Abstract
Background and Purpose
GPCRs undergo both homologous and heterologous regulatory processes in which receptor phosphorylation plays a critical role. The protein kinases responsible for each pathway are well established; however, other molecular details that characterize each pathway remain unclear. In this study, the molecular mechanisms that determine the differences in the functional roles and intracellular trafficking between homologous and PKC-mediated heterologous internalization pathways for the dopamine D2 receptor were investigated.
Experimental Approach
All of the S/T residues located within the intracellular loops of D2 receptor were mutated, and the residues responsible for GRK- and PKC-mediated internalization were determined in HEK-293 cells and SH-SY5Y cells. The functional role of receptor internalization and the cellular components that determine the post-endocytic fate of internalized D2 receptors were investigated in the transfected cells.
Key Results
T134, T225/S228/S229 and S325 were involved in PKC-mediated D2 receptor desensitization. S229 and adjacent S/T residues mediated the PKC-dependent internalization of D2 receptors, which induced down-regulation and desensitization. S/T residues within the second intracellular loop and T225 were the major residues involved in GRK-mediated internalization of D2 receptors, which induced receptor resensitization. ARF6 mediated the recycling of D2 receptors internalized in response to agonist stimulation. In contrast, GASP-1 mediated the down-regulation of D2 receptors internalized in a PKC-dependent manner.
Conclusions and Implications
GRK- and PKC-mediated internalizations of D2 receptors occur through different intracellular trafficking pathways and mediate distinct functional roles. Distinct S/T residues within D2 receptors and different sorting proteins are involved in the dissimilar regulation of D2 receptors by GRK2 and PKC.
Keywords: dopamine D2 receptor, GRK, PKC, intracellular trafficking, desensitization, ARF6, GASP-1
Introduction
Desensitization or tolerance is defined as the attenuation of receptor responsiveness by prolonged or previous stimulation. Homologous and heterologous desensitization are two representative regulatory patterns for GPCRs (Freedman and Lefkowitz, 1996; Okamoto et al., 1998). Receptor phosphorylation is believed to be a critical cellular event involved in these regulatory processes, and differential GPCR phosphorylation can trigger distinct responses (Tobin, 2008; Tobin et al., 2008).
Homologous desensitization occurs in an agonist-specific manner; that is, the response of one receptor is selectively diminished by previous exposure to its agonist without effect on the responsiveness of other receptors that are expressed in the same cell. GPCR kinases (GRKs) and arrestins are two critical players in homologous desensitization. Arrestins bind activated receptors to uncouple them from G-proteins and facilitate subsequent internalization. Some GRK subtypes, such as GRK2 and GRK3, phosphorylate agonist-occupied GPCRs and increase their affinity for arrestins.
Heterologous desensitization occurs in an agonist-nonspecific manner. PKA and PKC are the most common second messenger-dependent protein kinases involved in the heterologous desensitization (Freedman and Lefkowitz, 1996; Okamoto et al., 1998). When activated, these kinases phosphorylate not only the receptors that are activated by the cognate agonist, but also different types of receptors if they are the substrates for these protein kinases.
In previous studies, differences in the regulatory mechanisms between homologous and heterologous pathways have been reported for various GPCRs. For example, different serine (S) and threonine (T) residues are phosphorylated in the homologous and heterologous regulation of β2-adrenoceptors (Lefkowitz et al., 1990; Yuan et al., 1994; Fredericks et al., 1996) or δ-opioid receptors (Guo et al., 2000; Xiang et al., 2001); GRK-mediated desensitization occurs more rapidly than desensitization through PKA (Roth et al., 1991); and PKA- and GRK-mediated internalization of β1-adrenoceptors occurs in caveolae and clathrin-coated pits (CCP), respectively (Rapacciuolo et al., 2003). These previous studies, however, mainly focused on a particular regulatory step in a molecular cascade involved in complicated regulatory processes. A more complete and mechanistic study is needed to delineate the differences between the two pathways throughout the whole regulatory process, which includes receptor phosphorylation, endocytic processes and their functional roles.
Dopamine D2 receptors are important targets for the treatment of various diseases related to motor, emotional and endocrine functions (Parkinson's disease, schizophrenia and pituitary tumours) (for review, see Missale et al., 1998; Cho et al., 2010b). The regulatory properties of D2 receptors have been reported for both homologous and heterologous desensitization pathways. The D2 receptor is a substrate for both GRKs and PKCs and is internalized when phosphorylated by either kinase (Kim et al., 2001; Namkung and Sibley, 2004). Our preliminary studies also showed that GRK- and PKC-mediated intracellular trafficking of the D2 receptor has different characteristics (Cho et al., 2007). In this sense, the D2 receptor is an excellent experimental system for a comparative study of homologous and PKC-mediated heterologous regulatory pathways of GPCRs.
In this study, we wanted to understand the molecular mechanisms that determine the differences in the functional roles and intracellular trafficking between the homologous and heterologous regulatory pathways of GPCRs. Our results revealed that GRK- and PKC-mediated regulatory pathways show different post-endocytic behaviours and mediate distinct functional roles that are caused by differential involvement of S/T residues located within the intracellular loops of the D2 receptor and vesicular sorting proteins that determine the fate of the internalized receptors.
Methods
Materials
HEK-293 and SH-SY5Y cells were obtained from the American Type Culture Collection (Manassas, VA). Cell culture reagents were obtained from Invitrogen Life Technologies, Inc. (Carlsbad, CA). [3H]-Sulpiride (84 Ci·mmol−1) and [3H]-spiperone (25.5 Ci·mmol−1) were purchased from PerkinElmer Life Sciences (Boston, MA, USA). Dopamine (DA), (–) quinpirole, forskolin, PMA, 4α-PMA, sucrose, antibodies to FLAG and GFP and anti-FLAG antibody-conjugated agarose beads were obtained from Sigma/Aldrich Chemical Co. (St. Louis, MO). Antibodies to actin, ARF6, HA epitope and HRP-labelled secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Alexa 594-labelled anti-mouse antibodies were purchased from Invitrogen. Gö6976 and Gö6983 were purchased from EMD Chemicals (Gibbstown, NJ). Antibodies to β-arrestin were kindly provided by Dr Lefkowitz (Duke University, NC). The drug/molecular target nomenclature (e.g. receptors, ion channels) conforms to BJP's Guide to Receptors and Channels (Alexander et al., 2011).
Cell culture and transfection
HEK-293 and SH-SY5Y cells were cultured in minimal essential medium (MEM) supplemented with 10% FBS, 100 U·mL−1 penicillin and 100 mg·mL−1 of streptomycin in a humidified atmosphere containing 5% CO2. The transfections were performed using the calcium phosphate precipitation method or polyethylenimine (Polyscience, Warrington, PA).
Plasmid constructs
Human short alternatively spliced form of D2 receptor in the mammalian expression vector pCMV5 or in pcDNA 3.1 Zeo (+) was used. Some D2 receptor constructs were tagged at the N-terminus with the M2-FLAG epitope or at the C-terminus with GFP. The putative phosphorylation sites (S/T residues in the first, second and third intracellular loops) were mutated to alanine or valine residues by site-directed mutagenesis (Table 1). Rat β-arrestin2 was as described elsewhere (Barak et al., 1997). Small hairpin RNA constructs for human β-arrestin1 and β-arrestin2 were provided by Dr Lan Ma (Fundan University, China). HA-ARF6/T157N in pCMV5 was from Dr Jacek Jaworski (International Institute of Molecular and Cell Biology, Warsaw, Poland). GFP-tagged Q67L- and -T27N-ARF6 were prepared by site-directed mutagenesis. GFP-tagged GPCR-associated sorting protein-1 (GASP-1) and cGASP (the C-terminal part of GASP-1) were provided by Dr von Zastro (University of California at San Francisco). WT-Rab5, S34N-Rab5, WT-Rab23 and S23V-Rab23 were prepared by RT-PCR. Small hairpin RNA construct for ARF6 was provided by Dr Heike Fölsch (Northwestern University), and GST-GGA3-PBD was provided by Dr Michael Famulok (University of Bonn, Germany).
Table 1.
Notation and descriptions of mutants of the possible phosphorylation sites in the intracellular regions of the dopamine D2 receptor
| D2 receptor mutants | Description | Characteristics |
|---|---|---|
| #1 | T67V, T68V, T69V | - Cell surface expression level is decreased to ∼50% of wild-type |
| - No change in receptor internalization | ||
| #2 | T134V | PMA-induced desensitization is inhibited |
| #3 | T144V, S147A, S148Aa | |
| #4 | T225Va, S228A, S229Ab | - DA-induced internalization is reduced (T225) |
| - PMA-induced internalization and desensitization is reduced | ||
| #5 | S256A, S257A, T258V, S259A | Possible casein kinase II site |
| #6 | T264V, S267A | |
| #7 | S272A, T277V | |
| #8 | S282A | S282C is associated with schizophrenia (Glatt et al., 2003) |
| #9 | S288A, T289V, S292A | |
| #10 | T316V | |
| #11 | T322V, T324V, S325Aa | PMA-induced desensitization is abolished (S325) |
| #12 | T328V, S330Aa | |
| #13 | S335Ab | |
| #14 | T343Vb | |
| PKCX | T134V, S228A, S229A, S325A | PMA-induced desensitization and down-regulation was abolished |
| IC2 | T134V, T144V, S147A, S148A | - PMA-induced desensitization is abolished |
| - DA-induced internalization is reduced | ||
| IC2/T225 | T134V, T144V, S147A, S148A, T225V | - DA-induced internalization was reduced |
| IC3 | T225V, S228A, S229A, S256A, S257A, T258V, S259A, T264V, S267A, S272A, T277V, S282A, S288A, T289V, S292A, T316V, T322V, T324V, S325A, T328V, S330A, S335A, T343V | - DA-induced internalization is moderately reduced |
| - PMA-induced desensitization is inhibited | ||
| IC23 | IC2 + IC3 | - Receptor phosphorylation is abolished |
| - Receptor internalization is inhibited | ||
| IC123 | IC23 + T67V, T68V, T69V | Cell surface expression is reduced |
The values represent the position of the amino acid residues starting from the N-terminal end, Met.
These regions contain the putative phosphorylation sites for PKC.
These regions contain the putative phosphorylation sites for PKA.
Receptor internalization assay
Internalization of the D2 receptor was measured based on the hydrophilic properties of 3H-sulpiride (Kim et al., 2001). HEK-293 cells expressing D2 receptors were seeded 1 day after transfection at a density of 1.5 × 105 cells per well in 24-well plates. The following day, cells were rinsed once and pre-incubated for 15 min with 0.5 mL of pre-warmed serum-free medium containing 10 mM HEPES, pH 7.4, at 37°C. Cells were stimulated with 10 μM DA or 1 μM PMA for 0–120 min as indicated. Cells were then incubated with 250 μL of [3H]-sulpiride (final concentration 2.2 nM) at 4°C for 150 min in the absence and presence of unlabelled competitive inhibitor (10 μM haloperidol). Cells were washed three times with the same medium, and 1% SDS was added. Samples were mixed with 2 mL Lefkofluor scintillation fluid and counted on a liquid scintillation analyzer (Perkin Elmer, Waltham, MA).
