Abstract
Understanding the impact of antimicrobial use on the emergence of resistant bacteria is imperative to prevent its emergence. For instance, activation of the AcrAB efflux pumps is responsible for the emergence of antimicrobial-resistant Salmonella strains. Here, we examined the expression levels of acrB and its multiple regulator genes (RamA, SoxS, MarA, and Rob) in 17 field isolates of S. Choleraesuis by using quantitative PCR methods. The expression of acrB increased in eight of the field isolates (P < 0.05). The expression of acrB was associated with that of ramA in one isolate, soxS in one isolate, and both these genes in six isolates. Thereafter, to examine the effect of selected antimicrobials (enrofloxacin, ampicillin, oxytetracycline, kanamycin, and spectinomycin) on the expression of acrB and its regulator genes, mutants derived from five isolates of S. Choleraesuis were selected by culture on antimicrobial-containing plates. The expression of acrB and ramA was higher in the mutants selected using enrofloxacin (3.3–6.3- and 24.5–37.7-fold, respectively), ampicillin (1.8–7.7- and 16.1–55.9-fold, respectively), oxytetracycline (1.7–3.3- and 3.2–31.1-fold, respectively), and kanamycin (1.6–2.2- and 5.6–26.4-fold, respectively), which are AcrAB substrates, than in each of the parental strains (P < 0.05). In contrast, in AcrAB substrate-selected mutants, the expression of soxS, marA, and rob remained similar to that in parental strains. Of the four antimicrobials, the level of ramA expression was significantly higher in the enrofloxacin- and ampicillin-selected mutants than in the oxytetracycline- and kanamycin-selected mutants (P < 0.05), whereas the expression levels of acrB and multiple regulator genes in spectinomycin-selected mutants were similar to those in each parental strain. These data suggest that exposure to antimicrobials that are AcrAB substrates enhance the activation of the AcrAB efflux pump via RamA, but not via SoxS, MarA, or Rob in S. Choleraesuis.
Keywords: AcrAB efflux pump, antimicrobial resistance, RamA, Salmonella Choleraesuis, SoxS
Introduction
Salmonella enterica subspecies enterica serovar Choleraesuis is a bacterial pathogen that causes severe diarrhea, pneumonia, and septicemia in pigs and in elderly and immunocompromised humans (Chiu et al., 2004). These bacteria sometimes cause severe infections, necessitating antimicrobial treatment of affected patients. A high rate of multidrug resistance among S. Choleraesuis has been reported in several countries (Lee et al., 2009; Asai et al., 2010), raising a concern for public and animal health.
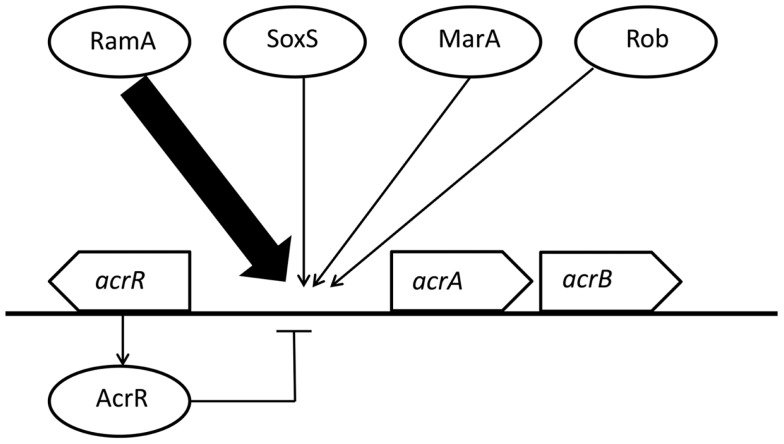
Activation of an efflux system that removes the drug from the cell is one antimicrobial resistance mechanism in bacteria (Chen et al., 2007; Li and Nikaido, 2009). The AcrAB-TolC system is the main multidrug efflux system in Gram-negative bacteria (Giraud et al., 2000). The expression level of acrAB mRNAs correlates with efflux activities and is regulated by global regulatory proteins and local repressors (Rosenberg et al., 2003; Li and Nikaido, 2004; Olliver et al., 2004; Abouzeed et al., 2008). In particular, the expression of acrAB is regulated by the global regulators, RamA, SoxS, MarA, and Rob (Rosenberg et al., 2003; Abouzeed et al., 2008). Moreover, mutations within the local repressor AcrR contribute to the overexpression of acrAB (Olliver et al., 2004). In S. Typhimurium and S. Haardt, RamA mainly regulates the expression of acrAB (Figure 1; Nikaido et al., 2008; Zheng et al., 2009; Kim and Woo, 2011). Other regulators, SoxS and MarA, are also known to regulate acrAB expression, but the contributions of SoxS and MarA to antimicrobial susceptibilities are lower than that of RamA (Abouzeed et al., 2008). Little is known about Rob and its contribution to the enhancement of AcrAB in Salmonella (Rosenberg et al., 2003). In S. Pullorum, the expression of acrB, which shifts from sensitivity to resistance against bile salt (deoxycholate, an AcrAB substrate), was independent of ramA, soxS, marA, and rob expression (Usui et al., 2011).
