Abstract
Diabetic conditions promote glomerulosclerosis by mesangial cells but the mechanisms are not fully elucidated. The present study evaluated the expression of toll-like receptor 4 in glomerular endothelial cells in the streptozotocin (STZ)-induced type 1 diabetic mouse (ICR-STZ) and the type 2 diabetic KK/TaJcl mouse which were fed a high fat diet feed (KK/Ta-HF). In the ICR-STZ and KK/Ta-HF almost glomeruli were immunostained with anti-TLR4 but there was no glomerulus immunostained by ani-TLR4 in the control ICR and KK/Ta. Laser-scanning confocal microscopy showed that the TLR4-positive region did not coincide with the podoplanin-positive region but coincide with the PECAM-1- and VE-cadherin-positive regions in the glomeruli of the ICR-STZ and KK/Ta-HF. The in situ hybridization showed that almost signals for TLR4 mRNA were present in the glomerulus of the ICR-STZ and KK/Ta-HF to a stronger extent than in the control ICR and KK/Ta. These suggest that glomerular endothelial cells usually express the TLR4 gene and hyperglycemia in the diabetic condition induces the TLR4 protein expression in the glomerular capillary endothelial cells. Cytokine productions through the TLR signaling pathway in glomerular endothelial cells may allow mesangial cells to produce extracellular matrix proteins in the diabetic milieu.
Keywords: diabetes, glomerular endothelial cells, toll-like receptor 4, streptozotocin, KK/TaJcl
I. Introduction
Diabetes mellitus is a common disease in Japan affecting nearly 2.7 million people. Type 2 diabetes mellitus results in a group of metabolic disorders and comprises 95% of diabetes mellitus cases while type 1 diabetes mellitus is a failure of glucose metabolism that results from insulin deficiencies by autoimmune destruction of insulin-secreting β cells. Since elevated glucose results in the harmful effects leading to increased proinflammatory cytokine productions and oxidative stress to cardiovascular and nervous systems, diabetes mellitus causes retinopathy, nephropathy, neuropathy by microvascular complications, and cardiovascular complications to the brain, heart, and peripheral arteries [2]. Characteristics of diabetic nephropathy include mesangial expansion, loss of podocytes, glomerular hyperplasia, and thickening of the glomerular basement membrane, and tubular epithelial cell dysfunction. The mesangium is composed of mesangial cells and extracellular matrix proteins secreted by mesangial cells. Glomerular mesangial cells usually function as central supports for glomerular capillaries but under a diabetic milieu they causes the occlusion of glomerular capillaries and diabetic glomerulosclerosis by excess accumulation of extracellular matrix [10, 18, 19, 25].
It has been thought that diabetic conditions promote overproduction of extracellular matrix protein by mesangial cells with increased formation of advanced glycation end products (AGEs) like Nε-(carboxymethyl) lysine (CML), and secretion of various cytokines such as transforming growth factor from podocytes, glomerular endothelial cells, mesangial cells, and leukocytes in glomeruli, but the mechanisms of the development and progression of renal injury are not fully elucidated [9, 30].
Toll-like receptors (TLRs) initiate a series of innate immune mechanisms against various microorganism infections by sensing the presence of pathogen-associated molecular patterns [15, 26]. The TLRs are sensors that trigger immune responses against bacterial components, extracellular matrix degradation products, fatty acids, and AGEs, and the ligand engagements finally result in the production of downstream inflammatory cytokines and leukocyte adhesion molecules by the activation of the NF-kB and AP-1-dependent transcriptional pathways [4, 24, 27–29]. The TLR ligands stimulate leukocytes and blood endothelial cells through the recognition systems followed by a myleoid differentiation primary response protein-dependent or independent pathway that culminates in the activation of the IkB kinase complex and p38 mitogen-activated protein kinases. Recently, it has been reported that monocytes and glomerular mesangial cells increase the expression of TLR4 under diabetic conditions [4–6, 20]. It is thought that the hyperglycemia may induce the damage to the glomeruli through the TLR signaling directly triggered by its ligand like AGEs in the blood but TLR expression in the glomerular capillary endothelial cells has not been clearly established. The present study evaluated the expression of TLRs in glomerular endothelial cells in diabetic mice.
