Abstract
Study Design
Prospective experimental study.
Purpose
To evaluate bacterial contamination during surgery.
Overview of Literature
The participants of surgery and ventilation system have been known as the most significant sources of contamination.
Methods
Two pairs of air culture blood agar plate for G(+) bacteria and MacConkey agar plate for G(-) bacteria were placed at 3 different locations in a conventional operation room: in the surgical field, under the airflow of local air conditioner, and pathway to door while performing spine surgeries. One pair of culture plates was retrieved after one hour and the other pair was retrieved after 3 hours. The cultured bacteria were identified and number of colonies was counted.
Results
There was no G(-) bacteria identified. G(+) bacteria grew on all 90 air culture blood agar plates. The colony count of one hour group was 14.5±5.4 in the surgical field, 11.3±6.6 under the local air conditioner, and 13.1±8.7 at the pathway to the door. There was no difference among the 3 locations. The colony count of 3 hours group was 46.4±19.5, 30.3±12.9, and 39.7±15.2, respectively. It was more at the surgical field than under the air conditioner (p=0.03). The number of colonies of one hour group was 13.0±7.0 and 3 hours group was 38.8±17.1. There was positive correlation between the time and the number of colonies (r=0.76, p=0.000).
Conclusions
Conventional operation room was contaminated by G(+) bacteria. The degree of contamination was most high at the surgical field. The number of bacteria increased right proportionally to the time.
Keywords: Bacterial contamination, Surgical site infection, Source of contamination
Introduction
Surgical site infection is one of the most serious complications in any kind of surgery. The infection rate of spine surgery was lower than that of arthroplasty at the early developing period [1]. However, the rate of surgical infection surpassed arthroplasty recently [2]. There are several presumptive factors that increase the infection rate. Surgery associated factors include an increase in scale and complexity of spine surgery and higher frequency of instrumentation [3]. Patient associated factors include old age and increased prevalence in coexistence of metabolic diseases which decrease host immunity. The advent of antibiotics made the recalcitrant surgical site infection as an amenable complication. The prophylactic use of antibiotics can reduce the surgical site infection effectively, but given that Methicillin-resistant Staphylococcus aureus (MRSA) emerged within 2 years after Methicillin began to be used, it is not everything to control surgical site infection [4,5]. With the help of disinfectants and ultraclean air technology, bacterial contamination of the operation environment has been reduced. Unfortunately, it is not easy to introduce the total body exhaust gown system to spine surgery because the application of surgical microscope and bright illumination is troublesome under such system.
The most consequential factors for surgical site infection is bacterial contamination which overwhelms the host immunity. Herein, the control of bacterial contamination and appropriate reduction of the soiled bacteria would be the most effective method to prevent a surgical site infection and the use of antibiotics could be minimized, so to retard the advent of resistant strains. An epidemiologic study of the routes and sources of contamination should be conducted to determine preventive measures. The presumptive source of contamination is the direct surgical participants, include the surgeon and scrub nurse. In addition, the traffic in the operation theater and the ventilation system can influence contamination. These factors would be related with the surgical time. The quantitative analysis, however, has not been reported.
We investigated the degree of bacterial contamination according to the locations which concern the surgical participants and ventilation system, and according to the elapsed time. With the results, the most significant source of contamination was presumed. In the light of above study, the pertinent method to minimize the bacterial contamination while performing spine surgery can be developed.
Materials and Methods
The experiment was conducted in the same operation room while performing instrumented posterior spinal fusion in a consecutive series of patients, between July 1, 2010 and July 31, 2010. The operation room had an ordinary wall mounted air-conditioner and a high efficiency paticulate air (HEPA) filter. To check the condition of the air in operation room, the concentration of floating microorganisms was measured 3 times at 5 days before the commencement. In each case, there were 4 direct participants: main surgeon, first assistant, and scrub nurse (who stood at the surgical field), and a second assistant (who joined 30 minutes after the beginning to handle the local bone stood at the distal portion of the instrument table). There were 3 indirect participants who included an anesthesiologist, a circulating nurse, and a technician for radiologic examination (who came into the room twice each surgery). All participants were not the same throughout the experiment.
