Abstract
Iptakalim is a new ATP-sensitive potassium (KATP) channel opener, and it inhibits the proliferation of pulmonary arterial smooth muscle cells (PASMCs) and pulmonary vascular remodeling. However, the underlying mechanism remains unclear. In the present study, we found that iptakalim significantly decreased pulmonary artery pressure, inhibited pulmonary ariery remodeling and PKC-α overexpression in chronic hypoxia in a rat pulmonary hypertension model. Iptakalim reduced hypoxia-induced expression of PKC-α, and abolished the effect of hypoxia on PASMC proliferation significantly in a dose-dependent manner in vitro. Moreover, these effects were abolished by glibenclamide, a selective KATP channel antagonist. These results indicate that iptakalim inhibits PASMC proliferation and pulmonary vascular remodeling induced by hypoxia through downregulating the expression of PKC-α. Iptakalim can serve as a novel promising treatment for hypoxic pulmonary hypertension.
Keywords: iptakalim, pulmonary arterial smooth muscle cells (PASMCs), pulmonary hypertension, protein kinase C-α (PKC-α), hypoxia, proliferation
INTRODUCTION
Hypoxia-induced pulmonary hypertension is a common complication of residence at high altitudes and chronic lung diseases such as chronic obstructive pulmonary disease (COPD), various interstitial lung diseases, bronchiectasis, asthma, and sleep apnea[1],[2]. It is widely accepted that hypoxic pulmonary hypertension is strongly associated with increased morbidity and reduced survival[3]. Hypoxic pulmonary vascular structural remodeling is the key pathological basis of chronic hypoxic pulmonary hypertension[4],[5], although the underlying mechanism is not fully understood. Numerous studies have demonstrated that hypoxia-induced abnormal proliferation of pulmonary arterial smooth muscle cells (PASMCs) may be a key component of pulmonary vascular remodeling[6]–[9].
The protein kinase C (PKC) is one component of the crucial intracellular signal transduction systems and a key enzyme in the regulation of cell permeability, contraction, proliferation, differentiation, apoptosis, and secretion[10]. There are at least 12 distinct isoforms in the PKC family. Among them, PKC-α is a conventional isoform, which requires calcium, phosphatidylserine, and diacylglycerol (DAG), or phorbol esters for full activation. PKC-α is widely expressed and plays important roles in several cellular processes and pathologies, involving cancer, cardiovascular disorders, atherogenesis, thrombosis and pulmonary disease. Therefore, selectively targeted modulation of PKC-α has potential therapeutic value in the future treatment and management of these diseases[11]. PKC-α is also found to be distributed in the cytosol of PASMCs[12]. Previous works suggested that PKC-α was closely associated with the abnormal proliferation of PASMCs and pulmonary vascular remodeling induced by cigarette smoking[13] or hypoxia[14],[15]. The expression of PKC-α was also significantly increased in the lung tissues from idiopathic pulmonary arterial hypertension patients[16].
Potassium channel dysfunction is evident in pulmonary hypertension. Potassium channels contribute to the regulation of pulmonary vascular tone and vascular remodeling through their effects on cytoplasmic K+ and Ca2+ concentrations and membrane potential, thereby regulating cell migration, proliferation, and apoptosis[17]. There are at least four types of potassium channels. Among them, ATP-sensitive potassium (KATP) channels belong to the Kir (inward rectifier) superfamily of potassium channels, and have received intense scrutiny for their biophysical properties, regulation, and pharmacology during the past two decades[18]. KATP channels are widely expressed in the cardiovascular system including the pulmonary circulation. KATP channels contribute to the maintenance of membrane potential (Em) in PASMCs. Closure of KATP channels could lead to increased [Ca2+]i and enhanced contraction and proliferation of PASMCs. Opening of KATP channels causes an increase of K+ efflux, membrane hyperpolarization, inhibition of Ca2+ influx, and subsequent vascular smooth muscle relaxation[19]. KATP channels appears suppressed in proliferating PASMCs[20]. Therefore, pharmacological manipulation of KATP channels in pulmonary vascular cells represents a potential therapeutic target in the treatment of pulmonary hypertension[21].
Iptakalim, a fatty para-amino compound with low molecular weight, has been confirmed by substantial pharmacological, biochemical, and electrophysiological studies, as well as receptor-combining tests as a selective KATP channel opener[22]. Our previous studies have revealed that iptakalim could alleviate pulmonary artery remodeling and pulmonary hypertension in chronic hypoxic rats and inhibit the proliferation of rabbit or human PASMCs induced by endothelin-1 (ET-1)[23]–[25].
However, the underlying mechanisms are not fully understood. It is known that overexpression of PKC-α in pulmonary arterioles is involved in the development of pulmonary vascular remodeling in pulmonary hypertension[13],[26] and the KATP channel opener cromakalim attenuated the pulmonary vasoconstrictory effect of PKC activation[27]. Therefore, in the present study, we used a rat chronic hypoxia exposure model to study whether iptakalim could inhibit the proliferation of PASMCs and pulmonary vascular remodeling by regulating the expression of PKC-α in PASMCs.
MATERIALS AND METHODS
Animals and administration
Sixty young male Sprague-Dawley rats (180-220 g) were obtained from Shanghai Laboratory Animal Center, Chinese Academy of Sciences (Certificate No. SCXK 2007-0005, Grade II) and allowed 1 week to acclimate. All rats were maintained on a 12 h light-dark cycle (light on from 8:00 to 20:00) at ambient temperature (22°C-24°C) with free access to standard rodent chow and tap water. All animal works were approved by the Animal Care and Use Committee of Nanjing Medical University.