II. Materials and Methods
Experimental animals
To investigate the expression of TLR4 in glomerular blood vessels under diabetic conditions, we used two kinds of diabetic mice; one is KK/TaJcl (KK/Ta) mice, a mouse model with type 2 diabetes, and the other is streptozotocin (STZ)-induced diabetic mice (ICR-STZ), a mouse model with type 1 diabetes [16, 22]. The protocol for the animal use was reviewed and approved by the animal experiment committee of Fukuoka Dental College in accordance with the principles of the Helsinki Declaration. Five-week-old male ICR mice (n=5) (Japan Clea Inc., Osaka, Japan) were given a single intraperitoneal injection of STZ (200 mg/kg body weight) (Sigma, St. Louis) in a 0.05 M citric acid buffer at pH 4.5 (20 mg/ml). One week after the STZ injection, diabetes was confirmed by measurement of the blood glucose using a Glutest Sensor (Sanwa Kagaku Kenkyusyo CO., LTD., Nagoya, Japan), and we designated ICR-STZ with blood glucose above 600 mg/dl for four months as insulin-dependent type 1 diabetic mice. Nine-week-old male KK/Ta mice (n=5) (Japan Clea Inc., Osaka, Japan) with a genetic background of insulin-independent diabetes were fed a high fat diet feed for diabetes mellitus studies (HFD32, Japan Clea) for five months, and we designated KK/Ta mice with blood glucose above 600 mg/dl as type 2 diabetic mice (KK/Ta-HF). The ICR mice given a 0.05 M citric acid buffer and Nine-week-old male KK/Ta mice before feeding HFD32 were used as controls.
Immunohistochemistry
The mouse monocyte cell line J774A.1 (JCRB9108, Osaka, Japan) was cultured in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum. Cells were harvested, seeded onto the slide glasses, and air-dried. The collection of the mouse tissue was conducted after euthanasia by induction anesthesia of the mice with 2% Isoflurane (1 l/min) and intraperitoneal injection with sodium pentobarbital (10 ml/kg, Nembutal, Abbott Laboratories, North Chicago, IL). The cells and frozen 10 µm sections cut in a cryostat on the slide glasses were fixed in 100% methanol for 5 min at –20°C. The sections were treated with 0.1% goat serum for 30 min at 20°C and then the sections were treated with PBS containing 0.1% goat serum and primary antibodies (1 µg/ml): hamster monoclonal anti-mouse podoplanin (AngioBio Co., Del Mar, CA) as a podocyte marker, rat monoclonal anti-mouse CD284 (TLR4)/MD-2 complex (BD Biosciences, San Jose, CA), rabbit polyclonal anti-mouse vascular endothelial (VE)-cadherin (Abcam plc., Cambridge, UK), and rabbit polyclonal anti-mouse platelet endothelial cell adhesion molecule-1 (PECAM-1, Abcam) for 8 hr at 4°C. After the treatment with primary antibodies the sections were washed three times in PBS for 10 min and immunostained for 0.5 hr at 20°C with 0.1 µg/ml of second antibodies: Alexa Fluor (AF) 488 or 568-conjugated goat anti-hamster, goat anti-rabbit, or goat anti-rat IgGs (Probes Invitrogen Com., Eugene, OR). The immunostained sections were mounted in 50% polyvinylpyrrolidone solution and examined by fluorescence microscopy (BZ-8100, Keyence Corp., Osaka, Japan) or laser-scanning microscopy (LSM710, Carl Zeiss, Jena, Germany) with a ×63 oil planapochromatic objective lens (numerical aperture ×1.3).
In situ hybridization
Frozen 10 µm sections cut in a cryostat on the slide glasses were fixed in 4% paraformaldehyde-PBS and air dried in an oven at 60°C for 1 hr. All procedures of the in situ hybridization for mouse TLR4 mRNA and 3,3'-Diaminobenzidine (DAB) staining on the hybridized genes were performed by RNAscope 2.0 FFPE Assay (Advanced Cell Diagnostics, Inc., Hayward, CA) and probe for mouse TLR4 mRNA (NM_021297.2, 2404-3775, Advanced Cell Diagnostics) including negative and positive controls according the manufacturer’s instructions. Several negative controls (e.g. sense probe and no probe) were run in parallel with the experimental reaction.
