Abstract
Objective: Weed pollens are common sources of allergens worldwide. The prevalence of weed pollen sensitization is not yet fully known in China. The purpose of this study was to investigate the prevalence of sensitization to weed allergens from Artemisia, Ambrosia, and Humulus in northern China. Methods: A total of 1 144 subjects (aged from 5 to 68 years) visiting our clinic from June to October 2011 underwent intradermal testing using a panel of 25 allergen sources. Subjects with positive skin responses to any pollen were further tested for their serum concentrations of IgE antibodies against Artemisia vulgaris, Ambrosia artemisiifolia, and Humulus scandens, and against the purified allergens, Art v 1 and Amb a 1. Results: Of 1 144 subjects, 170 had positive intradermal reactions to pollen and 144 donated serum for IgE testing. The prevalence of positive intradermal responses to pollens of Artemisia sieversiana, Artemisia annua, A. artemisiifolia, and H. scandens was 11.0%, 10.2%, 3.7%, and 6.6%, respectively. Among the intradermal positive subjects, the prevalence of specific IgE antigens to A. vulgaris was 58.3%, to A. artemisiifolia 14.7%, and to H. scandens 41.0%. The prevalence of specific IgE antigens to the allergen Art v 1 was 46.9%, and to Amb a 1 was 11.2%. The correlation between the presence of IgE antibodies specific to A. vulgaris and to the Art v 1 antigen was very high. Subjects with A. artemisiifolia specific IgE also had A. vulgaris specific IgE, but with relatively high levels of A. vulgaris IgE antibodies. There were no correlations between the presence of IgE antibodies to H. scandens and A. vulgaris or to H. scandens and A. artemisiifolia. Conclusions: The intradermal prevalence of weed pollen sensitization among allergic subjects in northern China is about 13.5%. Correlations of specific IgE antibodies suggest that pollen allergens from Artemisia and Humulus are independent sources for primary sensitization.
Keywords: Humulus scandens, Artemisia vulgaris, Ambrosia artemisiifolia, Intradermal test, Specific IgE, Sensitization
1. Introduction
Pollens from weeds are common sources of allergens worldwide. The most well described wind-pollinated genera are Artemisia (mugwort) and Ambrosia (ragweed) belonging to the family Asteraceae. Japanese hop (Humulus scandens) belonging to the family Cannabaceae, is also a wind-pollinated weed. It has recently spread across large parts of northern China. A recent cross-sectional survey of 6 304 Chinese allergic subjects showed that Artemisia vulgaris and Ambrosia artemisiifolia sensitizations are associated with the severity of intermittent rhinitis (Li et al., 2011).
Mugwort comprises several closely related species, of which A. vulgaris is most widespread across Europe, North America, and parts of Asia, and is one of the main causes of pollen allergy late in summer and in autumn. In Central Europe, mugwort pollination starts at the end of July and lasts until the end of August (Oberhuber et al., 2008), whereas in the Mediterranean area the flowering period shifts to September and the beginning of October (Spieksma et al., 1980). The prevalence of sensitization caused by mugwort pollen is 17% in Italy (Verini et al., 2001). Mugwort is an important cause of sensitization and allergy in Germany and Switzerland (Schmid-Grendelmeier, 1998; Schäfer et al., 1999; Krämer et al., 2001). A multicenter study showed that about 24% of subjects with asthma and/or rhinitis had positive skin prick tests (SPTs) to A. vulgaris in northern China (Li et al., 2009). Allergen microarray analyses of sera from 100 Chinese allergic subjects showed that the highest specific IgE-reactivity towards the pollen allergens investigated was against allergens of Art v (Zheng et al., 2011). An A. vulgaris specific IgE prevalence of 88% was found among 50 sera derived from weed-allergic rhinitis patients from Beijing (China) as well as a strong correlation between A. vulgaris specific IgE and Art v 1 specific IgE (Han et al., 2011).
Ragweed is the common name for several closely related species of which A. artemisiifolia is most widespread. Ragweed is an invasive species in North America, Europe, Australia, and Asia (European Aeroallergen Network https://ean.polleninfo.eu/Ean and European Pollen Information http://www.polleninfo.org). The flowering period lasts from August to October in Central Europe (Brandes and Nitzsche, 2006). In America, 45% of asthma patients were sensitized to ragweed (Kang et al., 1993). In northern China, about 13% of patients with asthma and/or rhinitis had a positive SPT reaction to A. artemisiifolia (Li et al., 2009). The prevalence of ragweed specific IgE among SPT weed pollen positive Chinese subjects has been reported to be 38% (Han et al., 2011).
