic problems. The underlying mechanisms of scar formation are complicated, and the process may be affected by multiple factors.
Angiogenesis and vascularization are the important processes during wound healing. These events ensure abundant microvascular perfusion for the regeneration of the neo-tissue (Kirsner and Eaglstein, 1993). However, excessive angiogenesis and vascularization can lead to pathological HSs (Kischer, 1992). Microvascular abnormalities have been observed in pathological scars. Compared with normal skin, such scars have vessels of greater density and diameter and blood flow is increased as measured by laser Doppler. This implies the presence of increased vascular density in HSs compared with normotrophic scars. Amadeu et al. (2003) have reported increased microvessel numbers in HSs. Thomas et al. (1994) confirmed that HSs have an increased vascular component. Ehrlich and Kelley (1992) also demonstrated increased blood flow in HSs. Thus, we propose that inhibition of the growth of excessive new blood vessels in the scar might be a promising new strategy for the suppression of HS formation.
Currently, surgical excision, intralesional injection of steroids, silicone, and laser are the most frequently used treatments for HSs (Leventhal et al., 2006). However, no single treatment appears to have the desired curative effect. As a result, new strategies for prevention of HSs are needed to avoid completely their formation. Antiangiogenic therapy may be used as an effective strategy in HS therapy. There have been some studies in the area of antiangiogenic therapy for HSs (Song et al., 2008a; 2008b; 2009); however, the antiangiogenic agents used in these reports are topically administered, which can lead to local pain and poor compliance.
Endostatin, the C-terminal fragment of collagen XVIII, has been clinically applied to suppress tumor growth and angiogenesis by inhibiting endothelial cell proliferation and migration (Song et al., 2009; Zheng, 2009). We propose that this anti-angiogenesis of endostatin may also be effective in scar-treatment.
The aim of this study was to investigate our hypothesis that endostatin may be successful in the therapeutic modulation of scar formation in vivo.
2. Materials and methods
2.1. HS model
Eight male adult New Zealand white rabbits weighing between 2.2 and 2.5 kg were used in this study. All animal handling procedures were approved by the Zhejiang University Animal Care and Use Committee. Animals were anaesthetized with 1% (10 g/L) pentobarbital sodium (1 mg/kg). Afterwards, the HS model was made following standardized protocol, as previously described (Morris et al., 1997; Saulis et al., 2002b). Six wounds were created down to bare cartilage on the ventral surface of each ear by means of a 7-mm punch biopsy at standardized locations (Tandara and Mustoe, 2008). An operating loupe was used to ensure removal of the epidermis, dermis, and perichondrium in each wound (Tandara and Mustoe, 2008). Hemostasis was then obtained by applying pressure with cotton balls.
2.2. Animal groups and drug administration
Eight rabbits were randomly assigned to either the intervention group or the control group such that each group had four members. Human recombinant endostatin (Endostar™, purchased from SIMCERE PHARMACEUTICAL Company, 15 mg/3 ml per dosage) was diluted with 47 ml physiological saline. Endostar™ solution was given to the intervention group rabbits by intraperitoneal injection every day from the Day 15 after operation when the wound had finished epithelialization. The dosage was calculated based on body surface area following the users’ instructions for Endostar™. The animal body surface area calculation formula is S=K×W 2/3 (S: surface area, K: constant, W: weight); for the rabbit K=10. A 2.5 kg rabbit has a body surface area of 0.2 m2 (S=10×2 5002/3=1 842 cm2≈0.2 m2), and 5 ml of Endostar™ solution containing 1.5 mg endostatin was injected each day.
2.3. Tissue preparation
On Day 35 post wounding, the rabbits of each group were sacrificed and the scars harvested. To prevent scar shrinkage in paraffin sections, the samples contained the rabbit ear full-thickness scar skin and the cartilage, the latter acting as a tissue pedestal. One half of each scar was fixed in 4% neutral-buffered formaldehyde, dehydrated, embedded in paraffin, cut in 4-mm sections, and stained with haematoxylin and eosin (H&E). The other half was frozen for Western blot analysis.