Figure 1.
Regulation of acrAB expression by multiple regulators in Salmonella Typhimurium and S. Haardt. This figure was produced using data from the literature (see main text). Functional interactions are represented as arrows for activation/induction and as “⊣” for repression. The size of arrows indicates the estimated impact.
We have previously recorded the various efflux activity levels of S. Choleraesuis under field conditions, suggesting that elevated efflux activities are related to the emergence of fluoroquinolone resistance (Usui et al., 2009). In fluoroquinolone-selected S. Choleraesuis, efflux pumps including AcrAB were enhanced and resulted in decreasing susceptibilities to several antimicrobials (Usui et al., 2010). However, little is known about roles of regulator genes for efflux pumps in S. Choleraesuis. In this study, we examined the expression levels of acrAB and multiple regulator genes in 17 clinical swine isolates of S. Choleraesuis and antimicrobial-selected mutants in order to understand the impact of antimicrobial exposure on the efflux activities in this organism.
Materials and Methods
Bacterial strains
Seventeen strains of S. Choleraesuis, obtained from different diseased pigs between 2001 and 2005 (Asai et al., 2010), were used in this study (Table 1). These strains comprised 6 nalidixic acid-resistant strains (low enrofloxacin-accumulation), and 11 nalidixic acid-susceptible strains (two strains: low enrofloxacin-accumulation; three strains: intermediate enrofloxacin-accumulation; six strains: high enrofloxacin-accumulation) as previously recorded (Usui et al., 2009). The intracellular concentration of enrofloxacin was significantly lower in nalidixic acid-resistant isolates, and the nalidixic acid-susceptible isolates ZSC-8 and 582 as compared to other susceptible isolates with the exception of isolates 143, ZSC-12, and 1002 (P < 0.1; Usui et al., 2009). The intracellular enrofloxacin concentrations of isolates 143, ZSC-12, and 1002 were at an intermediate level. Minimum inhibitory concentrations (MIC) of enrofloxacin, ampicillin, oxytetracycline, and kanamycin had been determined in our previous study (Asai et al., 2010). MIC of spectinomycin was determined by the broth microdilution method according to CLSI guidelines (Clinical and Laboratory Standard Institute, 2008).
Table 1.
Characterization of Salmonella Choleraesuis used in this study.