Japanese hop is widespread in China and Korea with a flowering period lasting from August to October (Yin et al., 1996; Park J.W. et al., 1999; Park H.S. et al., 2001). Aerobiological studies in Beijing (China) and Korea showed that H. scandens pollen counts are larger than those of both mugwort and ragweed and account for about 18% of total pollen during the pollination period (Park J.W. et al., 1999). The flowering periods of mugwort, ragweed, and Japanese hop almost overlap, making it difficult to distinguish which pollen species is responsible for sensitization and allergic reactions. Specific diagnosis and identification of primary sensitizing weed species is also hampered because many allergy clinics in China do not have access to species specific IgE testing (Han et al., 2011). Thus, the true prevalence of weed pollen sensitization is probably not yet fully known. The purpose of this study was to investigate the prevalence of sensitization to mugwort, ragweed, and Japanese hop as measured by intradermal testing of 1 144 allergic subjects visiting an allergy department in the city of Tangshan in Hebei Province in northern China, and by specific IgE testing of 144 subjects having positive intradermal reactivity against weed pollen allergens.
2. Materials and methods
Subjects with a clinical history of allergic rhinitis/asthma and/or allergic dermatitis, who visited the Department of Allergy, Tangshan Gongren Hospital, China, from mid-June to mid-October 2011, were included in this study. A total of 1 144 subjects, from 5 to 68 years old, underwent intradermal testing using a panel of 25 allergen sources (1:5 to 1:50 (w/v) extract dependent on allergens and with 100 times dilution for intradermal tests; Beijing Macro-Union Pharmaceutical Co. Ltd., China), including Artemisia sieversiana, Artemisia annua, A. artemisiifolia, and H. scandens (1 000 times dilution) (Table 1). Histamine (0.01 mg/ml) and saline were used as positive and negative controls, respectively. None of the subjects included in the study took medications, such as antihistamines or steroids for at least two weeks before the intradermal tests. The means of the longest diameter and the length of a perpendicular line through the middle of the wheal after 15 min were measured. Positive reaction was defined as a wheal size of >5 mm. All the subjects (N=170) having positive skin responses to any pollen (Table 1) were asked to donate blood for specific IgE tests. All subjects provided informed consent. A total of 144 serum samples were collected and stored in a freezer before specific IgE measurements were made.
Table 1.
Overall prevalence of positive skin responses in intradermal tests
| Allergen source | Positive patienta | Wheal size (mm)b |
| House dust | 361 (31.6%) | 7.2 (6.0; 3.0–15.5) |
| Dermatophagoides pteronyssinus | 466 (40.7%) | 8.8 (8.0; 3.0–19.0) |
| Dermatophagoides farinae | 453 (39.6%) | 8.4 (8.0; 3.0–20.0) |
| Cotton | 143 (12.5%) | 6.1 (6.0; 5.0–10.0) |
| Silk | 103 (9.0%) | 3.6 (6.0; 5.0–12.0) |
| Buckwheat shell | 139 (12.2%) | 6.5 (6.0; 5.0–10.0) |
| Ricinus communis | 101 (8.8%) | 6.5 (6.0; 5.0–10.0) |
| Cat | 164 (14.3%) | 6.5 (6.0; 5.0–12.0) |
| Dog | 163 (14.2%) | 6.3 (6.0; 5.0–10.0) |
| Cockroach | 301 (26.3%) | 6.1 (6.0; 5.0–12.5) |
| Mold mix I | 227 (19.8%) | 7.5 (7.0; 5.0–12.0) |
| Mold mix II | 186 (16.3%) | 7.0 (6.0; 4.0–15.0) |
| Animal hair mix | 100 (8.7%) | 6.5 (6.0; 5.0–15.0) |
| Feather mix | 132 (11.5%) | 6.5 (6.0; 5.0–12.0) |
| Tobacco | 165 (14.4%) | 5.9 (6.0; 5.0–10.0) |
| Spring pollen mix I* | 55 (4.8%) | 8.3 (8.0; 4.5–16.0) |
| Spring pollen mix II* | 54 (4.7%) | 9.9 (10.0; 5.0–22.0) |
| Spring pollen mix III* | 59 (5.2%) | 9.9 (10.0; 4.5–21.0) |
| Summer/autumn pollen mix I* | 116 (10.1%) | 11.0 (10.0; 3.5–21.0) |
| Summer/autumn pollen mix II* | 58 (5.1%) | 9.5 (9.75; 5.0–17.0) |
