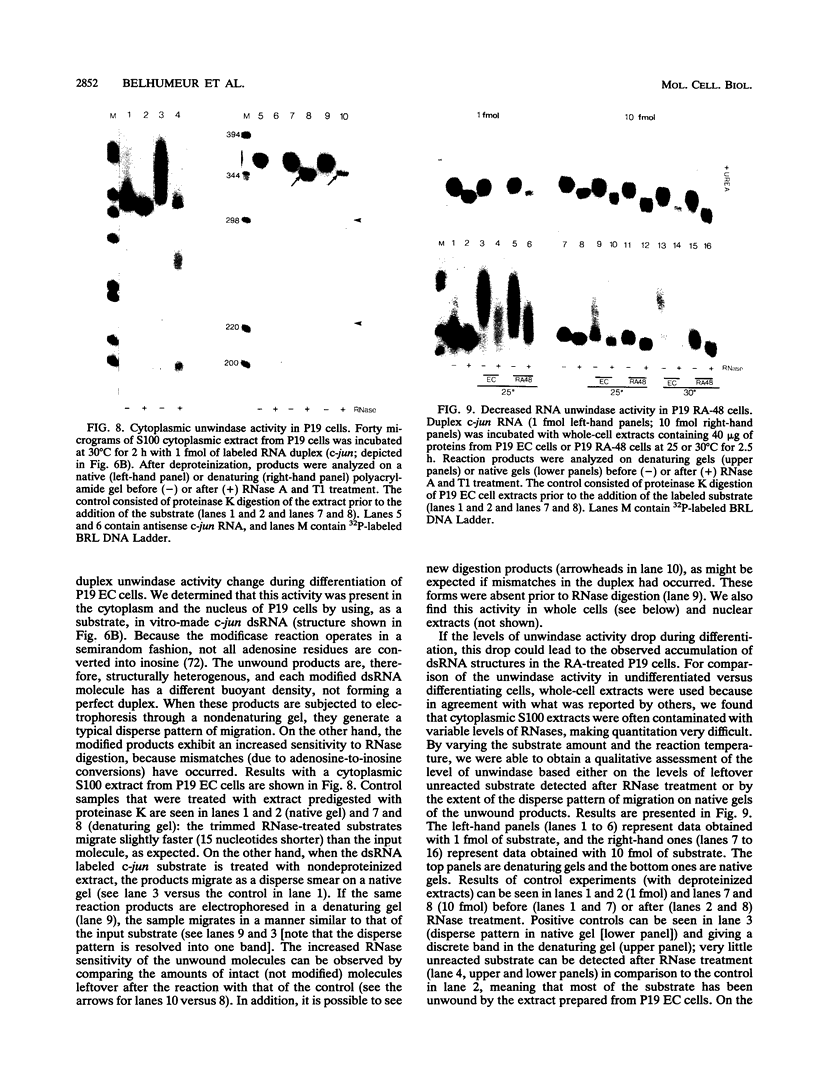
Abstract
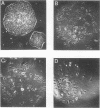
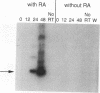
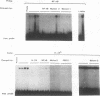
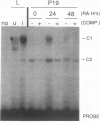
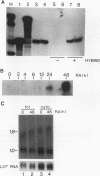
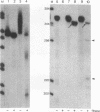
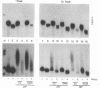
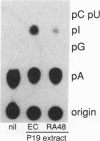
In the current study, we have addressed the role of interferons (IFNs) in controlling the differentiation of pluripotent P19 embryonal carcinoma (EC) cells. Blocking IFN activity in the culture medium of differentiating cells with antibodies leads to a strong decrease in the degree of differentiation. The antibodies are active for a relatively short time. During this time, IFN-beta mRNA can be detected in the differentiating cells, as can increases of IFN stimulation response element-binding activity and NF-KB. The timing of IFN action also coincides with the accumulation of cytoplasmic double-stranded RNA (dsRNA) and with a drop in dsRNA unwindase-modificase activity. A model for the involvement of autoinduction of IFN by intracellular dsRNA in the control of differentiation in this system is presented.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin A. S., Jr, Sharp P. A. Two transcription factors, NF-kappa B and H2TF1, interact with a single regulatory sequence in the class I major histocompatibility complex promoter. Proc Natl Acad Sci U S A. 1988 Feb;85(3):723–727. doi: 10.1073/pnas.85.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow D. P., Randle B. J., Burke D. C. Interferon synthesis in the early post-implantation mouse embryo. Differentiation. 1984;27(3):229–235. doi: 10.1111/j.1432-0436.1984.tb01433.x. [DOI] [PubMed] [Google Scholar]
- Bass B. L., Weintraub H. A developmentally regulated activity that unwinds RNA duplexes. Cell. 1987 Feb 27;48(4):607–613. doi: 10.1016/0092-8674(87)90239-x. [DOI] [PubMed] [Google Scholar]
- Bass B. L., Weintraub H. An unwinding activity that covalently modifies its double-stranded RNA substrate. Cell. 1988 Dec 23;55(6):1089–1098. doi: 10.1016/0092-8674(88)90253-x. [DOI] [PubMed] [Google Scholar]
- Bass B. L., Weintraub H., Cattaneo R., Billeter M. A. Biased hypermutation of viral RNA genomes could be due to unwinding/modification of double-stranded RNA. Cell. 1989 Feb 10;56(3):331–331. doi: 10.1016/0092-8674(89)90234-1. [DOI] [PubMed] [Google Scholar]
- Belhumeur P., Lussier M., Skup D. Expression of naturally occurring RNA molecules complementary to the murine L27' ribosomal protein mRNA. Gene. 1988 Dec 10;72(1-2):277–285. doi: 10.1016/0378-1119(88)90153-9. [DOI] [PubMed] [Google Scholar]
- Belhumeur P., Paterno G. D., Boileau G., Claverie J. M., Skup D. Isolation and characterisation of a murine cDNA clone highly homologous to the yeast L29 ribosomal protein gene. Nucleic Acids Res. 1987 Feb 11;15(3):1019–1029. doi: 10.1093/nar/15.3.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. W., McBurney M. W. Cell density and cell cycle effects on retinoic acid-induced embryonal carcinoma cell differentiation. Dev Biol. 1990 Mar;138(1):123–135. doi: 10.1016/0012-1606(90)90182-i. [DOI] [PubMed] [Google Scholar]
- Birnbaum M., Trink B., Shainberg A., Salzberg S. Activation of the interferon system during myogenesis in vitro. Differentiation. 1990 Nov;45(2):138–145. doi: 10.1111/j.1432-0436.1990.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Burke D. C., Graham C. F., Lehman J. M. Appearance of interferon inducibility and sensitivity during differentiation of murine teratocarcinoma cells in vitro. Cell. 1978 Feb;13(2):243–248. doi: 10.1016/0092-8674(78)90193-9. [DOI] [PubMed] [Google Scholar]