| Strain no. | Susceptibility to nalidixic acida |
Enrofloxacin-accumulation |
mRNA expression |
Mutations |
||||
|---|---|---|---|---|---|---|---|---|
| Categoryb | MIC (mg/L)b | Categoryc | (ng/105cfu)c | acrAd | acrBd | AcrRe | Binding site | |
| 13-PLS-6 | Resistance | 256 | Low | 8.2 | 1.6 ± 0.2* | 1.8 ± 0.2* | WT | WT |
| 14-PLS-21 | Resistance | >512 | Low | 8.7 | 3.9 ± 0.3* | 2.7 ± 0.2* | WT | WT |
| 16-PLS-45 | Resistance | >512 | Low | 6.5 | 1.5 ± 0.1* | 1.9 ± 0.3* | Q78ter | WT |
| 16-PLS-46 | Resistance | >512 | Low | 6.6 | 2.4 ± 0.2* | 2.4 ± 0.4* | Q78ter | WT |
| sal-1372 | Resistance | >512 | Low | 6.3 | 1.8 ± 0.1* | 2.2 ± 0.2* | WT | WT |
| 16-PLS-33 | Resistance | >512 | Low | 5.1 | 1.5 ± 0.1* | 1.9 ± 0.2* | Q78ter | WT |
| ZSC-8 | Susceptible | 8 | Low | 8.1 | 1.7 ± 0.1* | 1.8 ± 0.2* | Q78ter | WT |
| 582 | Susceptible | 8 | Low | 7.2 | 1.4 ± 0.1* | 1.4 ± 0.2* | Q78ter | WT |
| 143 | Susceptible | 8 | Intermediate | 9.1 | 1.1 ± 0.1 | 1.3 ± 0.1 | Q78ter | WT |
| ZSC-12 | Susceptible | 4 | Intermediate | 9.5 | 1.0 ± 0.1 | 1.0 ± 0.1 | Q78ter | WT |
| 1002 | Susceptible | 4 | Intermediate | 9.8 | 1.1 ± 0.2 | 1.2 ± 0.2 | Q78ter | WT |
| 916 | Susceptible | 4 | High | 10.7 | 1.1 ± 0.1 | 1.1 ± 0.1 | Q78ter | WT |
| 03-197-1 | Susceptible | 2 | High | 11.4 | 0.9 ± 0.1 | 0.8 ± 0.1 | Q78ter | WT |
| 03-228-1 | Susceptible | 2 | High | 10.7 | 1.0 ± 0.2 | 1.1 ± 0.1 | Q78ter | WT |
| ZSC-14-1 | Susceptible | 4 | High | 11.0 | 0.9 ± 0.1 | 1.1 ± 0.1 | Q78ter | WT |
| ZSC-40 | Susceptible | 4 | High | 10.5 | 0.9 ± 0.2 | 0.9 ± 0.2 | WT | WT |
| 419 | Susceptible | 4 | High | 12.7 | 1.0 ± 0.2 | 0.9 ± 0.1 | Q78ter | WT |
MIC, minimum inhibitory concentration; WT, wild-type sequence.
aMIC break point of nalidixic acid is 32 mg/L.
bData from a previous report (Asai et al., 2010).
cData from a previous report (Usui et al., 2009).
dRelative expression level compared to the average for six high enrofloxacin-accumulating nalidixic acid-susceptible strains.
eQ78ter, stop codon mutation, glutamine-78-termination.
*Indicates mRNA expression is significantly higher than mRNA expression of six high enrofloxacin-accumulating nalidixic acid-susceptible strains (P < 0.05).
Laboratory-selected mutants
Five strains (582, 143, 916, 1002, and 419), susceptible to all of the antimicrobial agents evaluated in this study, were selected as parent strains from which mutants were isolated. Cultures grown on agar containing various agents was employed, including three AcrAB substrates (ampicillin, oxytetracycline, and kanamycin) and one non-substrate (spectinomycin; Bailey et al., 2008; Ricci and Piddock, 2009a,b). For selection of mutants, 0.1 mL of each strain, adjusted to 108–109cfu/mL were exposed to 4 × MIC of the respective substance for each strain in the agar medium, as described previously (Ricci and Piddock, 2009b). A single strain was selected for each combination. In addition, as fluoroquinolone is an AcrAB substrates, enrofloxacin-selected mutants obtained in our previous report were used as control (Usui et al., 2010). MICs of antimicrobials for mutants were determined as described above.
Quantitative PCR analysis of expression of 16S rRNA, acrAB, and multiple global regulators
To evaluate the genes conferring efflux pump activity and their multiple global regulators, quantitative PCR was applied to the detection and quantification of mRNAs (Usui et al., 2010, 2011). The preparation of cDNA was performed as described by Zheng et al. (2009), with slight modifications. In brief, extraction of total RNA from 5 mL of bacterial suspensions was carried out with an ISOGEN kit (Nippon Gene, Tokyo, Japan). cDNA was synthesized from these RNA samples using the PrimeScript RT reagent kit (TaKaRa, Shiga, Japan). Quantitative PCR was performed with SYBR premix EX TaqII (TaKaRa) on a One Step real-time system (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. The oligonucleotide primers used for the detection of cDNA are listed in Table 2. The yields of amplicons from acrA, acrB, ramA, soxS, marA, and rob were normalized to those originating from 16S rRNA. Within bacterial cells, 16S rRNA was assumed to be transcribed at a constant rate throughout the growth conditions used in this study.
Table 2.