Immunoprecipitation
After 48 h of transfection, the cells were lysed in RIPA buffer (150 mM NaCl, 50 mM Tris, pH 7.4, 1% NP-40, 0.5% deoxycholate, 0.1% SDS) on a rotation wheel for 1 h at 4°C. The supernatants were mixed with 35 μL of 50% slurry of anti-Flag-agarose beads for 2–3 h on the rotation wheel. The beads were washed with washing buffer (50 mM Tris, pH 7.4, 137 mM NaCl, 10% glycerol, 1% NP-40) three times for 10 min each. The immunoprecipitates were analysed by immunoblotting.
Measurement of GTP-bound ARF6
ARF, a small GTPase, cycles between an inactive GDP-bound form and an active GTP-bound form. GTP-bound ARF1 and ARF6 bind specifically to the protein-binding domain (PBD) of GGA3. GGA3-PBD was bacterially expressed as a fusion protein with glutathione-S-transferase (GST-GGA3-PBD). BL21 bacterial cells expressing GST or GST-GGA3-PBD were treated with 0.5 mM IPTG for 2 h, lysed, centrifuged and the resulting supernatant was aliquoted and stored at −70°C until use. Supernatants from cell lysates obtained from HEK-293 cells transfected with D2 receptor and GFP-tagged ARF6 cDNA were added to the column containing GST-GGA3-PBD pre-coupled to glutathione–agarose beads and incubated overnight with continuous shaking at 4°C. Beads were washed four times with GST-binding buffer then treated with Laemmli sample buffer.
Whole cell cAMP assays
Cellular cAMP was measured by an indirect method (Cho et al., 2011) using a reporter plasmid containing the firefly luciferase gene under the control of multiple cAMP responsive elements (CRE) and with pRL-TK control vector (Promega, Madison, WI). Transfected cells were seeded in 24-well plates and each transfection set was organized into three identical groups. The cells were treated with 2 μM forskolin and quinpirole (10−12–10−8) for 4 h and harvested, and the relative luciferase expression was measured using the dual luciferase assay kit (Promega). In some experiments, HEK-293 cells expressing D2 receptors plated in 12-well dishes were labelled overnight with 1 μCi·mL−1 [3H]-adenine in MEM containing 10% FBS and gentamicin. Accumulated [3H]-cAMP was determined by the sequential chromatography method of Salomon (Johnson and Salomon, 1991). Data were normalized by expressing cAMP levels as a percentage of the forskolin-stimulated cAMP for each experiment. Dose–response curves were fitted in GraphPad Prism (GraphPad software, San Diego, CA).
Determination of receptor desensitization
Homologous (agonist-induced) desensitization of D2 receptors was measured as reported previously (Kim et al., 2005). Cells were pretreated either with vehicle or DA, and then the dose–response curves were constructed for the inhibition of cAMP production in response to the stimulation of D2 receptors with quinpirole, an agonist of D2 receptors. PKC-mediated heterologous desensitization of D2 receptors was measured as reported previously (Namkung and Sibley, 2004; Cho et al., 2007). Cells were treated either with vehicle or PMA, and then the dose–response curves were determined for each experimental group. The extent of agonist- or PMA-induced desensitization was determined by comparing the dose–response curves of vehicle-pretreated and DA- or PMA-pretreated experimental groups.
Determination of constitutive recycling of internalized D2 receptors
HEK-293 cells that stably express D2 receptors were pretreated with 50 μg·mL−1 cyclohexamide, followed by treatment with 10 μM DA for 1 h or 1 μM PMA for 2 h. After being washed with serum-free medium, cells were incubated at 37°C for the indicated period of time, washed, and incubated with 2.2 nM [3H]-supiride for 150 min at 4°C. Cells were washed three times with serum-free media, dissolved in 1% SDS, and counted with a liquid scintillation counter. The recycling of the internalized D2 receptor was represented by a vertical bar chart or by line and scatter plot. For line and scatter plot, the fraction of D2 receptors internalized by treatment with 10 μM DA or 1 μM PMA for 1–2 h was converted to 100%, and the number of constitutively recycled D2 receptors was calculated as a % of total internalized D2 receptors.
Knockdown of endogenous proteins by shRNAs
HEK-293 cells were prepared that stably expressed shRNA constructs of scrambled sequence or β-arrestin2. Some of the selected clones whose expression levels of endogenous β-arrestin2 were reduced were again stably transfected with the shRNA construct of β-arrestin1. Levels of endogenous β-arrestins were detected by immunoblotting. Similar procedures were employed for knockdown of ARF6.
Immunocytochemistry and confocal microscopy
One day after transfection, the cells were seeded onto 35 mm dishes containing a centered, 1 cm well that was formed from a glass coverslip-sealed hole in plastic (confocal dishes) and allowed to recover for one day. Next day, the cells were fixed with 4% paraformaldehyde, for 15 min at room temperature and permeabilized with 0.25% Triton X-100. Cells were labelled with antibodies raised against the target protein. The cells were examined by laser scanning confocal microscope (TCS SP5/AOBS/Tandem, Leica, Germany; supported by Korea Basic Science Institute) or Nikon Ti 2000 live cell imaging system (Nikon Instruments Inc., Melville, NY).
Statistics
All results are expressed as mean ± SEM. Comparisons between experimental groups were performed using ANOVA. For some results, Student's t-test was used.
Results
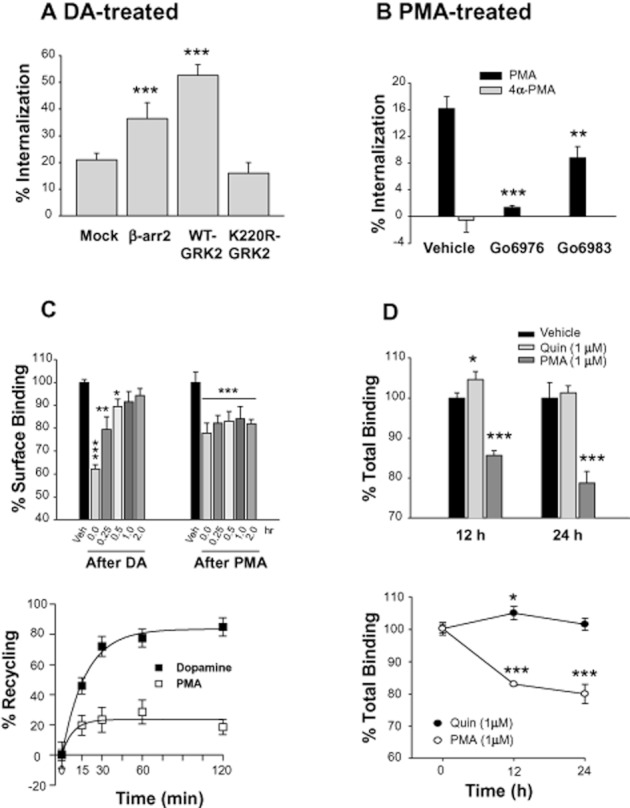
Agonist- and PMA-induced internalization of D2 receptor display different intracellular trafficking properties
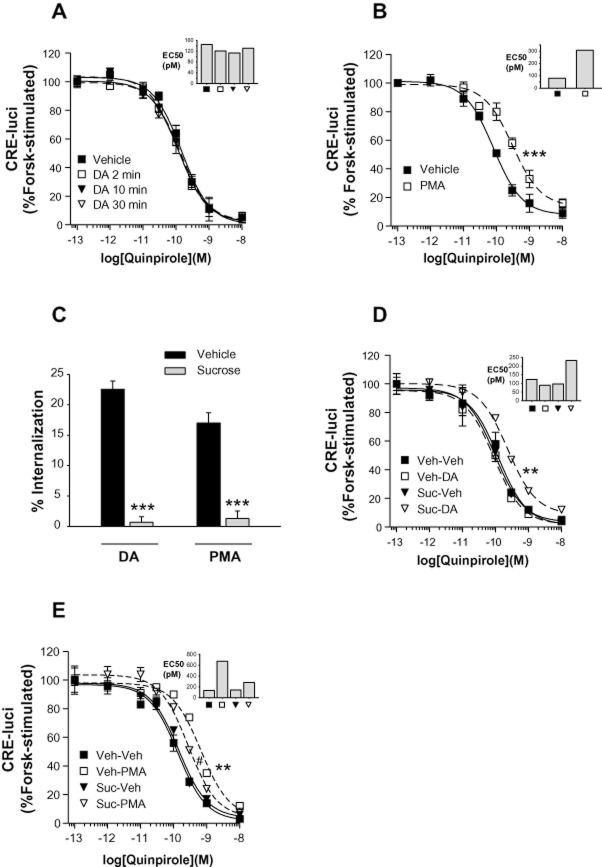
Internalization and desensitization of GPCRs are intimately related. To better understand the functional differences between the homologous and heterologous regulatory processes of D2 receptors, the DA-induced (homologous) and PKC-mediated (heterologous) internalization of D2 receptors were comparatively characterized. As reported previously (Kim et al., 2001), agonist-induced internalization of D2 receptors is known to be dependent on GRK and β-arrestin (Figure 1A). Heterologous internalization of D2 receptors was induced by PMA, a PKC activator, but not by its inactive isomer, 4α-PMA, and was blocked by Gö6976, a specific PKC inhibitor (Figure 1B). Gö6983, another specific PKC inhibitor, exerted less intense but still significant inhibitory effects. In addition, activation of PKC through stimulation of the Gq-coupled M1 muscarinic receptor resulted in the phosphorylation of D2 receptors (Namkung and Sibley, 2004), suggesting PMA-induced phosphorylation of D2 receptors has a physiological role.
Figure 1.
Characterization of homologous and heterologous internalization of D2 receptors. (A) Effects of GRK2 and β-arrestins on the homologous internalization of D2 receptors. Cells transiently transfected with D2 receptors with or without 2 μg GRK2-pRK5, β-arrestin2-pCMV5, or K220R-GRK2-pRK5 per 100 mm culture dish were treated with 10 μM DA for 1 h. Internalization assay was conducted as described in Methods. Receptor expression levels were maintained around 1.2 pmol·mg−1 protein. Each data point represents mean ± SEM. ***P < 0.001 compared with the ‘Mock’ group. (B) Characterization of PMA-induced internalization of D2 receptors. Cells transiently transfected with D2 receptors were treated with 1 μM PMA or 4α-PMA for 2 h. To study the effects of PKC inhibitors on PMA-induced internalization of D2 receptors, cells were pretreated with 1 μM Gö6976 or Gö6983 for 20 min and then treated with 1 μM PMA for 2 h. Receptor expression levels were maintained around 1.2 pmol·mg−1 protein. **P < 0.01, ***P < 0.001 compared with the vehicle group. (C) Comparison of post-endocytic behaviours of D2 receptors after treatment with DA or PMA. HEK-293 cells that stably express D2 receptors were treated with 10 μM DA for 1 h or 1 μM PMA for 2 h. Receptor recycling was determined as described in Methods. In the lower graph, the % of D2 receptors internalized by treatment with 10 μM DA or 1 μM PMA for 1–2 h was normalized to 100%, and the number of constitutively recycled D2 receptors was calculated as % of total internalized D2 receptors. Receptor expression levels were maintained around 0.9 pmol·mg−1 protein. *P < 0.05, **P < 0.01, ***P < 0.001 compared with vehicle group. (D) Comparisons of quinpirole- and PMA-induced down-regulation of D2 receptors. HEK-293 cells stably expressing D2 receptors were treated with 50 μg·mL−1 cyclohexamide, followed by treatment with vehicle, 1 μM quinpirole or 1 μM PMA for 0, 12 and 24 h. The total number of D2 receptor was measured by binding with 2 nM [3H]-spiperone. Receptor expression levels were maintained around 0.9 pmol·mg−1 protein. *P < 0.05, ***P < 0.001 compared with each vehicle group.