2.4. Microcirculation perfusion assessment
On Days 15, 21, 28, and 35 post wounding, scar microcirculatory perfusion was assessed by laser Doppler flowmetry (PeriFlux5000s, PF 5010 standard probe; Perimed, Stockholm, Sweden) according to the manufacturer’s instructions as previously described (Song et al., 2009). In short, a 1-mW laser source with a wavelength of 780 nm was used, and the time duration was set at 60 s for each measurement spot in each scar. The output signal was analyzed using PSW 2.0 software, and the average value was obtained for a total of 96 scars at each time point of investigation.
2.5. Scar elevation index (SEI)
The degree of dermal hypertrophy of each scar was expressed as the SEI (Morris et al., 1997; Tandara and Mustoe, 2008). This index is the ratio of the area of newly formed dermis of the scar to the area of surrounding normal dermis. An SEI value greater than 1.0 defines a hypertrophic dermis (Morris et al., 1997; Marcus et al., 2000; Saulis et al., 2002a; Tandara and Mustoe, 2008).
2.6. Immunohistochemistry and microvessel density (MVD)
Specific immunochemical staining of endothelial cells was performed using a monoclonal antibody to CD34. Mouse-anti-rabbit-CD34 monoclonal antibody was purchased from Santa Cruz Biotechnology. In each scar, the total number of CD34 positive blood vessels was counted in five randomly chosen high power fields, which were distributed evenly inside the scar. The counts from the five fields (200×) were averaged and used for comparisons.
2.7. Collagen organization and cellularity of the dermis
The dermis was examined for collagen organization in a semi-quantitative manner in Masson trichrome-stained slides as described previously (Saulis et al., 2002b; Tandara and Mustoe, 2008). The collagen organization was described by its alignment and thickness.
2.8. Western blot analysis
Western blot was performed using a previously described method (Kim et al., 2007; 2011). Scar samples were lysed in solutions containing 250 mmol/L sucrose, 1 mmol/L ethylenediaminetetraacetic acid (EDTA), 0.1 mmol/L phenylmethylsulfonyl fluoride (PMSF), and 20 mmol/L potassium phosphate buffer at pH 7.6 with a homogenizer at 3 000 r/min. Equal amounts of protein (50 mg/lane) were subjected to immunoblotting with the indicated antibodies. The antibodies used were type I collagen, Bcl-2, and β-actin (200 μg/ml, Santa Cruz Biotechnology, CA, USA). The bound horseradish peroxidase conjugated secondary antibody was detected using an enhanced chemiluminescence detection system (iNtRON Biotechnology, Korea). Protein expression levels were determined by analyzing the signals captured on the nitrocellulose membranes using an image analyzer (Las-3000, Fuji photo, Tokyo, Japan).
2.9. Statistical analysis
Each sample was evaluated twice at each time points using a calibrated eyepiece reticule. SPSS Version 18 (SPSS Inc.) was used for statistical analysis. Continuous variables are expressed as mean±standard deviation (SD). Enumeration data are expressed as percentages. A p-value of <0.05 was chosen to indicate statistical significance. We first checked the normality and homogeneity of the continuous variables, and then adopted an independent sample t-test or nonparametric independent sample test to analyze the differences between variables of both groups, depending on the distribution of the variables.
3. Results
3.1. Appearance of scars
Macroscopic view of scar samples was taken at each time point by digital camera (Fig. 1).
Fig. 1.
Macroscopic views of scar samples
(a) Control group (Day 21); (b) Intervention group (Day 21); (c) Control group (Day 35); (d) Intervention group (Day 35)
One week after complete healing (Day 21), the scars in the control group were severely congested and appeared purple and swollen (Fig. 1a). Since the perfusion of scars in the intervention group was dramatically depressed due to the injection of endostatin, these scars appeared pink and less swollen (Fig. 1b). Three weeks after healing, the intra scar microvascular perfusion in both groups had decreased, and the scars had become less red. However, scars in the control group were still thick, and had even become hardened (Fig. 1c), while scars in the intervention group were completely flat and had softened (Fig. 1d).