- Cattaneo R., Schmid A., Eschle D., Baczko K., ter Meulen V., Billeter M. A. Biased hypermutation and other genetic changes in defective measles viruses in human brain infections. Cell. 1988 Oct 21;55(2):255–265. doi: 10.1016/0092-8674(88)90048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R., Schmid A., Spielhofer P., Kaelin K., Baczko K., ter Meulen V., Pardowitz J., Flanagan S., Rima B. K., Udem S. A. Mutated and hypermutated genes of persistent measles viruses which caused lethal human brain diseases. Virology. 1989 Dec;173(2):415–425. doi: 10.1016/0042-6822(89)90554-0. [DOI] [PubMed] [Google Scholar]
- Cordell-Stewart B., Taylor M. W. Effect of double-stranded viral RNA on mammalian cells in culture. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1326–1330. doi: 10.1073/pnas.68.6.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigneault L., Coulombe B., Skup D. Regulation of interferon gene expression: mechanism of action of the If-1 locus. J Virol. 1988 Apr;62(4):1125–1131. doi: 10.1128/jvi.62.4.1125-1131.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigneault L., Skup D. Increased levels of interferon regulatory element-binding activities in nuclei of high interferon-producing If-1h mice. Cell Growth Differ. 1992 Feb;3(2):93–100. [PubMed] [Google Scholar]
- De Benedetti A., Baglioni C. Inhibition of mRNA binding to ribosomes by localized activation of dsRNA-dependent protein kinase. Nature. 1984 Sep 6;311(5981):79–81. doi: 10.1038/311079a0. [DOI] [PubMed] [Google Scholar]
- Dirks W., Mittnacht S., Rentrop M., Hauser H. Isolation and functional characterization of the murine interferon-beta 1 promoter. J Interferon Res. 1989 Feb;9(1):125–133. doi: 10.1089/jir.1989.9.125. [DOI] [PubMed] [Google Scholar]
- Fujita T., Miyamoto M., Kimura Y., Hammer J., Taniguchi T. Involvement of a cis-element that binds an H2TF-1/NF kappa B like factor(s) in the virus-induced interferon-beta gene expression. Nucleic Acids Res. 1989 May 11;17(9):3335–3346. doi: 10.1093/nar/17.9.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T., Ohno S., Yasumitsu H., Taniguchi T. Delimitation and properties of DNA sequences required for the regulated expression of human interferon-beta gene. Cell. 1985 Jun;41(2):489–496. doi: 10.1016/s0092-8674(85)80022-2. [DOI] [PubMed] [Google Scholar]
- Fujita T., Shibuya H., Hotta H., Yamanishi K., Taniguchi T. Interferon-beta gene regulation: tandemly repeated sequences of a synthetic 6 bp oligomer function as a virus-inducible enhancer. Cell. 1987 May 8;49(3):357–367. doi: 10.1016/0092-8674(87)90288-1. [DOI] [PubMed] [Google Scholar]
- Goodbourn S., Burstein H., Maniatis T. The human beta-interferon gene enhancer is under negative control. Cell. 1986 May 23;45(4):601–610. doi: 10.1016/0092-8674(86)90292-8. [DOI] [PubMed] [Google Scholar]
- Goodbourn S., Zinn K., Maniatis T. Human beta-interferon gene expression is regulated by an inducible enhancer element. Cell. 1985 Jun;41(2):509–520. doi: 10.1016/s0092-8674(85)80024-6. [DOI] [PubMed] [Google Scholar]
- Haggarty A., Camato R., Paterno G., Cohen L., Hiscott J., Skup D. A developmentally regulated octamer-binding activity in embryonal carcinoma cells which represses beta-interferon expression. Cell Growth Differ. 1991 Oct;2(10):503–510. [PubMed] [Google Scholar]