Since the post-endocytic behaviours of GPCRs determine the functional features of receptor internalization, the short-term and long-term recycling of D2 receptors was compared for the two regulatory pathways. As shown in Figure 1C, D2 receptors internalized through GRK- and PKC-mediated pathways showed opposite post-endocytic behaviours. Most homologously internalized D2 receptors (∼90%) were recycled back to the plasma membrane in 2 h; however, the heterologously internalized D2 receptors did not recycle. Long-term treatment with 1 μM quinpirole, a specific agonist of D2 receptors, for 12–24 h, did not induce down-regulation of D2 receptors. On the other hand, treatment with 1 μM PMA resulted in the down-regulation of D2 receptors (Figure 1D).
GRK- and PKC-mediated internalization of D2 receptors mediate opposite functional roles
Since DA- and PMA-induced internalization of D2 receptors resulted in different post-endocytic behaviour, the functional significance of internalization through each pathway was determined. Agonist-induced inhibition of cAMP production was employed as the measure of D2 receptor signalling and was measured by either direct determination of cellular cAMP (Supporting Information Figure S1A) or indirect reporter gene assay (Figure 2A). These two assay methods yielded essentially the same results. Homologous and heterologous desensitization of D2 receptors was induced by pretreatment with DA and PMA respectively. As reported previously (Cho et al., 2010a; Westrich and Kuzhikandathil, 2007), the signalling of D2 receptors was unaffected by pretreatment with DA (10 μM, between 2 and 30 min) (Figure 2A). On the other hand, pretreatment with 1 μM PMA induced evident desensitization of D2 receptors; the EC50 value increased from 81 to 310 pM (Figure 2B). As in the PMA-induced internalization of D2 receptors, the specific PKC inhibitor Gö6976 blocked the PMA-induced desensitization of D2 receptors more extensively than Gö6983 (Supporting Information Figure S1B and C). Also the inactive PMA analogue, 4α-PMA, did not induce desensitization of D2 receptors (Supporting Information Figure S1D), showing that PMA-induced desensitization is indeed mediated by PKC.
Figure 2.
Functional roles of homologous and heterologous internalization of D2 receptors. (A) Determination of homologous desensitization of D2 receptors. HEK-293 cells transiently transfected with D2 receptors were pretreated for 2–30 min with serum-free medium containing 100 μM ascorbic acid (vehicle) or 10 μM DA dissolved in vehicle. Cells were washed three times with 1 mL of serum-free medium, treated with increasing concentrations of quinpirole, and the cellular levels of cAMP were measured using a reporter gene assay as described in Methods. ‘Forsk’ represents forskolin. Receptor expression levels were maintained around 1.8 pmol·mg−1 protein. Inserted vertical bar chart shows the EC50 values of each experimental group. (B) Effects of PMA treatment on the desensitization of D2 receptors. Cells transiently transfected with D2 receptors were treated with 1 μM PMA in 0.1% of DMSO (vehicle) for 15 min followed by increasing concentrations of quinpirole. Receptor expression levels were maintained around 1.7 pmol·mg−1 protein. ***P < 0.001 when the dose-response curve for the PMA group was compared with that of the vehicle group. (C) Inhibition of D2 receptor internalization by sucrose treatment. Cells were treated with vehicle or 0.45 M sucrose for 20 min then treated with 10 μM DA for 1 h or with 1 μM PMA for 2 h. After washing, the internalization of D2 receptors was determined as described in Methods. Receptor expression levels were maintained around 1.4 pmol·mg−1 protein. ***P < 0.001 compared with each vehicle group. (D) Relationship between agonist-induced internalization and the signalling of D2 receptors. The desensitization assay was conducted after pretreatment with 0.45 M sucrose for 20 min, followed by 10 μM DA, washed, and the reporter gene assay was conducted with increasing concentrations of quinpirole. **P < 0.01 when the ‘Suc/DA group’ was compared with other experimental groups. (E) Relationship between PMA-induced internalization and the signalling of D2 receptors. In the desensitization assay, cells were pretreated with 0.45 M sucrose for 20 min, treated with 1 μM PMA and then washed; and the reporter gene assay was conducted as in (D). #P < 0.05 when the ‘Suc-PMA group’ was compared with ‘Suc-Veh group’; **P < 0.01 when the ‘Veh-PMA group’ was compared with ‘Veh-Veh group’.
To determine the functional roles of agonist-induced and PKC-mediated internalization, the internalization of D2 receptors was blocked, and the consequent effects on signalling were determined. Both DA- and PMA-induced internalization of D2 receptors were blocked by treating cells with 0.45 M sucrose for 20 min (Daukas and Zigmond, 1985) (Figure 2C). The sucrose treatment itself did not interfere with the signalling of D2 receptors, but the dose–response curve of the sucrose-treated group was shifted to the right by pre-exposure to DA (Figure 2D). These results suggest that the resensitization of D2 receptors was prevented when the agonist-induced internalization was blocked. As opposed to agonist-induced internalization of D2 receptors, inhibition of PMA-induced internalization of D2 receptors partly blocked PMA-induced desensitization of D2 receptors (Figure 2E), suggesting that PKC-mediated desensitization of D2 receptors is caused by receptor internalization as well as by receptor phosphorylation, which probably blocks the coupling between D2 receptors and G-protein.
Determination of receptor regions responsible for PKC-mediated desensitization of D2 receptors
The intracellular trafficking and desensitization studies of D2 receptors showed that both GRK-and PKC-mediated regulations involve receptor internalization, but display opposite post-endocytic behaviours (Figure 1C and D) and functional roles (Figure 2). Given that receptor phosphorylation is the key cellular event that determines the regulatory processes of both pathways (Kim et al., 2001; Namkung and Sibley, 2004), different phosphorylation sites within the intracellular loops of D2 receptors could be involved in the differences between the two pathways.
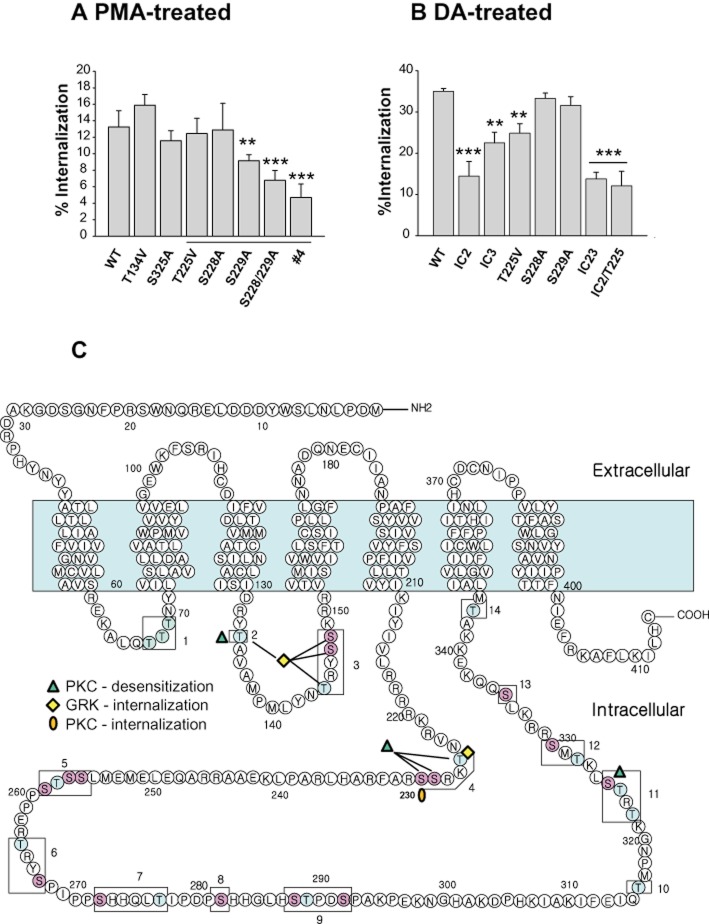
Using mutants in which every S/T residue within the intracellular loops was altered to an alanine (A)/valine (V) residue, a previous study reported that T225 is the key amino acid residue that drives the homologous internalization of D2 receptors (Cho et al., 2010a). Also, it was shown that several S/T residues located within the 3rd intracellular loop (S228, S229, T322, T324, S325) were phosphorylated in a PKC-dependent manner and that S325 was involved in the PKC-mediated desensitization of D2 receptors (Namkung and Sibley, 2004). However, this previous study did not identify the S/T residues responsible for PKC-mediated internalization. Furthermore, only a limited number of S/T residues were tested based on predicted consensus sites for PKC, and it is possible that other S/T residues located within the intracellular loops are also involved in the heterologous regulation of D2 receptors.
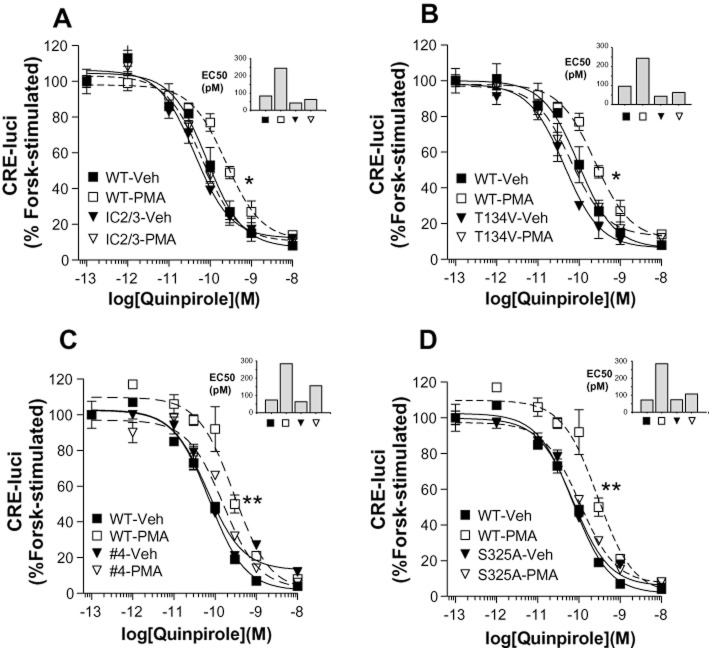
To locate potential phosphorylation sites responsible for PKC-mediated desensitization of D2 receptors, PMA-induced desensitization was tested in all D2 receptor mutants shown in Table 1 and Figure 4C. When all the S/T residues located within the second and third intracellular loops were mutated to A /V residues (D2R-IC23, Table 1), PMA-induced desensitization of D2 receptors was significantly inhibited (Figure 3A). The roles of individual S/T residues in the PKC-mediated desensitization of D2 receptors were further analysed. Mutation of T134 (#2, Figure 3B) but not other S/T residues located within the second intracellular loop [(T144V/S147A/S148A (#3, Supporting Information Figure S2A) or S147/8A (Supporting Information Figure S2B)] resulted in significant inhibition of PKC-mediated desensitization of D2 receptors. PMA-induced desensitization of D2 receptors was also tested for mutants within the third intracellular loop (from #4 to #14, Figure 4C). When these mutants were tested, a decrease in PMA-induced desensitization of D2 receptors was observed with D2R-#4 (T225V, S228A, S229A; Figure 3C) and D2R-#11 (T322V, T324V, S325A). When these S/T residues within D2R-#11 were subdivided, PMA-induced desensitization of D2 receptors was abolished with S325A-D2R (Figure 3D) but not with T322V/T324V-D2R (Supporting Information Figure S2C). The S/T residues responsible for PKC-mediated desensitization of D2 receptor are shown as triangles in Figure 4C.