3.2. Thickness of scars in the rabbit ear
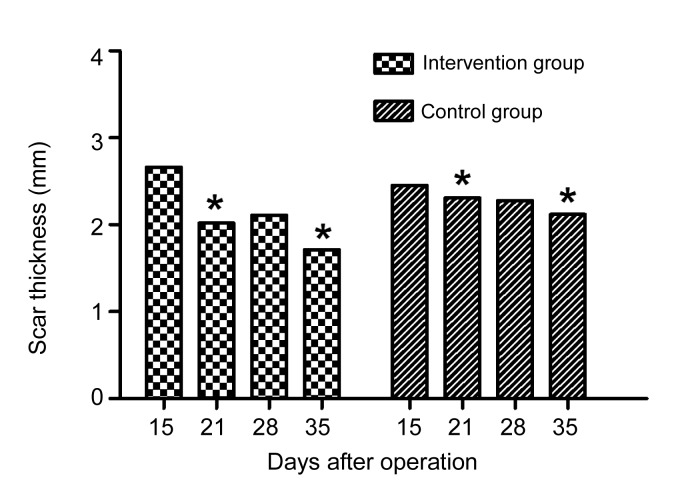
The thickness of scars in both groups was measured with a slide gauge (Table 1 and Fig. 2).
Table 1.
Thickness of scars measured with a slide gauge
| Day | Scar thickness (mm) |
|
| Control group | Intervention group | |
| 15 | 2.45±0.67 | 2.66±0.55 |
| 21 | 2.31±0.57 | 2.02±0.38* |
| 28 | 2.28±0.38 | 2.11±0.40 |
| 35 | 2.12±0.40 | 1.71±0.35* |
p<0.05, compared to the control group
Fig. 2.
Comparison of scar thickness
Scar thickness decreased much faster in the intervention group, and significant statistical differences were observed on Days 21 and 35 after modeling (* p<0.05)
On Day 15 the scar thickness in both groups had peaked, and there was no significant difference between the two groups (Table 1 and Fig. 2). After that, the thickness of scars in both groups decreased with time. However, the decrease was much faster in the intervention group than in the control group, and significant statistical differences were observed on Days 21 and 35 after modeling (p<0.05).
3.3. Perfusion of microcirculation
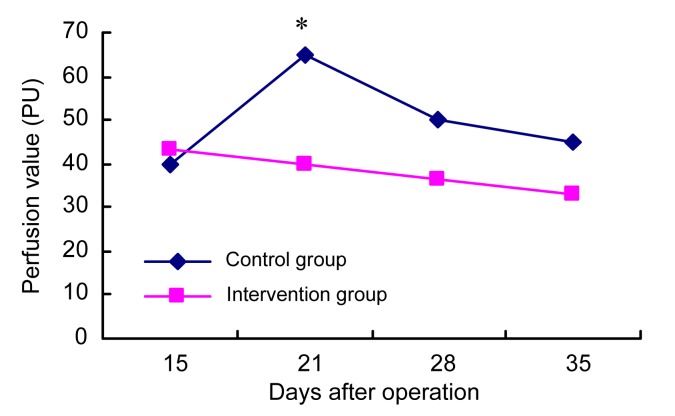
The scar microcirculatory perfusion was assessed by laser Doppler flowmetry (Fig. 3).
Fig. 3.
Changes in microcirculation perfusion in scars
* p<0.05, significant difference between the intervention and control groups on Day 21
After healing, the microcirculation perfusion in the control group went up on Day 21 and decreased slowly thereafter, but in the intervention group it continued to drop after endostatin administration. A significant difference between the two groups was observed on Day 21 (p<0.05).