| Platanus acerifolia * | 24 (2.1%) | 9.9 (10.0; 6.0–16.0) |
| Artemisia sieversiana * | 126 (11.0%) | 12.5 (12.0; 3.0–25.0) |
| Artemisia annua * | 117 (10.2%) | 12.5 (12.0; 5.0–22.0) |
| Ambrosia artemisiifolia * | 42 (3.7%) | 9.7 (8.0; 5.0–19.0) |
| Humulus scandens * | 75 (6.6%) | 13.0 (12.5; 5.0–24.5) |
Mold mix I: Penicillium chrysogenum, Aspergillus niger, Trichoderma koningii, Mucor racemosus, Rhizopus stolonifer; Mold mix II: Stemphylium spp., Curvularia lunata, Claclosporium macrocarpum, Helminthosporium sorokinanum, Saccharomyces cerevisiae; Animal hair mix: wool, camel hair, cony hair, pig hair, horse; Feather mix: chicken feather, duck feather, goose feather; Spring pollen mix I: Cryptomeria fortunei (Crypomeria japonica, sugi), Cunninghamia lanceolata (China fir), Populus deltoids (cottonwood), Ulmus pumila (Siberian elm), Salix caprea (willow); Spring pollen mix II: Betula verrucosa (birch), Platanus acerifolia (maple), Quercus alba (white oak), Juglans californica (walnut), Brassica campestris (rape); Spring pollen mix III: Morus alba (white mulberry), Ginkgo biloba (ginkgo), Spinacia oleracea (spinach), Typha angustifolia (bulrush); Summer/autumn pollen mix I: Helianthus annuus (sunflower), Xanthium sibiricum (cockle bur), Chenopodium album (goosefoot), Cannabis sativa (hemp fimble); Summer/autumn pollen mix II: Zea mays (corn), Sorghum vulgare (sorghum), Scirpus planiculmis (sedge), Ricinus communis (castor-oil-plant)
Pollen allergen sources used in intradermal tests
Number (percentage), total number is 1 144
Mean (median; range)
Specific IgE concentrations against A. vulgaris, Art v 1, A. artemisiifolia, Amb a 1, and H. scandens were measured using the ADVIA Centaur® (Siemens Medical Solutions, Diagnostics, NY, USA), which is a reverse sandwich immunoassay using direct chemo-luminescent technology (Petersen et al., 2004). Allergen extracts, calibrators, controls, and universal reagent packs were produced by ALK-Abelló (Hørsholm, Denmark). Single allergens Art v 1 and Amb a 1 were purified by ALK-Abelló as described by Jimeno et al. (2004). The assay procedure was automatically performed and the results are expressed as kU/L for H. scandens, A. vulgaris, and A. artemisiifolia, while for Art v 1 and Amb a 1 the results are reported in arbitrary units (AU/L) only. A result of ≥0.35 kU/L (or AU/L) was defined as positive: <0.35, class 0; 0.35–0.70, class 1; 0.70–3.50, class 2; 3.50–17.50, class 3; 17.50–50.00, class 4; 50.00–100.00, class 5; >100.00, class 6.
The linear regressions were plotted and P values were calculated at 95% confidence intervals using Graph Pad Prism Version 5.04.
3. Results
3.1. Intradermal reactivity against 25 allergen sources
Tangshan is the largest city in Hebei, a province with 68.5 million inhabitants located in the northern part of China. In the period mid-June to mid-October 2011, 1 144 subjects (aged 5–68 years; M/F, 612/532) with allergic symptoms were given intradermal tests. Of the 1 144 patients, 355 (31.0%) showed negative reactions to all allergens. Multiple sensitizations were common in the intradermal tests and 63.8% of subjects had positive reactions to more than three allergen sources. Positive reactions to the house dust mite were by far the largest group of positive reactions (40%). Of the 1 144 subjects tested, 170 (14.9%) had at least one positive reaction to pollens and 154 subjects (13.5%) were positive to weed pollens. The proportions of patients having skin positive responses to A. sieversiana, A. annua, A. artemisiifolia, and H. scandens were 11.0%, 10.2%, 3.7%, and 6.6%, respectively (Table 1). A significant correlation (P<0.001, N=174, R 2=0.69) of skin positive reaction (mm vs. mm, diameter) between A. sieversiana and A. annua was observed. No correlations were found by comparing intradermal test results among the other weed pollens.