Figure 4.
Determination of S/T residues responsible for homologous and heterologous internalization of D2 receptors. (A) Identification of S/T residues responsible for the PKC-mediated internalization of D2 receptors. Cells transiently transfected with wild-type or each S/T mutant of D2 receptor were treated with 1 μM PMA for 2 h. The underlined columns represent individual or combined mutations of S/T residues in mutant-#4 (T225/S228/S229). Receptor expression levels were maintained at around 1.2–1.5 pmol·mg−1 protein. **P < 0.01, ***P < 0.001 compared with wild type. (B) Identification of S/T residue responsible for the homologous internalization of D2 receptors. Cells transiently transfected with wild type or each S/T mutant of D2 receptor were treated with 10 μM DA for 1 h. Receptor expression levels were maintained at around 1.2–1.5 pmol·mg−1 protein. **P < 0.01, ***P < 0.001 compared with wild type. (C) Diagram showing the putative phosphorylation sites responsible for GRK- and PKC-mediated regulation of D2 receptors. The shaded region represents the transmembrane region. Site-directed mutagenesis was performed to change the designated serine (S) or threonine (T) residues within the cytoplasmic loops (#1–14) into alanine or valine residues respectively. T134, T225/S228/S229, and S325 are responsible for PKC-mediated desensitization of D2 receptors. S/T residues located within the second intracellular loop and T225 are responsible for agonist-induced internalization of D2 receptors. S229 is responsible for PMA-induced internalization of D2 receptors. More detailed information on the mutants is given in Table 1.
Figure 3.
Determination of the serine and threonine residues responsible for PKC-mediated desensitization of D2 receptors. HEK-293 cells transiently transfected with each mutant D2 receptor were treated with 1 μM PMA for 15 min, then dose–response curves were obtained for the inhibition of cAMP production. Receptor expression levels were maintained around 1.5–1.7 pmol·mg−1 protein. (A) Roles of the S/T residues located within the second and third intracellular loops in the PMA-induced desensitization of D2 receptors. (B) Roles of T134 in the PMA-induced desensitization of D2 receptors. (C, D) Determination of S/T residues in the third intracellular loop responsible for the PMA-induced desensitization of D2 receptors. *P < 0.05, **P < 0.01 when WT-PMA group was compared with WT-Veh group. Statistically significant differences were not observed between any of the mutants-PMA and mutants-Veh groups.
Relationship between PKC-mediated internalization and desensitization of D2 receptors
The results in Figure 2E show that PKC-mediated internalization is functionally related to desensitization of the D2 receptor. Site-directed mutagenesis analysis of S/T residues located within the intracellular loops revealed that three distinct receptor regions independently mediate the PKC-mediated desensitization of D2 receptors (Figure 3B–D). To test whether PKC-mediated internalization was necessary and sufficient for PKC-mediated desensitization of D2 receptors, the PMA-induced internalization was assessed in S/T mutants of the D2 receptor in which the PKC-mediated desensitization was abolished [T134V, T225V/S228A/S229A (#4), S325A]. Among these three D2 receptor mutants, only mutant-#4 showed significantly reduced PKC-mediated internalization (Figure 4A). To confirm whether T225/S228/S229 residues (mutant-#4) determine the PKC-mediated internalization of D2 receptor, we utilized another mutant, D2R-PKCX. In PKCX, all of the S/T residues responsible for PKC-mediated desensitization of the D2 receptor were mutated. PMA-induced internalization of D2 receptor was similarly reduced in the PKCX and mutant-#4 (Supporting Information Figure. S2D). These results show that phosphorylation of T134 or S325 mediates desensitization of D2 receptors independently of receptor internalization. On the other hand, phosphorylation of T225/S228/S229 may mediate receptor internalization and desensitization simultaneously, and the roles of individual S/T resides within mutant-#4 were further analysed.
Site-directed mutagenesis studies showed that the single point mutation of S229, but not of T225 or S228, inhibited PKC-mediated internalization of D2 receptors (Figure 4A). However, simultaneous mutations of T225/S228/S229 exerted a more marked inhibition of PKC-mediated internalization of D2 receptors. Similar results were obtained for PKC-mediated desensitization of D2 receptors. Mutants containing each individual mutation behaved like wild-type D2 receptors (Supporting Information Figure S3A–C), suggesting that these three residues are all needed for the PMA-induced desensitization of D2 receptors. S229 plays a major role in PKC-mediated internalization, and T225 and S228 potentiate the regulation through S229.
In contrast to PKC-mediated internalization and desensitization, GRK-mediated internalization of D2 receptor was significantly inhibited when the S/T residues located within IC2 or IC3 were mutated (Figure 4B). As reported previously (Cho et al., 2010a), the internalization of D2 receptor was significantly inhibited only when all four S/T residues within IC2 were simultaneously mutated (Figure 4B). Mutation of T225 resulted in a similar extent of inhibition as IC3, in which all S/T residues were mutated (Figure 4B). These results suggest that the S/T residues within the second intracellular loop and T225 are the major S/T residues responsible for the GRK-mediated internalization of D2 receptors. These results are summarized in Figure 4C.
ARF6 determines the recycling of homologously internalized D2 receptors
Next attempted to identify the cellular components that regulate the recycling of internalized D2 receptors. A previous study showed that β-arrestin2 is required for the recycling of internalized δ-opioid receptors (Zhang et al., 2008). Knockdown of β-arrestins, however, did not have noticeable effects on the recycling of internalized D2 receptors (Supporting Information Figure S4A and B), suggesting that β-arrestins are not involved in the recycling of D2 receptors.
Along with the large GTPase dynamin, various small GTPases such as Rab and ARF proteins have been proposed as regulators of vesicular transport (Segev, 2011). Some of the Rab proteins, such as Rab5 and Rab23, are known to regulate various steps of membrane trafficking in the route through which cell surface proteins traffic from the Golgi to the plasma membrane and recycle (Stenmark and Olkkonen, 2001; Evans et al., 2003). Co-expression of wild-type or dominant negative mutants of Rab5 or Rab23 did not have any effect on the recycling of internalized D2 receptors (Supporting Information Figure S4C and D), suggesting that these proteins are not involved in the constitutive recycling of the internalized D2 receptor in response to agonist stimulation.
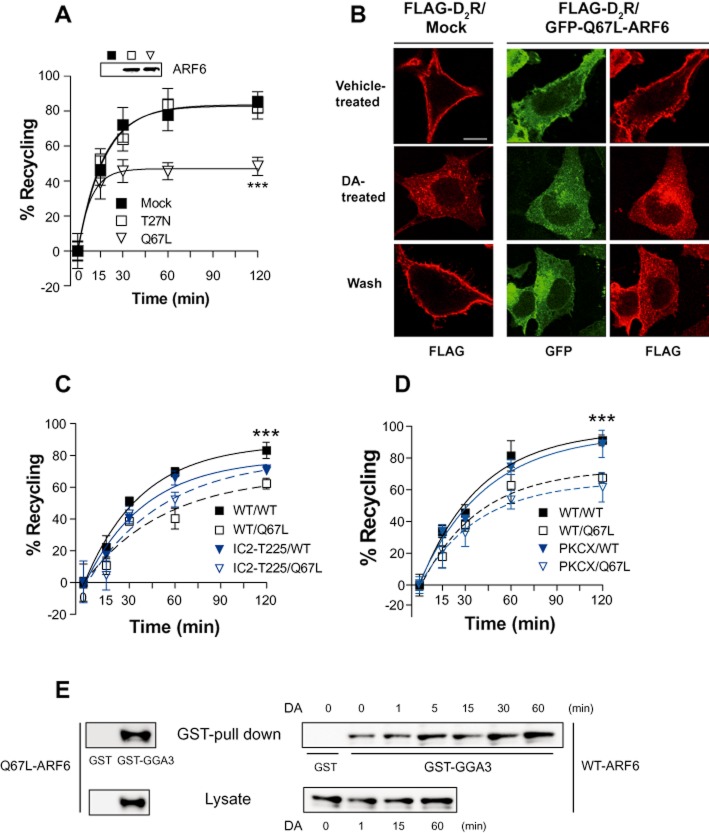
There are six members in the ARF (ADP-ribosylation factor) family of small GTPases; ARF6 is the best characterized for the regulation of intracellular trafficking of membrane proteins. ARF6 accumulates in clathrin-coated pits (CCPs) in a GTP-dependent manner and regulates fast recycling of plasma membrane receptors (Radhakrishna and Donaldson, 1997; Montagnac et al., 2011). The WT, constitutively active, or dominant-negative mutant of ARF6 did not have any effect on the agonist-induced internalization of D2 receptors (data not shown). Interestingly, a constitutively GTP-bound mutant of ARF6 (Q67L) inhibited the recycling of internalized D2 receptors in response to agonist stimulation (Figure 5A). These results were confirmed by immunocytochemical studies. The internalized D2 receptor readily recycled back to the plasma membrane in cells that do not express Q67L-ARF6 (Figure 5B, left panel). Q67L-ARF6 co-localized with D2 receptors on the plasma membrane and inhibited the recycling of internalized D2 receptors (Figure 5B, middle and right panel). Even though Q67L-ARF6 inhibited the recycling of D2 receptors, D2 receptors eventually returned to the plasma membrane (Supporting Information Figure S5A). These results suggest that Q67L-ARF6 retards the recycling of the internalized D2 receptor but does not increase the degradation of D2 receptors. The fast recycling ARF6 mutant (T157N) (Santy, 2002) did not affect the recycling of D2 receptors (Supporting Information Figure S5B), suggesting that GTP hydrolysis and ARF6 inactivation, rather than GDP/GTP exchange rate, are essential for the role of ARF6 in trafficking D2 receptors back to the plasma membrane.
Figure 5.