Iptakalim was synthesized and provided by the Institute of Pharmacology and Toxicology, Academy of Military Medical Sciences, China, and was dissolved in 0.9% saline to make a concentration of 0.30 g/mL for oral use. Pulmonary hypertension was induced by exposure to hypoxia (10% inspired O2 fraction) in a normobaric chamber as described previously[23]. Forty-five rats were placed into this hypoxia chamber for 8 h/d, 6 d/week for 4 weeks. Three subgroups of rats were treated with either iptakalim at a dose of 0.75 or 1.5 mg/(kg • d) or with vehicle alone (0.9% saline) by gavage tube, once a day, before the rats were exposed to the same hypoxic condition (n = 15, per group), while age- and weight-matched control animals were kept at normobaric pressure (760 mmHg) and room air (21%O2).
Measurement of mean pulmonary artery pressure
Mean pulmonary artery pressure (mPAP) was measured by the method as we previously described[23]. Briefly, after 4 weeks of hypoxia, all rats were intraperitoneally anesthetized with urethane (1.0 g/kg) and placed on the table. A small polyethylene catheter (PE10, Becton Dickinson, Frankin Lakes, NJ, USA) was advanced from the right jugular vein into the right ventricle (RV) and the main pulmonary artery under the guidance of the pressure tracing. After 20 min stabilization, mPAP was recorded using a miniature pressure transducer (TSD104A, BIOPAC Systems., Santa Barbara, CA, USA), digitized by a BIOPAC MP100 data acquisition system, and stored in the computer. After the completion of haemodynamic measurements, a thoracotomy was performed. The whole lungs and heart were rapidly removed and flushed with ice-cold normal saline to remove any blood.
Assessment of right ventricular hypertrophy
During harvest, the atria, the pulmonary trunk, and the aorta were removed from the heart. The right ventricular wall was separated from the left ventricular wall and ventricular septum. Wet weights of the right ventricle, free left ventricular wall, and ventricular septum were determined. Right ventricular hypertrophy was expressed as the ratio of weight of the right ventricular wall (RV) to that of the free left ventricular wall and ventricular septum (LV+S).
Morphometric analysis of the pulmonary arteries
The left lungs of the control, hypoxia and iptakalim treatment groups were cut and fixed with 4% phosphate-buffered formalin. Then, the lung specimens were embedded in paraffin, cut into 4-µm-thick sections and subjected to hematoxylin-eosin (H&E) and Weigert elastic staining before light microscopy. Medial thickness (MT) and external diameter (ED) were measured from 8 to 10 arterioles per animal using Image Pro-Plus 6.0 software (Media Cybernetics, USA) for assessing pulmonary vascular remodeling. ED was defined as distance between the external elastic laminae, while MT was determined as distance between the external and internal elastic laminae. The percentage of MT was calculated using the following formula: MT (%) = (2×MT/ED) × 100%.
Immunohistochemical staining
Immunohistochemical staining was performed by using the EnVision two-step detection method (EnVision Detection Kit, Gene Tech Co., Ltd, Shanghai, China), and the operations were carried out strictly following the manufacturer's instructions. In brief, the deparaffinized formalin-fixed sections were placed in EDTA retrieval agent at pH8.0, and autoclaved at 121°C for 20 min for antigen retrieval. The sections were washed in phosphate-buffered saline (PBS, pH7.6), and incubated overnight at 4°C with either a rabbit anti-PKC-α polyclonal antibody (1:100, Bioworld Technology, Inc., Minneapolis, MN, USA) or a mouse anti-PCNA primary antibody (1:50, Maxim Biotect, Fuzhou, China). After the sections were washed with PBS three times for 5 min, they were treated for 30 min at room temperature in ChemMate ™EnVision+/HRP. Subsequently, the sections were washed with PBS, and diaminobezidine (DAB) colorization was applied. Staining was monitored under a brightfield microscope, and slices were then counterstained with hematoxylin. Negative controls for each tissue section were prepared by omitting the primary antibody. The activity of PKC-α in arterioles was quantified by measuring the mean optical density (OD) using Image Pro-Plus 6.0 software. In each slide, 6 randomly selected muscular arterioles (diameter≤200 µm) were measured and the average was calculated as the representative value for each rat. The PCNA index, calculated as the number of cells staining positive divided by the total number of PASMCs in the muscular arterioles, was used as an indicator of PASMC proliferation.
Human pulmonary arterial smooth muscle cell (PASMC) culture and treatment
Primary PASMCs were isolated from human pulmonary arteries and cultured as we previously reported[25]. Briefly, after approval by the Ethical Review Board of the First Affiliated Hospital of Nanjing Medical University, we obtained specimens of human pulmonary arteries from the healthy segments of the lung of patients undergoing pulmonary resection. After the intrapulmonary arteries (3rd-4th division) were excised from the adventitia and opened longitudinally, the endothelial cells of the pulmonary arteries were removed mechanically with a scalpel blade, and the vessels were cut into 1-3 mm2 pieces. Pieces of the arteries were incubated at 37°C under humidified normoxia (21% O2, 5% CO2 and 74% N2) in the Medium 231(Cascade Biologics, Inc., Portlant, OR, USA) containing 20% fetal bovine serum (FBS), 100 µg/mL streptomycin, 100 U/mL penicillin, 2.5 µg/mL amphotericin B. Cells were confluent after 3-4 weeks, then digested with 0.25% trypsin and subcultured. Cells from passage 4 and 6 were used for the following experiments. Human PASMCs were identified by characteristic “hill-and-valley” morphology and the positive expression for α-smooth muscle actin demonstrated by immunocytochemical staining in more than 95% of cells.