III. Results
Immunohistochemical analysis
In mouse monocyte cell lines as controls, cells were immunostained with both anti-TLR4 and anti-PECAM-1 (Fig. 1). In the control mouse heart tissue, lymphatic vessels were immunostained by anti-podoplanin and blood vessels by anti-PECAM-1 and anti-VE-cadherin (Fig. 2). In all negative controls, there were no cross reactions with primary and secondary antibodies, and there were no differences in the fluorescence intensity of each target protein between the simple immunostaining with anti-TLR4, anti-PECAM-1, and anti-VE-cadherin, and their double immunostaining. In the cortex of the STZ-injected ICR mouse kidney, HE staining showed that glomeruli were subject to sclerosis and renal tubules were expanded, and that the tissue with nephropathy is collapsed by edema (Fig. 3). In the immunostaining for TLR4 and podoplanin in the ICR mouse kidney tissue, glomeruli were immunostained with an antibody for the podocyte marker, podoplanin, but there was no glomerulus immunostained by ani-TLR4. In the ICR-STZ mouse kidney tissue, almost all glomeruli were immunostained by anti-TLR4 whereas the Bowman capsules, distal and proximal tubules, collecting tubules, lymphatic vessels, and blood vessels outside glomeruli, were not immunostained (Fig. 3). In the cortex of the kidneys in the KK/Ta-HF mice which were fed the high fat diet feed, the HE staining showed that glomeruli were subject to sclerosis and renal tubules were expanded, and that the tissue with nephropathy is collapsed by edema as in the ICR-STZ mice (Fig. 4). In the immunostaining for TLR4 and podoplanin in KK/Ta mouse kidney tissue, glomeruli were immunostained with anti-podoplanin but there were no glomerulus immunostained by anti-TLR4. In the immunostaining for TLR4 and podoplanin in the KK/Ta-HF mouse kidney tissue, almost all glomeruli were immunostained by anti-TLR4 while distal and proximal tubules, collecting tubules, and blood vessels outside glomeruli were not immunostained, similar to the ICR-STZ mice (Fig. 4).
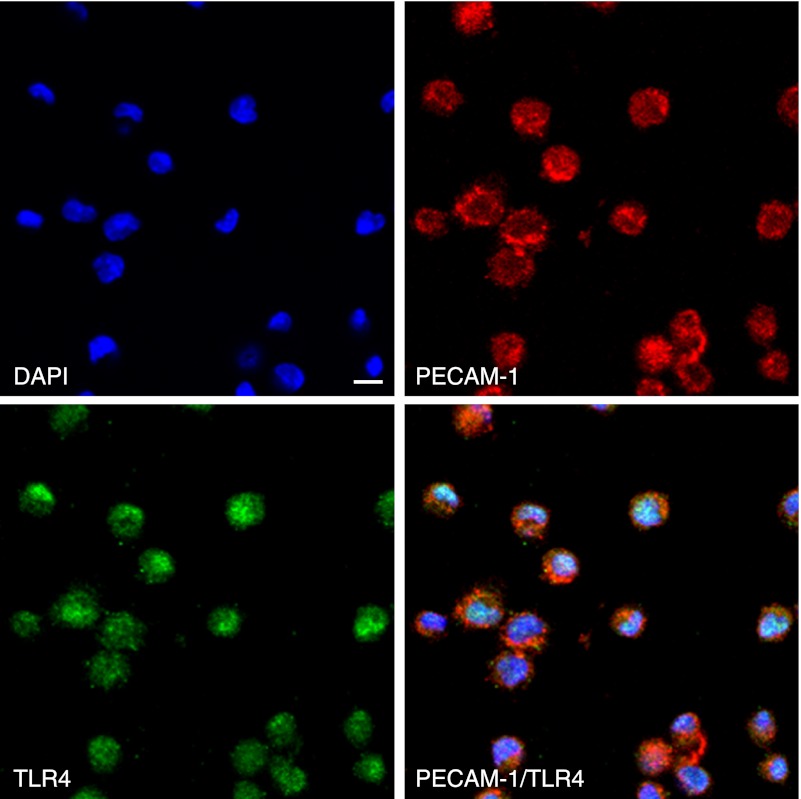
Fig. 1.
