Abstract
Background and Purpose
Chemotherapeutic agents, including 5-fluorouracil (5-FU), frequently cause intestinal mucositis resulting in severe diarrhoea and morphological mucosal damage. 5-HT3 receptor antagonists are clinically effective in the treatment of nausea and emesis during cancer chemotherapy. Therefore we here have examined the effects of 5-HT3 receptor antagonists on 5-FU-induced intestinal mucositis in mice.
Experimental Approach
Intestinal mucositis was induced in male C57BL/6 mice by daily administration of 5-FU (50 mg·kg−1) for 5 days. Effects of 5-HT3 receptor antagonists, ramosetron (0.01–0.1 mg·kg−1) and ondansetron (5 mg·kg−1), on the accompanying histology, cytokine production and apoptosis were assessed.
Key Results
Continuous administration of 5-FU to mice caused severe intestinal mucositis, which was histologically characterized by the shortening of villi and destruction of intestinal crypts, accompanied by body weight loss and diarrhoea. Daily ramosetron administration dose-dependently reduced the severity of intestinal mucositis, body weight loss and diarrhoea. Similar beneficial effects were observed with ondansetron. The number of apoptotic, caspase-3- and caspase-8-activated cells increased 24 h after the first 5-FU administration, and these responses were reduced by ramosetron. The up-regulation of TNF-α, IL-1β and IL-6 following 5-FU treatment was also attenuated by ramosetron.
Conclusions and Implications
5-HT3 receptor antagonists ameliorated 5-FU-induced intestinal mucositis in mice, and this action could result from suppression of apoptotic responses in the intestinal crypt cells via inhibition of cytokine expression. Thus, 5-HT3 receptor antagonists may be useful for preventing not only nausea and emesis but also intestinal mucositis during 5-FU chemotherapy.
Keywords: 5-fluorouracil, intestinal mucositis, 5-HT3 receptors, ramosetron, ondansetron, apoptosis, TNF-α, caspase-3, caspase-8
Introduction
Severe intestinal mucositis is a frequent side effect of clinical chemotherapy for cancer (Wadler et al., 1998; Benson et al., 2004). 5-Fluorouracil (5-FU), an anti-metabolite anticancer agent, is widely used for the treatment of malignant tumours, but causes mucositis in 50–80% of patients. The symptoms may include nausea, vomiting, anorexia and severe diarrhoea (Symonds, 1998; Wadler et al., 1998; Benson et al., 2004). These serious side effects are the major causes for discontinuation of treatment or reduction of drug dose, thereby compromising the success of the cancer chemotherapy. Chemotherapy-induced intestinal mucositis, characterized by the shortening of villi and disruption of crypt cell homeostasis, is considered to be a consequence of several processes, including abnormal inflammation and apoptosis, together with cellular hypoproliferation and direct cytotoxicity (Daniele et al., 2001; Duncan and Grant, 2003; Bowen et al., 2006).
5-HT exerts a variety of physiological functions not only in the central and peripheral nervous system but also in the gastrointestinal tract and cardiovascular system (Talley, 2001). In humans, approximately 90% of the total 5-HT is synthesized and localized in the enterochromaffin (EC) cells of the gastrointestinal mucosa (Talley, 2001). The many functions of 5-HT are generally mediated through interaction with a range of 5-HT receptor subtypes, classified into seven major groups (5-HT1 to 5-HT7), which also include several subgroups (Hannon and Hoyer, 2008; receptor nomenclature follows Alexander et al. (2011). The 5-HT3 receptor, which is a ligand-gated cation channel, is widely distributed in the neurons of the brain and spinal cord as well as in the gastrointestinal tract (Farber et al., 2004), and activation of this receptor results in intestinal secretions and peristaltic activity (Siriwardena et al., 1993; Hansen, 2003).
Antagonists of 5-HT3 receptors have been primarily used for the treatment of chemotherapy- and radiotherapy-induced nausea and emesis. These symptoms are believed to occur because of the release of 5-HT from the EC cells and the subsequent stimulation of peripheral 5-HT3 receptors in the vagal afferent neurons (Minami et al., 1997). Moreover, 5-HT3 antagonists have been shown to inhibit normal defecation and to increase the colonic perception threshold (Minami et al., 1997; Kozlowski et al., 2000), suggesting the involvement of 5-HT3 receptors in the pathogenesis of irritable bowel syndrome (IBS).
Recently, increasing evidence suggests that 5-HT is involved in modulating immune and inflammatory responses in mammals (Idzko et al., 2004; Walstab et al., 2010). The expression of 5-HT3 receptors has been shown in immune cells, such as monocytes, dendritic cells and T cells (Fiebich et al., 2004; Walstab et al., 2010). Indeed, tropisetron, a selective 5-HT3 receptor antagonist, has been shown to inhibit T-cell activation (Vega Lde et al., 2005). However, the role of 5-HT and 5-HT3 receptors in apoptotic and inflammatory responses in the intestinal mucosa during chemotherapy and the effects of 5-HT3 receptor antagonists on chemotherapy-induced intestinal mucositis remain unknown. In the present study, we examined the effect of 5-HT3 receptor antagonists, ramosetron and ondansetron, on 5-FU-induced intestinal mucositis in mice and attempted to elucidate the role of endogenous 5-HT and 5-HT3 receptors in the pathogenesis of intestinal mucositis.
Methods
Animals
All animal care and experimental procedures were approved by the Experimental Animal Research Committee of Kyoto Pharmaceutical University. Studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (McGrath et al., 2010). A total of 216 animals were used in the experiments described here. Male C57BL/6J mice (20–24 g, SLC Co., Shizuoka, Japan) were acclimated to standard laboratory conditions (12 h light–dark cycle, temperature of 22 ± 1°C). The experiments were performed using six un-anaesthetized mice per group.
Fluorescence immunohistochemistry for 5-HT3 receptors
Normal animals (5-FU-untreated) were killed under deep ether anaesthesia, and jejunum samples were removed, washed in cold PBS and immersed in 4% paraformaldehyde for 2 h at 4°C. After treatment with 20% sucrose solutions, the tissue samples were embedded in O.C.T. (Sakura Fintek, Tokyo, Japan) mounting medium and sectioned on a cryostat (Leica Instruments, Nussloch, Germany) at a thickness of 30 μm. The sections were thaw-mounted onto Superfrost Plus slides (Matsunami, Osaka, Japan), and immunohistochemical procedures were performed as described by Matsumoto et al. (2009). In brief, the slide-mounted sections were incubated with rabbit polyclonal anti-5-HT3 receptor antibody (Calbiochem, Darmstadt, Germany), rat monoclonal anti-CD11b antibody (R&D Systems, Minneapolis, MN, USA), rat monoclonal anti-CD68 antibody (Serotec, Oxford, UK) and rat monoclonal anti-CD45 antibody (BD Pharmingen, San Diego, CA, USA) for 40 h at room temperature, after treatment with 10% normal donkey serum for 1 h at room temperature. To visualize the expression, the sections were incubated with FITC-labelled donkey anti-rabbit IgG antibody and tetramethyl rhodamine isothiocyanate (TRITC)-labelled donkey anti-rat IgG antibody for 4 h. No specific immunostaining was observed in any of the control sections. Immunofluorescence was observed using a confocal microscope (FV-1000, Olympus, Tokyo, Japan) with an excitation wavelength appropriate for FITC (488 nm, Jackson Immunoresearch, West Grove, PA, USA) or TRITC (543 nm, Jackson Immunoresearch). Images were collected, and 50–60 optical sections were typically taken at intervals of 0.5 μm. Multiple images, in Z-stacks, were projected onto a single plane and reconstructed using Fluoview ver. 1.7a software (Olympus). The percentages of CD68-, CD11b- and CD45-immunopositive cells in 5-HT3 receptor-positive cells were determined at 20× magnification under a confocal microscope in the horizontal sections of the mucosa.
Induction of intestinal mucositis
Animals were administered 5-FU (50 mg·kg−1) i.p. once daily for 5 days (days 0–4), and saline (the vehicle for 5-FU) was administered to normal animals. Ramosetron (0.01, 0.03 and 0.1 mg·kg−1) and ondansetron (5 mg·kg−1) were administered orally (p.o.) twice daily for 5 days (days 0–4), and carboxymethylcellulose (CMC; vehicle for 5-HT3 receptor antagonists) was administered to control animals. The doses of ramosetron and ondansetron were chosen based on results from our recent study (Kato et al., 2012). Disease severity was assessed daily by measuring body weight and scoring the stool consistency: 0, normal; 1, slight diarrhoea (slightly wet and soft stool); 2, moderate diarrhoea (wet and unformed stool); and 3, severe diarrhoea (watery stool with severe perianal staining), as previously described by Kurita et al. (2000).