3.4. SEI
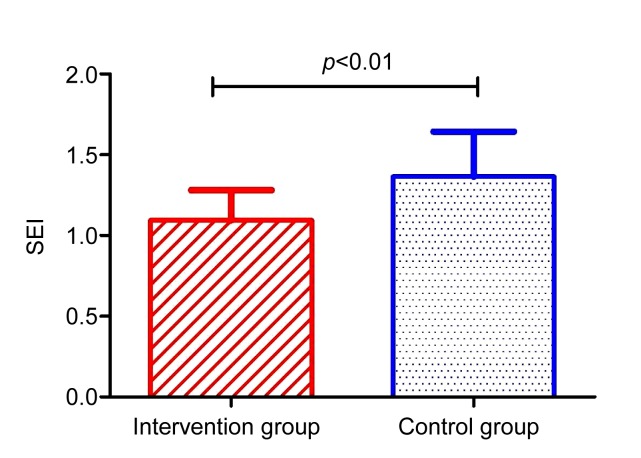
On Day 35 post wounding, the scars were harvested for SEI measurement (Fig. 4).
Fig. 4.
Comparison of scar elevation index on Day 35
The SEI in the control group was 1.36±0.28, compared to 1.09±0.19 in the intervention group. The difference was statistically significant (p<0.01).
3.5. MVD
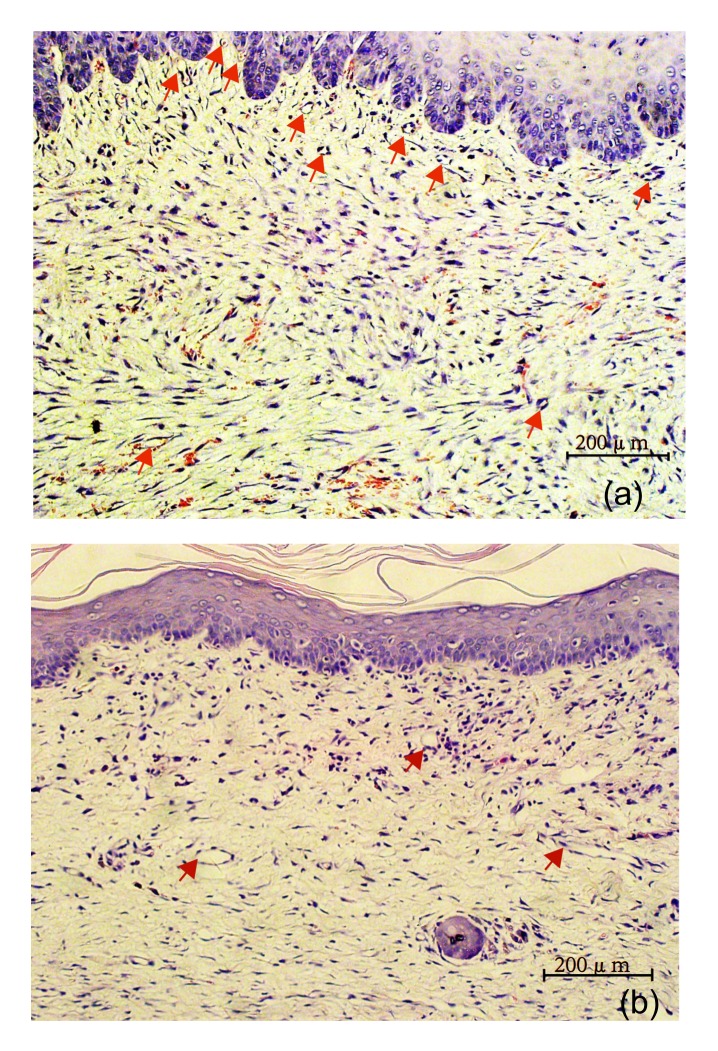
MVD within scar samples was measured by specific immunochemical staining of endothelial cells (CD34+). In each scar, the total number of CD34 positive blood vessels was counted in five randomly chosen high power fields, which were distributed evenly inside the scar. The counts from the five fields (200×) were averaged and used for comparisons (Fig. 5).