Immunostaining for TLR4 and PECAM-1 in mouse monocyte J774A.1. All cells were immunostained with both anti-TLR4 and anti-PECAM-1. The reaction products with anti-PECAM-1 are mainly on the cell surface and with anti-TLR4 are on the cell surface and in the cytoplasm. Nuclei were stained by DAPI. Bar=20 µm.
Fig. 2.
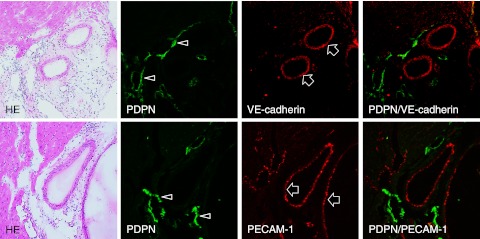
Immunostaining for podoplanin, PECAM-1, and VE-cadherin in the mouse heart sections. Narrow lymphatic vessels (arrows) are immunostained by anti-podoplanin. Blood vessels which opened in luminal circle (arrowheads) are immunostained by anti-PECAM-1 and anti-VE-cadherin, but not by anti-podoplanin. Bar=100 µm.
Fig. 3.
Immunostaining for TLR4 and podoplanin in the ICR and ICR-STZ mouse kidneys. The immunostained sections were re-stained by HE staining. The HE staining shows that the kidney tissue is collapsed by edema and renal tubules are expanding in the cortex of the STZ-injected ICR mouse kidney (ICR-STZ). In the ICR mouse kidney section, all glomeruli were immunostained by the antibody for the podocyte marker, podoplanin (PDPN), while there were no cells reacting with anti-TLR4. In the ICR-STZ mouse kidney sections, almost all podoplanin-positive glomeruli were immunostained by anti-TLR4 (arrowheads) whereas the Bowman capsules, distal and proximal tubules, collecting tubules, lymphatic vessels, and blood vessels outside the glomeruli, were not immunostained. Bar=100 µm.
Fig. 4.
Immunostaining for TLR4 and podoplanin in the KK/Ta and KK/Ta-HF mouse kidneys. The immunostained sections were re-stained by the H-E staining. The HE staining shows that the kidney tissue is collapsed by edema and renal tubules have expanded in the cortex of the kidneys in KK/Ta mice which have been fed the high fat diet feed (KK/Ta-HF). In the KK/Ta mouse kidney sections, all glomeruli were immunostained with an antibody for the podocyte marker, podoplanin (PDPN), while there were no cells reacting with ani-TLR4. In the KK/Ta-HF mouse kidney sections, almost all podoplanin-positive glomeruli were immunostained by anti-TLR4 (arrowheads) whereas the Bowman capsules, distal and proximal tubules, collecting tubules, lymphatic vessels, and blood vessels outside glomeruli, were not immunostained. Bar=100 µm.
In the laser-scanning confocal microscopy of the immunostaining on the glomeruli of the ICR-STZ and KK/Ta-HF mice, the region reacting with anti-podoplanin did not coincide with the region reacting with anti-TLR4 (Fig. 5) while the regions reacting with anti-PECAM-1 (Fig. 6) and VE-cadherin coincided with the regions reacting with anti-TLR4 (Fig. 7).
Fig. 5.
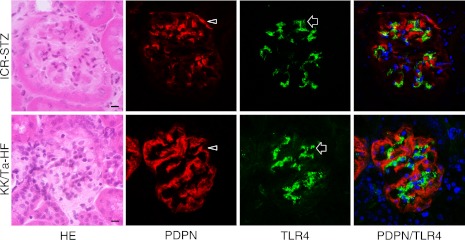
Laser-scanning confocal microscopy of immunostaining for podoplanin and TLR4 on the glomeruli of the ICR-STZ and KK/Ta-HF mouse kidneys. The HE staining showed that glomeruli were subject to sclerosis in the cortex of STZ-injected ICR mouse kidneys. The podocyte region reacting with anti-podoplanin (PDPN, arrowheads) did not coincide with the region reacting with anti-TLR4 (arrows) in the merged images (rightmost panels). Bar=20 µm.