Mechanistic and functional analysis of constitutive recycling of homologously internalized D2 receptors. (A) Roles of ARF6 in the recycling of homologously internalized D2 receptors determined by the radioligand binding assay. HEK-293 cells stably expressing D2 receptors (1.2 pmol·mg−1 protein) were transfected with EGFP, EGFP-tagged T27N- or Q67L-ARF6. Expression levels of ARF6 constructs were determined by immunoblotting the cell extracts with antibodies against GFP. The % of D2 receptor internalization after 1 h treatment with 10 μM DA was 37.8, 35.2 and 29.6 in the Mock, T27N-ARF6 and Q67L-ARF6 groups respectively. These internalization values were converted to 100% for each experimental group (e.g. 37.8–100), and the number of constitutively recycled D2 receptors was presented as a % of maximal internalization (e.g. % of 37.8). ***P < 0.001 when Q67L group was compared with other experimental groups. (B) Roles of ARF6 in the recycling of homologously internalized D2 receptors determined by immunocytochemistry. Cells were transfected with either FLAG-D2 receptor alone (left panel) or FLAG-D2 receptor/GFP-tagged Q67L-ARF6 (middle and right panel). Cells were treated with vehicle (top panel) or with 10 μM DA for 1 h (middle panel), followed by three washes with serum-free medium at 4°C, and incubation at 37°C for 1 h (lower panel). For immunocytochemistry, cells were fixed with ice-cold 4% paraformaldehyde in PBS, pH 7.4, for 10 min. Cells were incubated with PBS containing with 3% FBS and 1% BSA for 1 h and then incubated with FLAG antibody 1 h at room temperature. After three washes, cells were incubated with Alexa 594-conjugated secondary antibody for 1 h at room temperature. After three washes with washing buffer, the cells were mounted in Vectashield (Vector Laboratories, Burlingame, CA) and viewed with laser scanning confocal microscope (TCS SP5/ABOS/Tandem, Germany). The horizontal bar represents 10 μm. (C) Requirement for the S/T residues regulated by GRK2/3 in the sorting activities of ARF6. HEK-293 cells expressing WT-D2R or D2R-IC2/T225 were transfected either with WT-ARF6 or Q67L-ARF6, and the recycling rates of each receptor were measured. Receptor expression levels were maintained at around 1.2 pmol·mg−1 protein. ***P < 0.001 when WT-D2R/WT-ARF6 group was compared with WT-D2R/Q67L-ARF6 group. (D) PKC-mediated regulation of D2 receptors is not dependent on the sorting activities of ARF6. HEK-293 cells expressing WT-D2R or D2R-PKCX were transfected either with WT-ARF6 or Q67L-ARF6, and the recycling rates of each receptor were measured. Receptor expression levels were maintained at around 1.2 pmol mg−1 protein. ***P < 0.001 when WT/WT or PKCX/WT group was compared with WT/Q67L-ARF6 or PKCX/Q67L-ARF6 group. (E) Effects of agonist stimulation of D2 receptors on the activity of ARF6. (Left panel) Cells were transfected with Q67L-ARF6. (Right panel) Cells expressing D2 receptors were transfected with WT-ARF6, ad stimulated with 10 μM DA up to 60 min. GST pull-down assay was conducted as described in Methods. The data represent results from three independent experiments with similar outcomes.
To determine whether ARF6 specifically mediates one of these two components, two different D2 receptor mutants were utilized: D2R-IC2/T225 and D2R-PKCX. In D2R-IC2/T225, four S/T residues within the second intracellular loop and T225 were mutated. In D2R-PKCX, T134, S228, S229 and S325 were mutated. Therefore, the GRK2- and PKC-dependent component is absent in D2R-IC2/T225 and D2R-PKCX respectively. As shown in Figure 5C and D, the recycling of D2R-PKCX but not that of D2R-IC2/T225 was inhibited by Q67L-ARF6. In agreement with these results, ARF6 did not affect the PMA-induced down-regulation of D2 receptors (Supporting Information Figure S5C). These results show that ARF6 selectively mediates the recycling of D2 receptors, which are internalized in a GRK-dependent manner.
Next, endogenous ARF6 was knocked down and changes in D2 receptor recycling were determined. As shown in Supporting Information Figure S5D, a decrease in cellular ARF6 did not have significant effects on the recycling of D2 receptors, suggesting that certain cellular components other than ARF6 are also involved in the control of D2 receptor recycling.
Finally, the effects of agonistic stimulation of D2 receptors on ARF6 activity were determined through GST pull-down assay using GST-GGA3-PBD. As shown in Figure 5E, stimulation of D2 receptors activated ARF6, suggesting that persistent stimulation of the D2 receptor will retard the recycling of internalized D2 receptors, probably resulting in a decrease in D2 receptor activity in the plasma membrane.
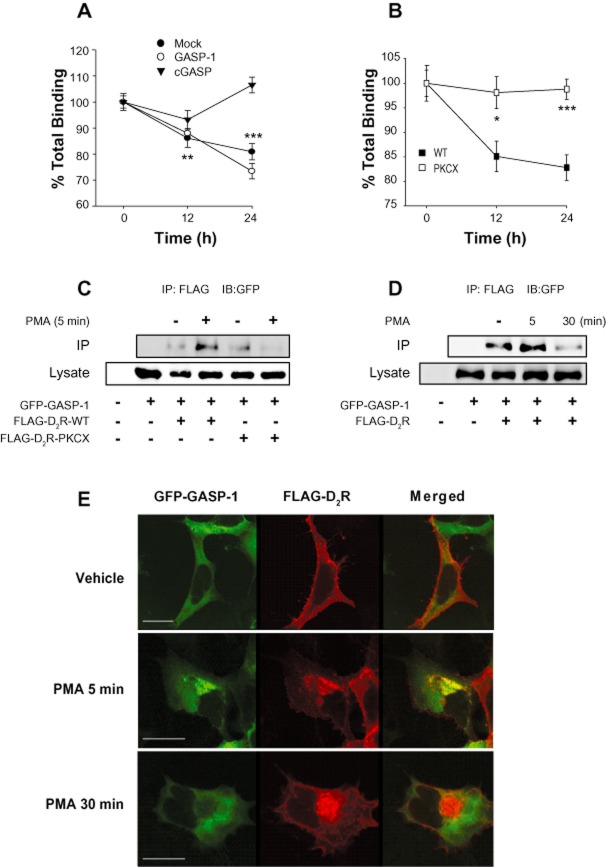
GASP-1 mediates the degradation of D2 receptors in a PKC-dependent manner
GASP-1 is a recently discovered sorting protein for GPCRs (Whistler et al., 2002; Moser et al., 2010) and seems to be involved in directing internalized GPCRs to lysosomes. The 497 amino-acid COOH terminal fragment of GASP-1 (cGASP) disrupts the interaction of GASP-1 with GPCRs and has been used as a dominant negative mutant of GASP-1.
Our results show that D2 receptors internalized in response to agonist treatment were constitutively recycled back to the plasma membrane when the agonist was removed from the culture media (Figure 1C), and that the receptor expression significantly increased when cells were treated with agonist between 12 and 24 h (Figure 1D). On the other hand, D2 receptors internalized in response to PKC stimulation were degraded (Figure 1C and D). Since GASP-1 was reported to interact with D2 receptors (Bartlett et al., 2005), we tested whether GASP-1 plays a specific role in differently sorting the D2 receptor internalized in response to agonist treatment or PKC activation. For this, the internalization of D2 receptors was induced by treating the cells with DA or PMA, and then their post-endocytic fate was assessed in the presence and absence of exogenous GASP-1 or cGASP. As shown in Figure 6A, the recycling of internalized D2 receptors in response to PKC stimulation was significantly retarded by co-expression of full length GASP-1 but significantly accelerated by co-expression of cGASP, the dominant-negative mutant of GASP-1. These results were confirmed by immunocytochemical studies. The D2 receptors internalized in response to PMA stimulation were more readily recycled back to the plasma membrane from cytosol by co-expression of cGASP (Figure 6B, compare the cells in the middle and right panels). In contrast, the recycling of D2 receptora internalized in response to agonistic stimulation was not altered by co-expression of GASP-1 or cGASP (Figure 6C). These results suggest that GASP-1 mediates the selective sorting of D2 receptors internalized in response to PKC activation into the degradation pathway. In accordance with this, GASP-1 interacted with D2 receptors, and this interaction was enhanced when cells were treated with PMA but not in DA-treated cells (Figure 6D). In agreement with these results, Gö6976, a specific PKC inhibitor, blocked the PMA-induced interaction between D2 receptors and GASP-1 (Supporting Information Figure S6A).
Figure 6.
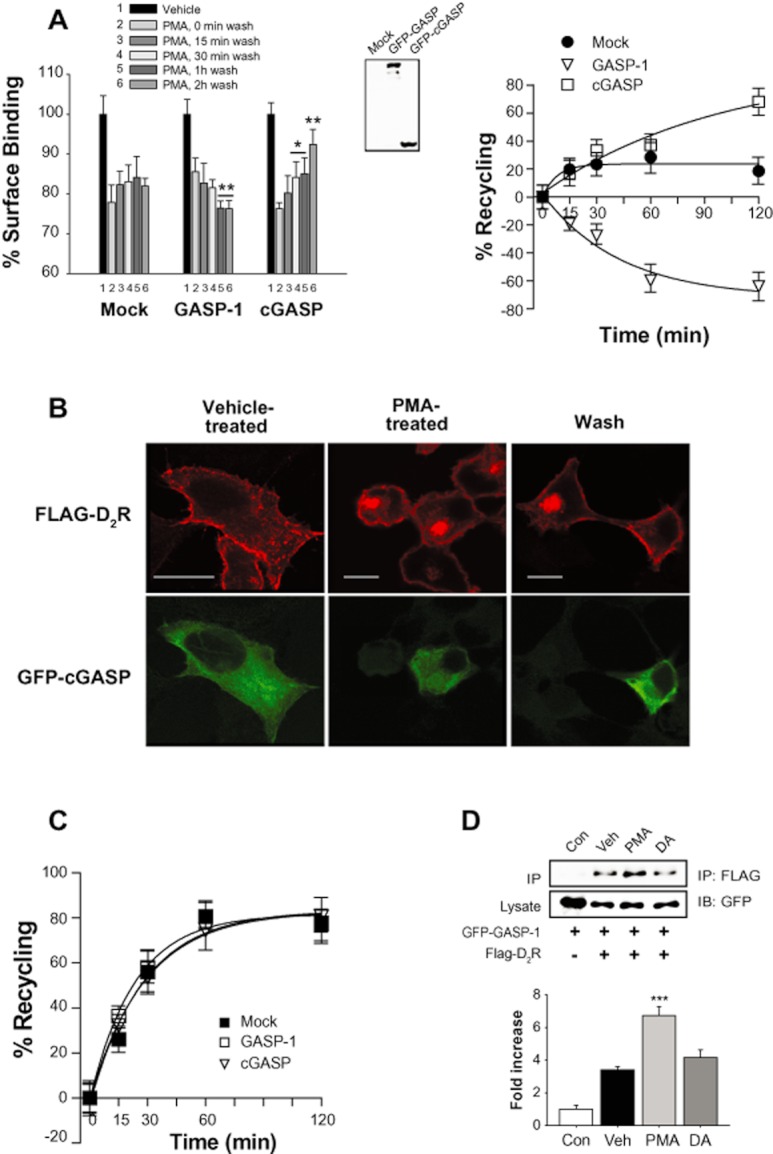
Effects of GASP-1 on the sorting of the internalized D2 receptor in response to PKC activation. (A) Roles of GASP-1 in the recycling of internalized D2 receptors in response to PKC activation. (Left panel) Cells stably expressing D2 receptors were transfected with EGFP, EGFP-tagged full-length GASP-1 or cGASP, stimulated with 1 μM PMA for 1 h, washed at 4°C and incubated with serum-free media for 0–120 min at 37°C. *P < 0.05, **P < 0.01 when compared with 0 min wash group. (Right panel) Results shown in left column were plotted for the recycling of D2 receptors. The maximum PKC-mediated internalization of D2 receptors, which was measured after PMA treatment and washing at 4°C, was converted to 100%. (B) Effects of GASP-1 on the recycling of heterologously internalized D2 receptors as determined by immunocytochemistry. Cells were transfected with FLAG-D2 receptor and GFP-tagged cGASP. Cells were treated with vehicle (left panel) or 1 μM PMA for 1 h (middle panel), followed by three washes with serum-free media at 4°C and incubation at 37°C for 1 h (right panel). Immunocytochemistry was conducted as in Figure 5B. The horizontal bars represent 10 μm. (C) Roles of GASP-1 in the recycling of internalized D2 receptors in response to agonist stimulation. Cells expressing D2 receptors and full-length GASP-1 or cGASP, were stimulated with 10 μM DA for 1 h and washed at 4°C. Receptor recycling was measured as in (A). (D) Selective interaction between D2 receptor and GASP-1 in response to PKC activation. Cells expressing GFP-tagged GASP-1 and FLAG-tagged D2 receptors were stimulated with 1 μM PMA or 10 μM DA for 5 min. Cell lysates were immunoprecipitated with FLAG antibodies and immunoblotted with antibodies to GFP. The data are representative of three independent experiments. ***P < 0.001 compared with vehicle group.