Primers used in this study.
| Primer name | Sequence (5′–3′) | Reference |
|---|---|---|
| acrA-F | AAAACGGCAAAGCGAAGGT | Usui et al. (2011) |
| acrA-R | GTACCGGACTGCGGGAATT | Usui et al. (2011) |
| acrB-rt1 | GGCATTGGGTATGACTGGAC | Zheng et al. (2009) |
| acrB-rt2 | GCATTACGGAGAACGGGATAG | Zheng et al. (2009) |
| ramA-rt1 | TTTCCGCTCAGGTTATCGAC | Zheng et al. (2009) |
| ramA-rt2 | CGGGCAATATCATCAATACG | Zheng et al. (2009) |
| soxS-rt1 | AAATCGGGCTACTCCAAG | Zheng et al. (2009) |
| soxS-rt2 | TACTCGCCTAATGTTTGATG | Zheng et al. (2009) |
| marA-rt1 | ATTCTCTATCTGGCGGAAC | Zheng et al. (2009) |
| marA-rt2 | CGGGTCAATGTTTGCTGTG | Zheng et al. (2009) |
| robA-rt1 | TATTCCGCCAGTGCTTTATG | Zheng et al. (2009) |
| robA-rt2 | CCTGCTCATCGTCTTTCTCC | Zheng et al. (2009) |
| 16S rRNA-F | CCAGCAGCCGCGGTAAT | Usui et al. (2010) |
| 16S rRNA-R | TTTACGCCCAGTAATTCCGATT | Usui et al. (2010) |
After normalization to the levels of 16S rRNA, gene expression was compared with the average values for the six high enrofloxacin-accumulating susceptible strains.
acrR DNA sequence analysis
Mutations in the local repressor gene, acrR, and the regulator-binding site were detected by direct DNA sequencing, using previously reported primer sets (Zheng et al., 2009). Amplification of the gene and purification of the resulting amplicon were performed as described before (Zheng et al., 2009). Nucleotide sequences were determined using a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) with a 3130 Genetic Analyzer (Applied Biosystems).
Statistical analysis
Student’s t-test was used to compare results between and within experiments. P-values < 0.05 were considered significant.
Results
Relationship between the enhancement of efflux pumps and the expression of acrAB
Expression of acrA and acrB in the six resistant strains and in the two low enrofloxacin-accumulation nalidixic acid-susceptible strains was significantly higher than that in the other susceptible strains (P < 0.05; Table 1). Expression levels of acrB correlated significantly with those of acrA in this study (correlation coefficient: 0.89).
Relationship between increased expression of acrB and global regulators
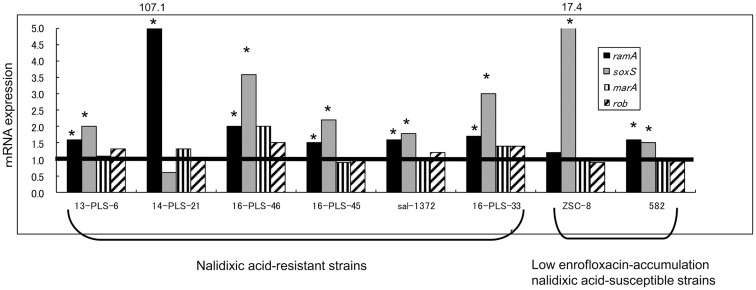
Of the eight strains with a high acrB expression level, six strains showed higher levels of both ramA and soxS expression compared with the average values for the six high accumulating susceptible strains (P < 0.05; Figure 2). The remaining strains showed a higher level of ramA or soxS expression (P < 0.05): the 14-PLS-21 strain showed only a higher expression level of ramA (107.1-fold ± 42.0-fold), while the ZSC-8 strain showed only a higher expression level of soxS (17.4-fold ± 2.7-fold). Neither the expression of marA nor that of rob changed in any of the strains tested.
Figure 2.
Expression of multiple regulator genes in field isolates of S. Choleraesuis. The expression of multiple regulator genes in resistant strains and low enrofloxacin-accumulating nalidixic acid-susceptible strains was compared to the average expression of the corresponding genes in high enrofloxacin-accumulating nalidixic acid-susceptible strains (n = 6). Horizontal line indicates the average gene expression in the six susceptible strains, which expressions were set to be 1.0. *Indicates that mRNA expression is significantly higher in a strain compared to mRNA expression in the six susceptible strains (P < 0.05).