Twenty-four hours after the final 5-FU injection (day 5), the animals were killed under deep ether anaesthesia, and the jejunum was removed and immersed in 10% neutralized formalin overnight. Tissue samples were excised and embedded in paraffin, and then cut into 4 μm thick sections for haematoxylin and eosin (H&E) staining. Measurements of villus height (from the top of the villus to the villus–crypt junction) and crypt damage (surviving crypts per millimetre and surviving crypt cells per crypt) were performed by light microscopy (BX-50, Olympus). Five intact and well-oriented villi and crypts were measured and averaged for each sample.
Determination of plasma 5-HT concentrations
Blood was collected 24 h after 5-FU treatment from the inferior vena cava of each animal, under ether anaesthesia. The blood samples, containing 5 mM EDTA-Na (Wako, Osaka, Japan) as an anticoagulant, were centrifuged at 1700× g for 30 min at 4°C. The concentration of 5-HT in the plasma supernatant was determined using an enzyme immunoassay (Immunotech, Marseille, France).
Apoptosis analysis
Animals were killed 24 and 72 h after initial 5-FU administration (days 1 and 3, respectively); and jejunum samples were fixed with 10% neutralized formalin, embedded in paraffin and cut into 4 μm sections. Apoptosis of enterocytes in the small intestine was detected by the TUNEL assay using an in situ Apoptosis Detection Kit (Takara, Shiga, Japan), according to the manufacturer's instructions. For each sample, the number of TUNEL-positive apoptotic cells was counted and averaged at a magnification of 500× under a light microscope (BX-50, Olympus).
Immunohistochemistry for determination of caspase activation and cell proliferation
Animals were killed 24 h after initial 5-FU administration (day 1); and jejunum samples were fixed with 10% neutralized formalin, embedded in paraffin and cut into 4 μm sections. Caspase-3 and caspase-8 activation, as well as cell proliferation, were determined immunohistochemically using rabbit-raised anti-cleaved caspase-3 (Cell Signaling Technology, Danvers, MI, USA), anti-cleaved caspase-8 (Imgenex, San Diego, CA, USA) and anti-Ki-67 antibodies (Novus Biologicals, Littleton, CO, USA), respectively, after activation with HistoVT One (Nacalai Tesque, Kyoto, Japan). The immunocomplex was visualized by the avidin-biotin-peroxide method using the Vecstatin Elite ABC Rabbit IgG kit (Vector Laboratories, Burlingame, CA, USA), according to the manufacturer's instructions. Sections were counter-stained with haematoxylin. The numbers of cleaved caspase-3-, cleaved caspase-8- and ki-67-positive cells were counted under a light microscope (BX-50, Olympus) from 10 crypts and averaged for each sample.
Determination of Bax and Bcl-2 expressions by Western blotting
Animals were killed 24 h after initial 5-FU administration (day 1); and the jejunum was removed, washed with cold PBS and homogenized in lysis buffer (pH 7.4) containing 50 mmol·L−1 Tris–HCl, 150 mmol·L−1 NaCl, 50 mmol·L−1 dithiothreitol, 1 mmol·L−1 EDTA, a protease inhibitor cocktail tablet (Complete mini, Roche, Penzberg, Germany) and 1% Triton X-100. After centrifugation at 20 000× g for 30 min at 4°C, the protein concentrations in the supernatants were determined using a BCA protein assay kit (Pierce, Rockford, IL, USA) and adjusted to 4 mg·mL−1 using lysis buffer. An appropriate volume of the sample was mixed with an equal volume of sample buffer (pH 6.8, 100 mmol·L−1 Tris-HCl, 4% SDS, 20% glycerol, 10% 2-mercaptoethanol and 0.02% bromophenol blue) and heated at 95°C for 3 min. The samples (20 μg·lane−1) were subjected to electrophoresis on 7.5% SDS-PAGE and transferred electrophoretically to PVDF membranes. The membranes were incubated with rabbit polyclonal anti-Bax antibody, rabbit polyclonal anti-Bcl-2 antibody (Cell Signaling Technology) or rabbit polyclonal β-actin (Novus Biologicals) and then treated with HRP-conjugated rabbit polyclonal anti-goat IgG antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The immune complex was visualized using an enhanced chemiluminescence detection system (NEN Life Science, Boston, MA, USA) and photographed (VersaDoc 5000, Bio-Rad Laboratories, Hercules, CA, USA). The expression levels of Bax and Bcl-2 proteins were determined densitometrically with Quantity One software (Bio-Rad Laboratories).
Determination of mRNA expression by real-time RT-PCR
Animals were killed under deep ether anaesthesia on days 0 (without 5-FU treatment), 0.5 (12 h), 1, 3, and 5 after initial 5-FU administration; and the jejunum was removed, washed with cold PBS and immersed in RNAlater (Ambion, Austin, TX, USA) at 4°C until use. Total RNA was extracted from the whole jejunum layer using Sepasol RNA-I Supper G (Nacalai Tesque), according to the manufacturer's instructions. Reverse transcription (RT) was performed using RevaTra Ace-alpha with random hexamers (Toyobo, Osaka, Japan). Real-time PCR) amplification was performed using SYBR Premix ExTaq (Takara) with specific primers sets, prepared using the Perfect real-time supporting system (Takara) for β-actin (Primer set ID: MA050368), TNF-α (Primer set ID: MA097070), IL-1β (Primer set ID: MA025939), IL-6 (Primer set ID: MA039013) and IFN-γ (Primer set ID: MA025911) using an ABI PRISM 7500 real-time PCR System (Applied Biosystems, Foster City, CA). Expression levels for each mRNA were standardized to that of β-actin mRNA, and normalized to the mean value for day 0 or normal (5-FU-untreated) mice at each time point.
Anti-tumour action of 5-FU in tumour-implanted mice
To examine the influence of ramosetron on the anti-tumour action of 5-FU, Colon 38 tumour (a mouse colon adenocarcinoma cell line created in C57BL/6 mice)-implanted mice were prepared, as previously described (Tomita et al., 2003). A Colon 38 tumour fragment (8 mm3) was implanted into the abdominal region of C57BL/6 mice under light ether anaesthesia. The volume (mm3) of the Colon 38 solid tumour and the weight of body were measured every 2 days, starting 7 days after implantation. The volume of the tumour was measured with a slide caliper (Mitsutoyo, Kanagawa, Japan) according to the following formula: V = L × W2 × 0.5236, where V = volume, L = length and W = width. The animals were given 5-FU (20 mg·kg−1, i.p.) once daily for 5 days, starting 7 days after implantation, and then 5-FU once daily for another 5 days after a 2-day, no-treatment period. Ramosetron (0.1 mg·kg−1, p.o.) was given twice daily for 14 days, starting 7 days after implantation. In this experiment, we used a lower dose of 5-FU (20 mg·kg−1) than that used in earlier studies because a daily dose of 50 mg·kg−1 of 5-FU for 3 weeks caused serious adverse events (severe weight loss and death) in about half of the animals.
Statistics
Data are presented as the mean ± SEM of six animals per group. Statistical analyses were performed using a two-tailed Dunnett's multiple comparison test, with P < 0.05 regarded as statistically significant.
Materials
The drugs used in this study were 5-FU (Sigma-Aldrich, St. Louis, MO), ramosetron (kindly supplied by Astellas Pharma Inc., Tokyo, Japan) and ondansetron (LKT Laboratories, St. Paul, MN, USA). 5-FU was dissolved in physiological saline. Ramosetron and ondansetron were dissolved and suspended in CMC respectively. All drugs were prepared immediately before use and administered i.p. or p.o. in a volume of 0.1 mL·(10 g)−1.
Results
Expression of 5-HT3 receptors in the normal intestinal mucosa
In transverse sections, strong immunoreactivity towards 5-HT3 receptors was found in cell-like structures located in the lamina propria of the intestinal villi and weak immunoreactivity in the nerve-like fibres around the submucosa of the small intestine (Figure 1A). The horizontal sections further showed that the immunoreactivity of the 5-HT3 receptors was localized in the lamina propria of the small intestine. To investigate the precise localization of 5-HT3 receptors, double-immunostaining of 5-HT3 receptors was performed with anti-CD68, -CD11 (macrophage markers) and -CD45 (a leukocyte marker) antibodies. Similar to the 5-HT3 receptors (green), the CD68-, CD11b- and CD45-positive immunoreactivities (red) were observed in cell-like structures localized in the lamina propria of the intestinal villi (Figure 1B). Some of the cells positive for 5-HT3 receptors were also immunopositive for CD68, CD11b or CD45 (yellow), as indicated by the arrows in Figure 1B. The percentage of CD68-, CD11b- and CD45-positive cells among the 5-HT3 receptor-positive cells located in the lamina propria was 34.8% ± 6.0%, 26.9% ± 2.4% and 10.1% ± 3.4% respectively.