The surgical field was disinfected by 10% povidone-iodine and 70% alcohol and covered by disposable surgical drape. Each exposed incision site was covered by 2% iodine impregnated drape (Ioban 2). Air culture blood agar plates for G(+) bacteria and MacConkey agar plates for G(-) bacteria were placed as a pair in the following places: surgical field, under the air conditioner where the plates face the air flow directly, and the pathway to the door where indirect participants frequently entered and exited. The plates under the local air-conditioner and pathway were placed about one meter away from floor. There were 2 pairs of plates at each place, total 12 plates in a case of surgery (Fig. 1). The plates were retrieved at 2 different times; 3 pairs from each place at one hour and the remaining 3 pairs at 3 hours after commencement. Room temperature was checked at 3 hours after commencement. The air conditioner was turned on when general anesthesia was induced and turned off from the moment when the instruments were unpacked to the moment the surgery was completed. The demography of the patients was not considered to be criteria for study inclusion and the cases that were finished earlier than 3 hours were excluded. Finally, 6 groups from the 15 cases were included. There were 180 plates in 90 pairs of 2. The plates were cultured for one day in 37℃ incubator (Forma Scientific, Vernon Hill, IL, USA). Each colony was re-implanted to another plate, cultured one day more, then inoculated at the discrimination kit (bioMerieux SA, Lyon, France). Bacterial identification was performed the next day. Total number of colonies was counted in original plates at day 7 (Fig. 2). All microbiologic studies were performed by a laboratory medicine doctor who didn't have information at all about the experiment. The types of bacteria and number of colonies were investigated according to their locations and collection times. The increasing rate, according to the elapsed time, was investigated as well. Statistical analysis was performed with SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA) package and details were as follows; Kolmogorov Smirnov test for the normal distribution of each group, one way analysis of variance and Turkey's b test for the difference according to the locations, paired t test, and Spearman correlation test for the difference according to the elapsed time.
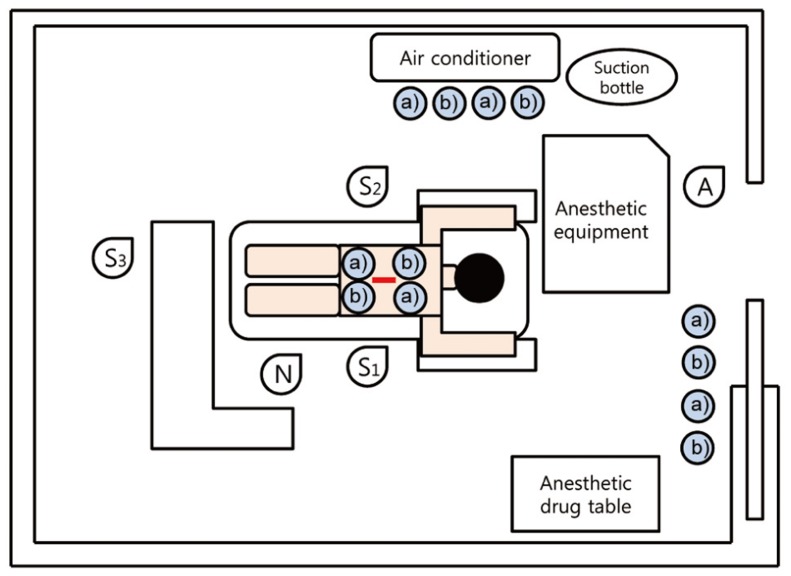
Fig. 1.
Two pairs of air culture blood agar plates for G(+) bacteria and MacConkey agar plates for G(-) bacteria were placed at surgical field, under the airflow of local air conditioner and pathway for indirect participants (total 12 plates). S1, operator; S2, 1st assistant; S3, 2nd assistant; N, scrub nurse; A, anesthesiologist. a)blood agar plate, b)MacConkey agar plate.
Fig. 2.

A total number of colonies was counted on the original plates at day 7.