Sequencing of the local repressor gene acrR and of the regulator-binding site
A stop codon mutation in AcrR (glutamine-78-to-terminal; Q78ter) was found in 13 strains (Table 1). No significant relationship was found between the Q78ter stop codon mutation in AcrR and the expression of acrB. None of the strains tested had a point mutation in the regulator-binding site, which affects the acrB expression, previously determined using the S. serovar Typhimurium strain BN18 (Olliver et al., 2004).
Gene expression in laboratory-selected mutants
Expression levels of both acrB and ramA increased significantly in AcrAB substrate-selected mutants compared to these levels in parental strains (P < 0.05; Table 3). Among four AcrAB substrates, a higher level of ramA expression was observed in the enrofloxacin- and ampicillin-selected mutants than in the oxytetracycline- and kanamycin-selected mutants (P < 0.05). In contrast, the expression of soxS decreased significantly in AcrAB substrate-selected mutants compared to that in parental strains (P < 0.05; Table 3). MICs of AcrAB substrates were increased in AcrAB substrate-selected mutants as compared with those in the parental strains (Table 3).
Table 3.
The MICs of several antimicrobials and gene expressions of acrB and multiple regulator genes in laboratory-selected mutants.
| Strain no. | Selecting agenta | MIC (mg/L) |
mRNA expressionc |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Enrofloxacin(2)b | Ampicillin (32)b | Oxytetracycline (16)b | Kanamycin (64)b | Spectinomycin(128)b | acrB | ramA | soxS | marA | rob | ||
| 582 | 0.0625 | 1 | 2 | 2 | 32 | 1.4 | 1.6 | 1.5 | 1.1 | 1.0 | |
| 582/E | Enrofloxacin | 0.25 | 4 | 4 | 2 | 32 | 6.9 | 39.2 | 0.8 | 1.2 | 0.6 |
| 582/A | Ampicillin | 0.25 | 4 | 8 | 2 | 32 | 7.7 | 89.5 | 0.3 | 1.2 | 0.8 |
| 582/O | Oxytetracycline | 0.125 | 4 | 4 | 2 | 32 | 4.2 | 9.5 | 0.3 | 1.1 | 0.9 |
| 582/K | Kanamycin | 0.125 | 2 | 4 | 4 | 32 | 3.1 | 9.0 | 0.5 | 1.3 | 0.6 |
| 582/S | Spectinomycin | 0.0625 | 1 | 2 | 2 | 128 | 1.7 | 1.4 | 1.5 | 1.2 | 0.8 |
| 143 | 0.0625 | 1 | 2 | 2 | 32 | 1.3 | 1.2 | 1.2 | 1.1 | 1.0 | |
| 143E | Enrofloxacin | 0.25 | 4 | 4 | 2 | 32 | 4.3 | 45.2 | 0.6 | 0.9 | 0.7 |
| 143/A | Ampicillin | 0.125 | 4 | 4 | 2 | 32 | 2.9 | 38.8 | 0.1 | 0.7 | 0.5 |
| 143/O | Oxytetracycline | 0.0625 | 2 | 8 | 2 | 32 | 2.2 | 10.2 | 1.3 | 1.2 | 0.3 |
| 143/K | Kanamycin | 0.0625 | 2 | 4 | 8 | 32 | 2.1 | 9.3 | 1.1 | 1.2 | 0.4 |
| 143/S | Spectinomycin | 0.0625 | 1 | 2 | 2 | 128 | 1.6 | 0.9 | 0.9 | 0.7 | 0.7 |
| 1002 | 0.0625 | 1 | 2 | 2 | 32 | 1.2 | 1.0 | 0.8 | 1.0 | 1.0 | |
| 1002/E | Enrofloxacin | 0.25 | 4 | 4 | 2 | 32 | 8.0 | 29.8 | 0.1 | 1.2 | 1.0 |
| 1002/A | Ampicillin | 0.125 | 4 | 2 | 2 | 32 | 2.4 | 16.1 | 0.1 | 0.3 | 0.5 |
| 1002/O | Oxytetracycline | 0.125 | 2 | 8 | 2 | 32 | 2.2 | 14.7 | 0.2 | 1.2 | 0.9 |
| 1002/K | Kanamycin | 0.125 | 2 | 4 | 4 | 32 | 2.4 | 14.2 | 0.3 | 1.0 | 0.9 |
| 1002/S | Spectinomycin | 0.0625 | 1 | 2 | 2 | 128 | 1.1 | 1.4 | 0.4 | 1.1 | 0.9 |