3.2. Weed pollen allergen specific IgE
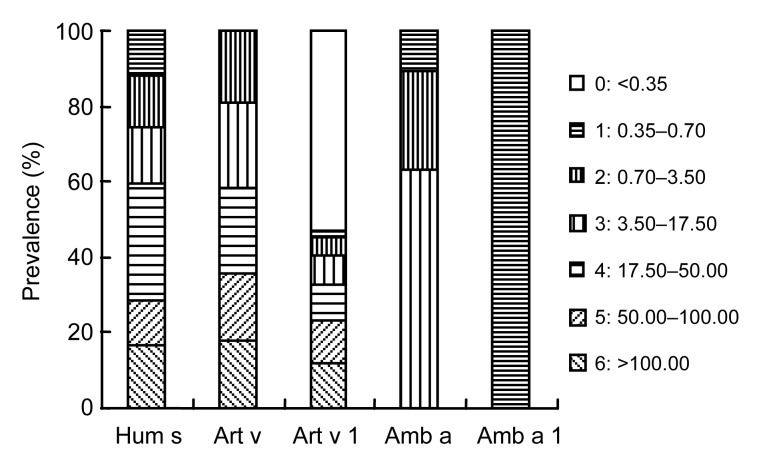
A total of 144 serum samples were collected from subjects with positive intradermal test results for pollens and analyzed for specific IgE against A. vulgaris, Art v 1, A. artemisiifolia, Amb a 1, and H. scandens. The results are shown in Fig. 1. The prevalence of IgE antibodies specific to A. vulgaris was 58.3%, to Art v 1 46.9%, to A. artemisiifolia 14.7%, to Amb a 1 11.2%, and to H. scandens 41.0%.
Fig. 1.
Prevalence of specific IgE reactivity (positive percentage in different classes) to weed pollen and individual major allergens (N=144)
Hum s: Humulus scandens
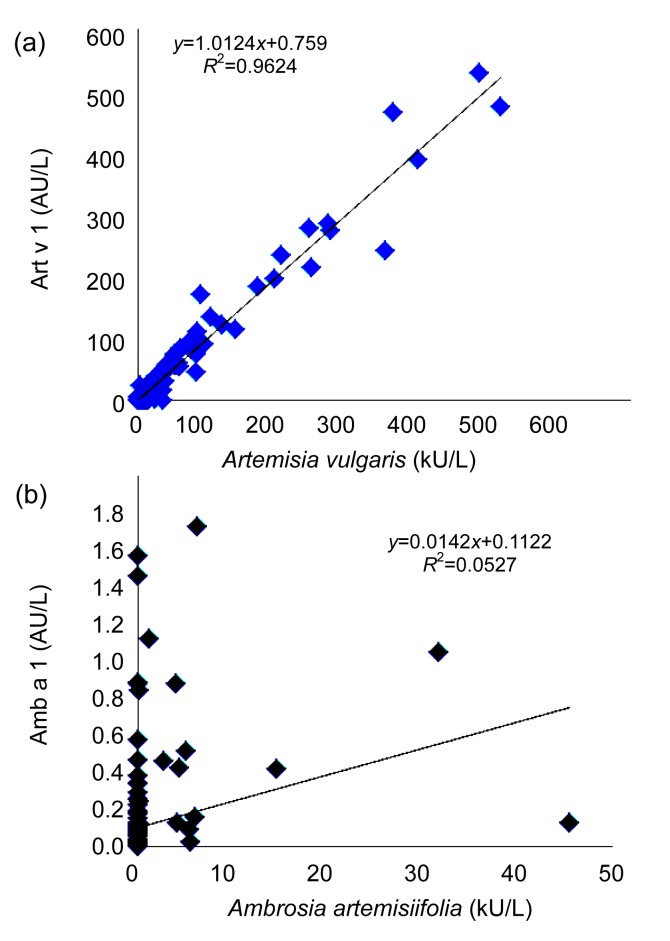
Table 2 shows that 64 of the 84 A. vulgaris specific IgE positive subjects (76.2%) were also found to be specific IgE positive to Art v 1. Only 3 of the 20 A. vulgaris negative subjects (15%) were IgE positive to Art v 1 (with low IgE levels). Among the 21 A. artemisiifolia IgE positive subjects, only 8 (38.1%) were IgE positive to Amb a 1. There were 16 subjects with specific IgE-reactivity towards Amb a 1 but only 8 of these subjects were specific IgE positive to A. artemisiifolia. The correlation for the presence of specific IgE between A. vulgaris and Art v 1 was very high (P<0.001, R 2=0.96, N=110). However, the correlation for the presence of specific IgE between A. artemisiifolia and Amb a 1 was low (R 2=0.05, Fig. 2). A. artemisiifolia specific IgE reactivity was strongly associated with A. vulgaris sensitivity. Almost all A. artemisiifolia sensitive subjects were sensitized to A. vulgaris, with a much higher IgE level. No subjects were found to be specific IgE mono-sensitized to A. artemisiifolia.