- Hall D. J., Jones S. D., Kaplan D. R., Whitman M., Rollins B. J., Stiles C. D. Evidence for a novel signal transduction pathway activated by platelet-derived growth factor and by double-stranded RNA. Mol Cell Biol. 1989 Apr;9(4):1705–1713. doi: 10.1128/mcb.9.4.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H., Fujita T., Miyamoto M., Kimura Y., Maruyama M., Furia A., Miyata T., Taniguchi T. Structurally similar but functionally distinct factors, IRF-1 and IRF-2, bind to the same regulatory elements of IFN and IFN-inducible genes. Cell. 1989 Aug 25;58(4):729–739. doi: 10.1016/0092-8674(89)90107-4. [DOI] [PubMed] [Google Scholar]
- Harada H., Willison K., Sakakibara J., Miyamoto M., Fujita T., Taniguchi T. Absence of the type I IFN system in EC cells: transcriptional activator (IRF-1) and repressor (IRF-2) genes are developmentally regulated. Cell. 1990 Oct 19;63(2):303–312. doi: 10.1016/0092-8674(90)90163-9. [DOI] [PubMed] [Google Scholar]
- Hiscott J., Alper D., Cohen L., Leblanc J. F., Sportza L., Wong A., Xanthoudakis S. Induction of human interferon gene expression is associated with a nuclear factor that interacts with the NF-kappa B site of the human immunodeficiency virus enhancer. J Virol. 1989 Jun;63(6):2557–2566. doi: 10.1128/jvi.63.6.2557-2566.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Hunt T., Jackson R. J., Robertson H. D. The characteristics of inhibition of protein synthesis by double-stranded ribonucleic acid in reticulocyte lysates. J Biol Chem. 1975 Jan 25;250(2):409–417. [PubMed] [Google Scholar]
- Jones-Villeneuve E. M., McBurney M. W., Rogers K. A., Kalnins V. I. Retinoic acid induces embryonal carcinoma cells to differentiate into neurons and glial cells. J Cell Biol. 1982 Aug;94(2):253–262. doi: 10.1083/jcb.94.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Villeneuve E. M., Rudnicki M. A., Harris J. F., McBurney M. W. Retinoic acid-induced neural differentiation of embryonal carcinoma cells. Mol Cell Biol. 1983 Dec;3(12):2271–2279. doi: 10.1128/mcb.3.12.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judware R., Petryshyn R. Partial characterization of a cellular factor that regulates the double-stranded RNA-dependent eIF-2 alpha kinase in 3T3-F442A fibroblasts. Mol Cell Biol. 1991 Jun;11(6):3259–3267. doi: 10.1128/mcb.11.6.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A. D., Maniatis T. Identification of an inducible factor that binds to a positive regulatory element of the human beta-interferon gene. Proc Natl Acad Sci U S A. 1988 May;85(10):3309–3313. doi: 10.1073/pnas.85.10.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelman D., Kirschner M. W. An antisense mRNA directs the covalent modification of the transcript encoding fibroblast growth factor in Xenopus oocytes. Cell. 1989 Nov 17;59(4):687–696. doi: 10.1016/0092-8674(89)90015-9. [DOI] [PubMed] [Google Scholar]
- Kostura M., Mathews M. B. Purification and activation of the double-stranded RNA-dependent eIF-2 kinase DAI. Mol Cell Biol. 1989 Apr;9(4):1576–1586. doi: 10.1128/mcb.9.4.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Dreyfuss G. RNA structure. Unwinding with a vengeance. Nature. 1989 Jan 5;337(6202):19–20. doi: 10.1038/337019a0. [DOI] [PubMed] [Google Scholar]
- Lawrence J. B., Singer R. H. Intracellular localization of messenger RNAs for cytoskeletal proteins. Cell. 1986 May 9;45(3):407–415. doi: 10.1016/0092-8674(86)90326-0. [DOI] [PubMed] [Google Scholar]