Fig. 6.
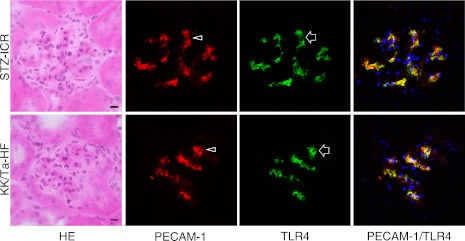
Laser-scanning confocal microscopy of immunostaining for PECAM-1 and TLR4 on the glomeruli of the ICR-STZ and KK/Ta mouse kidneys. The endothelial region reacting with anti-PECAM-1 (arrowheads) coincided with the region reacting with anti-TLR4 (arrows) in the merged images (rightmost panels). Bar=20 µm.
Fig. 7.
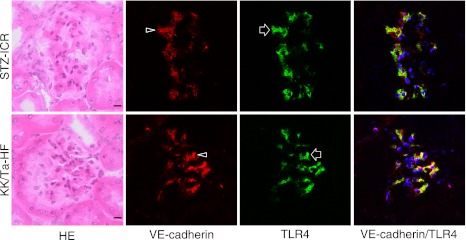
Laser-scanning confocal microscopy of immunostaining for VE-cadherin and TLR4 on the glomeruli of the ICR-STZ and KK/Ta mouse kidneys. The endothelial region reacting with anti-VE-cadherin (arrowheads) coincided with the region reacting with anti-TLR4 (arrows) in the merged images (rightmost panels). Bar=20 µm.
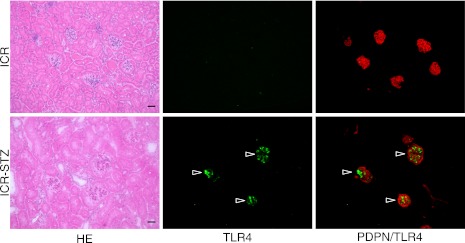
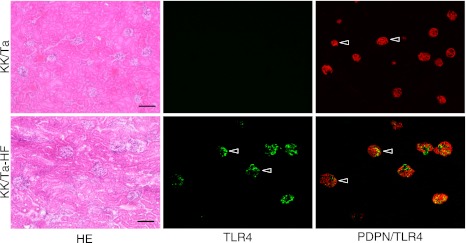
Gene analysis
In situ hybridization for TLR4 mRNA on the untreated control kidney tissues of the ICR and KK/Ta mice showed that almost all DAB signals on the genes hybridized with the probe for TLR4 mRNA were present in the glomerulus (Fig. 8). In the kidney tissue of the ICR-STZ and KK/Ta-HF mice, almost all DAB signals on the genes hybridized with probe for TLR4 mRNA were present in the glomerulus, and to a stronger extent than in the untreated control kidney tissue of ICR and KK/Ta mice. There were few signals for TLR4 mRNA in the Bowman capsules, distal and proximal tubules, collecting tubules, lymphatic vessels, or blood vessels outside glomeruli.
Fig. 8.
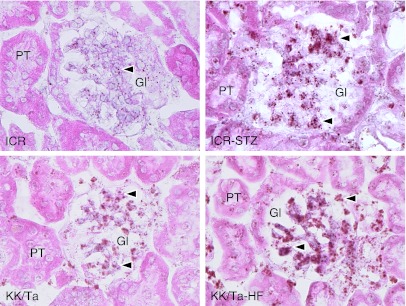
In situ hybridization for TLR4 mRNA to the ICR-STZ and KK/Ta-HF mouse kidneys. In the untreated control kidney tissues of ICR and KK/Ta mouse (left panels), almost all DAB signals of the genes hybridized with probe for TLR4 mRNA (arrowheads) are present in the glomerulus (Gl) but there are only few in the Bowman capsules and proximal tubules (PT). In the kidney tissue of the ICR-STZ and KK/Ta-HF mouse, almost all DAB signals on the genes hybridized with the probe for TLR4 mRNA (arrowheads) were present in the glomerulus to a stronger extent than the untreated control kidney tissue of the ICR and KK/Ta mice. Bar=20 µm.
