Abstract
Molecular genetic analyses of archival formalin-fixed, paraffin-embedded (FFPE) pathological specimens taken at biopsy or autopsy, are occasionally compromised because the DNA molecules therein are inevitably degraded. Furthermore, since these tissue samples comprise various cell types, the analyses based on mixtures of such heterogeneous populations often fail to reflect the nature of the affected cells. In the present study, to elucidate the contribution of β-catenin gene mutation to the fundic gland polyp and the heterotopic gastric mucosa in the duodenum, we successfully introduced an agarose-bead mediated technique as an effectual tool for retrospective morphology-oriented genetic analyses. Microdissected samples were embedded in low-melting agarose, and directly treated with proteinase K. A fragment of the agarose-bead was used as a template for polymerase chain reaction to analyze β-catenin mutation. Of the six cases of heterotopic gastric mucosa in the duodenum associated with fundic gland polyps, one showed a common 1-bp missense mutation at codon 37 shared by both the fundic gland polyp and the heterotopic gastric mucosa. Alternatively, a 1-bp silent mutation at codon 33 and missense mutation at codon 32 were identified only in the heterotopic gastric mucosa. Agarose-bead mediated technique shows superior sensitivity to the previously described techniques and is an effectual tool for retrospective morphology-oriented genetic analyses using a large number of archival pathological samples stored for long periods in the pathology laboratory.
Keywords: beta-catenin, duodenum, microdissection, DNA sequencing
I. Introduction
With the introduction of the polymerase chain reaction (PCR) technique, genetic alterations as minute as the point mutation of a specific gene can be detected from routine formalin-fixed, paraffin-embedded (FFPE) pathological specimens. Inevitably, however, the process, starting with tissue samples like pathological specimens taken at biopsy or autopsy, involves various cell types including infiltrating inflammatory and reactive stromal cells. Therefore, molecular genetic analyses of such mixed, heterogeneous cells sometimes fail to reflect the nature of the affected population. To overcome this drawback, the selection of target cells by microdissection is crucial to assessing the molecular pathogenesis of disease, especially when in situ evaluation is difficult. To date, however, although PCR has been sensitive enough to amplify target DNA from a single cell, the effectual genetic analysis of FFPE microdissected samples is often circumscribed.
The fundic gland polyp origination from gastric mucosa is the most common gastric polyp, and since it often occurs, besides being sporadic, in association with familial adenomatous polyposis (FAP), it is thought to carry an abnormality to the Wnt signaling pathway [1, 9]. Frequent somatic mutations of the β-catenin gene, one of the main components of the Wnt pathway, have recently been reported in sporadic fundic gland polyps [1, 9]. Furthermore, because patients with fundic gland polyp often have heterotopic gastric mucosa of the duodenum, we speculated that heterotopic gastric mucosa of the duodenum might carry the same genetic alterations as fundic gland polyp.
In this study we used a modified agarose-bead mediated technique for the genetic analysis of tissue from FFPE samples that had previously withstood assessment by routine DNA extraction procedures.
II. Materials and Methods
Samples
Routinely formalin-fixed (10%) and paraffin-embedded (FFPE) tissue samples were obtained from two patients manifesting both fundic gland polyps in the stomach and heterotopic gastric mucosa in the duodenum, and from four patients with only heterotopic gastric mucosa (Table 1). The samples archived in Ishikawa Hospital (Shikoku-chuo, Ehime) and the Division of Molecular Pathology, Ehime University (Toon, Ehime) were used in this study. All of the present cases, previously analyzed by commercially available DNA extraction kits and conventional DNA extraction protocols, were not informative by the PCR method. Written informed consent was obtained from all patients, and this study was reviewed and approved by the local ethics committee at Ehime University.
Table 1.
Summary of cases
| Case No. | Lesion(s) | Genetic alteration | Resulting AA* alteration |
|---|---|---|---|
| Case 1 | FGP*/HGM* | c.96C>A and c.109T>C/c.109T>C | p.D32E and p.S37P/p.S37P |
| Case 2 | FGP/HGM | None/c.96C>A | None/p.D32E |
| Case 3 | HGM | c.99T>C | p.S33S |
| Case 4 | HGM | None | None |
| Case 5 | HGM | None | None |
| Case 6 | HGM | None | None |
* FGP: fundic gland polyp; HGM: heterotopic gastric mucosa; AA: amino acid.