Fig. 5.
Microvessel density (MVD) within scar samples
(a) Control group; (b) Intervention group (immunohistochemistry)
The MVD in the control group (5.63±1.78) (Fig. 5a) was much higher than that of the intervention group (1.73±0.94) (Fig. 5b) on Day 35. The difference was statistically significant (p<0.05).
3.6. Collagen distribution in scars
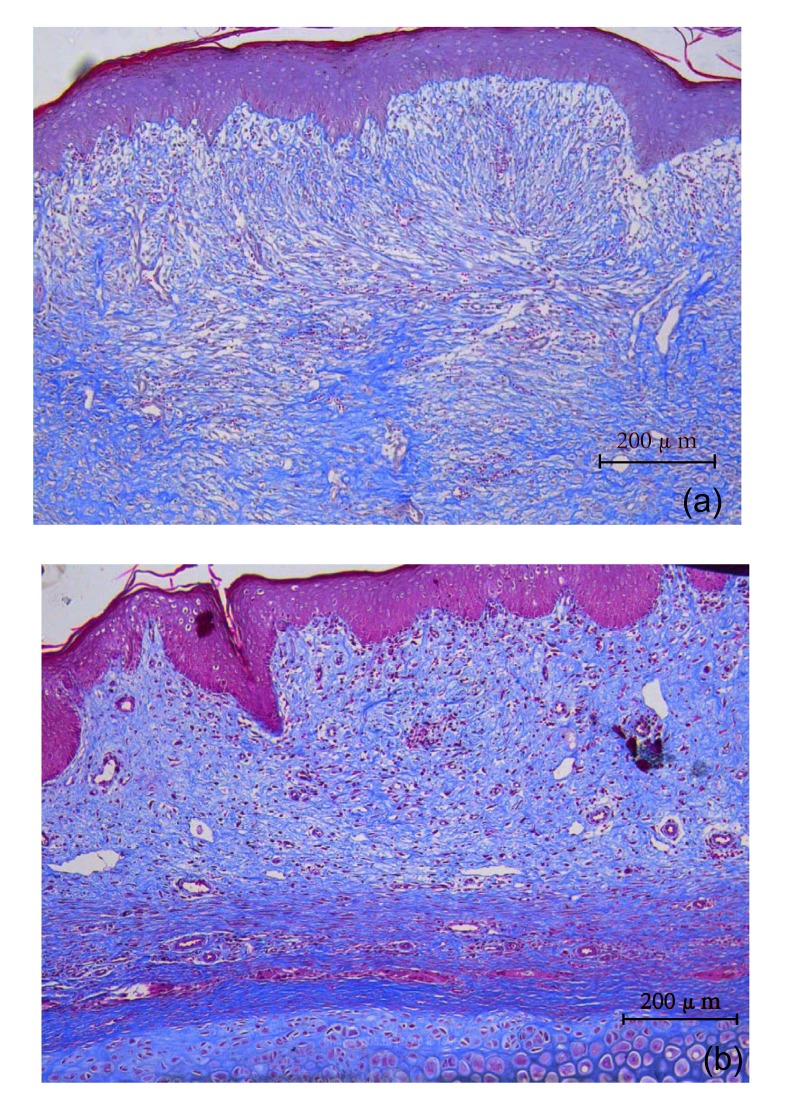
Collagen distribution in scar samples, harvested on Day 35 post wounding, was observed by Masson trichrome-stained slides (Fig. 6).
Fig. 6.
Collagen distribution in scars on Day 35
(a) Control group; (b) Intervention group (Masson)
The distribution of collagen fibers in the intervention group in Masson trichrome-stained slides showed regular alignment (Fig. 6b), while fibers were enlarged and disordered in the control group (Fig. 6a).