| 916* | 0.0625 | 1 | 2 | 2 | 32 | 1.1 | 1.1 | 0.8 | 0.9 | 0.9 | |
| 916/E | Enrofloxacin | 0.5 | 4 | 4 | 2 | 32 | 7.4 | 34.2 | 0.4 | 0.8 | 0.9 |
| 916/A | Ampicillin | 0.25 | 4 | 4 | 2 | 32 | 8.5 | 58.2 | 0.1 | 0.9 | 0.8 |
| 916/O | Oxytetracycline | 0.0625 | 1 | 4 | 2 | 32 | 2.2 | 3.5 | 0.2 | 1.0 | 0.9 |
| 916/K | Kanamycin | 0.0625 | 1 | 4 | 8 | 32 | 2.2 | 6.5 | 0.1 | 1.0 | 0.6 |
| 916/S | Spectinomycin | 0.0625 | 1 | 2 | 2 | 128 | 1.2 | 0.6 | 0.2 | 0.7 | 0.6 |
| 419* | 0.0625 | 4 | 2 | 2 | 32 | 0.9 | 0.7 | 1.0 | 1.0 | 1.0 | |
| 419/E | Enrofloxacin | 0.25 | 16 | 4 | 2 | 32 | 5.7 | 24.2 | 0.2 | 1.7 | 0.8 |
| 419/A | Ampicillin | 0.0625 | 8 | 2 | 2 | 32 | 1.6 | 32.5 | 0.3 | 0.7 | 0.9 |
| 419/O | Oxytetracycline | 0.125 | 8 | 8 | 2 | 32 | 3.0 | 21.8 | 0.8 | 3.4 | 1.6 |
| 419/K | Kanamycin | 0.0625 | 8 | 4 | 8 | 32 | 1.5 | 18.5 | 0.3 | 1.1 | 0.9 |
| 419/S | Spectinomycin | 0.0625 | 4 | 2 | 2 | 128 | 0.9 | 1.1 | 0.4 | 2.1 | 1.2 |
| 03-197-1* | 0.0625 | >128 | 256 | 2 | 32 | 0.8 | 1.1 | 1.3 | 1.2 | 1.2 | |
| 03-228-1* | 0.0625 | 1 | 256 | 8 | 32 | 1.1 | 1.1 | 1.0 | 0.9 | 0.9 | |
| ZSC-14-1* | 0.0625 | 1 | 256 | 2 | 32 | 1.1 | 1.0 | 1.0 | 1.0 | 0.8 | |
| ZSC-40* | 0.0625 | >128 | 256 | 2 | 32 | 0.9 | 0.8 | 0.8 | 1.1 | 0.9 | |
MIC, minimum inhibitory concentration.
aMutants were selected by exposing to fourth the MIC of the antimicrobials for each susceptible strain.
bMIC data of antimicrobials except for spectinomycin in parental strains from a previous report (Asai et al., 2010). Parenthesis indicates break points.
cRelative expression level of acrB and multiple regulator genes compared to average of six high accumulation susceptible strains represented as asterisks.
Expression of acrB and multiple regulator genes remained stable in spectinomycin-selected mutants. In spectinomycin-selected mutants, MICs of AcrAB substrates were of the same level as compared with those in the parental strains (Table 3).
Discussion
The current study showed that the expression of ramA and/or soxS was associated with acrAB overexpression in field isolates of S. Choleraesuis. In this study, expression of ramA, but not soxS, marA, or rob, was enhanced in laboratory-derived S. Choleraesuis mutants selected using antimicrobials that are AcrAB substrates. Several previous studies have reported that ramA, but not soxS, is involved in activation of the AcrAB-TolC system in S. Typhimurium (Ricci et al., 2006; Bailey et al., 2008; Zheng et al., 2009). Interestingly, in this study, soxS expression was downregulated in AcrAB substrate-selected mutants of S. Choleraesuis. In addition, enrofloxacin- and ampicillin-selected mutants derived from the ZSC-8 strain with a high level of soxS expression, showed increased expression of ramA, but not of soxS (data not shown). Several studies have suggested that increased expression of ramA downregulates soxS expression (Nikaido et al., 2008; O’Regan et al., 2009). These results suggest that the acrA and acrB expression in antimicrobial-selected mutants of S. Choleraesuis were dependent on ramA expression.