* c: complementary DNA.
* C>A: from C to A.
* p: protein.
Immunohistochemistry
The specimens of fundic gland polyps in the stomach and the heterotopic gastric mucosa in the duodenum were subjected to immunohistochemistry using diaminobenzidine as the chromogen. Deparaffinized sections of formalin-fixed tissue were stained with β-catenin antibody (rabbit monoclonal antibody; Cell Signaling Technology®) diluted at 1:200 after heat-induced antigen retrieval. HRP-conjugated anti-rabbit IgG was used as the secondary antibody (DAKO K4003). Non-immunized rabbit serum was used as negative controls.
Agarose-bead mediated template preparation
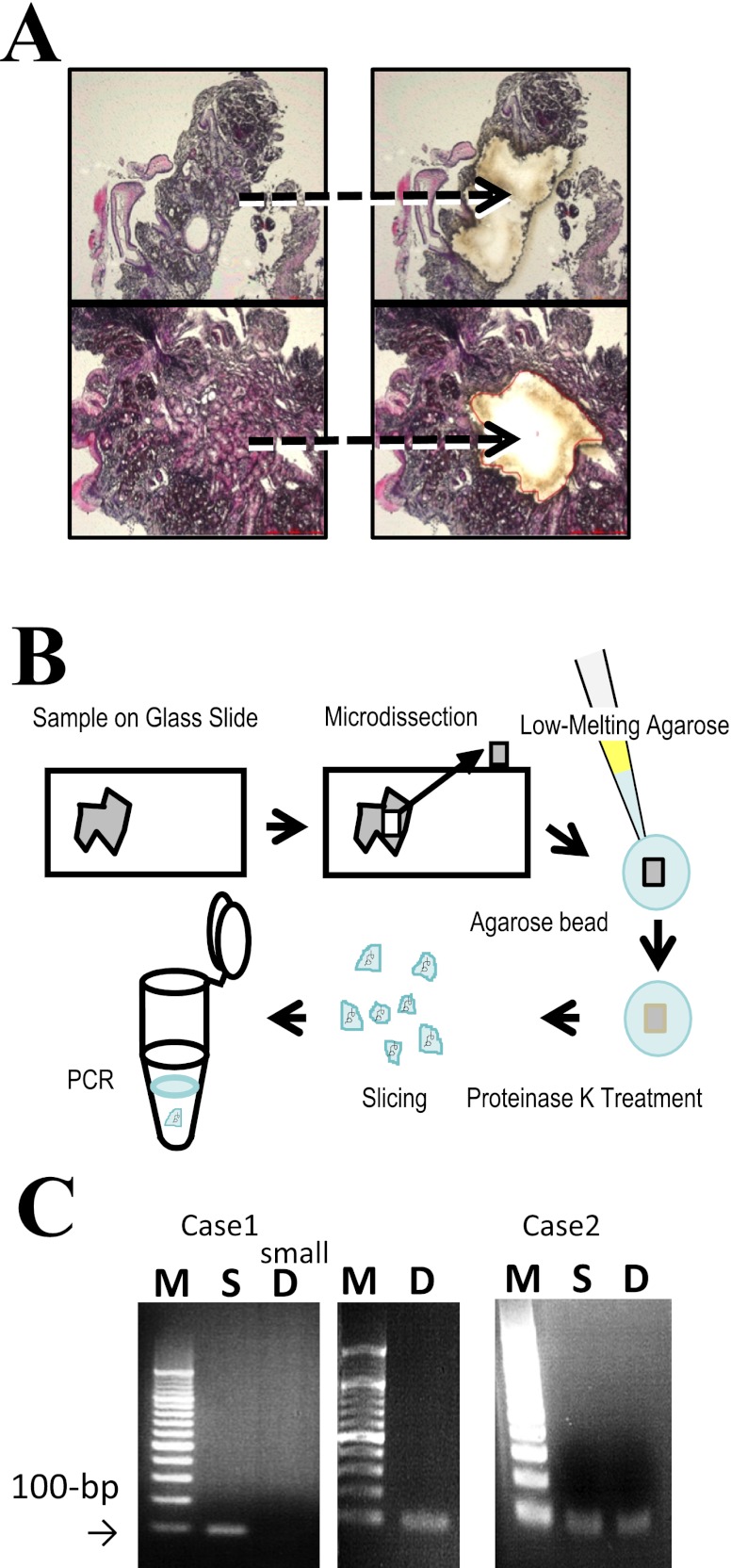
Paraffin-embedded samples from hematoxylin and eosin-stained sections were deparaffinized in xylene and subjected to microdissection under a light microscope (Leica Microsystems LMD7000). Typical areas consisting of hyperplastic fundic glands were selectively dissected from surrounding normal-appearing gastric and duodenal mucosa (Fig. 1A). The microdissected samples were suspended in 5 µl of 1×TE and then mixed with pre-warmed and liquefied low-melting agarose (3.2%) at 1:1. A total of 10 µl agarose beads containing 1×TE and tissue fragments was formed in pre-chilled 250 µl mineral oil and then incubated at 50°C overnight in 1000 µl of 200 µg/ml proteinase K, 10 mM Tris-HCl (pH 8.0) and 25 mM ethylene diamine tetraacetic acid (EDTA). Bead fragments were washed in 1×TE, sliced into several pieces, and then used directly as a template for PCR amplification. Figure 1B illustrates the agarose-bead mediated technique.