Table 2.
Combined specific IgE sensitizations
| Specific IgE | Sensitization (%) |
||||
| Humulus | Artemisia | Ambrosia | Art v 1 | Amb a 1 | |
| All (N=144) | 41.0 | 58.3 | 14.7 | 46.9 | 11.2 |
| Humulus IgE positive (N=59) | 100.0 | 49.2 | 20.3 | 33.9 | 11.9 |
| Artemisia IgE positive (N=84) | 34.5 | 100.0 | 22.6 | 76.2 | 19.1 |
| Ambrosia IgE positive (N=21) | 57.1 | 90.5 | 100.0 | 81.0 | 38.1 |
| Art v 1 IgE positive (N=67) | 29.9 | 99.5 | 25.4 | 100.0 | 23.9 |
| Amb a 1 IgE positive (N=16) | 43.8 | 100.0 | 50.0 | 100.0 | 100.0 |
Fig. 2.
Specific IgE correlations between Artemisia vulgaris and Art v 1 (a) and between Ambrosia artemisiifolia and Amb a 1 (b)
Of the 59 subjects with H. scandens specific IgE, 29 (49.2%) and 12 (20.3%) were found to be IgE positive to A. vulgaris and A. artemisiifolia, respectively. However, there were no correlations between H. scandens and A. vulgaris or between H. scandens and A. artemisiifolia for the presence of specific IgE.
A total of 144 serum samples were collected in this study. The highest number of sera with specific IgE against A. vulgaris was collected during August and September. For H. scandens, the highest number of sera was collected during August to October and, for A. artemisiifolia it was in September. However, the correlation (positive vs. negative) between intradermal tests and specific IgE tests was low in this study (data not shown).
4. Discussion
In this study, the sensitization of 1 144 subjects visiting an allergy clinic in northern China during the period June to October was tested by intradermal testing using a panel of 25 allergen sources. In agreement with other studies (Li et al., 2009; Yang et al., 2011), the highest prevalence of sensitization (40%) was found for house dust mites. The number of sera collected having specific IgE against weed pollens was highest during the period August to October, reflecting the period of peak levels of exposure to weed pollens in northern China. Of the 1 144 subjects visiting the clinic, 154 showed positive intradermal reactivity to weed pollens, suggesting a prevalence of weed pollen allergy of 13.5% among this selected group of subjects. The highest allergy prevalence among weed pollens was for the Artemisia species (10%–11%). A relatively high prevalence of 6.6% was also shown for Japanese hop, whereas the prevalence of ragweed allergy was below 4%. The percentages of positive reactions to mugwort and ragweed in intradermal tests in the present study were lower compared to those from SPT results obtained in a previous study (Li et al., 2009). The difference may be due to the methods used to measure skin sensitivity and/or differences in the allergenic extracts being used. Furthermore, in the present study all the subjects with allergic symptoms in the pollen season underwent intradermal tests whereas Li et al. (2009) investigated only selected subjects diagnosed with rhinitis and/or asthma throughout the year.