Human PASMCs were suspended in M231 containing 10% FBS, seeded in 96-well plates (1×104 cells per well) or T25 flask, grown until 80% to 90% confluent, and brought to quiescence by incubation with serum-free M231 for 24 h. Then, the cells were cultured in normoxia or hypoxia (5% O2, 5% CO2, and 90% N2) for 24 h. To determine whether hypoxia-induced human PASMC proliferation and PKC-α activation could be inhibited by iptakalim, the cells were pretreated with iptakalim (0, 0.1, 1.0, and 10 µmol/L, respectively) for 30 min prior to treatment with hypoxia. To determine whether the effects of iptakalim on hypoxia-induced human PASMC proliferation and PKC-α activation could be blocked by KATP channel antagonist, human PASMCs were preincubated with 0.1 or 1.0 or 10 µmol/L glibenclamide, a selective KATP channel antagonist, for 30 min prior to treatment with 10 µmol/L iptakalim and 24 h hypoxia.
[3H] thymidine incorporation assay for determination of cell proliferation
Agents were added into fresh M231 as indicated above and cells were incubated with this test substance for 24 h in normoxia or hypoxia as described above. [3H]Thymidine was added to each well at a final concentration of 0.5 Ci/well 6 h before termination of the cultures. At the end of the incubation period, the medium was removed, and the cell monolayer was washed with ice-cold PBS and digested with trypsin. DNA incorporated with [3H]thymidine was precipitated in cold 7.5% trichloroacetic acid, centrifuged, washed three times with 7.5% trichloroacetic acid, and then counted on a LKB Rackbeta scintillation counter. Experiments were performed for six times independently in duplicates.
Western blotting analysis
Protein was extracted from both pulmonary arterial tissues and cell lysates and the concentration of protein was determined by the BCA method. Protein samples were loaded in each lane and subjected to SDS-PAGE gel (10%) for separation. The PVDF membrane was used for immunoblotting. The membrane was blocked and incubated with antibodies against PKC-α (Bioworld, USA) or against GAPDH (KangChen Bio-tech Inc, Shanghai, China) overnight at 4°C. After washing with TBST, the membrane was incubated for 1 h with HRP-labeled anti-mouse IgG. The immunoreactive bands were visualized with enhanced chemiluminescence and captured on X-ray film. The optical density of the bands was measured with a gel imaging analysis system. The results were expressed as the ratio of the expression of PKC-α to that of GAPDH.
Statistical analysis
The results are expressed as mean±SD. Statistical analysis was performed using the unpaired Student's t-test or ANOVA and post hoc tests (Student–Newman–Keuls) as indicated. A value of P < 0.05 was considered significant.
RESULTS
Measurement of mean pulmonary artery pressure
After exposure to hypoxia for 4 weeks, mPAP in the hypoxia group was significantly increased by 69.95% compared with that of control group (P < 0.05). Treatment of rats with 0.75 and 1.5 mg/(kg • d) iptakalim decreased mPAP by 32.63% and 37.20% of the untreated hypoxia group, respectively (Table 1).
Table 1. Comparisons of mPAP and RV/(LV+S) in the four groups.
| Group | mPAP (mmHg) | RV/(LV+S) |
| Control | 18.90±1.30 | 0.26±0.02 |
| Hypoxia | 32.12±3.77* | 0.39±0.04* |
| Ipt treatment [0.75 mg/(kg·d)] | 21.64±2.29# | 0.29±0.02# |
| Ipt treatment [1.50 mg/(kg·d)] | 20.17±2.19# | 0.27±0.02# |
mPAP: mean pulmonary artery pressure, RV/(LV+S): right ventricular weight / left ventricular weight plus septal weight. Ipt: iptakalim. Data are mean±SD, n = 15. *P < 0.05 vs control; #P < 0.05 vs hypoxia.
Assessment of right ventricular hypertrophy
The animals subjected to chronic hypoxia for 4 weeks exhibited the expected right heart hypertrophy, the RV/(LV+S) ratio in the hypoxia group was increased by 32.42%, compared to the control group. Pretreatment with iptakalim significantly decreased the RV/(LV+S) ratio by 15.93% and 19.15%, respectively, as compared to the untreated hypoxia group (P < 0.05, Table 1).