Fig. 1.
Microdissection, agarose-bead mediated preparation for PCR template and PCR amplification. A) Sections of the fundic gland polyp and the heterotopic gastric mucosa were selected strictly and subjected to microdissection under a light microscope. B) Schema of procedures for agarose-bead mediated preparation for PCR template. Microdissected samples were embedded in low-melting agarose. Beads were then formed by chilling in ice-cold water. The beads were treated with proteinase K, heat inactivated and subjected to PCR using a portion of the bead fragment as a template. C) PCR products were electrophoresed on a 2% agarose gel, stained with ethidium bromide and visualized under ultraviolet light. Lane M: a molecular weight marker of 100-bp ladder. The PCR products obtained using sets of primers encompassing exon 3 of the β-catenin gene were visualized as 100-bp DNA bands. While the five cells microdissected from duodenum (D) of case 1 does not show clear PCR product (case 1: D, small), the rest of the samples containing at least 20 cells showed positive PCR amplification.
PCR amplification and sequencing
Bead fragments were analyzed by nested PCR using sets of primers encompassing exon 3 of the β-catenin gene. The primers for the first-step PCR amplification were BCAT-F-1 (5'-AGTCACTGGCAGCAACAGTC-3') and BCAT-R-1 (5'-CTCTTCCTCAGGATTGCCTT-3'). The PCR condition was as follows: 30 cycles at 98°C for 10 sec, 55°C for 15 sec, and 68°C for 30 sec. The PCR mixture contained Mighty AMP® DNA polymerase (Takara, Tokyo, Japan) and bead fragments in a final volume of 25 µl. The primers used for second-step PCR were BCAT-F-2 (5'-GGCAGCAACAGTCTTACCTG-3') and BCAT-R-2 (5'-CTCAGGATTGCCTTTACCAC-3'). The second-step PCR condition was as follows: 25 cycles at 98°C for 10 sec, 60°C for 15 sec, and 68°C for 30 sec. The PCR mixture contained Mighty AMP® DNA polymerase (Takara, Tokyo, Japan) and bead fragments in a final volume of 50 µl. The PCR products were electrophoresed in a 2% agarose gel, stained with ethidium bromide, and visualized under ultraviolet light. The products were cloned into the pMD20-T vector (Takara, Japan), transformed into Escherichia coli DH10B competent cells, and identified by blue white screening. Recombinant plasmids were purified using a NucleoSpin® Plasmid kit (Macherey-Nagel, place), and sequenced using ABI PRISM® on an ABI 310 Genetic Analyzer.