3.7. Expressions of type I collagen and Bcl-2 in scars
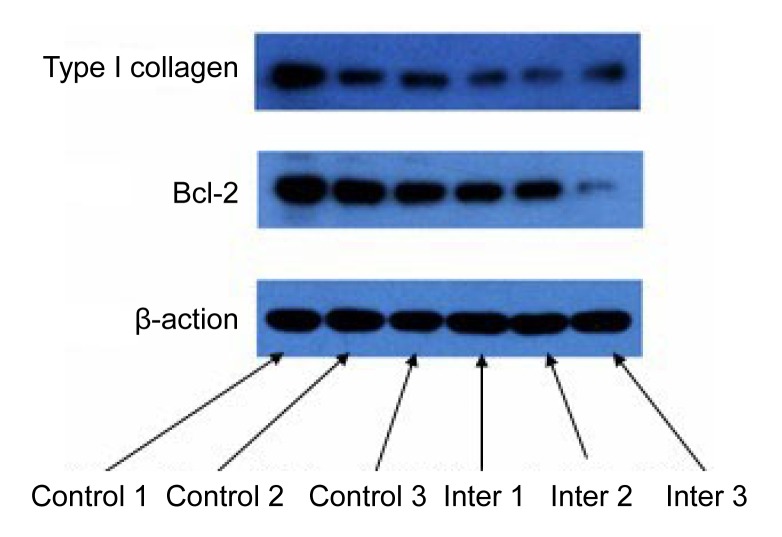
Expressions of type I collagen and Bcl-2 in scars were measured by Western blot analysis (Fig. 7).
Fig. 7.
Expressions of type I collagen and Bcl-2 in scars
Expressions of type I collagen and Bcl-2 were depressed by endostatin injection. Down-regulation of Bcl-2 was possibly related to the apoptosis of endothelium cells.
4. Discussion
Hypertrophic scarring is a common problem after injury and may cause functional and cosmetic deformities. Several treatment modalities have been applied for the prevention and treatment of HS, including pressure, silicone gel sheets, intralesional steroids, 5-fluorouracil (5-FU), cryotherapy, surgical excision, and lasers. Local pharmacological therapies such as repeat inter scar steroid injections, are painful and can result in complications including steroid atrophy. Systemic pharmacologic inhibitors of collagen synthesis such as penicillamine were previously used clinically for these lesions. However, significant systemic side effects of this therapy were reported (Camus and Koeger, 1986). Currently, there is a demand for systemically applied agent collagen synthesis inhibitors, which are considered an attractive strategy if they can be applied without obvious side effects.
Endostatin was first identified from murine hemangioendothelioma cell culture medium (O′Reilly et al., 1997). It has been systemically implicated in tumor therapy by reducing the number of newly-formed blood vessels (Rosca et al., 2011). Results from these clinical studies have proven the safety of this drug. Local injection of the anti-angiogenesis drug endostatin has been shown to significantly alleviate scar formation in rabbit ears (Wang et al., 2012). However, repeated local injections in the scar are painful for human patients, and lead to poor compliance.
This study, to our knowledge, is the first to confirm the hypothesis that systemic application of endostatin can also be effective in preventing and/or treating local HSs. Our results showed that the systematic injection of endostatin reduced scar congestion, thickness, SEI, and MVD significantly and produced fewer and regular collagen fibers compared to the control group. The effectiveness of endostatin in reducing the thickness and SEI of scars is possibly due to its ability to impede angiogenesis.
The relationships between the endothelial cells and other cells were described previously (Bao et al., 2009). The capillary endothelial cells can secrete fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF) which will lead to fibroblast hyperplasia (Bao et al., 2009). Bcl-2 is an anti-apoptotic molecule (Portt et al., 2011). A decrease in Bcl-2 would promote apoptosis in mammalian cells by directly activating the mitochondrial apoptotic pathway upstream of caspase-9, and caspase-9 naturally activates caspase-3 (Miller, 1997; Fan et al., 2005; Mazars et al., 2005). In the present study, Bcl-2 decreased following endostatin treatment. Thus, the decrease in Bcl-2 played an important role in the induction of endothelial cell apoptosis by endostatin. Our results indicated that endostatin may promote the apoptosis of endothelial cells and block their release of PDGF and FGF, thereby controlling the production of collagen by fibroblasts. Further research should be done to elucidate the mechanism of this process.