Figure 1.
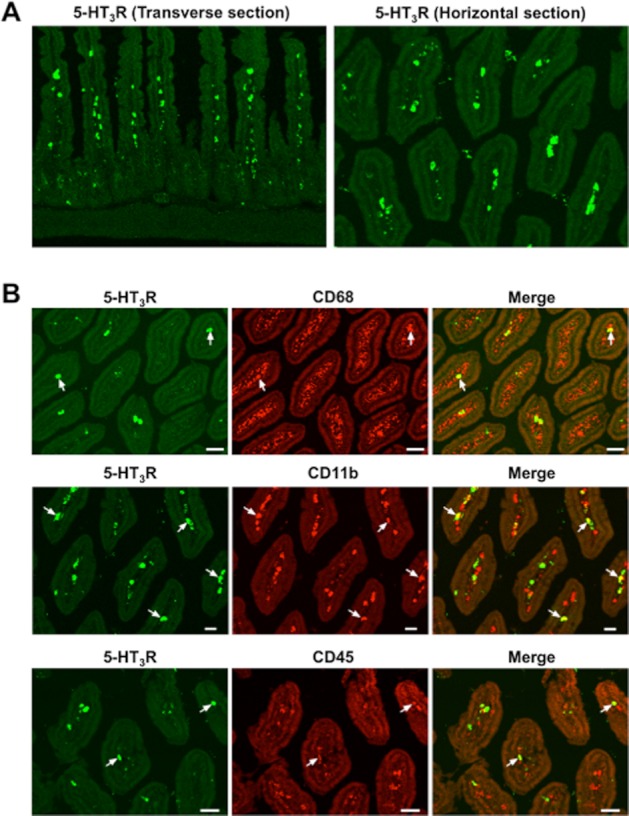
Fluorescent immunohistochemical study of 5-HT3 receptors in normal mouse intestinal mucosa. (A) 5-HT3 receptors (5-HT3R). (B) double-staining of 5-HT3 receptors for CD68, CD11b, and CD45. Double-positive cells are indicated by arrows.
The basal plasma concentration of 5-HT was 1384 ± 860 nmol·L−1. Administration of 5-FU (50 mg·kg−1) resulted in a significant rise in the plasma 5-HT concentration to 2940 ± 411 nmol·L−1 24 h later.
Effects of ramosetron and ondansetron on body weight loss and diarrhoea induced by 5-FU
Repeated administration of 5-FU (50 mg·kg−1) to the experimental animals caused body weight loss (Figure 2A) and diarrhoea (Figure 2B). Significant body weight loss was observed from day 1, and the mean body weight of treated animals was reduced to 78.2% ± 1.8% of the initial body weight by day 5, whereas prominent diarrhoea was observed from day 3, and the diarrhoea score reached 2.3 ± 0.2 on day 5 after the onset of 5-FU treatment. Twice-daily administration of ramosetron (0.01–0.1 mg·kg−1) suppressed body weight loss and diarrhoea induced by 5-FU, in a mostly dose-dependent manner, and a significant effect was observed at doses of 0.03 and 0.1 mg·kg−1. Similarly, ondansetron (5 mg·kg−1) significantly reduced body weight loss and diarrhoea during 5-FU treatment, and these effects were almost the same as those induced by ramosetron at doses of 0.03 and 0.1 mg·kg−1.
Figure 2.
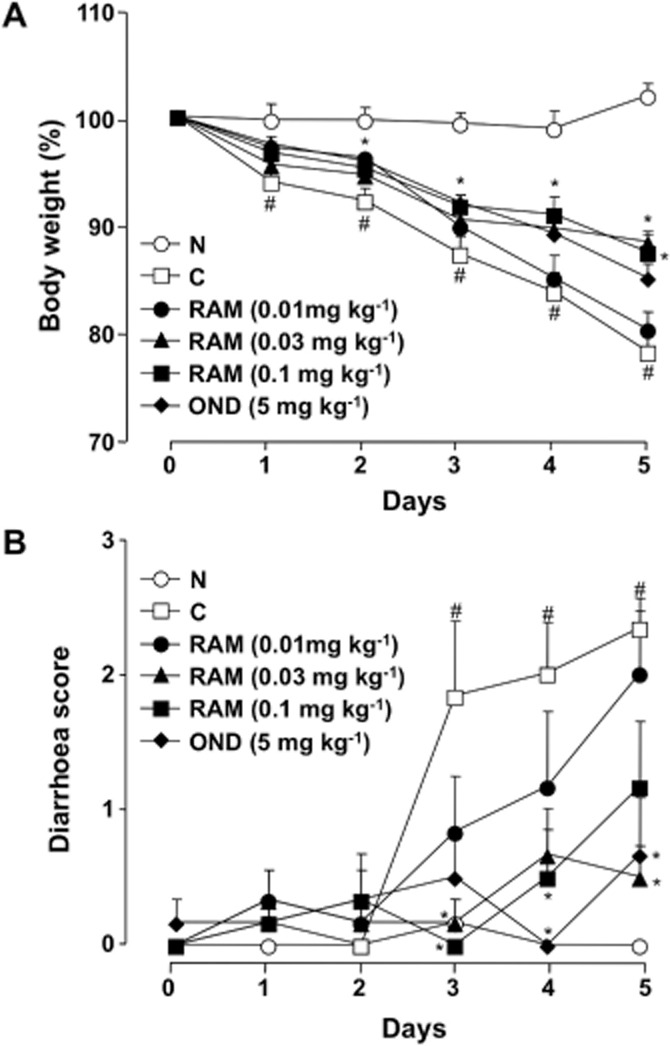
Effect of ramosetron and ondansetron on changes in body weight and diarrhoea during the course of 5-FU treatment in mice. Animals were administered 5-FU (50 mg·kg−1, i.p.) once daily for 5 days (days 0–4). Ramosetron (RAM, 0.01, 0.03, and 0.1 mg·kg−1) and ondansetron (OND, 5 mg·kg−1) were administered p.o. twice daily for 5 days. Body weight was monitored daily and is shown as a percentage of initial body weight (A), whereas severity of diarrhoea was scored daily using the four-grade scale (0 to 3), described in the Methods (B). Data are presented as the means ± SEM from six mice. Significant differences at P < 0.05; *from control (C, vehicle alone); #from normal (N, 5-FU-untreated).
Effects of ramosetron and ondansetron on the shortening of villus height and destruction of crypts induced by 5-FU in the mouse small intestines
Repeated administration of 5-FU (50 mg·kg−1) caused a shortening of villus height to less than half the normal value on day 5 after the onset of 5-FU treatment (Figure 3A and B). Twice-daily administration of ramosetron (0.01–0.1 mg·kg−1) prevented this shortening, in a dose-dependent manner, and the maximal effect was observed at a dose of 0.1 mg·kg−1. Similarly, ondansetron (5 mg·kg−1) significantly prevented the shortening of villus height, and this effect was comparable to that following ramosetron (0.1 mg·kg−1).
Figure 3.
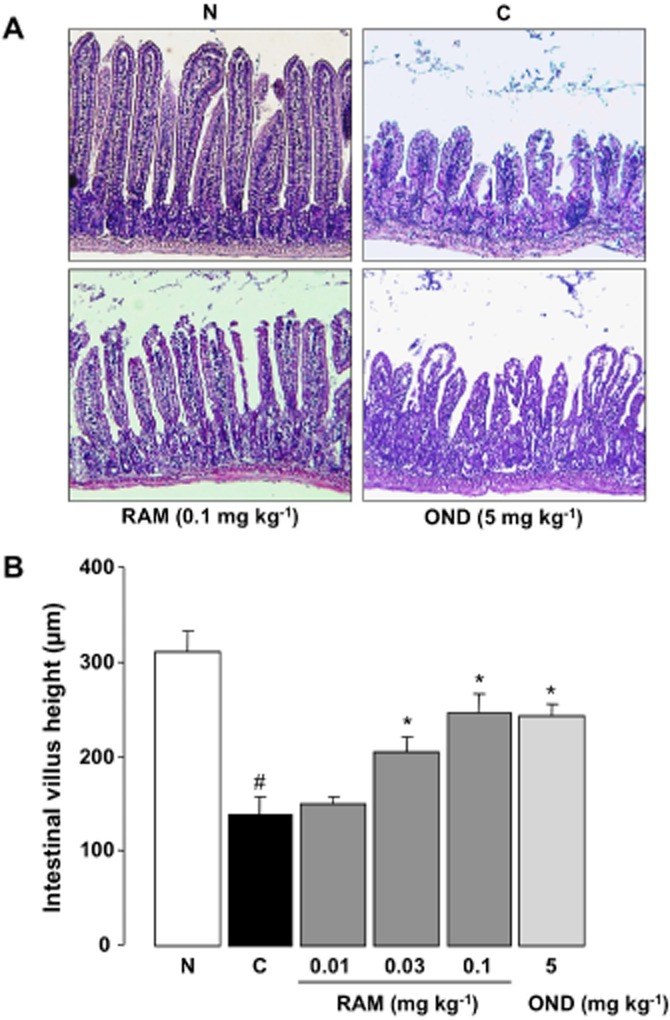
Effect of ramosetron and ondansetron on the shortening of intestinal villus height induced by 5-FU in mice. Animals were administered 5-FU (50 mg·kg−1, i.p.) once daily for 5 days (days 0 to 4) and killed 5 days after the initiation of 5-FU treatment (day 5). Ramosetron (RAM, 0.01, 0.03 and 0.1 mg·kg−1) and ondansetron (OND, 5 mg·kg−1) were administered p.o. twice daily for 5 days. Haematoxylin and eosin staining was performed (A, 100×), and the heights of the intestinal villi were measured (from the top of the villus to the villus–crypt junction) by light microscopy (B). Data are presented as the means ± SEM from six mice. Significant differences at P < 0.05; *From control (C, vehicle alone); #from normal (N, 5-FU-untreated).