IV. Discussion
The TLR4 and leukocyte adhesion molecule PECAM-1 are commonly expressed on the monocyte cell linage. The PECAM-1 and adherence junction molecule VE-cadherin are commonly expressed on vascular endothelial cells. Podoplanin is the marker of lymphatic endothelial cells and podocytes [13, 21]. Further, the expression of podoplanin has been reported on the tooth germ epithelial cells and salivary gland myoepithelial cells in the somatic tissue [1, 11, 12]. In the experiments here, it was confirmed that anti-TLR4 reacted with the mouse monocyte J774.1A cells; the anti-PECAM-1 reacted with the J774.1A cells and with blood vessels in the mouse heart tissue; the anti-VE-cadherin with blood vessels in the mouse heart tissue; the anti-podoplanin with lymphatic vessels in the mouse heart tissue and with podocytes in the mouse glomeruli (Figs. 1, 2). These results suggest that the immunostaining with these antibodies took place successfully.
In the ICR-STZ and KK/Ta-HF mouse kidneys where diabetic nephropathy had occurred based on the HE findings showing glomerulosclerosis and renal tubule expansion, almost all glomeruli in the cortex were immunostained with anti-TLR4 but there was no glomerulus immunostained by ani-TLR4 in the untreated control kidney tissue of ICR and KK/Ta mice (Figs. 3, 4). These results suggest that hyperglycemia in the diabetic condition results in the TLR4 expression at the protein level in the glomeruli. Further, in the ICR-STZ and KK/Ta-HF mouse kidneys, the Bowman capsules, distal and proximal tubules, collecting tubules, lymphatic vessels, and blood vessels outside glomeruli were not immunostained by anti-TLR4, suggesting that the expression of TLR4 may be an event specific to the glomeruli. Laser-scanning confocal microscopy on the TLR4 expression in the glomeruli of the ICR-STZ and KK/Ta-HF showed that the TLR4-positive region did not coincide with the podoplanin-positive region but coincided with both the PECAM-1- and VE-cadherin-positive regions (Figs. 5, 6, 7). The gene analysis for the expression of TLR4 mRNA by in situ hybridization showed that almost all DAB signals on the genes hybridized with the probe for TLR4 mRNA in the kidney tissue of the ICR-STZ and KK/Ta-HF mice were present in the glomerulus to a stronger extent than in the untreated control kidney tissue of ICR and KK/Ta mice, and that there were few signals for TLR4 mRNA in the Bowman capsules, distal and proximal tubules, collecting tubules, lymphatic vessels, and blood vessels outside glomeruli (Fig. 8). These suggest that glomerular endothelial cells usually express the TLR4 gene in the untreated control kidney and have the potential to produce TLR4, and that hyperglycemia in the diabetic condition induces the TLR4 protein expression in the glomerular capillary endothelial cells with sclerosis.
Enhanced expression of TLR4 has been reported in diabetes in both clinical and experimental conditions and it has been thought that the TLR signaling pathway in hyperglycemia induces cell damage. The TLR4 up-regulates in type 1 and type 2 diabetic patients in monocytes with increased glucose, A1C, CML, and free fatty acid, and the TLR4 expression is enhanced in patients with diabetic nephropathy [4–6, 20]. It has recently been shown that glucose exposure allows mouse mesangial cells to express TLR4 with the production of transforming growth factor-β, and that TLR4 knockout mice show less progression of nephropathy than wild-type diabetic mice [3, 7, 8, 14, 17, 23]. There is a possibility that not only endothelial cells but also mesangial cells have the potential to express TLR4 because of the distribution of TLR4 gene signals in the findings of in situ hybridization (Fig. 8). However, all TLR4-positive cells in glomeruli expressed PECAM-1 and VE-cadherin which are the endothelial markers (Figs. 6, 7), suggesting that glomerular TLR4-positive cells are capillary endothelial cells but not mesangial cells. Therefore, it is thought that cytokine productions through the TLR signaling pathway in glomerular endothelial cells may allow mesangial cells to produce extracellular matrix proteins in the diabetic milieu.