5. Conclusions
Our study demonstrates that the inhibition of angiogenesis by endostatin results in an obvious reduction of hypertrophic scarring in a rabbit ear model. To the best of our knowledge, this is the first report of the systemic application of endostatin for targeting the growth of new blood vessels to control local HS formation. By systematic application of endostatin, the inhibition of angiogenesis provides a potential approach for the prevention and treatment of HSs in humans.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 81272120) and the Health Department of the Zhejiang Province (No. 2007B086), China
References
- 1.Amadeu T, Braune A, Mandarim-de-Lacerda C, Porto LC, Desmouliere A, Costa A. Vascularization pattern in hypertrophic scars and keloids: a stereological analysis. Pathol Res Pract. 2003;199(7):469–473. doi: 10.1078/0344-0338-00447. [DOI] [PubMed] [Google Scholar]
- 2.Bao P, Kodra A, Tomic-Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res. 2009;153(2):347–358. doi: 10.1016/j.jss.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camus JP, Koeger AC. D-penicillamine and collagen. Ann Biol Clin (Paris) 1986;44(3):296–299. [PubMed] [Google Scholar]
- 4.Ehrlich HP, Kelley SF. Hypertrophic scar: an interruption in the remodeling of repair—a laser doppler blood flow study. Plast Reconstr Surg. 1992;90(6):993–998. doi: 10.1097/00006534-199212000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Fan TJ, Han LH, Cong RS, Liang J. Caspase family proteases and apoptosis. Acta Biochim Biophys Sin. 2005;37(11):719–727. doi: 10.1111/j.1745-7270.2005.00108.x. [DOI] [PubMed] [Google Scholar]
- 6.Kim YS, Jung DH, Kim NH, Lee YM, Jang DS, Song GY, Kim JS. KIOM-79 inhibits high glucose or AGEs-induced VEGF expression in human retinal pigment epithelial cells. J Ethnopharmacol. 2007;112(1):166–172. doi: 10.1016/j.jep.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 7.Kim YS, Kim J, Kim CS, Sohn EJ, Lee YM, Jeong IH, Kim H, Jang DS, Kim JS. KIOM-79, an inhibitor of AGEs-protein cross-linking, prevents progression of nephropathy in zucker diabetic fatty rats. Evid Based Compl Altern Med. 2011;2011:761859. doi: 10.1093/ecam/nep078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirsner RS, Eaglstein WH. The wound healing process. Dermatol Clin. 1993;11(4):629–640. [PubMed] [Google Scholar]
- 9.Kischer CW. The microvessels in hypertrophic scars, keloids and related lesions: a review. J Submicrosc Cytol Pathol. 1992;24(2):281–296. [PubMed] [Google Scholar]
- 10.Leventhal D, Furr M, Reiter D. Treatment of keloids and hypertrophic scars: a meta-analysis and review of the literature. Arch Fac Plast Surg. 2006;8(6):362–368. doi: 10.1001/archfaci.8.6.362. [DOI] [PubMed] [Google Scholar]
- 11.Marcus JR, Tyrone JW, Bonomo S, Xia Y, Mustoe TA. Cellular mechanisms for diminished scarring with aging. Plast Reconstr Surg. 2000;105(5):1591–1599. doi: 10.1097/00006534-200004050-00001. [DOI] [PubMed] [Google Scholar]
- 12.Mazars A, Geneste O, Hickman J. The bcl-2 family of proteins as drug targets. J Soc Biol. 2005;199(3):253. doi: 10.1051/jbio:2005027. (in French) [DOI] [PubMed] [Google Scholar]