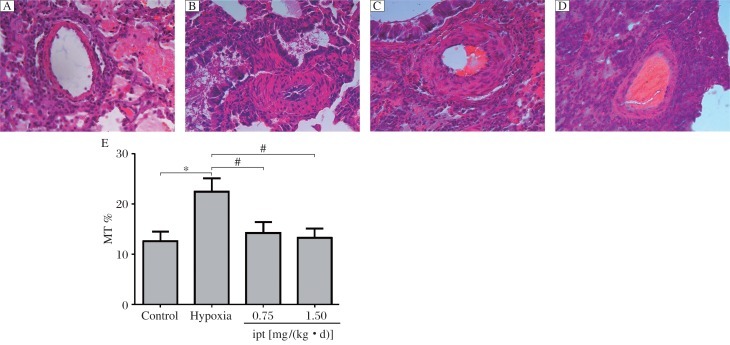
Histology of the lungs of rats with pulmonary hypertension
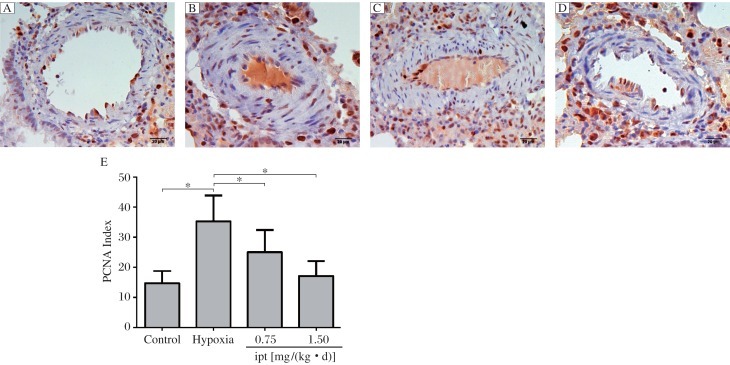
Morphometric analysis revealed that chronic hypoxia resulted in structural changes of small intrapulmonary arteries in rat lungs. These changes were mainly characterized by thickening of the medial layer (proliferation of vascular smooth muscle cells, Fig. 1A and Fig. 1B) and significant increase in the percentage of MT (%), (22.47±2.63)% vs (12.61±1.86)% for control (Fig. 1E). While the medial muscle hypertrophy between the external and the internal elastic lamina in iptakalim [0.75 and 1.5 mg/(kg • d), i.g.] treatment group were attenuated compared with the hypoxia group, the medial layer was close to that of the control group (Fig. 1C, Fig. 1D, and Fig. 1E). In hypoxic rats, PCNA labeling showed proliferation of smooth muscle cells in distal pulmonary vessel walls (Fig. 2B). PCNA-positive cells were (35.25±8.63)% in the muscularized pulmonary vessels of the hypoxia group compared with the control group (14.69±4.20)% (Fig. 2E). Compared to the hypoxia group of (35.25±8.63)%, rats treated with iptakalim [0.75 and 1.5 mg/(kg • d), i.g.] after 4 weeks also had a significant decrease in pulmonary artery PCNA index, (25.05±7.40)% vs. (17.09±4.98)%, respectively (Fig. 2C, Fig. 2D and Fig. 2E). These results indicate that iptakalim suppressed PASMC proliferation and attenuated pulmonary artery remodeling induced by hypoxia.
Fig.1. Effects of iptakalim on lung vascular morphology in hypoxia-induced pulmonary hypertension rats (Magnification ×400).
Lung and pulmonary arterioles were stained with hematoxylin and eosin (H&E). A: the control group. B: the hypoxia group. C: the iptakalim treatment group [0.75 mg/(kg • d)]. D: the iptakalim treatment group [1.50 mg/(kg • d)]. E: effects of iptakalim on the percentage of medial thickness (MT) in pulmonary arterioles of rats with chronic hypoxia-induced pulmonary hypertension. *P < 0.05, Ipt: iptakalim.
Fig. 2. Immunohistochemistry for proliferative cell nuclear antigen (PCNA) in rat lungs (Magnification ×400).
A: the control group, B: the hypoxia group, C: the iptakalim treatment group [0.75 mg/(kg • d)], D: the iptakalim treatment group [1.50 mg/(kg • d)]. E: Effects of iptakalim on the proliferation index in pulmonary arteries of rats with chronic hypoxic pulmonary hypertension. *P < 0.05. Ipt: iptakalim.
Effect of iptakalim on PKC-α expression in the lungs
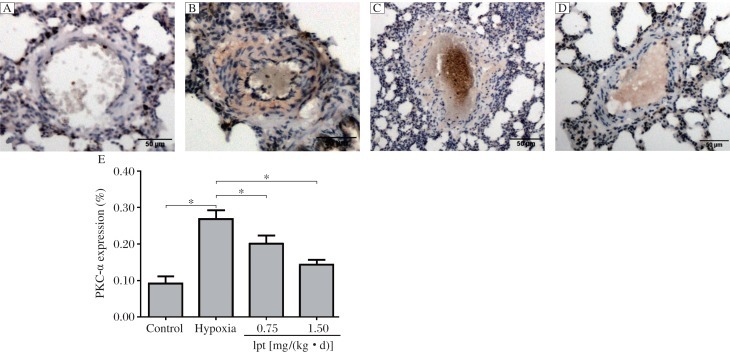
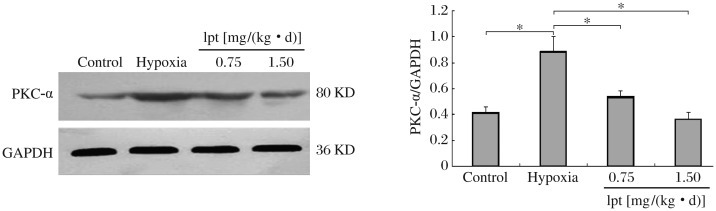
The expression of PKC-α protein in the lungs was detected by immunohistochemical staining and Western blot analysis. Immunohistochemical analysis showed that PKC-α was expressed weakly in the cytoplasm of smooth muscle cells in the medial layers of muscular arterioles in the control group. In contrast, the medial smooth muscule was strongly positive for PKC-α after exposure to hypoxia (Fig. 3). As shown in Fig. 3E, PKC-α expression, as the mean percentage changes in mean optical intensity, in rats kept in hypoxia increased significantly, compared with that in the control group after 4 weeks (P < 0.05). Immunohistochemical analysis showed that the expression of PKC-α was significantly decreased in PASMCs of the iptakalim treatment group [0.75 and 1.5 mg/(kg • d)] compared with those of the hypoxia group (P < 0.05). Similarly, Western blotting of the lung tissues showed an increase in the expression of PKC-α in the hypoxia group compared with the control group (P < 0.05, Fig. 4A). Iptakalim reversed hypoxia-induced increase in PKC-α expression in a dose-dependent manner (P < 0.05, Fig. 4B).
Fig. 3. The expression of PKC-α in the lungs was detected by immunohistochemical staining (Magnification: ×200).