The number of surviving crypts and crypt cells in the control (5-FU-untreated) mice are shown in Figure 4A and B respectively (Figure 4A and C). Repeated administration of 5-FU decreased the number of surviving crypts to about 30% of this value and the crypt cells were reduced to less than 20% of the control, on day 5 after the onset of 5-FU treatment. Twice-daily administration of ramosetron (0.01–0.1 mg·kg−1) inhibited the decrease in the numbers of surviving crypts and crypt cells, in a dose-dependent manner, with a maximal effect observed at 0.1 mg·kg−1. Similarly, ondansetron (5 mg·kg−1) significantly inhibited the decrease in these numbers, to almost the same extent as ramosetron (0.1 mg·kg−1).
Figure 4.

Effect of ramosetron and ondansetron on the destruction of intestinal crypts induced by 5-FU in mice. Animals were administered 5-FU (50 mg·kg−1, i.p.) once daily for 5 days (days 0 to 4) and killed 5 days after the onset of 5-FU treatment (Day 5). Ramosetron (RAM, 0.01, 0.03, and 0.1 mg·kg−1) and ondansetron (OND, 5 mg·kg−1) were administered p.o. twice daily for 5 days. Haematoxylin and eosin staining was performed (A, 500×), and the number of surviving crypts per millimetre (B) and surviving crypt cells per crypt (C) were measured by light microscopy. Data are presented as the means ± SEM from six mice. Significant differences at P < 0.05; *From control (C, vehicle alone); #from normal (N, 5-FU-untreated).
Effect of ramosetron on apoptotic responses to 5-FU in the intestinal crypt
Only a few TUNEL-positive apoptotic cells were found in the intestinal crypt of normal (5-FU-untreated) mice (Figure 5A). The administration of 5-FU (50 mg·kg−1) markedly increased the number of apoptotic cells in the intestinal crypt on days 1 and 3; however, this response was more evident on day 1 than on day 3 (Figure 5A and B). The administration of ramosetron (0.1 mg·kg−1) significantly reduced the increase in the number of apoptotic cells induced by 5-FU on both days 1 and 3.
Figure 5.

Effect of ramosetron on apoptosis in the intestinal crypt induced by 5-FU in mice. Animals were administered 5-FU (50 mg·kg−1, i.p.) once daily and killed 1 and 3 days after the initiation of 5-FU treatment. Ramosetron (RAM, 0.1 mg·kg−1) was administered p.o. twice daily. Apoptosis was assessed using the TUNEL assay (A, 500×). The number of TUNEL-positive cells was counted in the intestinal crypt using a light microscope (B). Data are presented as the means ± SEM from six mice. Significant differences at P < 0.05; *from control (C, vehicle alone); #from normal (N, 5-FU-untreated).
To investigate the mechanism of 5-FU-induced apoptosis, the extent of caspase-3 and caspase-8 activation, within 24 h of the first administration of 5-FU, were assessed immunohistochemically using antibodies raised against cleaved caspase-3 and caspase-8 respectively. The administration of 5-FU (50 mg·kg−1) caused an 8.3-fold increase in the number of caspase-3-activated cells in the intestinal crypt 24 h after initial inoculation; however, this response was significantly attenuated by ramosetron (0.1 mg·kg−1) (Figure 6A and B). Similarly, although 5-FU caused a 5.9-fold increase in the number of caspase-8-activated cells, this response was significantly suppressed by ramosetron (0.1 mg·kg−1) (Figure 6A and C).
Figure 6.
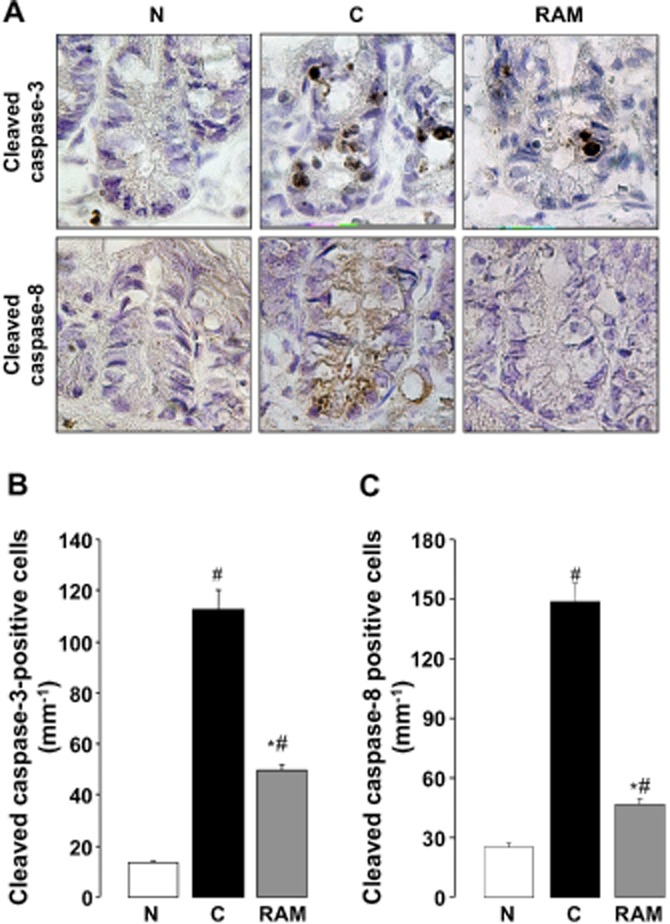
Effect of ramosetron on activation of caspase-3 and caspase-8 in the intestinal crypt induced by 5-FU in mice. Animals were administered 5-FU (50 mg·kg−1, i.p.) and killed 1 day (24 h) after the administration of 5-FU. Ramosetron (RAM, 0.1 mg·kg−1) was administered p.o. twice, 0.5 h before and 8 h after the administration of 5-FU. The activation of caspase-3 and caspase-8 (A, 500×) were determined immunohistochemically using anti-cleaved caspase-3 and caspase-8 antibodies respectively. The numbers of cells immunopositive for caspase-3 (B) and caspase-8 (C) were counted in the intestinal crypt using a microscope. Data are presented as the means ± SEM from six mice. Significant differences at P < 0.05; *from control (C, vehicle alone); #from normal (N, 5-FU-untreated).
Effect of ramosetron on proliferation and expression of Bax and Bcl-2 induced by 5-FU in intestinal crypt cells
To confirm the influence of ramosetron on the anti-proliferative action of 5-FU in the intestinal crypt cells, the proliferative activity was determined immunohistochemically using anti-Ki-67 antibody. A large number of Ki-67-positive proliferative cells were observed in the normal (5-FU-untreated) intestinal crypt (Figure 7A and C). Treatment with 5-FU (50 mg·kg−1) markedly reduced the number of Ki-67-positive cells to 41.3 ± 6.0 cells·mm−1 24 h later. The administration of ramosetron (0.1 mg·kg−1) failed to affect the anti-proliferative action of 5-FU.
Figure 7.

Effect of ramosetron on the suppression of cell proliferation and expression of Bax and Bcl-2 induced by 5-FU in mouse small intestine. Animals were administered 5-FU (50 mg·kg−1, i.p.) and killed 1 day (24 h) after the administration of 5-FU. Ramosetron (RAM, 0.1 mg·kg−1) was administered p.o. twice, 0.5 h before and 8 h after the administration of 5-FU. The cell proliferative activity was determined immunohistochemically using anti-Ki-76 antibody (A, 500×), whereas the expression of Bax and Bcl-2 was analysed by Western blotting (B). The numbers of Ki-67-positive cells were counted in the intestinal crypts using a light microscope (C), whereas the expression level of Bax and Bcl-2 proteins was densitometrically determined. Data are presented as the means ± SEM from six mice. Significant difference at P < 0.05; *from control (C, vehicle alone); #from normal (N, 5-FU-untreated).