As expected from their effects on the recycling of D2 receptors in response to PKC activation, the PKC-mediated down-regulation of D2 receptors was enhanced or inhibited by co-expression of GASP-1 or cGASP, respectively (Figure 7A). In agreement with our hypothesis that D2 receptors phosphorylated in response to PKC activation undergo down-regulation, D2R-PKCX did not undergo down-regulation in response to PMA treatment (Figure 7B). The finding that GASP-1 is specifically involved in PKC-mediated phosphorylation of D2 receptors was further confirmed by protein interaction studies. The interaction between GASP-1 and WT-D2R but not D2R-PKCX increased in response to PMA treatment (Figure 7C), suggesting that GASP-1 is involved in PKC-mediated regulation of D2 receptors. The interaction between D2 receptors and GASP-1 was increased at 5 min after PMA treatment but decreased at 30 min after PMA treatment (Figure 7D). These results were confirmed by immunocytochemical studies. GASP-1 co-localized with D2 receptors at 5 min with PMA treatment (Figure 7E, middle panel) but showed exclusive subcellular distribution at 30 min with PMA treatment (Figure 7E, bottom panel). Essentially, the same results were obtained from SH-SY5Y cells, dopaminergic neuroblastoma cells. As shown in the two upper panels of Supporting Information Figure S6B, cGASP promoted the recycling of internalized D2 receptors. In addition, D2R-PKCX, which did not undergo PMA-induced internalization (Supporting Information Figure S2D) and did not bind with GASP-1 (Figure 7C) in HEK-293 cells, showed the same patterns of internalization and recycling as in SH-SY5Y dopaminergic neuroblastoma cells (Supporting Information Figure S6B). These results show that GASP-1 quickly interacts with D2 receptors to guide the intracellular trafficking pathway and then returns back to the cytoplasm.
Figure 7.
Roles of GASP-1 in the selective sorting of the internalized D2 receptor into the degradation pathway after PKC activation. (A) Roles of GASP-1 in the down-regulation of internalized D2 receptors in response to PKC activation. Cells expressing D2 receptors and GASP-1 or cGASP were treated with 1 μM PMA for 12 or 24 h, washed at 4°C. **P < 0.01, ***P < 0.001 when the experimental group at 12 or 24 h were compared with corresponding vehicle group for the cells expressing Mock vector or GASP-1. (B) Selective contribution of S/T residues involved in the PKC-mediated regulation of D2 receptor to the sorting activity of GASP-1. Cells expressing GASP-1 and WT-D2R or D2R-PKCX were treated as in (A). *P < 0.05, ***P < 0.001 compared with the WT group. (C) Selective contribution of S/T residues involved in the PKC-mediated regulation of D2 receptor to the protein interaction of GASP-1 with D2 receptors. Cells expressing GFP-tagged GASP-1 and FLAG tagged WT-D2R or D2R-PKCX, were treated with vehicle or 1 μM PMA for 5 min. Cell lysates were immunoprecipitated with FLAG antibodies and immunoblotted with antibodies to GFP. The data are representative of three independent experiments. (D) Time course of the interaction between D2 receptors and GASP-1 in response to PMA stimulation. Cells expressing GFP-tagged GASP-1 and FLAG-tagged WT-D2 receptors were treated with vehicle or 1 μM PMA for 5 and 30 min. Cell lysates were immunoprecipitated with FLAG antibodies and immunoblotted with antibodies to GFP. The data are representative of three independent experiments. (E) Time course of the co-localization of D2 receptors and GASP-1. Cells were transfected with FLAG-D2 receptor and GFP-tagged GASP-1. Cells were treated with vehicle (top panel), 1 μM PMA for 5 min (middle panel) or 30 min (lower panel). Immunocytochemistry was conducted as in Figure 5B. The horizontal bars represent 10 μm.
Discussion
GRK and PKC/PKA are representative regulators of homologous and heterologous desensitization of GPCRs in which receptor phosphorylation is the key cellular event. The involvement of GRK2/3 and PKC in the DA- and PMA-induced phosphorylation of D2 receptors has been well-documented (Kim et al., 2001; Namkung and Sibley, 2004). The elucidation of the molecular mechanisms that determine the differences between the two pathways has been a fundamental issue in this research area. Even though studies from β2-adrenoceptors have shown that different S/T residues are involved in GRK- and PKA-dependent regulation, the detailed molecular mechanisms that differentiate the two pathways are not clear. Here we performed a comparative study of the homologous and heterologous regulation of D2 receptors in terms of differences in the S/T residues involved, intracellular trafficking properties and differential usage of sorting proteins.
A previous study showed that phosphorylation of some of S/T residues located within the third intracellular loop (S256, S257, T258, S259, T264, S282, S288, S292) is required for the recycling but not the internalization of D2 receptors (Namkung et al., 2009). In our study, other S/T residues located in the second intracellular loop (T134, T144, S147, S148) and third intracellular loop (T225) are involved in the receptor internalization. It is interesting that different sets of S/T residues mediate distinct intracellular trafficking processes, and elucidation of underlying molecular mechanisms such as involvement of intermediate proteins could reveal novel regulatory processes that mediate different intracellular trafficking processes of GPCRs.
A working model is proposed in Figure 8 based on the experimental results obtained in this study. Our results show that S/T residues within IC2 and T225 are the major phosphorylation sites involved in the GRK2-mediated internalization of D2 receptors. On the other hand, T225, S228 and S229 are the major phosphorylation sites involved in the PKC-mediated internalization of D2 receptors. Therefore, differences in the phosphorylation patterns around IC2 and T225/S228/S229 could be critical factors that determine their post-endocytic fates. It is not clear, at this point, how the differently phosphorylated D2 receptors are selectively sorted into endocytic vesicles that either recycle back to the plasma membrane or are degraded in lysosomal vesicles.
Figure 8.
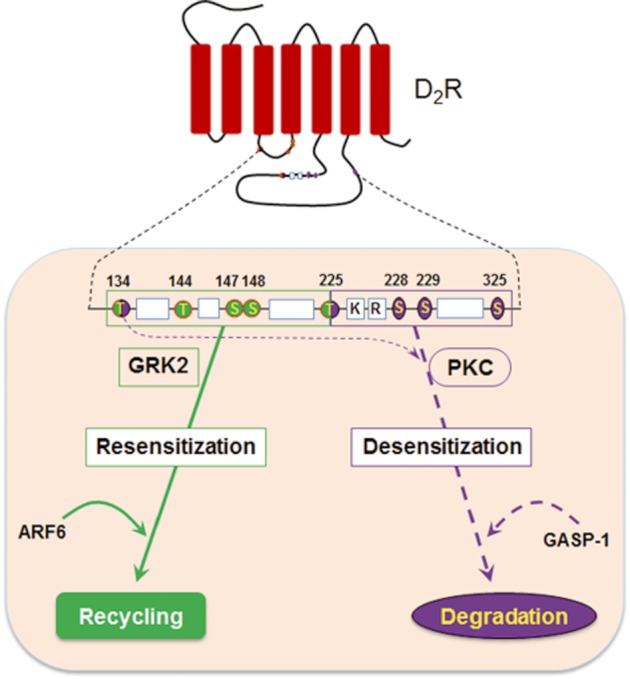
Diagram showingthe endocytic motifs and sorting proteins involved in the intracellular trafficking of D2 receptors. S/T residues located within the second intracellular loop and T225 are involved in the agonist-induced internalization. S229 is the main amino acid residue responsible for PMA-induced internalization, and T225/S228 is needed to enhance the PMA-induced internalization in collaboration with S229. T134 and T225 are involved both in agonist-induced and PMA-induced internalization of D2 receptors. ARF6 mediates the recycling of D2 receptors internalized in a GRK2-dependent manner, which results in the resensitization of D2 receptors. GASP-1 is involved in the lysosomal sorting of D2 receptors internalized in a PKC-dependent manner and mediates the desensitization of D2 receptors.
The fate of internalized receptors will be determined by sorting between recycling and degradation pathways. Thus, the post-endocytic fate of a receptor could be an important factor in determining the role of endocytosis in signal transduction. If the internalized receptors recycle back to the plasma membrane, the internalization could mediate receptor resensitization either through receptor dephosphorylation or dissociation from arresting proteins. Alternatively, if the internalized receptor is targeted to the lysosomes, internalization could be the initial step towards receptor desensitization through down-regulation. Our results show that the post-endocytic fate of D2 receptors is oppositely determined by ARF6 and GASP-1, depending on the nature of the stimulus, that is, either dopamine agonist or activation of cellular PKC activity.
Our results are different from a previous study with δ-opioid receptors in which GRK2-mediated receptor phosphorylation and β-arrestins play critical roles in the constitutive recycling of internalized receptor proteins (Zhang et al., 2008). We have shown that mutation of all of serine and threonine residues located within the intracellular loop of D2 receptors slightly delayed recycling but it still occurred (Cho et al., 2010a). In addition, knockdown of β-arrestin1/2 did not have any effect on the recycling of internalized D2 receptors (Supporting Information Figure S4B).