The present study showed that increased levels of both ramA and soxS expression were demonstrated by some field isolates of S. Choleraesuis upon activation of the AcrAB-TolC system. There was a marked difference in soxS expression between the field isolates and the laboratory-derived strains. As all of the strains used in this study were isolated from diseased pigs, these isolates would have been exposed to antimicrobials and disinfectants used in disease treatment and hygiene management. This suggests that the resistance in some field isolates was mediated through a different mechanism, and therefore could have arisen from a different selective pressure.
Activation of the AcrAB efflux pump is responsible for the emergence of fluoroquinolone-resistant Salmonella strains (Ricci et al., 2006; Usui et al., 2009). In Japan, the description of veterinary fluoroquinolone drugs includes an explanation that the drug is considered as a second-line drug. In the AcrAB substrate-selected mutants, the level of acrB expression generally increased, depending on ramA expression. It is possible that the use of an AcrAB substrate as a first-line drug crucially selects strains of S. Choleraesuis with activated AcrAB efflux systems. Among AcrAB substrates used in this study, the degree of acrB expression may be different due to exposure to the various antimicrobial agents. Therefore, the antimicrobial class used as first-line drugs may be associated with the frequency of fluoroquinolone resistance in bacterial strains. Further studies are needed in order to clarify the effects of each antimicrobial on acrB expression in these bacteria.
In conclusion, the AcrAB-TolC efflux system in field isolates of S. Choleraesuis is likely regulated by external factors in the environment, such as antimicrobials and drug residues via ramA and soxS. In particular, exposure of AcrAB substrates enhances activation of AcrAB via RamA in S. Choleraesuis.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the staff at the Prefectural Livestock Hygiene Service Center for providing S. Choleraesuis isolates. This work was supported in part by a Grant-in Aid from the Ministry of Health, Labor and Welfare of Japan (H21-Shokuhin-Ippan-013).
References
- Abouzeed Y. M., Baucheron S., Cloeckaert A. (2008). ramR mutations involved in efflux-mediated multidrug resistance in Salmonella enterica serovar Typhimurium. Antimicrob. Agents Chemother. 52, 2428–2434 10.1128/AAC.00084-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai T., Namimatsu T., Osumi T., Kojima A., Harada K., Aoki H., et al. (2010). Molecular typing and antimicrobial resistance of Salmonella enterica subspecies enterica serovar Choleraesuis isolates from diseased pigs in Japan. Comp. Immunol. Microbiol. Infect. Dis. 33, 109–119 10.1016/j.cimid.2008.08.004 [DOI] [PubMed] [Google Scholar]
- Bailey A. M., Paulsen I. T., Piddock L. J. (2008). RamA confers multidrug resistance in Salmonella enterica via increased expression of acrB, which is inhibited by chlorpromazine. Antimicrob. Agents Chemother. 52, 3604–3611 10.1128/AAC.00661-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Cui S., McDermott P. F., Zhao S., White D. G., Paulsen I., et al. (2007). Contribution of target gene mutations and efflux to decreased susceptibility of Salmonella enterica serovar Typhimurium to fluoroquinolones and other antimicrobials. Antimicrob. Agents Chemother. 51, 535–542 10.1128/AAC.00600-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C. H., Su L. H., Chu C. (2004). Salmonella enterica serotype Choleraesuis: epidemiology, pathogenesis, clinical disease, and treatment. Clin. Microbiol. Rev. 17, 311–322 10.1128/CMR.17.2.311-322.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical and Laboratory Standard Institute. (2008). Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, Third Edition: Approved Standard M31-A3. Wayne, PA: Clinical and Laboratory Standard Institute [Google Scholar]
