Abstract
To report keratitis with Elizabethkingia meningoseptica, which occurred in a healthy patient after wearing contact lenses for 6 months. A 24-year-old male patient visited our hospital with ocular pain. This patient had a history of wearing soft contact lenses for 6 months, about 10 hours per day. At initial presentation, slit lamp examination showed corneal stromal infiltrations and small epithelial defect. Microbiological examinations were performed from corneal scrapings, contact lenses, and the contact lens case and solution. The culture results from contact lenses, contact lens case and solution were all positive for Elizabethkingia meningoseptica. Thus, we could confirm that the direct cause of keratitis was contamination of the contact lenses. The patient was treated with 0.3% gatifloxacin. After treatment, the corneal epithelial defect was completely healed, and a slight residual subepithelial corneal opacity was observed. We diagnosed keratitis with Elizabethkingia meningoseptica in a healthy young male wearing soft contact lenses. We conclude that Elizabethkingia meningoseptica should be considered as a rare but potential pathogen for lens-related keratitis in a healthy host.
Keywords: Contact lenses, Elizabethkingia meningoseptica, Keratitis
Elizabethkingia meningoseptica is a Gram-negative bacillus that occasionally causes neonatal meningitis and infections with a wide variety of locations in immunologically compromised patients. This ubiquitous bacterium, formerly known as Flavobacterium meningosepticum, was recently termed Elizabethkingia meningosepticum (or meningoseptica) by some authors. It belongs to the family of Flavobacteriae and inhabits both natural and hospital environments [1,2]. It can exist in fresh water, salt water, or soil. It may be normally present in fish and frog but not human microflora [3]. In general, the Flavobacterium species posses low virulence and is only rarely a pathogen in humans, mainly infecting newborns and immunologically compromised hosts [4-6]. The clinical information on infections with Elizabethkingia meningoseptica is mostly limited to the occasional case reports of patients with various presentations, including meningitis, endocarditis, cellulitis, bacteremia, abdominal abscess, peritonitis, and endophthalmitis. The first known isolation of Elizabethkingia meningoseptica from the eye was reported in a case of endophthalmitis in the immediate postoperative period, following penetrating keratoplasty [7]. Herein, we report one case of keratitis with Elizabethkingia meningoseptica in a healthy young man who had worn soft contact lens for 6 months prior to diagnosis.
Case Report
A 24-year-old male patient visited our hospital due to right ocular pain. He had been wearing soft contact lenses for six months, for approximately 10 hours per day. He had no medical history of ocular injury, surgery, or treatment with either ophthalmic or systemic medications. No abnormality was found in the blood test. On our initial examination, visual acuity was 20 / 25 in the right eye and 20 / 20 in the left eye. Slit lamp examination revealed hyperemia of the right bulbar conjunctiva near the lesion and a corneal epithelial defect sized 0.5 mm × 0.5 mm accompanied by intra-stromal infiltration and peripheral neovascularization toward the 7 o'clock direction of the limbus (Fig. 1A). The lens, vitreous body, and retina were all normal. Corneal scraping was performed for microbiologic investigation. A bacteriological test was conducted on the corneal scrapings, contact lens, lens case and lens washing agent. The patient was treated as follows: 3% gatifloxacin (Gatiflo; Handok, Seoul, Korea) with an initial one-hour interval and a later reduced frequency; 2% homatropine (Ocuhomapine Eye; Samil, Seoul, Korea), three times a day; and 0.3% unpreserved hyaluronic acid (Hyalein mini; Santen, Osaka, Japan), four times a day. Three days later, when the patient revisited the hospital, the symptoms had improved. The corneal epithelial defect was nearly healed, and the corneal intra-stromal infiltration was also decreased. After the initial bacterial culture, bacteria were inoculated and cultured in blood agar at an intermediate level, in all the prelevated specimens (Fig. 2). Elizabethkingia meningoseptica was identified using the Vitek 2 GN colorimetric identification card (Biomerieux Vitek, Hazelwood, MO, USA). Elizabethkingia meningoseptica were sensitive to ciprofloxacin, trimethoprim/sulfamethoxazole, levofloxacin, gentamycin, piperacillin/tazobactam and amikacin. The patient was continued on 3% gatifloxacin (Gatiflo) and 0.3% unpreserved hyaluronic acid (Hyalein mini), four times a day. However, 2% homatropine (Ocuhomapine Eye) was discontinued. Complete re-epithelialization of the lesion was observed within the sub-epithelial corneal opacity on the seventh day after treatment (Fig. 1B). After two weeks, the patient was being treated only with 0.3% unpreserved hyaluronic acid (Hyalein mini), four times a day. His final corrected visual acuity in the right eye was 20 / 20.
Fig. 1.