The present study shows a close correlation in intradermal skin sensitization between A. sieversiana and A. annua, suggesting a strong IgE-mediated cross-reactivity among the Artemisia species, in agreement with the findings of Katial et al. (1997). For Artemisia specific IgE testing, we chose to use A. vulgaris since it is best characterized of the Artemisia species.
Specific IgE testing showed that many weed pollen sensitized subjects are sensitized against two or more different weeds. A strong correlation between the presence of A. vulgaris and A. artemisiifolia specific IgE together with the finding that all subjects with ragweed IgE also had specific IgE against mugwort, suggests a high degree of IgE-mediated cross-reactivity between ragweed and mugwort. This is also in agreement with previous findings (Han et al., 2011). For Japanese hop, even if multiple sensitizations were evident, the lack of significant correlations between the presence of specific IgE of H. scandens and A. vulgaris and A. artemisiifolia, suggests a low degree of cross-reactivity towards other weed pollen allergens. This is further supported by the fact that the major allergen of Japanese hop appears to be a protein of 10 kDa that is unique to Japanese hop, and that its binding to H. scandens specific IgE cannot be inhibited by extracts from mugwort or ragweed (Park J.W. et al., 1999). Given its specific sensitization and the relatively high specific IgE prevalence of 41%, it is likely that pollen from Japanese hop is an important source for primary sensitization in northern parts of China.
Specific IgE tests in the present study suggest that mugwort and Japanese hop are important primary sensitizing allergen sources whereas the apparent ragweed sensitization is largely a result of IgE-mediated cross-reactivity primarily towards mugwort. This observation is supported by previous findings among allergic subjects from Beijing, China (Han et al., 2011). The reason why ragweed seems to be of minor importance as a primary sensitizing source in China compared to other parts of the world is probably related to the levels of pollen exposure in different geographical regions. Short ragweed is abundant in Europe and USA, but has also spread in northern China (Qiao and Ye, 2005). However, it grows best under warm and moist conditions, while low temperatures and inadequate water supply delay its development (Brandes and Nitzsche, 2006). The relatively dry climate and cold winters in northern China may hamper its development.
Movérare et al. (2011) showed that the prevalence of A. vulgaris specific IgE was significantly higher in serum samples from North Europe than in those from North America, but A. artemisiifolia specific IgE was more common in North American than in European samples. Asero et al. (2006) showed that in Italy only 7% of A. vulgaris sensitive patients were not sensitized to A. artemisiifolia, whereas 62% of A. artemisiifolia sensitive patients were not sensitized to A. vulgaris. In this study, the prevalence of specific IgE against tree and grass pollens has not been investigated, in part due to the complexity of tree and grass pollen mixtures used for intradermal testing, and to the relatively low skin-prick and specific IgE prevalence reported elsewhere (Li et al., 2009; Zheng et al., 2011).
As the flowering periods of Japanese hop, mugwort, and ragweed are partly overlap in northern China, accurate weed pollen specific diagnosis is very difficult during routine clinical practice. The poor correlation between intradermal tests and specific IgE measurements in this work indicates that diagnosis of specific pollen allergies cannot be achieved solely by intradermal testing. For example, there were 21 subjects who showed positive reactions to H. scandens in intradermal tests, but no specific IgE to H. scandens. On the other hand, 10 subjects with negative skin reactions to Humulus had high Humulus specific IgE concentrations. The use of major allergens as diagnostic tools may be of help to facilitate proper diagnosis. Art v 1 is a major allergen with a prevalence of specific IgE in 79%–95% of Artemisia allergic patients (Himly et al., 2003; Oberhuber et al., 2008). The present study showed that 76% of patients positive to Artemisia were also positive to Art v 1; only 15% of Artemisia negative patients were Art v 1 positive. There was a very strong correlation between the levels of specific IgE to Art v 1 and to A. vulgaris, in agreement with Han et al. (2011), indicating that generally Art v 1 can be regarded as a good indicator of true ragweed sensitization. A previous study also showed that Amb a 1 was a major allergen in ragweed and more than 95% of ragweed allergic patients have been reported to have IgE antibodies to Amb a 1 (Gadermaier et al., 2008). However, in this study the IgE positive reaction against ragweed was likely due to cross-reactivity with mugwort. Similar specific molecular indicators for Japanese hop sensitization still need to be identified.