It is interesting that differential phosphorylation of D2 receptors by GRK and PKC results in selective functional association with distinct sorting proteins. It has been suggested that receptor phosphorylation not only influences endocytosis but also influences the post-endocytic fate of a receptor (Namkung et al., 2009; Cho et al., 2010a). A recent study showed that GASP-1 interacts with dysbindin, a cytoplasmic protein that is known to function in the biogenesis of specialized lysosome-related organelles (Marley and von Zastrow, 2010). Therefore, it is speculated that the conformational status of D2 receptors induced by phosphorylation on different S/T residues might differently interact with different sorting proteins.
Small GTPases, such as Rab, ARF and Rho, and a large GTPase, dynamin, are known to regulate various steps of vesicular transport, including vesicle formation, scission, targeting and fusion (Segev, 2011). These GTPases have their own authentic roles in vesicular transport. For example, vesicle formation is regulated by ARF, vesicle scission by dynamin, vesicle motility by Rabs, vesicle tethering by Rabs and Rhos, and vesicle fusion by Rhos (Segev, 2011). Small GTPases slowly switch between the GDP- and GTP-bound forms, and this process is greatly accelerated with the help of guanine nucleotide exchange factors and GTPase activating proteins. In addition, GTPases cycle between the cytoplasm and membranes, and the GTP-bound forms of GTPases on membranes interact with their specific effectors, which mediate vascular transport (Seabra and Wasmeier, 2004). Results in Figure 5A and C are in agreement with these molecular schemes. The GTP-bound form of ARF6 (Q67L), but not the GDP-bound form of ARF6 (T27N) or the fast cycling form of ARF6 (T157N), interfered with the recycling. Proper conversion of ARF6 from GTP- to GDP-bound form seems to be a critical factor for normal recycling of internalized vesicles.
However, caution still needs to be taken when drawing definite conclusions. As shown in Supporting Information Figure S5D, knockdown of endogenous ARF6 did not have significant effects on the recycling of internalized D2 receptors (Supporting Information Figure S5D). Therefore, it is possible that certain cellular components other than ARF6 are also involved in the recycling of D2 receptors, or it can be speculated that ARF6, in a GTP-dependent manner, regulates other cellular components that are responsible for the recycling of D2 receptors.
GASP is known to be involved in directing internalized GPCRs to lysosomes, leading to their degradation (Whistler et al., 2002). A subsequent study in HEK-293 cells showed that GASP mediates the degradation of internalized D2 receptors in response to dopamine treatment (Bartlett et al., 2005). In contrast, previous studies in HEK-293 cells and C6 glioma cells showed that D2 receptors are up-regulated by agonist treatment (Filtz et al., 1993; Starr et al., 1995). Our studies in HEK-293 cells showed that the D2 receptors internalized in response to agonistic stimulation readily recycle back to the plasma membrane, while long-term treatment with agonist up-regulated the D2 receptor levels (Figure 1D). It is not clear what caused the opposite results for the same receptor in the same cell types. Furthermore, one study group, from measuring the expression of D2 receptors on the cell surface by immunocytochemical labelling of the N-terminus, reported that agonistic stimulation resulted in internalization and degradation of D2 receptors. Whereas other study groups measured the surface D2 receptor levels by radioligand binding assay and reported that D2 receptors are increased by long-term treatment with agonist. Further studies are needed to clarify the technical differences between the two approaches.
Another question is whether PKC-mediated internalization is directly associated with PKC-mediated desensitization of D2 receptors. The results in Figures 3 and 4C show that PKC-mediated desensitization of D2 receptors is mediated by S/T residues located within three independent regions (T134, T225/S228/S229, S325) of intracellular loops, but the internalization is mediated by only one of three locations (T225/S228/S229, Figure 4A and C). The results in Figure 2C and E also show that the blockade of PKC-mediated internalization of D2 receptors only partly inhibits the PKC-mediated desensitization. These results suggest that the PKC-mediated desensitization of D2 receptors is collaboratively controlled by receptor phosphorylations through which receptor internalization might or might not be induced.
Our results show that T134, T225/S228/S229 and S325 collaboratively mediate PKC-mediated desensitization of D2 receptors, and that alteration of any of these S/T residues abolishes PKC-mediated desensitization of D2 receptors. These results suggest that PKC-mediated desensitization of D2 receptors will occur only when all of these S/T residues are simultaneously phosphorylated. In addition, agonist-induced internalization mediates the resensitization of D2 receptors (Figure 2D). These results suggest that both homologous and heterologous desensitization of D2 receptors are tightly regulated and the responsiveness of a D2 receptor is strictly preserved. Agonists for D2 receptors have been used for the clinical management of Parkinson's disease (Calne et al., 1974) and prolactin-secreting adenomas (Cunnah and Besser, 1991). Since these treatments involve long-term administration of D2 receptor agonists, the maintenance of receptor responsiveness is critical for their successful use.
In conclusion, both homologous and heterologous pathways are involved in the regulatory processes of D2 receptors. Different post-endocytic fates and opposite functional roles of GRK- and PKC-mediated endocytic pathways of D2 receptor are mediated by differential involvement of phosphorylation sites and selective involvement of sorting factors such as ARF6 and GASP-1. However, the relevance of the endogenous mechanisms revealed in this study remains to be demonstrated, since all of the data discussed were obtained from transfected cells.
Acknowledgments
This work was funded by KRF-2011-0016396. We thank the Korea Basic Science Institute for technical support.
Glossary
Abbreviations
- ARF6
ADP-ribosylation factor 6
- DA
dopamine
- GRK
GPCR kinase
- GASP
GPCR- associated sorting protein
- GG3A
golgi associated, γ adaptin ear containing, ARF binding protein 3
- PMA
phorbol myristate acetate
Conflict of interest
The authors state no conflict of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Figure S1 Characterization of PMA-induced desensitization of D2 receptors. (A) Dose–response curve for the inhibition of cAMP production in D2 receptors. cAMP was measured by column chromatography as described in Methods. Receptor expression levels were maintained at around 1.4 pmol·mg−1 protein. (B) Effects of PKC inhibitor Gö6976 on the PMA-induced desensitization of D2 receptors. Cells were pretreated with 1 μM Gö6976 for 20 min and then treated with 1 μM PMA for 15 min, followed by determination of dose-response curves. ***P < 0.001 when Veh/PMA group was compared with Veh/Veh group. (C) Effects of the PKC inhibitor Gö6983 on the PMA-induced desensitization of D2 receptors. Cells were pretreated with 1 μM Gö6983 for 20 min and then treated with 1 μM PMA for 15 min. **P < 0.01 when ‘Veh/PMA’ group was compared to ‘Veh/Veh’ group. (D) Involvement of PKC in the desensitization of D2 receptors. Cells expressing D2 receptors were treated with 1 μM PMA or 4α-PMA for 15 min, followed by determination of dose–response curves. ***P < 0.001 when PMA group was compared with vehicle or 4α-PMA group.
Figure S2 Characterization of effects of mutants of D2 receptors at S/T residues on the PMA-induced desensitization of D2 receptors. (A) Effects of mutations of T144/S147/S148 (#3) on the PMA-induced desensitization of D2 receptors. *P < 0.05 when PMA group was compared with each vehicle group. Receptor expression levels were maintained around 1.8 pmol·mg−1 protein. (B) Effects of mutation of S147 and S148 on PMA-induced desensitization of D2 receptors. *P < 0.05 when PMA group was compared with each vehicle group. (C) Effects of mutations of T322 and T324 on the PMA-induced desensitization of D2 receptors. *P < 0.05 when PMA group was compared with each vehicle group. (D) Effects of mutations of S/T residues involved in the PKC-mediated desensitization on the PMA-induced internalization of D2 receptors. ***P < 0.001 compared with WT group.
Figure S3 Functional analysis of S/T residues located within the endocytic motif of the third intracellular loop and associated plasma membrane microdomain in which PKC-mediated internalization of the D2 receptor occurs. Effects of point mutation of T225, S228 and S229 on the PKC-mediated desensitization of D2 receptors. Cells expressing wild-type or each S/T mutant of D2 receptor were treated with 1 μM PMA for 15 min, and the dose–response curves were determined. *P < 0.05 when PMA-treated group was compared with each vehicle group.
Figure S4 Roles of β-arrestins and Rab5/Rab23 in the recycling of homologously internalized D2 receptors. (A) Preparation of double knockout cell lines of β-arrestin1/2. Double knockdown of β-arrestins was conducted as described in Methods. (B) Roles of β-arrestins in the recycling of D2 receptors. Cells expressing D2 receptors (around 0.9 pmol·mg−1 protein) were treated with 50 μg·mL−1 cyclohexamide, followed by 10 μM DA for 60 min. After being washed with serum-free medium, cells were incubated at 37°C for the indicated period of time. (C) Roles of Rab5 in the recycling of D2 receptors. Cells stably expressing D2 receptors (∼1.1 pmol·mg−1 protein) were transfected with Mock, WR-Rab5 or S34N-Rab5. (D) Roles of Rab23 in the recycling of D2 receptors. Cells stably expressing D2 receptors (∼1.1 pmol·mg−1 protein) were transfected with Mock, WR-Rab23 or S23V-Rab23.
Figure S5 Roles of ARF6 in the intracellular trafficking of D2 receptors. (A) Roles of ARF6 in the induced down-regulation of D2 receptors. HEK-293 cells stably expressing D2 receptor were co-expressed with empty vector, ARF6-WT, ARF6-T27N or ARF-Q67L, treated with 1 μM quinpirole for 12 and 24 h. Receptor binding was determined as in Figure 1D. (B) Effects of fast cycling ARF6 mutant on the recycling of homologously internalized D2 receptors. Cells were transfected with wild type (WT), T157N- or Q67L-ARF6. The recycling of homologously internalized D2 receptors was determined as in Figure 5A. ***P < 0.001 when Q67L group was compared to WT or T157N group. (C) Effects of ARF6 on the down-regulation of D2 receptors in response to long-term PMA stimulation. HEK-293 cells expressing ARF6 constructs were treated with vehicle or 1 μM PMA for 12 or 24 h, and receptor binding was conducted as in Figure 1D. (D) Effects of knockdown of endogenous ARF6 on the recycling of internalized D2 receptors. HEK-293 cells stably expressing scrambled shRNA or ARF6 shRNA were transfected with D2 receptors. Receptor recycling was determined as in Figure 1C.
Figure S6 Roles of GASP-1 in the recycling of D2 receptors internalized in response to PMA stimulation. (A) Effects of PKC inhibitors on the interaction between D2 receptors and GASP-1. HEK-293 cells expressing FLAG-D2R and GFP-GASP-1 were pretreated with 1 μM Gö6976 or Gö6983 for 20 min, followed by 1 μM PMA for 5 min. Immunoprecipitation was conducted as in Figure 1D. **P < 0.01, ***P < 0.001 when PMA group was compared with corresponding Veh group. (B) Roles of GASP-1 in the recycling of D2 receptors in SH-SY5Y dopaminergic neuroblastoma cells. (Upper two panels) Roles of GASP-1 in the recycling of D2 receptors. Cells transfected with FLAG-D2R and GFP-GASP-1 were stimulated with 1 μM PMA for 60 min (middle panel), followed by washing and incubation at 37°C for 60 min (right panel). Immunocytochemistry was conducted as in Figure 5B. (Lower two panels) Cells were transfected either with D2R-GFP or D2R-PKCX-GFP and processed as in the upper two panels. The horizontal bars represent 10 μm.