A: the control group. B: the hypoxia group. C: the iptakalim treatment group [0.75 mg/(kg • d)], D: the iptakalim treatment group [1.5 mg/(kg • d)]. E: effects of iptakalim on PKC-α expression (represented by the mean percentage changes in mean optical intensity) in the vessel wall of pulmonary arterioles of rats with chronic hypoxia-induced pulmonary hypertension. *P < 0.05. Ipt: iptakalim.
Fig. 4. Effects of iptakalim on the expression of PKC-α in the pulmonary arteries of rats with chronic hypoxia induced pulmonary hypertension.
*P < 0.05. lpt: iptakalim.
Iptakalim inhibits hypoxia-induced proliferation of human PASMCs by opening KATP channel
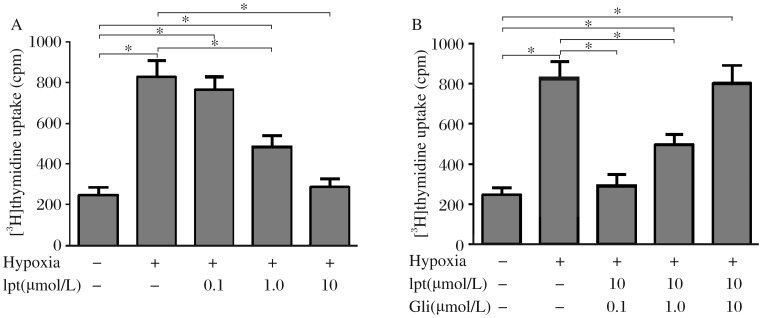
As shown in Fig. 5, [3H]thymidine incorporation was increased 3.38 folds in cells treated with hypoxia compared to cells in normoxia. When the cells were treated with 0.1, 1.0 or 10 µmol/L iptakalim, [3H]thymidine incorporation was decreased by 7.40%, 41.26% and 65.28%, respectively, compared with those treated with hypoxia alone. Consequently, iptakalim inhibited the proliferation of human PASMCs induced by hypoxia in a dose-dependent manner. When cells were pre-incubated with 0.1, 1.0 or 10 µmol/L glibenclamide for 30 min prior to the addition of 10 µmol/L iptakalim and 24 h hypoxia treatment, [3H]thymidine uptake was increased by 21.41%, 103.93%, 228.52% and 263.57%, respectively, compared to normoxic conditions. Consequently, glibenclamide abolished the inhibition of iptakalim on hypoxia-induced proliferation of human PASMCs in a dose-dependent manner. These results showed that iptakalim almost completely inhibited the proliferation of PASMCs induced by hypoxia by opening KATP channel.
Fig. 5. Effect of iptakalim (Ipt) with or without glibenclamide (Gli) on hypoxia-induced proliferation of human PASMCs.
A: The human PASMCs were treated with hypoxia for 24 h with or without iptakalim at 0.1-10 µmol/L and iptakalim dose-dependently inhibited the proliferation of human PASMCs induced by hypoxia. B: Pretreatment with glibenclamide at 0.1-10 µmol/L 30 min before Ipt treatment (10 µmol/L) and hypoxia dose-dependently blocked the anti-proliferation effect of iptakalim. The proliferation of human PASMCs was determined by [3H]thymidine incorporation assay. *P < 0.05, n = 6.
Iptakalim downregulates the expression of PKC-α in human PASMCs under hypoxia by opening KATP channel
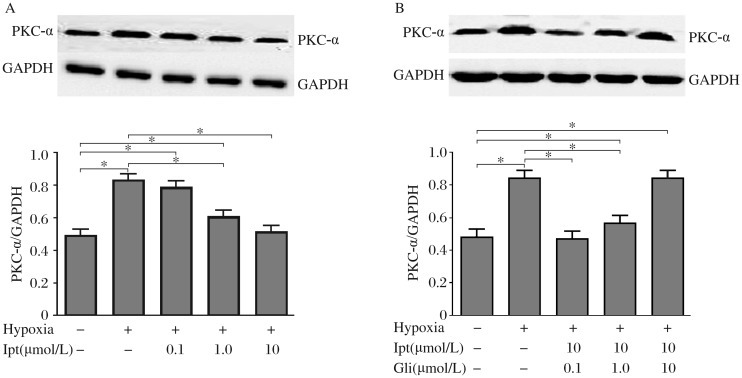
Fig. 6 showed that iptakalim inhibited the proliferation of PASMCs in a dose-depandent wanner, and the effect of inhibition could be reversed by gliben clamide. our results confirmed that hypoxia increases the expression of PKC-α, and human PASMCs treated with iptakalim (0.1-10 µmol/L) for 24 h decreased PKC-α expression dose-dependently (Fig. 6A). glibenclamide abolished the effect of iptakalim on the expression of PKC-α in human PASMCs activated by hypoxia in a dose-dependent manner (Fig. 6B). Moreover, glibenclamide (10 µmol/L) completely reversed the decreases in the expression of PKC-α induced by iptakalim. These results demonstrated that hypoxia activated PKC-α and promoted human PASMC proliferation, and iptakalim inhibited the expression of PKC-α by opening KATP channel.
Fig. 6. Effect of iptakalim (Ipt) with or without glibenclamide (Gli) on the upregulation of PKC-α expression in human PASMCs induced by hypoxia.
A: The human PASMCs were treated with hypoxia for 24 h with or without iptakalim at 0.1-10 µmol/L and iptakalim dose-dependently decreased the expression of PKC-α in human PASMCs induced by hypoxia. B: Pretreatment with glibenclamide at 0.1-10 µmol/L 30 min before iptakalim treatment (10 µmol/L) dose-dependently blocked the downregulation effect of iptakalim. Each value is the mean±SD of 3 independent determinations in each group. *P < 0.05.