V. Acknowledgments
This work was supported in part by Scientific Research (B) 22390345 and Exploratory Research 23659884 from Japan Society for the Promotion of Science.
VI. References
- 1.Amano I., Imaizumi Y., Kaji C., Kojima H., Sawa Y. Expression of podoplanin and classical cadherins in salivary gland epithelial cells of klotho-deficient mice. Acta Histochem. Cytochem. 2011;44:267–276. doi: 10.1267/ahc.11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonetti D. A., Klein R., Gardner T. W. Mechanisms of disease: Diabetic retinopathy. N. Engl. J. Med. 2012;366:1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 3.Dasu M. R., Devaraj S., Zhao L., Hwang D. H., Jialal I. High glucose induces toll-like receptor expression in human monocytes: mechanism of activation. Diabetes. 2008;57:3090–3098. doi: 10.2337/db08-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dasu M. R., Devaraj S., Park S., Jialal I. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care. 2010;33:861–868. doi: 10.2337/dc09-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devaraj S., Dasu M. R., Rockwood J., Winter W., Griffen S. C., Jialal I. Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: further evidence of a proinflammatory state. J. Clin. Endocrinol. Metab. 2008;93:578–583. doi: 10.1210/jc.2007-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devaraj S., Dasu M. R., Park S. H., Jialal I. Increased levels of ligands of Toll-like receptors 2 and 4 in type 1 diabetes. Diabetologia. 2009;52:1665–1668. doi: 10.1007/s00125-009-1394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devaraj S., Jialal I., Yun J. M., Bremer A. Demonstration of increased toll-like receptor 2 and toll-like receptor 4 expression in monocytes of type 1 diabetes mellitus patients with microvascular complications. Metabolism. 2011;60:256–259. doi: 10.1016/j.metabol.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devaraj S., Tobias P., Jialal I. Knockout of toll-like receptor-4 attenuates the pro-inflammatory state of diabetes. Cytokine. 2011;55:441–445. doi: 10.1016/j.cyto.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 9.Forbes J. M., Cooper M. E., Oldfield M. D., Thomas M. C. Role of advanced glycation end products in diabetic nephropathy. J. Am. Soc. Nephrol. 2003;14:S254–S258. doi: 10.1097/01.asn.0000077413.41276.17. [DOI] [PubMed] [Google Scholar]
- 10.Haneda M., Koya D., Isono M., Kikkawa R. Overview of glucose signaling in mesangial cells in diabetic nephropathy. J. Am. Soc. Nephrol. 2003;14:1374–1382. doi: 10.1097/01.asn.0000064500.89551.76. [DOI] [PubMed] [Google Scholar]
- 11.Hata M., Amano I., Tsuruga E., Kojima H., Sawa Y. Immunoelectron microscopic study of podoplanin localization in mouse salivary gland myoepithelium. Acta Histochem. Cytochem. 2010;43:77–82. doi: 10.1267/ahc.10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imaizumi Y., Amano I., Tsuruga E., Kojima H., Sawa Y. Immunohistochemical examination for the distribution of podoplanin-expressing cells in developing mouse molar tooth germs. Acta Histochem. Cytochem. 2010;43:115–121. doi: 10.1267/ahc.10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaji C., Tsujimoto Y., Kaneko M. K., Kato Y., Sawa Y. Immunohistochemical examination of novel rat monoclonal antibodies against mouse and human podoplanin. Acta Histochem. Cytochem. 2012;45:227–237. doi: 10.1267/ahc.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaur H., Chien A., Jialal I. Hyperglycemia induces Toll like receptor 4 expression and activity in mouse mesangial cells: relevance to diabetic nephropathy. Am. J. Physiol. Renal Physiol. 2012;303:F1145–1150. doi: 10.1152/ajprenal.00319.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 16.Kiguchi S., Imamura T., Ichikawa K., Kojima M. Oxcarbazepine antinociception in animals with inflammatory pain or painful diabetic neuropathy. Clin. Exp. Pharmacol. Physiol. 2004;31:57–64. doi: 10.1111/j.1440-1681.2004.03950.x. [DOI] [PubMed] [Google Scholar]