Conversely, the expression of Bax and Bcl-2 proteins was clearly detected in the normal (5-FU-untreated) small intestine (Figure 7B). Treatment with 5-FU (50 mg·kg−1) failed to affect the expression of these proteins in the presence or absence of ramosetron (0.1 mg·kg−1) (Figure 7B, D, E).
Effect of ramosetron on up-regulation of mRNA for TNF-α, IL-1β, IL-6 and IFN-γ in the intestinal mucosa
Up-regulation of mRNA for TNF-α was observed as early as 12 h after administration of 5-FU (50 mg·kg−1) and reached 5-6-fold the values in normal (5-FU-untreated) animals on days 1 and 3, respectively (Figure 8A). Although the expression of IL-1β, IL-6 and INF-γ was also increased, these responses were apparently delayed with only slight increases observed over the first 24 h after the initiation of 5-FU treatment. The increased expression of TNF-α and IL-1β mRNA was significantly suppressed by ramosetron (0.1 mg·kg−1) at all time points (Figure 8B, C, D).
Figure 8.
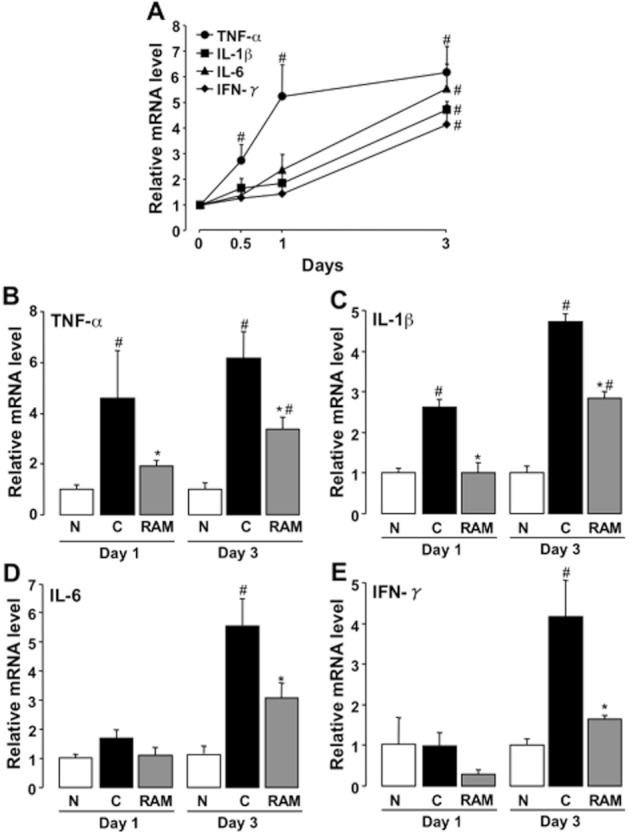
Effect of ramosetron on up-regulation of TNF-α, IL-1β, IL-6, IFN-γ mRNA expression in the small intestine induced by 5-FU in mice. Animals were administered 5-FU (50 mg·kg−1, i.p.) and killed 0.5, 1 and 3 days later. Ramosetron (RAM, 0.1 mg·kg−1) was administered p.o. twice daily. The expression of TNF-α, IL-1β and IL-6 was quantified by real-time RT-PCR. Expression levels of each mRNA were standardized to that of β-actin mRNA, and normalized to the mean value for day 0 or normal (5-FU-untreated) mice at each time point. (A) Changes in TNF-α, IL-1β, IL-6 and IFN-γ expression during the course of 5-FU treatment. Effect of ramosetron on the up-regulation of TNF-α (B), IL-1β (C), IL-6 (D), and IFN-γ (E) induced by 5-FU. Data are presented as the means ± SEM from six mice. Significant differences at P < 0.05; *from control (C, vehicle alone); #from normal (N, 5-FU-untreated).
Effect of ondansetron on apoptotic response and cytokine up-regulation induced by 5-FU
The administration of ondansetron (5 mg·kg−1) significantly reduced the increase in the number of apoptotic cells induced by 5-FU (50 mg·kg−1) on day 1 (Figure 9A and B). Similarly, the administration of ondansetron significantly suppressed 5-FU-induced up-regulation of TNF-α and IL-1β expression on day 1 (Figure 9C).
Figure 9.
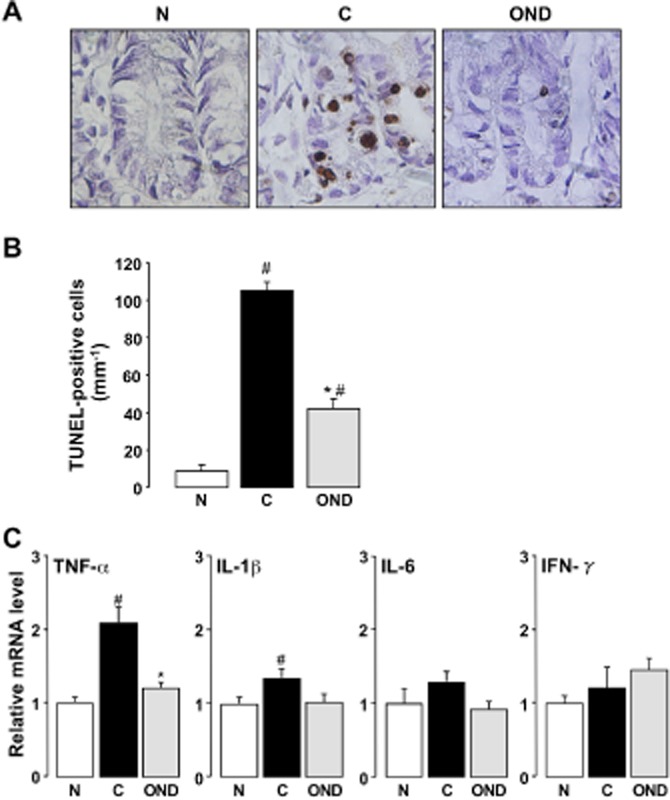
Effect of ondansetron on apoptosis in the intestinal crypt and up-regulation of cytokine mRNA in the intestine induced by 5-FU in mice. Animals were injected with 5-FU (50 mg·kg−1, i.p.) and killed 1 day (24 h) later. Ondansetron (OND, 5 mg·kg−1) was administered p.o. twice 0.5 h before and 8 h after the administration of 5-FU. Apoptosis was assessed using the TUNEL assay (A, 500×). The number of TUNEL-positive cells was counted in the intestinal crypt using a light microscope (B). The expression of TNF-α, IL-1β, IL-6 and IFN-γ was quantified by real-time RT-PCR (C). Expression levels of each mRNA were standardized to that of β-actin mRNA and normalized to the mean value for normal (5-FU-untreated) mice. Data are presented as the means ± SEM from six mice. Significant differences at P < 0.05; *from control (C, vehicle alone); #from normal (N, 5-FU-untreated).
Effect of ramosetron on anti-proliferative action of 5-FU in tumour-implanted mice
Colon 38 tumour fragments, implanted s.c. in the abdominal region of mice, developed single, solid tumours over the course of the experiment. Each solid tumour gradually grew and reached an average volume of just over 1000 mm3 by 21 days after tumour implantation (Figure 10A). Repeated administration of 5-FU (20 mg·kg−1) fully suppressed tumour growth, and the tumour volume did not increase significantly by day 21 after implantation. Twice-daily administration of ramosetron (0.1 mg·kg−1) affected neither spontaneous tumour growth nor the anti-growth effect of 5-FU. On the other hand, repeated administration of 5-FU (20 mg·kg−1) caused a reduction in body weight, although the reduction was less than that accompanying treatment with 5-FU (50 mg·kg−1) (Figure 10B). Twice-daily administration of ramosetron clearly showed the tendency to avoid the 5-FU-induced body weight loss.
Figure 10.