III. Results
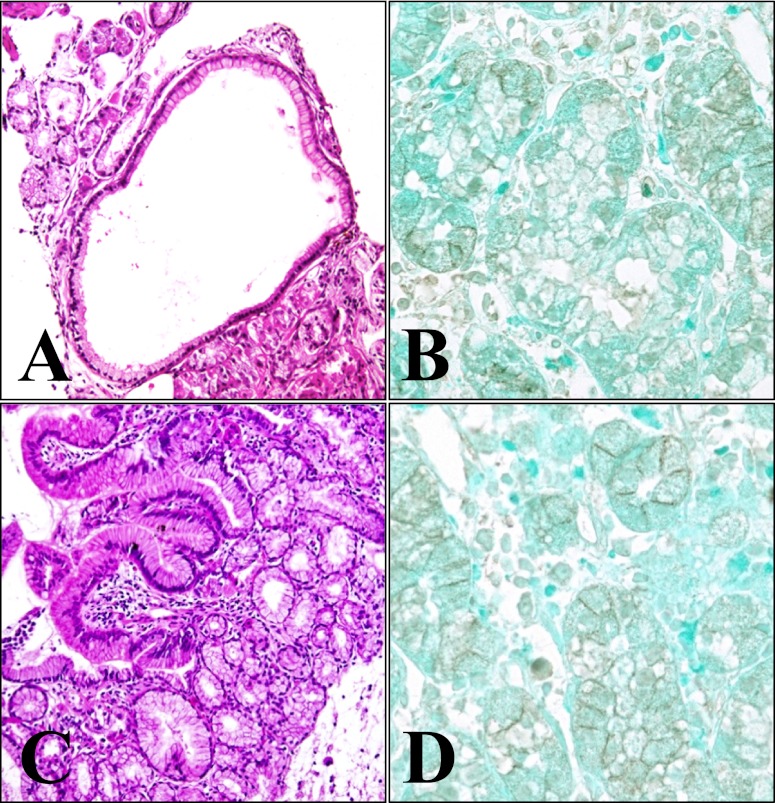
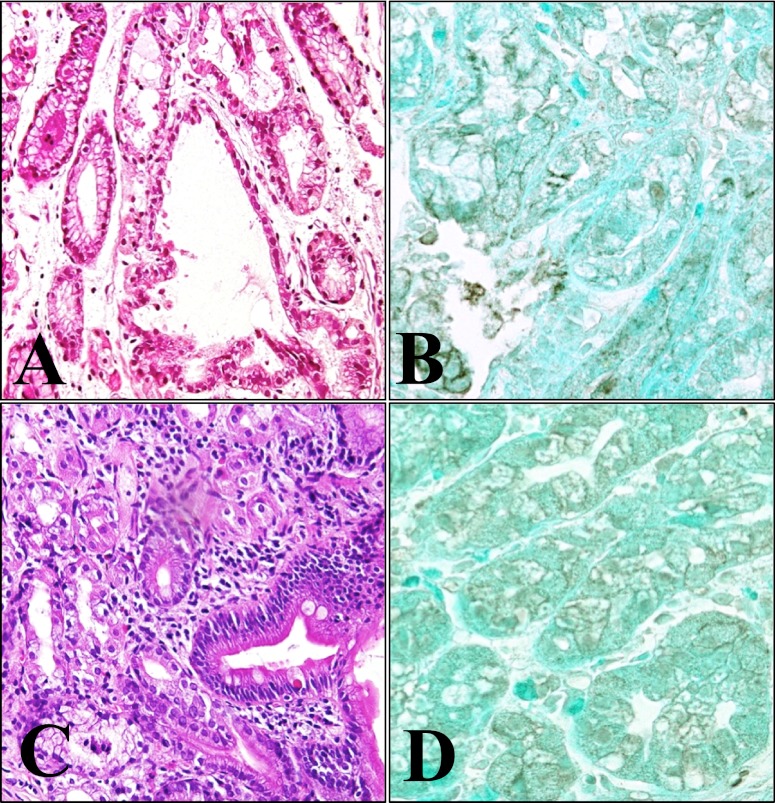
Specimens from two of the six patients showed both typical histopathological features of fundic gland polyps and heterotopic gastric mucosa; the other four cases showed only heterotopic gastric mucosa. Dilated glands lined by oxyntic epithelium were noted in two cases of fundic gland polyps of the stomach (Figs. 2A and 3A). In all six cases of heterotopic gastric mucosa of the duodenum, the oxyntic epithelium was typically nondysplastic, and the junction between the gastric-type and the duodenal surface epithelium was apparent (Figs. 2C and 3C). As in the normal fundic gland, immunohistochemistry of β-catenin in the fundic gland polyp and in the heterotopic gastric mucosa (Figs. 2B and 3B) showed predominantly membranous staining in the epithelial cells lining the dilated fundic glands. Although much less extensive, cytoplasmic staining of β-catenin was also observed (Figs. 2B and 3B). In the heterotopic gastric mucosa of the duodenum, β-catenin immunostaining was observed on the membrane, and only weak cytoplasmic staining of β-catenin was noted cytoplasm (Figs. 2D and 3D), similar to that of the fundic gland polyp. All cases of heterotopic gastric mucosa, either associated with fundic gland polyp or sporadic, showed similar β-catenin immunostaining. Irrespective of the presence or absence of the β-catenin gene mutation, there were no significant differences in β-catenin immunostaining of the normal fundic gland, the fundic gland polyp, and the heterotopic gastric mucosa.