- Leblanc J. F., Cohen L., Rodrigues M., Hiscott J. Synergism between distinct enhanson domains in viral induction of the human beta interferon gene. Mol Cell Biol. 1990 Aug;10(8):3987–3993. doi: 10.1128/mcb.10.8.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo M. J., Fan C. M., Maniatis T., Baltimore D. The involvement of NF-kappa B in beta-interferon gene regulation reveals its role as widely inducible mediator of signal transduction. Cell. 1989 Apr 21;57(2):287–294. doi: 10.1016/0092-8674(89)90966-5. [DOI] [PubMed] [Google Scholar]
- Lengyel P. Biochemistry of interferons and their actions. Annu Rev Biochem. 1982;51:251–282. doi: 10.1146/annurev.bi.51.070182.001343. [DOI] [PubMed] [Google Scholar]
- Li J., Petryshyn R. A. Activation of the double-stranded RNA-dependent eIF-2 alpha kinase by cellular RNA from 3T3-F442A cells. Eur J Biochem. 1991 Jan 1;195(1):41–48. doi: 10.1111/j.1432-1033.1991.tb15673.x. [DOI] [PubMed] [Google Scholar]
- Luo G. X., Chao M., Hsieh S. Y., Sureau C., Nishikura K., Taylor J. A specific base transition occurs on replicating hepatitis delta virus RNA. J Virol. 1990 Mar;64(3):1021–1027. doi: 10.1128/jvi.64.3.1021-1027.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. R., Evans M. J. Differentiation of clonal lines of teratocarcinoma cells: formation of embryoid bodies in vitro. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1441–1445. doi: 10.1073/pnas.72.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurney M. W., Jones-Villeneuve E. M., Edwards M. K., Anderson P. J. Control of muscle and neuronal differentiation in a cultured embryonal carcinoma cell line. Nature. 1982 Sep 9;299(5879):165–167. doi: 10.1038/299165a0. [DOI] [PubMed] [Google Scholar]
- McBurney M. W., Rogers B. J. Isolation of male embryonal carcinoma cells and their chromosome replication patterns. Dev Biol. 1982 Feb;89(2):503–508. doi: 10.1016/0012-1606(82)90338-4. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson J., Glasgow L. A. The in vitro and in vivo effects of cortisol on interferon production and action. J Immunol. 1966 Feb;96(2):345–352. [PubMed] [Google Scholar]
- Meurs E., Chong K., Galabru J., Thomas N. S., Kerr I. M., Williams B. R., Hovanessian A. G. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell. 1990 Jul 27;62(2):379–390. doi: 10.1016/0092-8674(90)90374-n. [DOI] [PubMed] [Google Scholar]
- Miyamoto M., Fujita T., Kimura Y., Maruyama M., Harada H., Sudo Y., Miyata T., Taniguchi T. Regulated expression of a gene encoding a nuclear factor, IRF-1, that specifically binds to IFN-beta gene regulatory elements. Cell. 1988 Sep 9;54(6):903–913. doi: 10.1016/s0092-8674(88)91307-4. [DOI] [PubMed] [Google Scholar]
- Morrissey L. M., Kirkegaard K. Regulation of a double-stranded RNA modification activity in human cells. Mol Cell Biol. 1991 Jul;11(7):3719–3725. doi: 10.1128/mcb.11.7.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S., Langer J. A., Zoon K. C., Samuel C. E. Interferons and their actions. Annu Rev Biochem. 1987;56:727–777. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- Petryshyn R., Chen J. J., London I. M. Detection of activated double-stranded RNA-dependent protein kinase in 3T3-F442A cells. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1427–1431. doi: 10.1073/pnas.85.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryshyn R., Chen J. J., London I. M. Growth-related expression of a double-stranded RNA-dependent protein kinase in 3T3 cells. J Biol Chem. 1984 Dec 10;259(23):14736–14742. [PubMed] [Google Scholar]