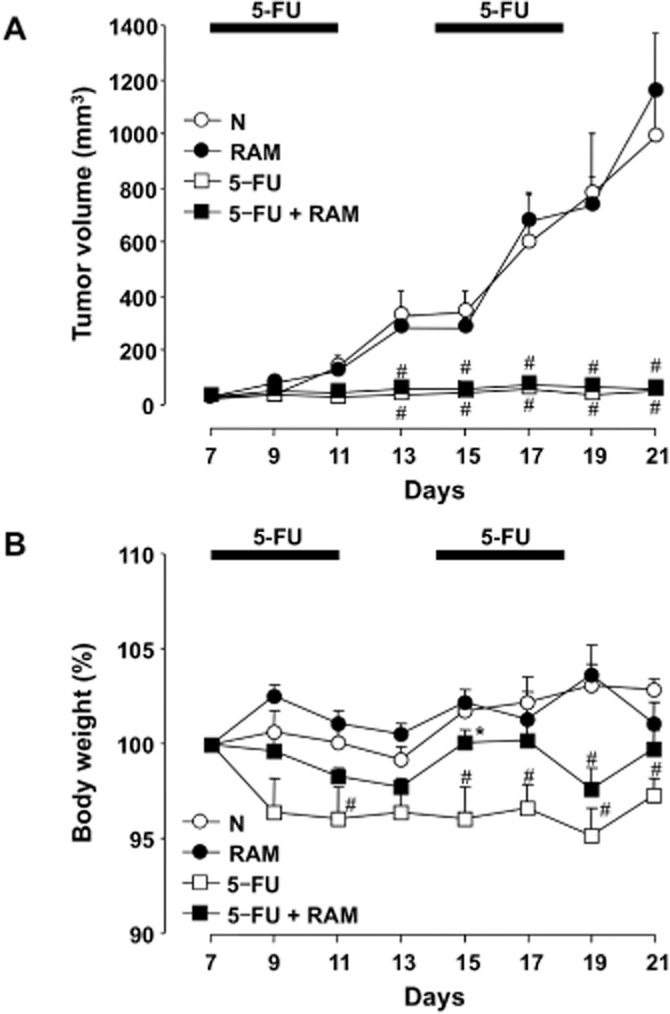
Effect of ramosetron on the anti-tumour action and body weight loss induced by 5-FU in tumour-implanted mice. A Colon 38 tumour fragment (8 mm3) was implanted into the abdominal region of mice and the volume (mm3) of the Colon 38 solid tumour was measured every 2 days, starting 7 days after implantation. The animals were administered 5-FU (20 mg·kg−1, i.p.) once daily for 5 days, and then administered 5-FU once daily for 5 days after a 2-day no-treatment period. Ramosetron (RAM, 0.1 mg·kg−1) was administered p.o. twice daily for 14 days, starting 7 days after implantation. The tumour volume (A) and body weight (B) were measured every 2 days, starting 7 days after the implantation. Data are presented as the means ± SEM from six mice. N, vehicle alone (5-FU-untreated); C, vehicle + 5-FU; RAM, ramosetron + 5-FU; RAM alone, ramosetron alone (5-FU-untreated). #Significant difference from normal (N) at P < 0.05.
Discussion and conclusions
In the present study, we have demonstrated the immunohistochemical localization of 5-HT3 receptors in the normal small intestine. Immunoreactive 5-HT3 receptors were found mostly in cell-like structures located in the lamina propria of the intestinal villi. Interestingly, some of these 5-HT3-immunopositive structures were also positive for CD68 and CD11b (markers for macrophages), as well as for CD45 (a marker of leucocytes). These findings suggest the 5-HT3 receptor is mostly expressed in inflammatory and immune cells, localized in the small intestine and may modulate the inflammatory responses. Furthermore, administration of 5-FU increased plasma 5-HT levels, suggesting that 5-HT was being released, probably from the EC cells. Thus, it is possible that activation of 5-HT3 receptors, via the release of 5-HT from EC cells, may be a critical event in the pathogenesis of 5-FU-induced intestinal mucositis.
The results obtained from the present study indicated that 5-HT3 antagonists ameliorated 5-FU-induced intestinal mucositis. Consistent with the findings from many previous studies (Symonds, 1998; Wadler et al., 1998; Benson et al., 2004), repeated dosing of mice with 5-FU caused severe intestinal mucositis, morphologically characterized by the shortening of villi and destruction of intestinal crypts, accompanied by systemic symptoms that included diarrhoea and body weight loss. These morphological changes and systemic symptoms were suppressed, in a dose-dependent manner, by daily administration of ramosetron. A similar ameliorative effect was observed following administration of ondansetron, another 5-HT3 receptor antagonist. Since diarrhoea and body weight loss during 5-FU treatment are considered to be closely linked to the severity of intestinal mucositis (Symonds, 1998), these findings strongly suggest that the 5-HT3 receptor antagonists can be effective against 5-FU-induced intestinal mucositis.
Although the pathogenesis of chemotherapeutic agent-induced intestinal mucositis is not fully understood, it is considered to be a consequence of various processes, including abnormal inflammation, apoptosis, cell hypoproliferation and direct cytotoxicity (Daniele et al., 2001; Duncan and Grant, 2003; Bowen et al., 2006). Apoptosis is a particularly critical event in intestinal mucositis induced by chemotherapeutic agents (Anilkumar et al., 1992; Pritchard et al., 1998; Keefe et al., 2000; Inomata et al., 2002). In the present study, the number of TUNEL-positive apoptotic cells markedly increased in the intestinal crypt after administration of 5-FU. Interestingly, the increased number of apoptotic cells was more evident on day 1 than on day 3 of the 5-FU treatment. As the morphological changes in the small intestine were not visible on day 1, it is likely that apoptosis was triggered within 24 h after the first administration of 5-FU and may be a critical event in the development of intestinal mucositis. Ramosetron and ondansetron significantly reduced 5-FU-induced apoptosis of the intestinal crypt cells, suggesting that the ameliorative effect of these agents in intestinal mucositis may be attributed to suppression of the apoptosis induced by 5-FU.
Several studies have indicated that activation of caspase-3 is involved in the intestinal cell apoptosis that occurs during the treatment with chemotherapeutic agents, including 5-FU (Bowen et al., 2005; Li et al., 2009; Wu et al., 2011). Caspase-3, the main downstream effector, plays a key role in the process of apoptosis by cleaving the majority of cellular substrates (Perner et al., 2003; Kumar, 2007). In the present study, the number of cells immunopositive for cleaved caspase-3, the active form of caspase-3, was markedly increased in intestinal crypt cells during 5-FU treatment. The observed pattern of immunopositive cells was consistent with the localization of TUNEL-positive apoptotic cells. The activation of this enzyme was also significantly suppressed by ramosetron, indicating that the anti-apoptotic effect of 5-HT3 receptor antagonists was mediated by the inhibition of caspase-3 activation.
Inomata et al. (2002) and Wu et al. (2011) have shown that the apoptosis and caspase-3 activation induced in intestinal crypt cells by 5-FU are accompanied by alterations in Bax and Bcl-2 expression. In the present study, however, as 5-FU failed to affect the expression of these proteins, it is unlikely that the alteration of these proteins is involved in the activation of caspase-3 induced by 5-FU. To induce intestinal mucositis in mice, these authors administered high doses (130–200 mg·kg−1) of 5-FU in a single injection (Inomata et al., 2002; Wu et al., 2011), whereas the present method required repeated administration of 5-FU (50 mg·kg−1). Therefore, these discrepancies may be due to the different experimental conditions and/or measurements of the expression.
In the present study, caspase-8 was found to be potently activated in intestinal crypt cells, similar to caspase-3, after 5-FU treatment, and to be inhibited by ramosetron. Caspase-8 is an initiator that promotes caspase-3 activation, and the activation of this enzyme is initiated by members of the TNF family through recruitment of the death-inducing signalling complex (DISC) via binding to the adaptor protein (FADD) (Boldin et al., 1996; Muzio et al., 1996). Several studies have demonstrated the increased expression and production of inflammatory cytokines, such as TNF-α, IL-1β, IL-6 and IFN-γ, during chemotherapy-induced intestinal mucositis (Itoh et al., 2002; Sonis et al., 2004; Elsea et al., 2008; Huang et al., 2009). In the present study, we also observed that the administration of 5-FU caused up-regulation of these cytokines in the small intestine. Interestingly, the up-regulation of TNF-α preceded that of IL-1β, IL-6 and IFN-γ, and also correlated with the induction of apoptosis and activation of caspases-3 and -8. Thus, TNF-α is likely to be an important mediator in the process of 5-FU-induced apoptosis. Indeed, Jin et al. (2006) showed that TNF-α induced apoptosis via activation of caspase-3/caspase-8 in intestinal epithelial cells. Further studies are needed to clarify the detailed association of these cytokines, especially TNF-α, with the pathogenesis of 5-FU-induced intestinal apoptosis, resulting in mucositis. Ramosetron and ondansetron significantly suppressed the up-regulation of TNF-α and other cytokines in response to 5-FU, suggesting that the anti-apoptotic effect of these agents is attributable to suppression of the caspase-3/caspase-8 pathways via inhibition of inflammatory cytokine up-regulation.