In conclusion, based on intradermal testing and specific IgE measurements, pollens of A. vulgaris and H. scandens are independent and important allergen sources giving rise to a relatively high prevalence of allergic sensitizations in northern parts of China.
Acknowledgments
The authors would like to thank Tian-tian LIU and Hai-feng ZHONG, from ALK-Abelló A/S, Guangzhou, China, for the IgE measurements.
References
- 1.Asero S, Wopfner N, Gruber P, Gadermaier G, Ferreira F. Artemisia and Ambrosia hypersensitivity: co-sensitization or co-recognition. Clin Exp Allergy. 2006;36(5):658–665. doi: 10.1111/j.1365-2222.2006.02477.x. [DOI] [PubMed] [Google Scholar]
- 2.Brandes D, Nitzsche J. Biology, introduction, dispersal, and distribution of common ragweed (Ambrosia artemisiifolia L.) with special regard to Germany. Nachrichtenbl Deut Pflanzenschutzd. 2006;58(11):286–291. [Google Scholar]
- 3.Gadermaier G, Wopfner N, Wallner M, Egger M, Didierlaurent A, Regl G, Aberger F, Lang R, Ferreira F, Hawranek T. Array-based profiling of ragweed and mugwort pollen allergens. Allergy. 2008;63(11):1543–1549. doi: 10.1111/j.1398-9995.2008.01780.x. [DOI] [PubMed] [Google Scholar]
- 4.Han D, Lai X, Gjesing B, Zhong NS, Zhang L, Spangfort M. The specific IgE reactivity pattern of weed pollen-induced allergic rhinitis patients. Acta Ota-Laryngol. 2011;131(5):533–538. doi: 10.3109/00016489.2010.539265. [DOI] [PubMed] [Google Scholar]
- 5.Himly M, Jahn-Schmid B, Dedic A, Kelemen P, Wopfner N, Altmann F, van Ree R, Briza P, Richter K, Ebner C, et al. Art v 1, the major allergen of mugwort pollen, is a modular glycoprotein with a defensin-like and a hydroyproline-rich domain. FASEB J. 2003;17(1):106–108. doi: 10.1096/fj.02-0472fje. [DOI] [PubMed] [Google Scholar]
- 6.Jimeno L, Duffort O, Serrano C, Barber D, Polo F. Monoclonal antibody-based ELISA to quantify the major allergen of Artemisia vulgaris pollen, Art v 1. Allergy. 2004;59(9):995–1001. doi: 10.1111/j.1398-9995.2004.00464.x. [DOI] [PubMed] [Google Scholar]
- 7.Kang BC, Johnson J, Veres-Thorner C. Atopic profile of inner-city asthma with a comparative analysis on the cockroach-sensitive and ragweed-sensitive subgroups. J Allergy Clin Immunol. 1993;92(6):802–811. doi: 10.1016/0091-6749(93)90057-M. [DOI] [PubMed] [Google Scholar]
- 8.Katial RK, Lin FL, Stafford WW, Ledoux RA, Westley CR, Weber RW. Mugwort and sage (Artemisia) pollen cross-reactivity: ELISA inhibition and immunoblot evaluation. Ann Allergy Asthma Immunol. 1997;79(4):340–346. doi: 10.1016/S1081-1206(10)63025-6. [DOI] [PubMed] [Google Scholar]
- 9.Krämer U, Link E, Behrendt H. Geographic and time trends of pollen count due to beeches, grass and mugwort (Artemisia) in Germany. Pneumologie. 2001;55(5):229–230. doi: 10.1055/s-2001-13945. (in German) [DOI] [PubMed] [Google Scholar]
- 10.Li J, Sun B, Huang Y, Lin X, Zhao D, Tan G, Wu J, Zhao H, Cao L, Zhong N Chinese Alliance of Research on Respiratory Allergic Disease (CARRAD) A multicenter study assessing the prevalence of sensitizations in patients with asthma and/or rhinitis in China. Allergy. 2009;64(7):1083–1092. doi: 10.1111/j.1398-9995.2009.01967.x. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Huang Y, Lin XP, Zhao D, Tan G, Wu J, Zhao C, Zhao J, Spangfort MD, Zhong N China Alliance of Research on Respiratory Allergic Disease (CARRAD) Influence of degree of specific allergic sensitivity on severity of rhinitis and asthma in Chinese allergic patients. Respir Res. 2011;12(1):95–103. doi: 10.1186/1465-9921-12-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Movérare R, Larsson H, Carlsson R, Holmquist L. Mugwort-sensitized individuals from North Europe, South Europe and North America show different IgE reactivity patterns. Int Arch Allergy Immunol. 2011;154(2):164–172. doi: 10.1159/000320231. [DOI] [PubMed] [Google Scholar]