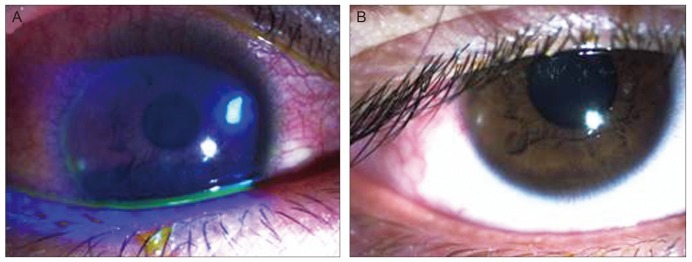
(A) The slit-lamp photography at the first visit showed a 0.5 mm × 0.5 mm-sized round corneal epithelial defect, with stromal infiltration. (B) Seven days after treatment, corneal lesion showed complete re-epithelialization within the sub-epithelial corneal opacity.
Fig. 2.
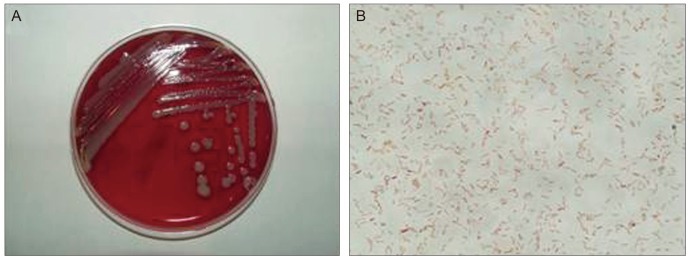
(A) Multiple colonies were formed on the blood agar medium, and each colony showed a very paleyellow pigmented, dome-shaped cluster. (B) Gram staining showed various sizes of gram-negative bacilli (×1,000).
Discussion
Elizabethkingia meningoseptica is a waterborne saprophytic bacterium that can be found in both hospital and natural environments, such as soil and seawater. Elizabethkingia meningoseptica has been found in the hospital environment at different sites, such as water supplies, the saline solution used for flashing procedures, disinfectants, and medical devices. The bacterium is an aerobic, Gram-negative rod of low virulence, traditionally associated with neonatal meningitis; however, it may also be responsible for pneumonia and sepsis in immunologically compromised and postoperative adults [1,8-10].
To the best of our knowledge, three cases of ocular infection with Elizabethkingia meningoseptica have been reported in the literature so far. The first patient was a 78-year-old woman who had trabecular trephination, complicated extracapsular cataract extraction, and resulting severe bullous keratopathy. After penetrating keratoplasty, the patient developed endophthalmitis. The anterior chamber tap, as well as the culture from the donor corneal rim, grew Elizabethkingia meningoseptica. The bacteria were multidrug resistant but sensitive to trimethoprim-sulfamethoxazole. Despite aggressive therapy, the eye progressed to phthisis. The authors suggested that the donor eye was the source of the infection [7].
The second patient was an 82-year-old woman with a corneal ulcer secondary to trichiasis and exposure. After the central corneal perforation occurred, a lamellar corneal graft was performed, followed by tarsorrhaphy. When the ulcer recurred, cultures revealed Elizabethkingia meningoseptica. The ulcer healed after topical therapy with trimethoprim-sulfamethoxazole, the only tested antibiotic to which the strain was sensitive [11].
The third patient, a 48-year-old man, presented with corneal ulcer in his right eye and was treated with ofloxacin and fortified bacitracin ophthalmic solution. Cultures grew Elizabethkingia meningoseptica, and the infection resolved [12].
In contrast to those three previous cases, this is the first report of ocular infection with Elizabethkingia meningoseptica in an eye with no significant comorbidities. In the present case, it was suspected that the contact lenses induced keratitis; therefore, microbiological examinations were performed on the corneal scrapings, contact lenses, the contact lens cases and the lens washing agents. The growth of Elizabethkingia meningoseptica from all of these specimens was confirmed; this made it possible to ascertain that the contact lenses were the direct cause of keratitis. To the best of our knowledge, Elizabethkingia meningoseptica has never been isolated in patients with contact lens-related microbial keratitis.
Contact lens-induced corneal hypoxia may predispose contact lens wearers to corneal infection due to compromised corneal epithelial integrity, impaired wound healing, and increased susceptibility of corneal epithelial cells to bacterial binding [13-17]. Similarly, the use of contaminated saline solution, inappropriate management of the contact lenses or direct contact with contaminated water increased the risk of corneal infection. For example, the use of extended-wear lenses, poor maintenance of the contact lens case, reuse of the contact lens solution and poor lens hygiene contribute to the contamination of contact lenses. Therefore, proper lens hygiene is very important to prevent corneal infection. Contact lens users need to wash their hands before handling their lenses, keep their lens cases clean at all times, replace their cases at regular intervals and replace their soft contact lenses at least every three months.
We believe that this occurrence of contact lens-induced keratitis with Elizabethkingia meningoseptica was only now reported for the first time in the literature because of the difficulty of positively identifying the organism. Our patient was healthy and had no underlying disease. Keratitis with Elizabethkingia meningoseptica is indeed rare in healthy people without risk factors. Therefore, this microbe should be considered a rare but potential pathogen for contact lens-induced keratitis in a healthy host.