It is well accepted that cisplatin, a platinum-containing chemotherapeutic agent, directly causes the release of 5-HT from EC cells (Schworer et al., 1991; Minami et al., 2003). However, the mechanism by which 5-FU causes the release of 5-HT from these cells remains unclear. Several studies have revealed that the number of EC cells, and the content of 5-HT in the small intestine are elevated in patients with inflammatory bowel disease (IBD) (El-Salhy et al., 1997) and in experimental animal models of colonic inflammation (Oshima et al., 1999; Linden et al., 2003; Khan et al., 2006; Bertrand et al., 2010). Furthermore, the release of 5-HT from EC cells has been reported to be enhanced by bacterial infection (Wang et al., 2007) and inflammatory cytokines (Khan et al., 2006; Kidd et al., 2009). Thus, the augmentation of 5-HT release in response to 5-FU may be associated with intestinal inflammation.
Several studies have shown that 5-HT stimulated the production of cytokines, such as TNF-α, IL-1β and IL-12p40, in isolated mouse macrophages and dendritic cells, although no study has examined the 5-HT receptor subtypes involved in this response (Ghia et al., 2009; Li et al., 2011). However, the molecular mechanisms by which activation of 5-HT3 receptors induces up-regulation of inflammatory cytokines remain obscure. A pathogenic role for NADPH oxidase 1 (NOX1)-derived reactive oxygen species (ROS) was recently described in 5-FU-induced intestinal mucositis (Yasuda et al., 2012). Administration of 5-FU increased the expression of inflammatory cytokines, accompanied by NOX1, in the small intestine. A preliminarily study also showed that the up-regulation of NOX1 in response to 5-FU was significantly prevented by ramosetron and ondansetron (data not shown). On the other hand, the immunohistochemical investigations in the present study also revealed that the 5-HT3 receptor was expressed in nerve fibres in the myenteric and submucosal plexus, as previously reported by Glatzle et al. (2002). Thus, it is possible that the NOX1/ROS pathway and an indirect pathway via neuronal 5-HT3 receptors may be involved in 5-HT3 receptor-induced inflammatory responses. However, further studies are required to clarify the molecular mechanisms underlying 5-HT3 receptor-mediating inflammatory responses.
Finally, the influence of ramosetron on the anti-tumour action of 5-FU was examined in Colon 38-implanted mice. Repeated treatment with 5-FU completely prevented the growth of solid tumours implanted s.c. in the animals. However, daily administration of ramosetron did not affect the anti-tumour action of 5-FU. In addition, daily administration of ramosetron failed to affect the 5-FU-induced decrease in the number of Ki-67-positive proliferative cells in the intestinal crypt. These findings suggest that ramosetron does not affect the anti-proliferative action of 5-FU. Therefore, we suggest that ramosetron can ameliorate intestinal mucositis without any negative impact on the anti-tumour activity of 5-FU during chemotherapy. On the other hand, we observed that twice daily administration of ramosetron clearly prevented the reduction of body weight induced by 5-FU with a lower dose (20 mg·kg−1) over a longer period (3 weeks). This result may also suggest that ramosetron is effective against 5-FU-induced intestinal mucositis.
Taken together, these results indicate that endogenous 5-HT plays a critical role in the pathogenesis of 5-FU-induced intestinal mucositis via activation of 5-HT3 receptors. Therefore, 5-HT3 receptor antagonists may be useful for the prevention of not only nausea and emesis but also intestinal mucositis during cancer chemotherapy.
Acknowledgments
We thank Astellas Pharma Inc. for supplying ramosetron.
Glossary
Abbreviations
- 5-FU
5-fluorouracil
- EC
enterochromaffin
- H&E
haematoxylin and eosin
- IBS
irritable bowel syndrome
- NOX1
NADPH oxidase 1
- ROS
reactive oxygen species
Conflict of interest
KT received grant support from Astellas Pharma Inc. The remaining authors have declared no financial interests.
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anilkumar TV, Sarraf CE, Hunt T, Alison MR. The nature of cytotoxic drug-induced cell death in murine intestinal crypts. Br J Cancer. 1992;65:552–558. doi: 10.1038/bjc.1992.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson AB, 3rd, Ajani JA, Catalano RB, Engelking C, Kornblau SM, Martenson JA, Jr, et al. Recommended guidelines for the treatment of cancer treatment-induced diarrhea. J Clin Oncol. 2004;22:2918–2926. doi: 10.1200/JCO.2004.04.132. [DOI] [PubMed] [Google Scholar]
- Bertrand PP, Barajas-Espinosa A, Neshat S, Bertrand RL, Lomax AE. Analysis of real-time serotonin (5-HT) availability during experimental colitis in mouse. Am J Physiol Gastrointest Liver Physiol. 2010;298:G446–G455. doi: 10.1152/ajpgi.00318.2009. [DOI] [PubMed] [Google Scholar]
- Boldin MP, Goncharov TM, Goltsev YV, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- Bowen JM, Gibson RJ, Keefe DM, Cummins AG. Cytotoxic chemotherapy upregulates pro-apoptotic Bax and Bak in the small intestine of rats and humans. Pathology. 2005;37:56–62. doi: 10.1080/00313020400023461. [DOI] [PubMed] [Google Scholar]
- Bowen JM, Gibson RJ, Cummins AG, Keefe DM. Intestinal mucositis: the role of the Bcl-2 family, p53 and caspases in chemotherapy-induced damage. Support Care Cancer. 2006;14:713–731. doi: 10.1007/s00520-005-0004-7. [DOI] [PubMed] [Google Scholar]
- Daniele B, Secondulfo M, De Vivo R, Pignata S, De Magistris L, Delrio P, et al. Effect of chemotherapy with 5-fluorouracil on intestinal permeability and absorption in patients with advanced colorectal cancer. J Clin Gastroenterol. 2001;32:228–230. doi: 10.1097/00004836-200103000-00010. [DOI] [PubMed] [Google Scholar]
- Duncan M, Grant G. Oral and intestinal mucositis – causes and possible treatments. Aliment Pharmacol Ther. 2003;18:853–874. doi: 10.1046/j.1365-2036.2003.01784.x. [DOI] [PubMed] [Google Scholar]
- El-Salhy M, Danielsson A, Stenling R, Grimelius L. Colonic endocrine cells in inflammatory bowel disease. J Intern Med. 1997;242:413–419. doi: 10.1046/j.1365-2796.1997.00237.x. [DOI] [PubMed] [Google Scholar]
- Elsea CR, Roberts DA, Druker BJ, Wood LJ. Inhibition of p38 MAPK suppresses inflammatory cytokine induction by etoposide, 5-fluorouracil, and doxorubicin without affecting tumoricidal activity. PLoS ONE. 2008;3:e2355. doi: 10.1371/journal.pone.0002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber L, Haus U, Spath M, Drechsler S. Physiology and pathophysiology of the 5-HT3 receptor. Scand J Rheumatol Suppl. 2004;119:2–8. [PubMed] [Google Scholar]
- Fiebich BL, Akundi RS, Seidel M, Geyer V, Haus U, Muller W, et al. Expression of 5-HT3A receptors in cells of the immune system. Scand J Rheumatol Suppl. 2004;119:9–11. [PubMed] [Google Scholar]
- Ghia J-E, Li N, Wang H, Collins M, Deng Y, El-Sharkawy RT, et al. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology. 2009;137:1649–1660. doi: 10.1053/j.gastro.2009.08.041. [DOI] [PubMed] [Google Scholar]
- Glatzle J, Sternini C, Robin C, Zittel TT, Wong H, Reeve JR, Jr, et al. Expression of 5-HT3 receptors in the rat gastrointestinal tract. Gastroenterology. 2002;123:217–226. doi: 10.1053/gast.2002.34245. [DOI] [PubMed] [Google Scholar]
- Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Hansen MB. The enteric nervous system III: a target for pharmacological treatment. Pharmacol Toxicol. 2003;93:1–13. doi: 10.1034/j.1600-0773.2003.930101.x. [DOI] [PubMed] [Google Scholar]
- Huang TY, Chu HC, Lin YL, Ho WH, Hou HS, Chao YC, et al. Minocycline attenuates 5-fluorouracil-induced small intestinal mucositis in mouse model. Biochem Biophys Res Commun. 2009;389:634–639. doi: 10.1016/j.bbrc.2009.09.041. [DOI] [PubMed] [Google Scholar]
- Idzko M, Panther E, Stratz C, Muller T, Bayer H, Zissel G, et al. The serotoninergic receptors of human dendritic cells: identification and coupling to cytokine release. J Immunol. 2004;172:6011–6019. doi: 10.4049/jimmunol.172.10.6011. [DOI] [PubMed] [Google Scholar]