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edn. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak LS, Ferguson SS, Zhang J, Caron MG. A beta-arrestin/green fluorescent protein biosensor for detecting G protein-coupled receptor activation. J Biol Chem. 1997;272:27497–27500. doi: 10.1074/jbc.272.44.27497. [DOI] [PubMed] [Google Scholar]
- Bartlett SE, Enquist J, Hopf FW, Lee JH, Gladher F, Kharazia V, et al. Dopamine responsiveness is regulated by targeted sorting of D2 receptors. Proc Natl Acad Sci U S A. 2005;102:11521–11526. doi: 10.1073/pnas.0502418102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calne DB, Teychenne PF, Leigh PN, Bamji AN, Greenacre JK. Treatment of parkinsonism with bromocriptine. Lancet. 1974;2:1355–1356. doi: 10.1016/s0140-6736(74)92219-3. [DOI] [PubMed] [Google Scholar]
- Cho D, Zheng M, Min C, Ma L, Kurose H, Park JH, et al. Agonist-induced endocytosis and receptor phosphorylation mediate resensitization of dopamine D(2) receptors. Mol Endocrinol. 2010a;24:574–586. doi: 10.1210/me.2009-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho D, Min C, Jung K, Cheong S, Zheng M, Oak M, et al. N-terminal region of dopamine D(2) receptor, a rhodopsin family GPCR, regulates proper integration into plasma membrane and endocytic routes. Br J Pharmacol. 2011;166:659–675. doi: 10.1111/j.1476-5381.2011.01787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho DI, Zheng M, Kim KM. Current perspectives on the selective regulation of dopamine D and D receptors. Arch Pharm Res. 2010b;33:1521–1538. doi: 10.1007/s12272-010-1005-8. [DOI] [PubMed] [Google Scholar]
- Cho EY, Cho DI, Park JH, Kurose H, Caron MG, Kim KM. Roles of protein kinase C and actin-binding protein 280 in the regulation of intracellular trafficking of dopamine D3 receptor. Mol Endocrinol. 2007;21:2242–2254. doi: 10.1210/me.2007-0202. [DOI] [PubMed] [Google Scholar]
- Cunnah D, Besser M. Management of prolactinomas. Clin Endocrinol (Oxf) 1991;34:231–235. doi: 10.1111/j.1365-2265.1991.tb00299.x. [DOI] [PubMed] [Google Scholar]
- Daukas G, Zigmond SH. Inhibition of receptor-mediated but not fluid-phase endocytosis in polymorphonuclear leukocytes. J Cell Biol. 1985;101((5 Pt 1)):1673–1679. doi: 10.1083/jcb.101.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TM, Ferguson C, Wainwright BJ, Parton RG, Wicking C. Rab23, a negative regulator of hedgehog signaling, localizes to the plasma membrane and the endocytic pathway. Traffic. 2003;4:869–884. doi: 10.1046/j.1600-0854.2003.00141.x. [DOI] [PubMed] [Google Scholar]
- Filtz TM, Artymyshyn RP, Guan W, Molinoff PB. Paradoxical regulation of dopamine receptors in transfected 293 cells. Mol Pharmacol. 1993;44:371–379. [PubMed] [Google Scholar]
- Fredericks ZL, Pitcher JA, Lefkowitz RJ. Identification of the G protein-coupled receptor kinase phosphorylation sites in the human beta2-adrenergic receptor. J Biol Chem. 1996;271:13796–13803. doi: 10.1074/jbc.271.23.13796. [DOI] [PubMed] [Google Scholar]
- Freedman NJ, Lefkowitz RJ. Desensitization of G protein-coupled receptors. Recent Prog Horm Res. 1996;51:319–351. discussion 352–313. [PubMed] [Google Scholar]
- Glatt SJ, Faraone SV, Tsuang MT. Meta-analysis identifies an association between the dopamine D2 receptor gene and schizophrenia. Mol Psychiatry. 2003;8:911–915. doi: 10.1038/sj.mp.4001321. [DOI] [PubMed] [Google Scholar]
- Guo J, Wu Y, Zhang W, Zhao J, Devi LA, Pei G, et al. Identification of G protein-coupled receptor kinase 2 phosphorylation sites responsible for agonist-stimulated delta-opioid receptor phosphorylation. Mol Pharmacol. 2000;58:1050–1056. doi: 10.1124/mol.58.5.1050. [DOI] [PubMed] [Google Scholar]
- Johnson RA, Salomon Y. Assay of adenylyl cyclase catalytic activity. Methods Enzymol. 1991;195:3–21. doi: 10.1016/0076-6879(94)38005-8. [DOI] [PubMed] [Google Scholar]
- Kim KM, Valenzano KJ, Robinson SR, Yao WD, Barak LS, Caron MG. Differential regulation of the dopamine D2 and D3 receptors by G protein-coupled receptor kinases and beta-arrestins. J Biol Chem. 2001;276:37409–37414. doi: 10.1074/jbc.M106728200. [DOI] [PubMed] [Google Scholar]
- Kim KM, Gainetdinov RR, Laporte SA, Caron MG, Barak LS. G protein-coupled receptor kinase regulates dopamine D3 receptor signaling by modulating the stability of a receptor-filamin-beta-arrestin complex. A case of autoreceptor regulation. J Biol Chem. 2005;280:12774–12780. doi: 10.1074/jbc.M408901200. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Hausdorff WP, Caron MG. Role of phosphorylation in desensitization of the beta-adrenoceptor. Trends Pharmacol Sci. 1990;11:190–194. doi: 10.1016/0165-6147(90)90113-m. [DOI] [PubMed] [Google Scholar]
- Marley A, von Zastrow M. Dysbindin promotes the post-endocytic sorting of G protein-coupled receptors to lysosomes. PloS One. 2010;5:e9325. doi: 10.1371/journal.pone.0009325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Montagnac G, de Forges H, Smythe E, Gueudry C, Romao M, Salamero J, et al. Decoupling of activation and effector binding underlies ARF6 priming of fast endocytic recycling. Curr Biol. 2011;21:574–579. doi: 10.1016/j.cub.2011.02.034. [DOI] [PubMed] [Google Scholar]
- Moser E, Kargl J, Whistler JL, Waldhoer M, Tschische P. G protein-coupled receptor-associated sorting protein 1 regulates the postendocytic sorting of seven-transmembrane-spanning G protein-coupled receptors. Pharmacology. 2010;86:22–29. doi: 10.1159/000314161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namkung Y, Sibley DR. Protein kinase C mediates phosphorylation, desensitization, and trafficking of the D2 dopamine receptor. J Biol Chem. 2004;279:49533–49541. doi: 10.1074/jbc.M408319200. [DOI] [PubMed] [Google Scholar]
- Namkung Y, Dipace C, Javitch JA, Sibley DR. G protein-coupled receptor kinase-mediated phosphorylation regulates post-endocytic trafficking of the D2 dopamine receptor. J Biol Chem. 2009;284:15038–15051. doi: 10.1074/jbc.M900388200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing ‘preassembled signaling complexes’ at the plasma membrane. J Biol Chem. 1998;273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- Radhakrishna H, Donaldson JG. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J Cell Biol. 1997;139:49–61. doi: 10.1083/jcb.139.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapacciuolo A, Suvarna S, Barki-Harrington L, Luttrell LM, Cong M, Lefkowitz RJ, et al. Protein kinase A and G protein-coupled receptor kinase phosphorylation mediates beta-1 adrenergic receptor endocytosis through different pathways. J Biol Chem. 2003;278:35403–35411. doi: 10.1074/jbc.M305675200. [DOI] [PubMed] [Google Scholar]
- Roth NS, Campbell PT, Caron MG, Lefkowitz RJ, Lohse MJ. Comparative rates of desensitization of beta-adrenergic receptors by the beta-adrenergic receptor kinase and the cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1991;88:6201–6204. doi: 10.1073/pnas.88.14.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santy LC. Characterization of a fast cycling ADP-ribosylation factor 6 mutant. J Biol Chem. 2002;277:40185–40188. doi: 10.1074/jbc.C200481200. [DOI] [PubMed] [Google Scholar]
- Seabra MC, Wasmeier C. Controlling the location and activation of Rab GTPases. Curr Opin Cell Biol. 2004;16:451–457. doi: 10.1016/j.ceb.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Segev N. GTPases in intracellular trafficking: an overview. Semin Cell Dev Biol. 2011;22:1–2. doi: 10.1016/j.semcdb.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr S, Kozell LB, Neve KA. Drug-induced up-regulation of dopamine D2 receptors on cultured cells. J Neurochem. 1995;65:569–577. doi: 10.1046/j.1471-4159.1995.65020569.x. [DOI] [PubMed] [Google Scholar]
- Stenmark H, Olkkonen VM. The Rab GTPase family. Genome Biol. 2001;2:REVIEWS3007. doi: 10.1186/gb-2001-2-5-reviews3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin AB. G-protein-coupled receptor phosphorylation: where, when and by whom. Br J Pharmacol. 2008;153(Suppl. 1):S167–S176. doi: 10.1038/sj.bjp.0707662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin AB, Butcher AJ, Kong KC. Location, location, location … site-specific GPCR phosphorylation offers a mechanism for cell-type-specific signalling. Trends Pharmacol Sci. 2008;29:413–420. doi: 10.1016/j.tips.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westrich L, Kuzhikandathil EV. The tolerance property of human D3 dopamine receptor is determined by specific amino acid residues in the second cytoplasmic loop. Biochim Biophys Acta. 2007;1773:1747–1758. doi: 10.1016/j.bbamcr.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Whistler JL, Enquist J, Marley A, Fong J, Gladher F, Tsuruda P, et al. Modulation of postendocytic sorting of G protein-coupled receptors. Science. 2002;297:615–620. doi: 10.1126/science.1073308. [DOI] [PubMed] [Google Scholar]
- Xiang B, Yu GH, Guo J, Chen L, Hu W, Pei G, et al. Heterologous activation of protein kinase C stimulates phosphorylation of delta-opioid receptor at serine 344, resulting in beta-arrestin- and clathrin-mediated receptor internalization. J Biol Chem. 2001;276:4709–4716. doi: 10.1074/jbc.M006187200. [DOI] [PubMed] [Google Scholar]
- Yuan N, Friedman J, Whaley BS, Clark RB. cAMP-dependent protein kinase and protein kinase C consensus site mutations of the beta-adrenergic receptor. Effect on desensitization and stimulation of adenylylcyclase. J Biol Chem. 1994;269:23032–23038. [PubMed] [Google Scholar]
- Zhang X, Wang F, Chen X, Chen Y, Ma L. Post-endocytic fates of delta-opioid receptor are regulated by GRK2-mediated receptor phosphorylation and distinct beta-arrestin isoforms. J Neurochem. 2008;106:781–792. doi: 10.1111/j.1471-4159.2008.05431.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.