Acknowledgements
This study was supported by research fund from Chosun University, 2013.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Holmes B. Identification and distribution of Flavobacterium meningosepticum in clinical material. J Appl Bacteriol. 1987;62:29–41. doi: 10.1111/j.1365-2672.1987.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 2.Kim KK, Kim MK, Lim JH, et al. Transfer of Chryseobacterium meningosepticum and Chryseobacterium miricola to Elizabethkingia gen. nov. as Elizabethkingia meningoseptica comb. nov. and Elizabethkingia miricola comb. nov. Int J Syst Evol Microbiol. 2005;55(Pt 3):1287–1293. doi: 10.1099/ijs.0.63541-0. [DOI] [PubMed] [Google Scholar]
- 3.King EO. Studies on a group of previously unclassified bacteria associated with meningitis in infants. Am J Clin Pathol. 1959;31:241–247. doi: 10.1093/ajcp/31.3.241. [DOI] [PubMed] [Google Scholar]
- 4.Gungor S, Ozen M, Akinci A, Durmaz R. A Chryseobacterium meningosepticum outbreak in a neonatal ward. Infect Control Hosp Epidemiol. 2003;24:613–617. doi: 10.1086/502261. [DOI] [PubMed] [Google Scholar]
- 5.Adachi A, Mori T, Shimizu T, et al. Chryseobacterium meningosepticum septicemia in a recipient of allogeneic cord blood transplantation. Scand J Infect Dis. 2004;36:539–540. doi: 10.1080/00365540410020587. [DOI] [PubMed] [Google Scholar]
- 6.Lin JT, Wang WS, Yen CC, et al. Chryseobacterium indologenes bacteremia in a bone marrow transplant recipient with chronic graft-versus-host disease. Scand J Infect Dis. 2003;35:882–883. doi: 10.1080/00365540310016637. [DOI] [PubMed] [Google Scholar]
- 7.LeFrancois M, Baum JL. Flavobacterium endophthalmitis following keratoplasty: use of a tissue culture medium-stored cornea. Arch Ophthalmol. 1976;94:1907–1909. doi: 10.1001/archopht.1976.03910040617007. [DOI] [PubMed] [Google Scholar]
- 8.Bloch KC, Nadarajah R, Jacobs R. Chryseobacterium meningosepticum: an emerging pathogen among immunocompromised adults: report of 6 cases and literature review. Medicine (Baltimore) 1997;76:30–41. doi: 10.1097/00005792-199701000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Di Pentima MC, Mason EO, Jr, Kaplan SL. In vitro antibiotic synergy against Flavobacterium meningosepticum: implications for therapeutic options. Clin Infect Dis. 1998;26:1169–1176. doi: 10.1086/520309. [DOI] [PubMed] [Google Scholar]
- 10.Lee CC, Chen PL, Wang LR, et al. Fatal case of community-acquired bacteremia and necrotizing fasciitis caused by Chryseobacterium meningosepticum: case report and review of the literature. J Clin Microbiol. 2006;44:1181–1183. doi: 10.1128/JCM.44.3.1181-1183.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bucci FA, Jr, Holland EJ. Flavobacterium meningosepticum keratitis successfully treated with topical trimethoprim-sulfamethoxazole. Am J Ophthalmol. 1991;111:116–118. doi: 10.1016/s0002-9394(14)76916-8. [DOI] [PubMed] [Google Scholar]
- 12.Bloom AH, Perry HD, Donnenfeld ED, Davis RG. Chryseobacterium meningosepticum keratitis. Am J Ophthalmol. 2003;136:356–357. doi: 10.1016/s0002-9394(03)00153-3. [DOI] [PubMed] [Google Scholar]
- 13.Madigan MC, Holden BA. Reduced epithelial adhesion after extended contact lens wear correlates with reduced hemidesmosome density in cat cornea. Invest Ophthalmol Vis Sci. 1992;33:314–323. [PubMed] [Google Scholar]
- 14.Mauger TF, Hill RM. Corneal epithelial healing under contact lenses: quantitative analysis in the rabbit. Acta Ophthalmol (Copenh) 1992;70:361–365. doi: 10.1111/j.1755-3768.1992.tb08580.x. [DOI] [PubMed] [Google Scholar]
- 15.Imayasu M, Petroll WM, Jester JV, et al. The relation between contact lens oxygen transmissibility and binding of Pseudomonas aeruginosa to the cornea after overnight wear. Ophthalmology. 1994;101:371–388. doi: 10.1016/s0161-6420(94)31326-1. [DOI] [PubMed] [Google Scholar]
- 16.Cavanagh HD, Ladage P, Yamamoto K, et al. Effects of daily and overnight wear of hyper-oxygen transmissible rigid and silicone hydrogel lenses on bacterial binding to the corneal epithelium: 13-month clinical trials. Eye Contact Lens. 2003;29(1 Suppl):S14–S16. doi: 10.1097/00140068-200301001-00005. [DOI] [PubMed] [Google Scholar]
- 17.Latkovic S, Nilsson SE. The effect of high and low Dk/L soft contact lenses on the glycocalyx layer of the corneal epithelium and on the membrane associated receptors for lectins. CLAO J. 1997;23:185–191. [PubMed] [Google Scholar]