DISCUSSION
Our results from both in vivo and in vitro studies demonstrated that hypoxia increased the expression of PKC-α in PASMCs and promoted PASMC proliferation, which could be prevented by iptakalim. We also found that iptakalim prevented hypoxia–induced pulmonary hypertension and vascular remodeling in rats, which may be due to suppressing the expression of PKC-α in PASMCs. Furthermore, this study showed that the inhibitory effects of iptakalim on PKC-α were completely abolished by glibenclamide, which indicated that the effects of iptakalim on overexpression of PKC-α and pulmonary hypertension induced by hypoxia is associated with the activation of KATP channels.
Hypoxic vasoconstriction and hypoxia-induced pulmonary vascular remodeling characterized by proliferation and hypertrophy of smooth muscle cells are the key pathophysiological features of hypoxic pulmonary hypertension[28],[29]. In the present study, we observed that hypoxia led to the increased proliferation of PASMCs in vivo and in vitro, which is in accordance with previous studies[7],[30]. The mechanism of hypoxic pulmonary vascular remodeling is highly complex, and there are still no truly effective drugs for treatments[5]; therefore, we need to search for newer and more effective therapeutic interventions.
PKC-α, a classical Ca2+-dependent PKC isotype, plays an important role in smooth muscle cell contraction as well as in the proliferation and apoptotic response of several different cell types[31]. PKC-α is regarded as a key target in the future treatment and management of cardiovascular dysfunction, arterial thrombosis and cancer[11]. Some studies have shown that PKC-α could induce the proliferation of PASMCs, and play important roles in mediating vascular remodeling in the formation of pulmonary hypertension[15],[32].
We observed that the expression of PKC-α increased obviously at the protein level in pulmonary arterioles of chronic hypoxic rats, and PKC-α overexpression was positively correlated with the increase in mPAP and RV/(LV+S) and pulmonary morphological changes after exposure to chronic hypoxia comparing with the control group. In our in vitro study, we also found that hypoxia-induced PASMC proliferation was associated with PKC-α overexpression in the hypoxia group compared with the control group, which is in accordance with the results of Dempsey et al.[9],[14]. Thus, our results confirmed that hypoxia promotes PASMC proliferation, pulmonary vascular remodeling and pulmonary hypertension, which may be activated by the PKC-α pathway.
The KATP channels are widely distributed in many tissues and cell types including vascular smooth muscle cells, pancreatic β-cells, neurons, skeletal muscle cells, and cardiac myocytes[33]. Like KATP channels in other tissues, vascular smooth muscle KATP channels are now thought to play a vital role as mediators of the response of vascular smooth muscle to a variety of pharmacological and endogenous vasodilators, as well as to changes in metabolic activity that can directly influence blood flow in various tissues[19]. Previous studies have demonstrated that cellular signaling pathways involving serine/threonine kinases, such as cAMP-dependent protein kinase A (PKA) and Ca2+ and PKC play a vital role in the amplitude of whole-cell KATP currents of isolated vascular smooth muscle cells[19],[34]. Activation of PKC-α inhibits potassium current leads to PASMC depolarization and increase of [Ca2+]i, which may lead to PASMC contraction and proliferation and pulmonary vascular remodeling [14].
It is well demonstrated that K+ channel dysfunction plays an important role in the development of pulmonary hypertension. KATP channel also is involved in hypoxic pulmonary vasoconstriction[35],[36] and proliferation of PASMCs and pulmonary vascular remodeling[23],[37]. It has also been confirmed that opening of KATP channel by cromakalim attenuated the pulmonary vasoconstrictory effect of PKC activation[27]. With their multiple pulmonary hypertension-related physiological functions, KATP channels represent promising drug targets[20],[38]. Iptakalim, 2, 3-dimethyl-N-(1-methylethyl)-2-butanamine hydrochloride, has been established as a novel selective KATP opener with preferential activation of the SUR2B/Kir6.1 subtypes of KATP[22] and shown to be a promising drug for treatment of cardiovascular disease. Our previous studies have shown that iptakalim has an inhibitory effect on [Ca2+]i increase and proliferation of PASMCs induced by ET-1 via activating KATP channels[24],[25]. Moreover, chronic treatment with iptakalim attenuates vascular remodeling and control pulmonary hypertension in rats induced by hypoxia[23]. Recently, Wang et al.[39] and Long et al.[40] demonstrated that iptakalim protects against endothelial dysfunction through activating KATP channels in endothelial cells. So, iptakalim is a promising drug for treatment of pulmonary hypertension.
In the present study, we showed that the expression of PKC-α in PASMCs under hypoxia was higher than that of the control group and those treated with iptakalim, indicating that iptakalim could inhibit hypoxia-induced PKC-α activity in PASMCs. These results suggested that iptakalim alleviated hypoxia-induced pulmonary vascular remodeling contributed to the inhibition of PASMC proliferation through the PKC signaling pathway. Possible mechanism for these may be that iptakalim activates KATP channel, leading to K+ efflux and promoting PASMC membrane hyperpolarization, which inhibits Ca2+ influx, thereby decreasing [Ca2+]i in PASMC. while PKC-α is activated by a variety of stimuli in a Ca2+-dependent manner[31]. Therefore, iptakalim may decrease the expression of PKC-α and proliferation of PASMCs by reducing [Ca2+]i in PASMCs. Iptakalim may also inhibit abnormal elevation of PKC-α via correcting the dysfunction of translocation of PKC-α induced by hypoxia.
Therefore, the results of this study could have significant clinical implications and may aid in developing treatments for pulmonary hypertension by providing novel targets of iptakalim for therapeutic intervention.
Acknowledgments
Iptakalim was kindly provided by Dr. Hai Wang (Institute of Pharmacology and Toxicology, Academy of Military Medical Sciences, Beijing, China).