- 13.Miller DK. The role of the caspase family of cysteine proteases in apoptosis. Semin Immunol. 1997;9(1):35–49. doi: 10.1006/smim.1996.0058. [DOI] [PubMed] [Google Scholar]
- 14.Morris D, Wu L, Zhao L, Bolton L, Roth S, Ladin D, Mustoe T. Acute and chronic animal models for excessive dermal scarring: quantitative studies. Plast Reconstr Surg. 1997;100(3):674. doi: 10.1097/00006534-199709000-00021. [DOI] [PubMed] [Google Scholar]
- 15.O′Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88(2):277–285. doi: 10.1016/S0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 16.Portt L, Norman G, Clapp C, Greenwood M, Greenwood MT. Anti-apoptosis and cell survival: a review. Biochim Biophys Acta. 2011;1813(1):238–259. doi: 10.1016/j.bbamcr.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Rosca EV, Koskimaki JE, Rivera CG, Pandey NB, Tamiz AP, Popel AS. Anti-angiogenic peptides for cancer therapeutics. Curr Pharm Biotechnol. 2011;12(8):1101–1016. doi: 10.2174/138920111796117300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saulis AS, Mogford JH, Mustoe TA. Effect of mederma on hypertrophic scarring in the rabbit ear model. Plast Reconstr Surg. 2002;110(1):177–183. doi: 10.1097/00006534-200207000-00029. disscussion 184-186. [DOI] [PubMed] [Google Scholar]
- 19.Saulis AS, Chao JD, Telser A, Mogford JE, Mustoe TA. Silicone occlusive treatment of hypertrophic scar in the rabbit model. Aesthet Surg J. 2002;22(2):147–153. doi: 10.1067/maj.2002.123023. [DOI] [PubMed] [Google Scholar]
- 20.Song B, Lu K, Zhang Y, Guo S, Han Y, Ma F, Li H. Angiogenesis in hypertrophic scar of rabbit ears and effect of extracellular protein with metalloprotease and thrombospondin 1 domains on hypertrophic scar. Chin J Repar Reconstr Surg. 2008;22(1):70–74. [PubMed] [Google Scholar]
- 21.Song BQ, Lu KH, Guo SZ, Zhang Y, Peng P, Ma FC, Li HY. Effect of METH1 gene transfection on the proliferation of rabbit’s ear scar. Chin J Plast Surg. 2008;24(2):148–150. (in Chinese) [PubMed] [Google Scholar]
- 22.Song B, Zhang W, Guo S, Han Y, Zhang Y, Ma F, Zhang L, Lu K. Adenovirus-mediated METH1 gene expression inhibits hypertrophic scarring in a rabbit ear model. Wound Repair Regen. 2009;17(4):559–568. doi: 10.1111/j.1524-475X.2009.00514.x. [DOI] [PubMed] [Google Scholar]
- 23.Tandara AA, Mustoe TA. The role of the epidermis in the control of scarring: evidence for mechanism of action for silicone gel. J Plast Reconstr Aesthet Surg. 2008;61(10):1219–1225. doi: 10.1016/j.bjps.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 24.Thomas DW, Hopkinson I, Harding KG, Shepherd JP. The pathogenesis of hypertrophic/keloid scarring. Int J Oral Maxillof Surg. 1994;23(4):232–236. doi: 10.1016/S0901-5027(05)80377-7. [DOI] [PubMed] [Google Scholar]
- 25.Wang ZY, Fei S, Lianju X, Yingkai L, Chun Q, Shuliang L, Xiqiao W. Endostar injection inhibits rabbit ear hypertrophic scar formation. Int J Low Extrem Wounds. 2012;11(4):271–276. doi: 10.1177/1534734612463698. [DOI] [PubMed] [Google Scholar]
- 26.Zheng MJ. Endostatin derivative angiogenesis inhibitors. Chin Med J (Engl) 2009;122(16):1947–1951. [PubMed] [Google Scholar]