- 17.Lin M., Yiu W. H., Wu H. J., Chan L. Y., Leung J. C., Au W. S., Chan K. W., Lai K. N., Tang S. C. Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy. J. Am. Soc. Nephrol. 2012;23:86–102. doi: 10.1681/ASN.2010111210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mason R. M., Wahab N. A. Extracellular matrix metabolism in diabetic nephropathy. J. Am. Soc. Nephrol. 2003;14:1358–1373. doi: 10.1097/01.asn.0000065640.77499.d7. [DOI] [PubMed] [Google Scholar]
- 19.Mauer S. M., Steffes M. W., Ellis E. N., Sutherland D. E., Brown D. M., Goetz F. C. Structural functional relationships in diabetic nephropathy. J. Clin. Invest. 1984;74:1143–1155. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohammad M. K., Morran M., Slotterbeck B., Leaman D. W., Sun Y., Grafenstein H., Hong S. C., McInerney M. F. Dysregulated Toll-like receptor expression and signaling in bone marrow-derived macrophages at the onset of diabetes in the non-obese diabetic mouse. Int. Immunol. 2006;18:1101–1113. doi: 10.1093/intimm/dxl045. [DOI] [PubMed] [Google Scholar]
- 21.Noda Y., Amano I., Hata M., Kojima H., Sawa Y. Immunohistochemical examination on the distribution of cells expressed lymphatic endotheial marker podoplanin and LYVE-1 in the mouse tongue tissue. Acta Histochem. Cytochem. 2010;43:61–68. doi: 10.1267/ahc.10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phichitrasilp T., Hondo E., Rerkamnuaychoke W., Wakitani S., Sugiyama M., Terakawa J., Kiso Y. Reproductive performance in diabetes mice with a special reference to uterine natural killer cells and placental growth factor. J. Vet. Med. Sci. 2009;71:519–523. doi: 10.1292/jvms.71.519. [DOI] [PubMed] [Google Scholar]
- 23.Sherry C. L., O’Connor J. C., Kramer J. M., Freund G. G. Augmented lipopolysaccharide-induced TNF-alpha production by peritoneal macrophages in type 2 diabetic mice is dependent on elevated glucose and requires p38 MAPK. J. Immunol. 2007;178:663–670. doi: 10.4049/jimmunol.178.2.663. [DOI] [PubMed] [Google Scholar]
- 24.Shi H., Kokoeva M. V., Inouye K., Tzameli I., Yin H., Flier J. S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steffes M. W., Osterby R., Chavers B., Mauer S. M. Mesangial expansion as a central mechanism for loss of kidney function in diabetic patients. Diabetes. 1987;38:1077–1081. doi: 10.2337/diab.38.9.1077. [DOI] [PubMed] [Google Scholar]
- 26.Takeda K., Kaisho T., Akira S. Toll-like receptors. Annu. Rev. Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 27.Tsan M. F., Gao B. Endogenous ligands of Toll-like receptors. J. Leukoc. Biol. 2004;76:514–519. doi: 10.1189/jlb.0304127. [DOI] [PubMed] [Google Scholar]
- 28.van Beijnum J. R., Buurman W. A., Griffioen A. W. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1) Angiogenesis. 2008;11:91–99. doi: 10.1007/s10456-008-9093-5. [DOI] [PubMed] [Google Scholar]
- 29.Wong F. S., Wen L. Toll-like receptors and diabetes. Ann. N. Y. Acad. Sci. 2008;1150:123–132. doi: 10.1196/annals.1447.063. [DOI] [PubMed] [Google Scholar]
- 30.Yan S. F., Ramasamy R., Schmidt A. M. Mechanisms of disease: advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat. Clin. Pract. Endocrinol. Metab. 2008;4:285–293. doi: 10.1038/ncpendmet0786. [DOI] [PubMed] [Google Scholar]