- 13.Oberhuber C, Ma Y, Wopfner N, Gadermaier G, Dedic A, Niggemann B, Maderegger B, Gruber P, Ferreira F, Scheiner O, et al. Prevalence of IgE-binding to Art v 1, Art v 4 and Amb a 1 in mugwort-allergic patients. Int Arch Allergy Immunol. 2008;145(2):94–101. doi: 10.1159/000108134. [DOI] [PubMed] [Google Scholar]
- 14.Park HS, Jung KS, Jee SY, Hong SH, Kim HY, Nahm DH. Are there any links between Hop Japanese pollen and other weed pollens or food allergens on skin prick tests? Allergy Asthma Proc. 2001;22(1):43–46. doi: 10.2500/108854101778249186. [DOI] [PubMed] [Google Scholar]
- 15.Park JW, Ko SK, Kim CW, Jeoung BJ, Hong CS. Identification and characterization of the major allergen of the Humulus japonicus pollen. Clin Exp Allergy. 1999;29(8):1080–1086. doi: 10.1046/j.1365-2222.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 16.Petersen AB, Gudmann P, Milvang-Gronager P, Mørkeberg R, Bøgestrand S, Linneberg A, Johansen N. Performance evaluation of a specific IgE assay developed for the ADVIA Centaur_immunoassay system. Clin Biochem. 2004;37(10):882–892. doi: 10.1016/j.clinbiochem.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Qiao B, Ye S. Color Atlas of Air-Borne Pollens and Plants in China. Beijing, China: Peking Union Medical College Press; 2005. p. 194. (in Chinese) [Google Scholar]
- 18.Schäfer T, Krämer U, Dockery D, Vieluf D, Behrendt H, Ring J. What makes a child allergic? Analysis of risk factors for allergic sensitisation in preschool children from East and West Germany. Allergy Asthma Proc. 1999;20(1):23–27. doi: 10.2500/108854199778681477. [DOI] [PubMed] [Google Scholar]
- 19.Schmid-Grendelmeier P. Pollinosis: clinical aspects and epidemiology. Contribution of the Allergy Clinic 1948-1998. Schweiz Rundsch Med Prax. 1998;87:1300–1308. (in German) [PubMed] [Google Scholar]
- 20.Spieksma FT, Charpin H, Nolard N, Stix E. City spore concentrations in the European Economic Community (EEC). IV. Summer weed pollen (Rumex, Plantogo, Chenopodiaceae, Artemisia), 1976 and 1977. Clin Exp Allergy. 1980;10:319–329. doi: 10.1111/j.1365-2222.1980.tb02114.x. [DOI] [PubMed] [Google Scholar]
- 21.Verini M, Rossi N, Verrotti A, Pelaccia G, Nicodemo A, Chiarelli F. Sensitization to environmental antigens in asthmatic children from a central Italian area. Sci Total Environ. 2001;270(1-3):63–69. doi: 10.1016/S0048-9697(00)00798-1. [DOI] [PubMed] [Google Scholar]
- 22.Yang Y, Zhao Y, Wang CS, Wang XD, Zhang L. Prevalence of sensitization to aeroallergens in 10 030 patients with allergic rhinitis. Chin J Otolaryngol Head Neck Surg. 2011;46:914–920. (in Chinese) [PubMed] [Google Scholar]
- 23.Yin J, Ye S, Gu R. A preliminary investigation into main allergenic pollens during autumnal seasons in Beijing area. Chin J Microbiol Immunol. 1996;16:31–36. (in Chinese) [Google Scholar]
- 24.Zheng YW, Li J, Lai XX, Zhao DY, Liu XF, Lin XP, Gjesing B, Palazzo P, Mari A, Zhong NS, et al. Allergen micro-array detection of specific IgE reactivity in Chinese allergic patients. Chin Med J. 2011;124:4350–4354. [PubMed] [Google Scholar]