Results
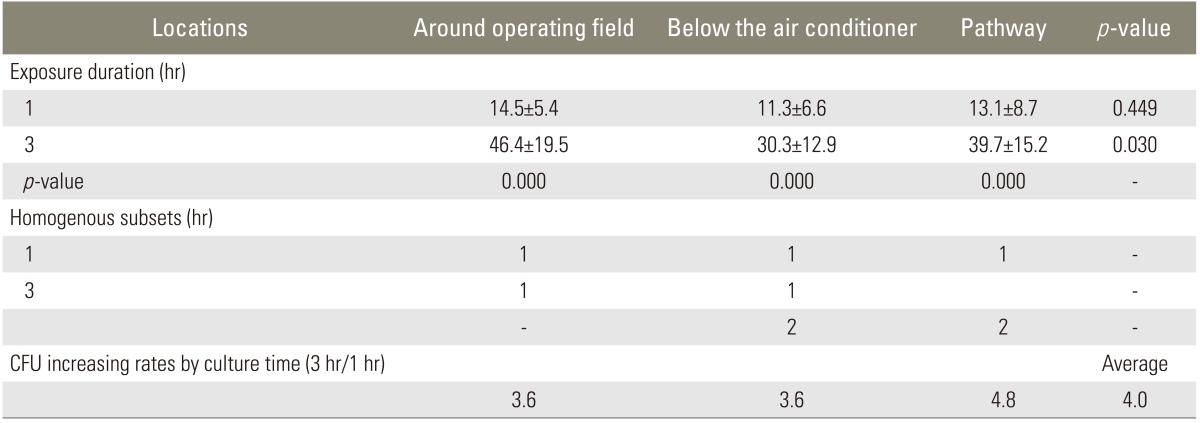
The quality of operation room air was better than the official standard (800 colony forming unit [CFU]/m2) (Table 1). The room temperature under the air-flow of air conditioner was significantly lower (p=0.00) at 20.9±2.3℃, while the surgical field and pathway to the door were similar (p=0.15), as 27.8±3.1℃ and 25.9±2.0℃, respectively. G(-) bacteria were not cultured at all 90 MacConkey agar plates while G(+) bacteria were cultured at all 90 air culture blood agar plates. The sorts of bacteria were as follows; S. aureus on 19 out of 90 plates (21%), S. epidermidis on 90 out of 90 plates (100%), micrococcus on 79 out of 90 plates (88%), and S. capitis on 17 out of 90 plates (19%). All 6 groups had a normal distribution. In the one hour retrieval groups, the number of colonies was 14.5±5.4 at the surgical field, 11.3±6.6 under the air-flow of local air conditioner, and 13.1±8.7 at the pathway to the door. The differences were not statistically significant. In the 3 hour retrieval group, the number of colonies was 46.4±19.5 at the surgical field, 30.3±12.9 under the air-flow of air conditioner, and 39.7±15.2 at the pathway to the door. The surgical field showed more colonies than under the air-flow of air conditioner (p=0.03). The average number of colonies of one hour retrieval group was 13.0±7.0 and the average number of colonies of the 3-hour retrieval group was 38.8±17.1 (Table 2). There was significant difference between the two (p=0.006) and positive correlation between the number of colonies and elapsed time (r=0.76, p=0.000). The increasing rate of the 3 places were not different (p=0.475).
Table 1.
Air quality of the operating room
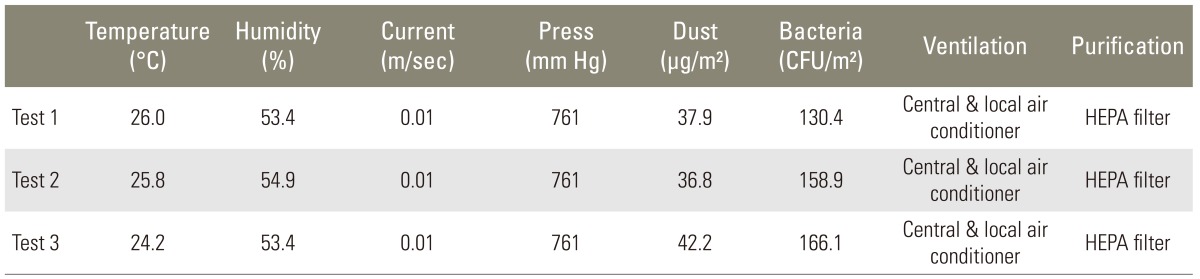
CFU, colony forming unit; HEPA, high efficiency particulate air filter.
Table 2.