- Inomata A, Horii I, Suzuki K. 5-Fluorouracil-induced intestinal toxicity: what determines the severity of damage to murine intestinal crypt epithelia? Toxicol Lett. 2002;133:231–240. doi: 10.1016/s0378-4274(02)00204-7. [DOI] [PubMed] [Google Scholar]
- Itoh N, Nishimura H, Matsuguchi T, Yajima T, Mokuno Y, Hiromatsu T, et al. CD8 alpha-deficient mice are highly susceptible to 5-fluorouracil-induced lethality. Clin Diagn Lab Immunol. 2002;9:550–557. doi: 10.1128/CDLI.9.3.550-557.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S, Ray RM, Johnson LR. Rac1 mediates intestinal epithelial cell apoptosis via JNK. Am J Physiol Gastrointest Liver Physiol. 2006;291:G1137–G1147. doi: 10.1152/ajpgi.00031.2006. [DOI] [PubMed] [Google Scholar]
- Kato S, Matsuda N, Matsumoto K, Wada M, Onimaru N, Yasuda M, et al. Dual role of serotonin in the pathogenesis of indomethacin-induced small intestinal ulceration: pro-ulcerogenic action via 5-HT3 receptors and anti-ulcerogenic action via 5-HT4 receptors. Pharmacol Res. 2012;66:226–234. doi: 10.1016/j.phrs.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Keefe DM, Brealey J, Goland GJ, Cummins AG. Chemotherapy for cancer causes apoptosis that precedes hypoplasia in crypts of the small intestine in humans. Gut. 2000;47:632–637. doi: 10.1136/gut.47.5.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan WI, Motomura Y, Wang H, El-Sharkawy RT, Verdu EF, Verma-Gandhu M, et al. Critical role of MCP-1 in the pathogenesis of experimental colitis in the context of immune and enterochromaffin cells. Am J Physiol Gastrointest Liver Physiol. 2006;291:G803–G811. doi: 10.1152/ajpgi.00069.2006. [DOI] [PubMed] [Google Scholar]
- Kidd M, Gustafsson BI, Drozdov I, Modlin IM. IL1beta- and LPS-induced serotonin secretion is increased in EC cells derived from Crohn's disease. Neurogastroenterol Motil. 2009;21:439–450. doi: 10.1111/j.1365-2982.2008.01210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski CM, Green A, Grundy D, Boissonade FM, Bountra C. The 5-HT(3) receptor antagonist alosetron inhibits the colorectal distention induced depressor response and spinal c-fos expression in the anaesthetised rat. Gut. 2000;46:474–480. doi: 10.1136/gut.46.4.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S. Caspase function in programmed cell death. Cell Death Differ. 2007;14:32–43. doi: 10.1038/sj.cdd.4402060. [DOI] [PubMed] [Google Scholar]
- Kurita A, Kado S, Kaneda N, Onoue M, Hashimoto S, Yokokura T. Modified irinotecan hydrochloride (CPT-11) administration schedule improves induction of delayed-onset diarrhea in rats. Cancer Chemother Pharmacol. 2000;46:211–220. doi: 10.1007/s002800000151. [DOI] [PubMed] [Google Scholar]
- Li N, Ghia JE, Wang H, McClemens J, Cote F, Suehiro Y, et al. Serotonin activates dendritic cell function in the context of gut inflammation. Am J Pathol. 2011;178:662–671. doi: 10.1016/j.ajpath.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Ito K, Sumi S, Fuwa T, Horie T. Protective effect of aged garlic extract (AGE) on the apoptosis of intestinal epithelial cells caused by methotrexate. Cancer Chemother Pharmacol. 2009;63:873–880. doi: 10.1007/s00280-008-0809-4. [DOI] [PubMed] [Google Scholar]
- Linden DR, Chen JX, Gershon MD, Sharkey KA, Mawe GM. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G207–G216. doi: 10.1152/ajpgi.00488.2002. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Kurosawa E, Terui H, Hosoya T, Tashima K, Murayama T, et al. Localization of TRPV1 and contractile effect of capsaicin in mouse large intestine: high abundance and sensitivity in rectum and distal colon. Am J Physiol Gastrointest Liver Physiol. 2009;297:G348–G360. doi: 10.1152/ajpgi.90578.2008. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami M, Nemoto M, Endo T, Hamaue N, Kohno Y. Effects of talipexole on emesis-related changes in abdominal afferent vagal activity and ileal serotonin metabolism in rats. Res Commun Mol Pathol Pharmacol. 1997;95:67–82. [PubMed] [Google Scholar]
- Minami M, Endo T, Hirafuji M, Hamaue N, Liu Y, Hiroshige T, et al. Pharmacological aspects of anticancer drug-induced emesis with emphasis on serotonin release and vagal nerve activity. Pharmacol Ther. 2003;99:149–165. doi: 10.1016/s0163-7258(03)00057-3. [DOI] [PubMed] [Google Scholar]
- Muzio M, Chinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, Ni J, et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death – inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- Oshima S, Fujimura M, Fukimiya M. Changes in number of serotonin-containing cells and serotonin levels in the intestinal mucosa of rats with colitis induced by dextran sodium sulfate. Histochem Cell Biol. 1999;112:257–263. doi: 10.1007/s004180050445. [DOI] [PubMed] [Google Scholar]
- Perner A, Andresen L, Pedersen G, Rask-Madsen J. Superoxide production and expression of NAD(P)H oxidases by transformed and primary human colonic epithelial cells. Gut. 2003;52:231–236. doi: 10.1136/gut.52.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard DM, Potten CS, Hickman JA. The relationships between p53-dependent apoptosis, inhibition of proliferation, and 5-fluorouracil-induced histopathology in murine intestinal epithelia. Cancer Res. 1998;58:5453–5465. [PubMed] [Google Scholar]
- Schworer H, Racke K, Kilbinger H. Cisplatin increases the release of 5-hydroxytryptamine (5-HT) from the isolated vascularly perfused small intestine of the guinea-pig: involvement of 5-HT3 receptors. Naunyn Schmiedebergs Arch Pharmacol. 1991;344:143–149. doi: 10.1007/BF00167211. [DOI] [PubMed] [Google Scholar]
- Siriwardena AK, Budhoo MR, Smith EP, Kellum JM. A 5-HT3 receptor agonist induces neurally mediated chloride transport in rat distal colon. J Surg Res. 1993;55:55–59. doi: 10.1006/jsre.1993.1108. [DOI] [PubMed] [Google Scholar]
- Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer-Jensen M, et al. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer. 2004;100:1995–2025. doi: 10.1002/cncr.20162. [DOI] [PubMed] [Google Scholar]
- Symonds RP. Treatment-induced mucositis: an old problem with new remedies. Br J Cancer. 1998;77:1689–1695. doi: 10.1038/bjc.1998.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley NJ. Serotoninergic neuroenteric modulators. Lancet. 2001;358:2061–2068. doi: 10.1016/S0140-6736(01)07103-3. [DOI] [PubMed] [Google Scholar]
- Tomita K, Izumi K, Okabe S. Roxatidine- and cimetidine-induced angiogenesis inhibition suppresses growth of colon cancer implants in syngeneic mice. J Pharmacol Sci. 2003;93:321–330. doi: 10.1254/jphs.93.321. [DOI] [PubMed] [Google Scholar]
- Vega Lde L, Munoz E, Calzado MA, Lieb K, Candelario-Jalil E, Gschaidmeir H, et al. The 5-HT3 receptor antagonist tropisetron inhibits T cell activation by targeting the calcineurin pathway. Biochem Pharmacol. 2005;70:369–380. doi: 10.1016/j.bcp.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Wadler S, Benson AB, 3rd, Engelking C, Catalano R, Field M, Kornblau SM, et al. Recommended guidelines for the treatment of chemotherapy-induced diarrhea. J Clin Oncol. 1998;16:3169–3178. doi: 10.1200/JCO.1998.16.9.3169. [DOI] [PubMed] [Google Scholar]
- Walstab J, Rappold G, Niesler B. 5-HT(3) receptors: role in disease and target of drugs. Pharmacol Ther. 2010;128:146–169. doi: 10.1016/j.pharmthera.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Wang H, Steeds J, Motomura Y, Deng Y, Verma-Gandhu M, El-Sharkawy RT, et al. CD4+ T cell-mediated immunological control of enterochromaffin cell hyperplasia and 5-hydroxytryptamine production in enteric infection. Gut. 2007;56:949–957. doi: 10.1136/gut.2006.103226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZQ, Han XD, Wang Y, Yuan KL, Jin ZM, Di JZ, et al. Interleukin-1 receptor antagonist reduced apoptosis and attenuated intestinal mucositis in a 5-fluorouracil chemotherapy model in mice. Cancer Chemother Pharmacol. 2011;68:87–96. doi: 10.1007/s00280-010-1451-5. [DOI] [PubMed] [Google Scholar]
- Yasuda M, Kato S, Yamanaka N, Iimori M, Utsumi D, Kitahara Y, et al. Potential role of the NADPH oxidase NOX1 in the pathogenesis of 5-fluorouracil-induced intestinal mucositis in mice. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1133–G1142. doi: 10.1152/ajpgi.00535.2011. [DOI] [PubMed] [Google Scholar]