Footnotes
This study was partially supported by the National Natural Science Foundation of China (No.30971319) and the “Six Talent Peak” Project of Jiangsu Province (No.08-B) and the grant from Open Project Program of the Key Disciplines of the Public Health Department of Jiangsu Province (No. XK13_200902).
References
- 1.Hopkins N, McLoughlin P. The structural basis of pulmonary hypertension in chronic lung disease: Remodelling, rarefaction or angiogenesis? J Anat. 2002;201:335–48. doi: 10.1046/j.1469-7580.2002.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219–63. doi: 10.1183/09031936.00139009. [DOI] [PubMed] [Google Scholar]
- 3.Weitzenblum E, Chaouat A, Canuet M, Kessler R. Pulmonary hypertension in chronic obstructive pulmonary disease and interstitial lung diseases. Semin Respir Crit Care Med. 2009;30:458–70. doi: 10.1055/s-0029-1233315. [DOI] [PubMed] [Google Scholar]
- 4.Voelkel NF, Tuder RM. Hypoxia-induced pulmonary vascular remodeling: A model for what human disease? J Clin Invest. 2000;106:733–8. doi: 10.1172/JCI11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: Cellular and molecular mechanisms. Circ Res. 2006;99:675–91. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- 6.Cooper AL, Beasley D. Hypoxia stimulates proliferation and interleukin-1alpha production in human vascular smooth muscle cells. Am J Physiol. 1999;277:H1326–37. doi: 10.1152/ajpheart.1999.277.4.H1326. [DOI] [PubMed] [Google Scholar]
- 7.Lu SY, Wang DS, Zhu MZ, Zhang QH, Hu YZ, Pei JM. Inhibition of hypoxia-induced proliferation and collagen synthesis by vasonatrin peptide in cultured rat pulmonary artery smooth muscle cells. Life Sci. 2005;77:28–38. doi: 10.1016/j.lfs.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 8.Schultz K, Fanburg BL, Beasley D. Hypoxia and hypoxia-inducible factor-1alpha promote growth factor-induced proliferation of human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2006;290:H2528–34. doi: 10.1152/ajpheart.01077.2005. [DOI] [PubMed] [Google Scholar]
- 9.Dempsey EC, McMurtry IF, O'Brien RF. Protein kinase C activation allows pulmonary artery smooth muscle cells to proliferate to hypoxia. Am J Physiol. 1991;260:L136–45. doi: 10.1152/ajplung.1991.260.2.L136. [DOI] [PubMed] [Google Scholar]
- 10.Dempsey EC, Newton AC, Mochly-Rosen D, Fields AP, Reyland ME, Insel PA, et al. Protein kinase C isozymes and the regulation of diverse cell responses. Am J Physiol Lung Cell Mol Physiol. 2000;279:L429–38. doi: 10.1152/ajplung.2000.279.3.L429. [DOI] [PubMed] [Google Scholar]
- 11.Konopatskaya O, Poole AW. Protein kinase Calpha: Disease regulator and therapeutic target. Trends Pharmacol Sci. 31:8–14. doi: 10.1016/j.tips.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dempsey EC, Cool CD, Littler CM. Lung disease and PKCs. Pharmacol Res. 2007;55:545–59. doi: 10.1016/j.phrs.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Zhu Z, Xu Y, Zou H, Zhang Z, Ni W, Chen S. Effects of puerarin on pulmonary vascular remodeling and protein kinase C-alpha in chronic cigarette smoke exposure smoke-exposed rats. J Huazhong Univ Sci Technolog Med Sci. 2008;28:27–32. doi: 10.1007/s11596-008-0107-8. [DOI] [PubMed] [Google Scholar]
- 14.Dempsey EC, Frid MG, Aldashev AA, Das M, Stenmark KR. Heterogeneity in the proliferative response of bovine pulmonary artery smooth muscle cells to mitogens and hypoxia: importance of protein kinase C. Can J Physiol Pharmacol. 1997;75:936–44. [PubMed] [Google Scholar]
- 15.Xu Y, Stenmark KR, Das M, Walchak SJ, Ruff LJ, Dempsey EC. Pulmonary artery smooth muscle cells from chronically hypoxic neonatal calves retain fetal-like and acquire new growth properties. Am J Physiol. 1997;273:L234–45. doi: 10.1152/ajplung.1997.273.1.L234. [DOI] [PubMed] [Google Scholar]
- 16.Do e Z, Fukumoto Y, Takaki A, Tawara S, Ohashi J, Nakano M, et al. Evidence for Rho-kinase activation in patients with pulmonary arterial hypertension. Circ J. 2009;73:1731–9. doi: 10.1253/circj.cj-09-0135. [DOI] [PubMed] [Google Scholar]
- 17.Bonnet S, Archer SL. Potassium channel diversity in the pulmonary arteries and pulmonary veins: Implications for regulation of the pulmonary vasculature in health and during pulmonary hypertension. Pharmacol Ther. 2007;115:56–69. doi: 10.1016/j.pharmthera.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 18.Cole WC, Clement-Chomienne O. ATP-sensitive K+ channels of vascular smooth muscle cells. J Cardiovasc Electrophysiol. 2003;14:94–103. doi: 10.1046/j.1540-8167.2003.02376.x. [DOI] [PubMed] [Google Scholar]
- 19.Brayden JE. Functional roles of KATP channels in vascular smooth muscle. Clin Exp Pharmacol Physiol. 2002;29:312–6. doi: 10.1046/j.1440-1681.2002.03650.x. [DOI] [PubMed] [Google Scholar]