Colony forming unit (CFU) on three different sites in the operating room
Discussion
Surgical site infection can be fatal or lead to a poor prognosis, causing critical damage to both patients and surgeons. Astagneau et al. [6] noted that 38% of post-surgical patient deaths are the direct consequences of surgical site infection. In orthopedic surgeries, the infection rate of total hip arthroplasty was 7%, however, Charnley [7] reduced it to 0.5% even without prophylactic antibiotics by using the ultraclean air technology. On the other hand Fitzgerald et al. [8] reduced it to 1%, by administration of prophylactic antibiotics in conventional operation rooms. From this point onward, the 2 methods have been accepted as mainstays to prevent surgical site infection after total hip arthroplasty. In 1960's, the infection rate of uninstrumented fusion surgeries for scoliosis was 0.9%, which was much lower than that of total hip arthroplasties at the same period [1]. After the advent of Harrington instrumentation the infection rate was increased up to 9.3% without antibiotics. However, the application of prophylactic antibiotics reduced the instrumentation infection rate to 2.8% [2] and even lower to 1% in adolescent idiopathic scoliosis surgery [9]. The infection rate of spine surgery, as a whole, rose to 5% again due to the increased magnitude and complexity of the surgery; prophylatic antibiotics were not able to prevent this rise in infection rate [3]. Prophylactic use of antibiotics has been accepted as a mainstay to prevent surgical site infection for more than 30 years [10], an acceptance that occurred after the study by Horwitz and Curtin [11]. Ultraclean air technology is composed of vertical exponential laminar flow and a total body exhaust gown. Though there was a study that applied ultraclean air technology to spine surgery [12], it is not easy to use the total body exhaust gown in spine surgery, which usually needs strong illumination and a surgical microscope. Meanwhile, the overuse of antibiotics has been followed by the immediate advent of resistant strains. Denmark surveyed transformation on S. aureus throughout the country for the 20 years after the first discovery of MRSA. The ratio of MRSA was 45% in 1967. It was reduced, however, to 6% in late 1980s. This was attributed to the reduced administration of antibiotics and strict control of hospital hygiene by epidemiologic quarantine of bacterial contamination [13]. Consequently, the resistant strains had become susceptible to treatment. Given the historical evidence, the overuse of antibiotics and the following advent of resistant strains seemed like an endless competition of armaments. Klekamp et al. [14] indicated that over use of antibiotics increased infection by MRSA and G(-) bacteria. It would be a better strategy to clean up the environment of operation rooms and control the contamination route of surgical field to prevent surgical site infection and bacterial mutation. There are patient factors, surgical factors and environmental factors that contribute to the development of surgical site infection. Patient factors, such as older age, and surgical factors, such as the expansion of complexity and magnitude of a surgery, create a higher risk of infections [15]. The largest source of bacterial contamination in surgical site infection is the bacteria of the patient's own skin, followed by the participants in a surgery [16]. There have been many studies that have investigated how to disinfect patient skin and to determine what kind of disinfectants is superior. There are very few studies on how the participants contaminate the operation room or how to control it. A combination of 10% povidone Iodine and 70% alcohol was used to disinfect the skin of patients in this experiment. One log CFU of bacteria, however, remain on average after this treatment [17]. Therefore, the area of skin exposure was sealed by 2% iodine impregnated drape (Ioban 2) to minimize bacterial transmission from the patient's skin.
For the purpose of investigating the influence of direct surgical participants, operation room traffic and ventilation system, the culture plates were placed at 3 different places near the surgeons, the ventilation system and the circulating participants. At first, we anticipated that the degree of contamination would be highest under the direct air flow of local air conditioner and least at the surgical field. Under the facility with ultraclean air technology vertical airflow should run vertically from directly above the surgical field. In the conventional operation room, where we performed this experiment, the air flow was turned down to avert a direct current which contains contaminated microorganisms. The results, however, were completely opposite in that bacterial contamination was less under direct air-flow. In light of the above evidence, we presume that bacterial soiling is more likely to occur under the static current. If the air-flow of local air conditioner is adjusted to run directly to the surgical field, bacterial contamination is expected to be reduced. Thus, we decided not to turn off the local air conditioner while performing instrumentation. The air quality of the operation room was maintained better than the official recommendation. It is not certain that the same result can be achieved under a more contaminated atmosphere.
Given that the degree of contamination of the surgical field and pathway were both highly contaminated, continuous, and close exposure to participants even under scrub state is considered to increase bacterial contamination unavoidable. It is considered very natural that the more participants join the surgery even under scrub state, the more bacteria contaminate the surgical field.