Fig. 2.
Histopathological features and immunohistochemical staining for β-catenin of case 1. Dilated glands lined by oxyntic epithelium are noted in the fundic gland polyp of the stomach (A, HE, ×100). The diffuse membranous and partly cytoplasmic distribution of β-catenin are noted (B, ×400). In the heterotopic gastric mucosa of the duodenum, the oxyntic epithelium is typically nondysplastic, and the junction between the gastric-type and duodenal surface epithelium is apparent (C, HE, ×100). β-catenin immunostaining shows mainly membranous staining (D, ×400).
Fig. 3.
Histopathological features and immunohistochemical staining for β-catenin of case 2. As in case 1, dilated glands lined by oxyntic epithelium are also observed in the fundic gland polyp of the stomach (A, HE, ×100). β-catenin in the fundic gland polyp showed predominantly membranous staining in the epithelial cells lining the dilated fundic glands. Small fractions of cytoplasmic staining and nuclear accumulation of β-catenin are also seen (B, ×400). In the heterotopic gastric mucosa of the duodenum, gastric-type glands are covered by duodenal mucosa (C, HE, ×100). Membranous β-catenin immunostaining is observed (D, ×400).
Sections of fundic gland polyps and heterotopic gastric mucosa were selected strictly and subjected to microdissection under a light microscope (Fig. 1A). Agarose beads were formed and all PCR procedures were successfully carried out using a portion of the bead fragment as a template (Fig. 1B). PCR products were electrophoresed on a 2% agarose gel, stained with ethidium bromide and visualized under ultraviolet light. Lane M shows a molecular weight marker of 100-bp ladder. The PCR products obtained using sets of primers encompassing exon 3 of the β-catenin gene were visualized as 100-bp DNA bands. While five cells microdissected from duodenum (D) of case 1 did not show clear PCR product (case 1: D, small), the rest of the samples containing at least 20 cells showed positive PCR amplification.
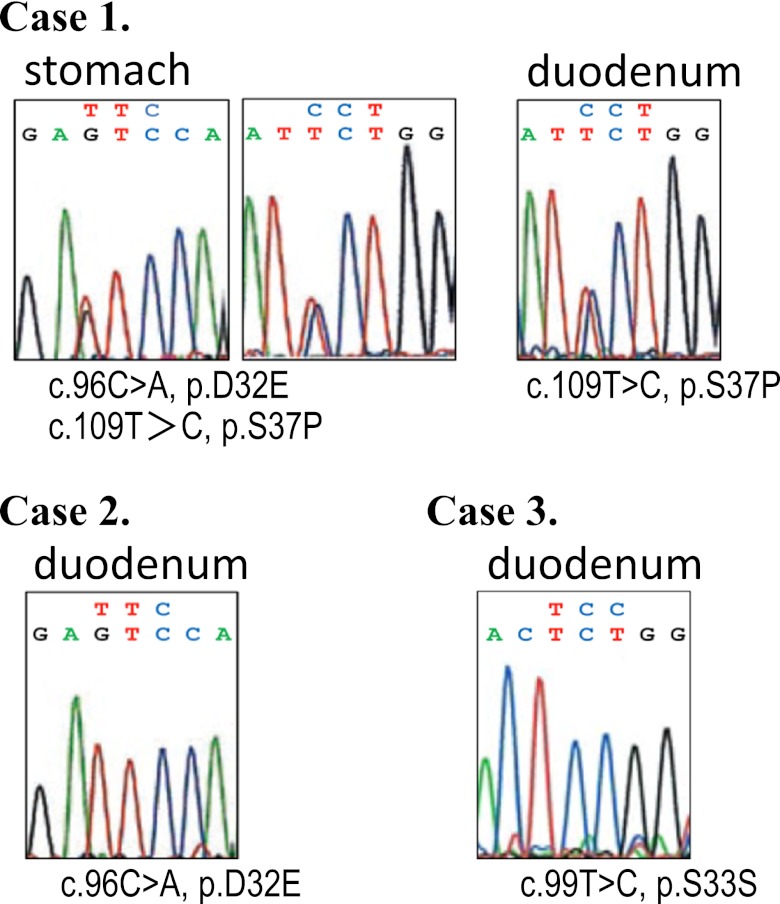
In case 1, two 1-bp missense mutations at codon 32 (GAC to GAA resulting in the replacement of aspartic acid by glutamic acid), and codon 37 (TCT to CCT resulting in the replacement of serine by proline) were identified in the fundic gland polyp of the stomach. The same 1-bp missense mutation at codon 37 was identified in the heterotopic gastric mucosa (Table 1 and Fig. 4, case 1). In case 2, a 1-bp missense mutation at codon 32, was identified in the heterotopic gastric mucosa, while no mutation was observed in the fundic gland polyp of the stomach (Table 1 and Fig. 4, case 2). In case 3, a 1-bp silent mutation at codon 33 was identified in the heterotopic gastric mucosa (Table 1 and Fig. 4, case 3). The other three cases of heterotopic gastric mucosa in the duodenum showed no DNA alterations in the portion of the β-catenin gene examined (Table 1).
Fig. 4.