- 20.Cui Y, Tran S, Tinker A, Clapp LH. The molecular composition of KATP channels in human pulmonary artery smooth muscle cells and their modulation by growth. Am J Respir Cell Mol Biol. 2002;26:135–43. doi: 10.1165/ajrcmb.26.1.4622. [DOI] [PubMed] [Google Scholar]
- 21.Guibert C, Marthan R, Savineau JP. Modulation of ion channels in pulmonary arterial hypertension. Curr Pharm Des. 2007;13:2443–55. doi: 10.2174/138161207781368585. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Tang Y, Wang L, Long CL, Zhang YL. ATP-sensitive potassium channel openers and 2,3-dimethyl-2-butylamine derivatives. Curr Med Chem. 2007;14:133–55. doi: 10.2174/092986707779313390. [DOI] [PubMed] [Google Scholar]
- 23.Xie W, Wang H, Hu G. Effects of iptakalim hydrochloride, a novel KATP channel opener, on pulmonary vascular remodeling in hypoxic rats. Life Sci. 2004;75:2065–76. doi: 10.1016/j.lfs.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 24.Xie W, Wang H, Ding J, Hu G. Anti-proliferating effect of iptakalim, a novel KATP channel opener, in cultured rabbit pulmonary arterial smooth muscle cells. Eur J Pharmacol. 2005;511:81–7. doi: 10.1016/j.ejphar.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Y, Zhang S, Xie W, Li Q, Zhou Y, Wang H. Iptakalim inhibited endothelin-1-induced proliferation of human pulmonary arterial smooth muscle cells through the activation of KATP channel. Vascul Pharmacol. 2008;48:92–9. doi: 10.1016/j.vph.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Tan X, Liu YJ, Li JC, Pan JQ, Sun WD, Wang XL. Activation of PKCalpha and pulmonary vascular remodelling in broilers. Res Vet Sci. 2005;79:131–7. doi: 10.1016/j.rvsc.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Barman SA. Potassium channels modulate canine pulmonary vasoreactivity to protein kinase C activation. Am J Physiol. 1999;277:L558–65. doi: 10.1152/ajplung.1999.277.3.L558. [DOI] [PubMed] [Google Scholar]
- 28.Presberg KW, Dincer HE. Pathophysiology of pulmonary hypertension due to lung disease. Curr Opin Pulm Med. 2003;9:131–8. doi: 10.1097/00063198-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Stenmark KR, McMurtry IF. Vascular remodeling versus vasoconstriction in chronic hypoxic pulmonary hypertension: A time for reappraisal? Circ Res. 2005;97:95–8. doi: 10.1161/01.RES.00000175934.68087.29. [DOI] [PubMed] [Google Scholar]
- 30.Yu TZ, Ma CT. Effects of angiotensin-converting enzyme and angiotensin II on hypoxia-induced proliferation of cultured intra-pulmonary artery smooth muscle cells. Acta Pharmacol Sin. 2000;21:381–4. [PubMed] [Google Scholar]
- 31.Nakashima S. Protein kinase C alpha (PKCalpha): Regulation and biological function. J Biochem. 2002;132:669–75. doi: 10.1093/oxfordjournals.jbchem.a003272. [DOI] [PubMed] [Google Scholar]
- 32.Itoh H, Yamamura S, Ware JA, Zhuang S, Mii S, Liu B, et al. Differential effects of protein kinase C on human vascular smooth muscle cell proliferation and migration. Am J Physiol Heart Circ Physiol. 2001;281:H359–70. doi: 10.1152/ajpheart.2001.281.1.H359. [DOI] [PubMed] [Google Scholar]
- 33.Rodrigo GC, Standen NB. ATP-sensitive potassium channels. Curr Pharm Des. 2005;11:1915–40. doi: 10.2174/1381612054021015. [DOI] [PubMed] [Google Scholar]
- 34.Teramoto N. Physiological roles of ATP-sensitive K+ channels in smooth muscle. J Physiol. 2006;572:617–24. doi: 10.1113/jphysiol.2006.105973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dumas JP, Bardou M, Goirand F, Dumas M. Hypoxic pulmonary vasoconstriction. Gen Pharmacol. 1999;33:289–97. doi: 10.1016/s0306-3623(99)00026-9. [DOI] [PubMed] [Google Scholar]
- 36.Clapp LH, Gurney AM. ATP-sensitive K+ channels regulate resting potential of pulmonary arterial smooth muscle cells. Am J Physiol. 1992;262:H916–20. doi: 10.1152/ajpheart.1992.262.3.H916. [DOI] [PubMed] [Google Scholar]
- 37.Oka M, Morris KG, McMurtry IF. Nip-121 is more effective than nifedipine in acutely reversing chronic pulmonary hypertension. J Appl Physiol. 1993;75:1075–80. doi: 10.1152/jappl.1993.75.3.1075. [DOI] [PubMed] [Google Scholar]
- 38.Mannhold R. KATP channel openers: Structure-activity relationships and therapeutic potential. Med Res Rev. 2004;24:213–66. doi: 10.1002/med.10060. [DOI] [PubMed] [Google Scholar]
- 39.Wang H, Long C, Duan Z, Shi C, Jia G, Zhang Y. A new ATP-sensitive potassium channel opener protects endothelial function in cultured aortic endothelial cells. Cardiovasc Res. 2007;73:497–503. doi: 10.1016/j.cardiores.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 40.Long CL, Qin XC, Pan ZY, Chen K, Zhang YF, Cui WY, et al. Activation of ATP-sensitive potassium channels protects vascular endothelial cells from hypertension and renal injury induced by hyperuricemia. J Hypertens. 2008;26:2326–38. doi: 10.1097/HJH.0b013e328312c8c1. [DOI] [PubMed] [Google Scholar]