It was already proved by several researchers that longer surgical times lead to higher infection rates [18-21]. We would like to investigate the quantitative correlation between the degree of contamination and the elapsed time. The number of colonies was increased 3 times after 3 hours comparing with 1 hour, a far more rapid increment than expected. It presented how important reducing surgery time is to prevent surgical site infection.
There are 2 modalities to reduce the number of contaminated bacteria thereby preventing it from reaching critical levels of infection development, administration of prophylactic antibiotics and dilution of bacteria by irrigation. With elapsed time, contaminated bacteria can resist against both antibiotics and irrigation by forming a biofilm [22]. Hence, there is the need for prophylactic antibiotics to be administered one hour prior to surgery [23]. Repeated irrigation from an early stage is important according to the report of Owen and Wenke [24], who reported that the effect of irrigation to reduce the number of bacteria decreased as time elapsed. In the case of instrumentation surgery or using local chip bone, which is achieved while performing decompression, it is unavoidable that the degree of contamination increases proportionally to the elapsed time. Therefore, it is very important not to expose the instruments until the moment of instrumentation and irrigation of local chip bone before being grafted to minimize contamination of bacteria [15].
Bacteria of the pathway were surely expected to be brought along the circulating participants. Massie et al. [21] reported that frequent come and go of non-scrub participants could increase the surgical site infection and Hodges et al. [25] indicated that the expertise of participants was an important factor in prevention of infection. This study could not reveal the difference of the operation field and the operation room traffic, however, limiting entry of non-scrub participants is not possible, control of unnecessary moving, disinfection and maintenance of cleanliness of the suits and portable X-ray machine can be done to decrease contamination. Moreover, identification of contaminated bacteria by frequent microbial culture of air and apparatus of operation room is necessary to forecast causative bacteria of surgical site infection and to decide what kind of prophylactic antibiotics should be used.
Conclusions
The air of operation room is contaminated by G(+) bacteria. The surgical field is more severely contaminated by bacteria than under the direct air-flow of local air conditioner. The results of this study did not reveal the influence of operation room traffic. Reducing surgical time is the cardinal factor. Direct air current did not increase the bacterial contamination.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Moe JH, Gustilo RB. Treatment of scoliosis: results in 196 patients treated by cast correction and fusion. J Bone Joint Surg Am. 1964;46:293–312. [PubMed] [Google Scholar]
- 2.Lonstein J, Winter R, Moe J, Gaines D. Wound infection with Harrington instrumentation and spine fusion for scoliosis. Clin Orthop Relat Res. 1973;(96):222–233. [PubMed] [Google Scholar]
- 3.Picada R, Winter RB, Lonstein JE, et al. Postoperative deep wound infection in adults after posterior lumbosacral spine fusion with instrumentation: incidence and management. J Spinal Disord. 2000;13:42–45. doi: 10.1097/00002517-200002000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Tomasz A. Accelerated evolution: emergence of multidrug resistant gram-positive bacterial pathogens in the 1990's. Neth J Med. 1998;52:219–227. doi: 10.1016/s0300-2977(98)00035-7. [DOI] [PubMed] [Google Scholar]
- 5.Tomasz A. Multiple-antibiotic-resistant pathogenic bacteria: a report on the Rockefeller University Workshop. N Engl J Med. 1994;330:1247–1251. doi: 10.1056/NEJM199404283301725. [DOI] [PubMed] [Google Scholar]
- 6.Astagneau P, Rioux C, Golliot F, Brucker G, INCISO Network Study Group Morbidity and mortality associated with surgical site infections: results from the 1997-1999 INCISO surveillance. J Hosp Infect. 2001;48:267–274. doi: 10.1053/jhin.2001.1003. [DOI] [PubMed] [Google Scholar]