- Giraud E., Cloeckaert A., Kerboeuf D., Chaslus-Dancla E. (2000). Evidence for active efflux as the primary mechanism of resistance to ciprofloxacin in Salmonella enterica serovar Typhimurium. Antimicrob. Agents Chemother. 44, 1223–1228 10.1128/AAC.44.5.1223-1228.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. Y., Woo G. J. (2011). Expression of acrB and ramA in fluoroquinolone resistant mutants from multi-drug resistant Salmonella enterica serovar Haardt. Lett. Appl. Microbiol. 52, 484–490 10.1111/j.1472-765X.2011.03013.x [DOI] [PubMed] [Google Scholar]
- Lee H. Y., Su L. H., Tsai M. H., Kim S. W., Chang H. H., Jung S. I., et al. (2009). High rate of reduced susceptibility to ciprofloxacin and ceftriaxone among nontyphoid Salmonella clinical isolates in Asia. Antimicrob. Agents Chemother. 53, 2696–2699 10.1128/AAC.01297-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. Z., Nikaido H. (2004). Efflux-mediated drug resistance in bacteria. Drugs 64, 159–204 10.2165/00003495-200464020-00004 [DOI] [PubMed] [Google Scholar]
- Li X. Z., Nikaido H. (2009). Efflux-mediated drug resistance in bacteria: an update. Drugs 69, 1555–1623 10.2165/11317030-000000000-00000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido E., Yamaguchi A., Nishino K. (2008). AcrAB multidrug efflux pump regulation in Salmonella enterica serovar Typhimurium by RamA in response to environmental signals. J. Biol. Chem. 283, 24245–24253 10.1074/jbc.M804544200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olliver A., Valle M., Chaslus-Dancla E., Cloeckaert A. (2004). Role of an acrR mutation in multidrug resistance of in vitro-selected fluoroquinolone-resistant mutants of Salmonella enterica serovar Typhimurium. FEMS Microbiol. Lett. 238, 267–272 10.1016/j.femsle.2004.07.046 [DOI] [PubMed] [Google Scholar]
- O’Regan E., Quinn T., Pages J. M., McCusker M., Piddock L., Fanning S. (2009). Multiple regulatory pathways associated with high-level ciprofloxacin and multidrug resistance in Salmonella enterica serovar enteritidis: involvement of RamA and other global regulators. Antimicrob. Agents Chemother. 53, 1080–1087 10.1128/AAC.01005-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci V., Piddock L. J. (2009a). Ciprofloxacin selects for multidrug resistance in Salmonella enterica serovar Typhimurium mediated by at least two different pathways. J. Antimicrob. Chemother. 63, 909–916 10.1093/jac/dkp054 [DOI] [PubMed] [Google Scholar]
- Ricci V., Piddock L. J. (2009b). Only for substrate antibiotics are a functional AcrAB-TolC efflux pump and RamA required to select multidrug-resistant Salmonella Typhimurium. J. Antimicrob. Chemother. 64, 654–657 10.1093/jac/dkp234 [DOI] [PubMed] [Google Scholar]
- Ricci V., Tzakas P., Buckley A., Piddock L. J. (2006). Ciprofloxacin-resistant Salmonella enterica serovar Typhimurium strains are difficult to select in the absence of AcrB and TolC. Antimicrob. Agents Chemother. 50, 38–42 10.1128/AAC.50.1.38-42.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E. Y., Bertenthal D., Nilles M. L., Bertrand K. P., Nikaido H. (2003). Bile salts and fatty acids induce the expression of Escherichia coli AcrAB multidrug efflux pump through their interaction with Rob regulatory protein. Mol. Microbiol. 48, 1609–1619 10.1046/j.1365-2958.2003.03531.x [DOI] [PubMed] [Google Scholar]
- Usui M., Asai T., Sato S. (2011). Low expression of acrB in the deoxycholate-sensitive strains of Salmonella enterica subspecies enterica serovar Pullorum. Microbiol. Immunol. 55, 366–368 10.1111/j.1348-0421.2011.00321.x [DOI] [PubMed] [Google Scholar]
- Usui M., Uchiyama M., Baba K., Nagai H., Yamamoto Y., Asai T. (2010). Contribution of enhanced efflux to reduced susceptibility of Salmonella enterica serovar Choleraesuis to fluoroquinolone and other antimicrobials. J. Vet. Med. Sci. 73, 279–282 10.1292/jvms.10-0309 [DOI] [PubMed] [Google Scholar]
- Usui M., Uchiyama M., Iwanaka M., Nagai H., Yamamoto Y., Asai T. (2009). Intracellular concentrations of enrofloxacin in quinolone-resistant Salmonella enterica subspecies enterica serovar Choleraesuis. Int. J. Antimicrob. Agents 34, 592–595 10.1016/j.ijantimicag.2009.07.009 [DOI] [PubMed] [Google Scholar]
- Zheng J., Cui S., Meng J. (2009). Effect of transcriptional activators RamA and SoxS on expression of multidrug efflux pumps AcrAB and AcrEF in fluoroquinolone-resistant Salmonella Typhimurium. J. Antimicrob. Chemother. 63, 95–102 10.1093/jac/dkn448 [DOI] [PubMed] [Google Scholar]