β-catenin mutations in fundic gland polyp and heterotopic gastric mucosa in duodenum. In case 1, two 1-bp missense mutations at codon 32 (GAC to GAA resulting in the replacement of aspartic acid by glutamic acid), and codon 37 (TCT to CCT resulting in the replacement of serine by proline) were identified in the fundic gland polyp of the stomach. The same 1-bp missense mutation at codon 37 was identified in the heterotopic gastric mucosa (case 1). In case 2, a 1-bp missense mutation at codon 32, resulting in the replacement of aspartic acid by glutamic acid, was identified in the heterotopic gastric mucosa, while no mutation was observed in the fundic gland polyp of the stomach (case 2). In case 3, a 1-bp silent mutation at codon 33 was identified in the heterotopic gastric mucosa. In cases 1 and 2, DNA sequences were read in an antisense direction.
IV. Discussion
Alterations of the β-catenin gene have been reported in various malignant and boundary-malignant neoplasms including colorectal carcinoma, hepatocellular carcinoma, endometrial adenocarcinoma, and desmoid tumors [5, 7, 9]. β-catenin gene alterations at phosphorylation sites protect the β-catenin from ubiquitination, resulting in the nuclear accumulation of β-catenin. Moreover, somatic β-catenin gene alterations have been found in the fundic gland polyp, a relatively common benign lesion in the stomach [9]. The frequent association of the fundic gland polyp with heterotopic gastric mucosa in the duodenum prompted us to investigate, with the use of FFPE samples, whether or not heterotopic gastric mucosa in the duodenum, either sporadic or the fundic gland polyp associated type, is attributable to alterations of the β-catenin gene.
The use of archival FFPE pathological specimens for molecular biological analysis has become increasingly important. Tissues stored in paraffin blocks can be potential sources of DNA for retrospective genetic analysis. Nonetheless, formalin-fixation causes various alterations of nucleic acids, including degradation attributed to endogenous nuclease and crosslinking between nucleic acids and proteins [2, 4]. Moreover, because pathological specimens inevitably comprise heterogeneous populations of cells, objective cell populations must be separated by microdissection. Several methods for DNA extraction from paraffin-embedded tissues have been introduced. Several commercial kits for rapid DNA extraction without DNA-cleaning steps, and a more conventional DNA-preparation protocol with DNA-cleaning are now widely used for handling FFPE samples [3, 6]. Yet, these commercial and conventional methods, sometimes fail to assess minute or microdissected FFPE samples mainly because of sample loss during the DNA extraction process. Indeed, all our microdissected samples from FFPE specimens were noninformative by our assays with commercial kits (DNA Isolater PS-Rapid Reagent®, Wako, Japan, Takara DEXPAT®, Takara, Japan) and conventional techniques. To minimize this DNA loss, we introduced an ‘en bloc’ treatment during proteinase K treatment, cleaning and buffer exchange processes with the use of the agarose-bead method.