- 7.Charnley J. Postoperative infection after total hip replacement with special reference to air contamination in the operating room. Clin Orthop Relat Res. 1972;87:167–187. doi: 10.1097/00003086-197209000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Fitzgerald RH, Jr, Nolan DR, Ilstrup DM, Van Scoy RE, Washington JA, 2nd, Coventry MB. Deep wound sepsis following total hip arthroplasty. J Bone Joint Surg Am. 1977;59:847–855. [PubMed] [Google Scholar]
- 9.Lenke LG, Bridwell KH, Baldus C, Blanke K, Schoenecker PL. Cotrel-Dubousset instrumentation for adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1992;74:1056–1067. [PubMed] [Google Scholar]
- 10.Hill C, Flamant R, Mazas F, Evrard J. Prophylactic cefazolin versus placebo in total hip replacement: report of a multicentre double-blind randomised trial. Lancet. 1981;1:795–796. doi: 10.1016/s0140-6736(81)92678-7. [DOI] [PubMed] [Google Scholar]
- 11.Horwitz NH, Curtin JA. Prophylactic antibiotics and wound infections following laminectomy for lumber disc herniation. J Neurosurg. 1975;43:727–731. doi: 10.3171/jns.1975.43.6.0727. [DOI] [PubMed] [Google Scholar]
- 12.Gruenberg MF, Campaner GL, Sola CA, Ortolan EG. Ultraclean air for prevention of postoperative infection after posterior spinal fusion with instrumentation: a comparison between surgeries performed with and without a vertical exponential filtered air-flow system. Spine (Phila Pa 1976) 2004;29:2330–2334. doi: 10.1097/01.brs.0000142436.14735.53. [DOI] [PubMed] [Google Scholar]
- 13.Westh H, Jarlov JO, Kjersem H, Rosdahl VT. The disappearance of multiresistant Staphylococcus aureus in Denmark: changes in strains of the 83A complex between 1969 and 1989. Clin Infect Dis. 1992;14:1186–1194. doi: 10.1093/clinids/14.6.1186. [DOI] [PubMed] [Google Scholar]
- 14.Klekamp J, Spengler DM, McNamara MJ, Haas DW. Risk factors associated with methicillin-resistant staphylococcal wound infection after spinal surgery. J Spinal Disord. 1999;12:187–191. [PubMed] [Google Scholar]
- 15.Ahn DK, Choi DJ, Park HS, Kim TW, Chun TH, Yang JH. Precautions against infection following posterior spinal fusion based on types of infection and risk factors. J Korean Soc Spine Surg. 2009;16:274–284. [Google Scholar]
- 16.Ayliffe GA. Role of the environment of the operating suite in surgical wound infection. Rev Infect Dis. 1991;13(Suppl 10):S800–S804. doi: 10.1093/clinids/13.supplement_10.s800. [DOI] [PubMed] [Google Scholar]
- 17.Kramer A, Assadian O, Lademann J. Prevention of postoperative wound infections by covering the surgical field with iodine-impregnated incision drape (Ioban 2) GMS Krankenhhyg Interdiszip. 2010;5:Doc08. doi: 10.3205/dgkh000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olsen MA, Mayfield J, Lauryssen C, et al. Risk factors for surgical site infection in spinal surgery. J Neurosurg. 2003;98:149–155. [PubMed] [Google Scholar]
- 19.Aydinli U, Karaeminogullari O, Tiskaya K. Postoperative deep wound infection in instrumented spinal surgery. Acta Orthop Belg. 1999;65:182–187. [PubMed] [Google Scholar]
- 20.Wimmer C, Gluch H, Franzreb M, Ogon M. Predisposing factors for infection in spine surgery: a survey of 850 spinal procedures. J Spinal Disord. 1998;11:124–128. [PubMed] [Google Scholar]
- 21.Massie JB, Heller JG, Abitbol JJ, McPherson D, Garfin SR. Postoperative posterior spinal wound infections. Clin Orthop Relat Res. 1992;(284):99–108. [PubMed] [Google Scholar]
- 22.Gristina AG, Naylor PT, Myrvik QN. Mechanisms of musculoskeletal sepsis. Orthop Clin North Am. 1991;22:363–371. [PubMed] [Google Scholar]
- 23.Fry DE. Surgical site infections and the surgical care improvement project (SCIP): evolution of national quality measures. Surg Infect (Larchmt) 2008;9:579–584. doi: 10.1089/sur.2008.9951. [DOI] [PubMed] [Google Scholar]
- 24.Owens BD, Wenke JC. Early wound irrigation improves the ability to remove bacteria. J Bone Joint Surg Am. 2007;89:1723–1726. doi: 10.2106/JBJS.F.01210. [DOI] [PubMed] [Google Scholar]
- 25.Hodges SD, Humphreys SC, Eck JC, Covington LA, Kurzynske NG. Low postoperative infection rates with instrumented lumbar fusion. South Med J. 1998;91:1132–1136. doi: 10.1097/00007611-199812000-00007. [DOI] [PubMed] [Google Scholar]