- Pratt G., Galpine A., Sharp N., Palmer S., Clemens M. J. Regulation of in vitro translation by double-stranded RNA in mammalian cell mRNA preparations. Nucleic Acids Res. 1988 Apr 25;16(8):3497–3510. doi: 10.1093/nar/16.8.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebagliati M. R., Melton D. A. Antisense RNA injections in fertilized frog eggs reveal an RNA duplex unwinding activity. Cell. 1987 Feb 27;48(4):599–605. doi: 10.1016/0092-8674(87)90238-8. [DOI] [PubMed] [Google Scholar]
- Resnitzky D., Yarden A., Zipori D., Kimchi A. Autocrine beta-related interferon controls c-myc suppression and growth arrest during hematopoietic cell differentiation. Cell. 1986 Jul 4;46(1):31–40. doi: 10.1016/0092-8674(86)90857-3. [DOI] [PubMed] [Google Scholar]
- Roberts W. K., Hovanessian A., Brown R. E., Clemens M. J., Kerr I. M. Interferon-mediated protein kinase and low-molecular-weight inhibitor of protein synthesis. Nature. 1976 Dec 2;264(5585):477–480. doi: 10.1038/264477a0. [DOI] [PubMed] [Google Scholar]
- Rollins B. J., Morrison E. D., Stiles C. D. Cloning and expression of JE, a gene inducible by platelet-derived growth factor and whose product has cytokine-like properties. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3738–3742. doi: 10.1073/pnas.85.11.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo G., Fiorucci G., Rossi G. B. Interferons in cell growth and development. Trends Genet. 1989 Jan;5(1):19–24. doi: 10.1016/0168-9525(89)90007-3. [DOI] [PubMed] [Google Scholar]
- Rutherford M. N., Hannigan G. E., Williams B. R. Interferon-induced binding of nuclear factors to promoter elements of the 2-5A synthetase gene. EMBO J. 1988 Mar;7(3):751–759. doi: 10.1002/j.1460-2075.1988.tb02872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryseck R. P., Hirai S. I., Yaniv M., Bravo R. Transcriptional activation of c-jun during the G0/G1 transition in mouse fibroblasts. Nature. 1988 Aug 11;334(6182):535–537. doi: 10.1038/334535a0. [DOI] [PubMed] [Google Scholar]
- Singh H., LeBowitz J. H., Baldwin A. S., Jr, Sharp P. A. Molecular cloning of an enhancer binding protein: isolation by screening of an expression library with a recognition site DNA. Cell. 1988 Feb 12;52(3):415–423. doi: 10.1016/s0092-8674(88)80034-5. [DOI] [PubMed] [Google Scholar]
- Visvanathan K. V., Goodbourn S. Double-stranded RNA activates binding of NF-kappa B to an inducible element in the human beta-interferon promoter. EMBO J. 1989 Apr;8(4):1129–1138. doi: 10.1002/j.1460-2075.1989.tb03483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. W., Nishikura K. Cell cycle expression of RNA duplex unwindase activity in mammalian cells. Mol Cell Biol. 1988 Feb;8(2):770–777. doi: 10.1128/mcb.8.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. W., Smith J. E., Cooperman B. S., Nishikura K. A double-stranded RNA unwinding activity introduces structural alterations by means of adenosine to inosine conversions in mammalian cells and Xenopus eggs. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2647–2651. doi: 10.1073/pnas.86.8.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. C., Ayata M., Hirano A., Yoshikawa Y., Tsuruoka H., Yamanouchi K. Generalized and localized biased hypermutation affecting the matrix gene of a measles virus strain that causes subacute sclerosing panencephalitis. J Virol. 1989 Dec;63(12):5464–5468. doi: 10.1128/jvi.63.12.5464-5468.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]
- Zullo J. N., Cochran B. H., Huang A. S., Stiles C. D. Platelet-derived growth factor and double-stranded ribonucleic acids stimulate expression of the same genes in 3T3 cells. Cell. 1985 Dec;43(3 Pt 2):793–800. doi: 10.1016/0092-8674(85)90252-1. [DOI] [PubMed] [Google Scholar]