In our current study of the six cases of heterotopic gastric mucosa in the duodenum associated with fundic gland polyps, one showed a common 1-bp missense mutation at codon 37 shared by both the fundic gland polyp and the heterotopic gastric mucosa. Alternatively, a 1-bp silent mutation at codon 33 and missense mutation at codon 32 were identified only in the heterotopic gastric mucosa. Since silent mutation sometimes causes alterations in protein expression levels by affecting translational and/or protein-folding efficiency, the genetic alteration can be pathogenic [8]. The other cases, however, showed no DNA alterations in either the fundic gland polyp or the heterotopic gastric mucosa in the duodenum. Besides β-catenin, because the Wnt signaling pathway consists of many components such as APC, GSK-3β and Axin, alteration of these genes may be involved in these cases. The controversy that genetic alterations seen in heterotopic gastric mucosa in the duodenum may derive from the same tissue progenitor cells bearing the same genetic alteration as that of fundic gland polyps, or may simply be an incidental event by exogenous carcinogens, suggests that the heterotopic gastric mucosa in the duodenum may be a heterogeneous population. Further studies are needed to resolve the controversy.
In conclusion, the agarose-bead mediated technique is an effectual tool for retrospective morphology-oriented genetic analyses using a large number of archival pathological samples stored for long periods in the pathology laboratory.
V. Acknowledgments
We wish to thank Ms Yuki Takaoka for technical assistance.
VI. References
- 1.Abraham S. C., Giardiello F. M., Hamilton S. R., Nobukawa B., Wu T. Sporadic fundic gland polyps: common gastric polyps arising through activating mutations in the β-catenin gene. Am. J. Pathol. 2001;158:1005–1010. doi: 10.1016/s0002-9440(10)64047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujimoto J., Hirano T., Ichikawa N., Okamoto E., Takeuchi M., Ueki T., Yamamoto H. Adverse effect of prolonged formalin fixation on DNA histograms in paraffin-embedded tissue. Acta Histochem. Cytochem. 1998;31:171–175. [Google Scholar]
- 3.Huijsmans C. J., Damen J., Hermans M. H., Linden J. C., Savelkoul P. H. Comparative analysis of four methods to extract DNA from paraffin-embedded tissues: effect on downstream molecular applications. BMC Res. Notes. 2010;3:239. doi: 10.1186/1756-0500-3-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitazawa S., Kitazawa R., Maeda S. Identification of methylated cytosine from archival formalin-fixed paraffin-embedded specimens. Lab. Invest. 2000;80:275–276. doi: 10.1038/labinvest.3780031. [DOI] [PubMed] [Google Scholar]
- 5.Koch A., Albrecht S., Denkhaus D., Leuschner I., Pietsch T., Schweinitz D. V. Childhood hepatoblastomas frequently carry a mutated degradation targeting box of the β-catenin gene. Cancer Res. 1999;59:269–273. [PubMed] [Google Scholar]
- 6.Kullmann F., Bocker T., Ruschoff J., Scholmerich J. A comparison of methods for DNA extraction from paraffin-embedded tissue for microsatellite instability analysis by polymerase chain reaction. Acta Biotechnol. 1998;1:77–83. [Google Scholar]
- 7.Lazar A. J. F., Bolshakov S., Habeeb S., Hajibashi S., Lev D., Lopez-Terrada D., Mayordomo-Aranda E., Pollock R. E., Tuvin D., Warneke C. L. Specific mutations in the β-catenin gene (CTNNB1) correlate with local recurrence in sporadic desmoid tumors. Am. J. Pathol. 2008;173:1518–1527. doi: 10.2353/ajpath.2008.080475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salzman D. W., Weidhaas J. B. miRNAs in the spotlight: making ‘silent’ mutations speak up. Nat. Med. 2011;17:934–935. doi: 10.1038/nm0811-934. [DOI] [PubMed] [Google Scholar]
- 9.Sekine S., Hirohashi S., Nakanishi Y., Sakamoto M., Shibata T., Shimoda T., Yamauchi Y. β-catenin mutations in sporadic fundic gland polyps. Virchows Arch. 2002;440:381–386. doi: 10.1007/s004280100527. [DOI] [PubMed] [Google Scholar]