Abstract
OBJECTIVE
To study the effect of the H2S-donating derivative of sildenafil (ACS6) compared to sildenafil citrate and sodium hydrosulphide (NaHS) on relaxation, superoxide formation and NADPH oxidase and type 5 phosphodiesterase (PDE5) expression in isolated rabbit cavernosal tissue and smooth muscle cells (CSMCs), and in vivo on indices of oxidative stress induced with buthionine sulphoximine (BSO).
MATERIALS AND METHODS
Relaxation was studied in an organ bath in response to carbachol and after incubation with interleukin-1β for 12 h. CSMCs were incubated with tumour-necrosis factor-α or the thromboxane A2 (TXA2) analogue, U46619, with or with no sildenafil citrate, ACS6 or NaHS for 16 h. Superoxide formation and the expression of p47phox (an active subunit of the NADPH oxidase complex) and PDE5 protein was then assessed using Western blotting. Rats were also treated with BSO (with or with no sildenafil citrate or ACS6) for 7 days; cavernosal cGMP, cAMP, glutathionine and plasma TXA2 and 8-isoprostane F2α was measured by enzyme-linked immunosorbent assay.
RESULTS
ACS6 and sildenafil citrate relaxed cavernosal smooth muscle equipotently; NaHS alone had little effect at up to 100 μM. The formation of superoxide and expression of p47phox and PDE5 was reduced by ACS6, sildenafil citrate and NaHS (order of potency: ACS6 > sildenafil citrate > NaHS). The effects of ACS6 were blocked by inhibitors of protein kinase A (PKA) and PKG. In rats treated with BSO, both ASC6 and sildenafil citrate reduced the increased plasma levels of TXA2 and 8-isoprostane F2α but increased cGMP, cAMP and glutathionine levels in corpus cavernosum.
CONCLUSIONS
By virtue of a dual action on PKA and PKG activation, ACS6 not only promotes erection, acutely, but might also have a long-term beneficial effect through inhibition of oxidative stress and downregulation of PDE5.
Keywords: erectile dysfunction, sildenafil, hydrogen sulphide
INTRODUCTION
The nitric oxide (NO)/cGMP pathway is central to the erectile response, in that it elicits relaxation of cavernosal smooth muscle (CSM) and pudendal arteries [1]. This pathway is controlled, in part, by the inactivation of cGMP by type 5 phosphodiesterase (PDE5) which hydrolyses cGMP to GMP, thereby stopping the dilation effects of NO [2]. The importance of PDE5 in erection is exemplified by the therapeutic use of PDE5 inhibitors to treat erectile dysfunction (ED) [3,4]. Although PDE5 inhibitors have proved to be effective in treating ED, significantly many patients (in particular diabetics) do not respond favourably to this therapy [4–6]. It is therefore important to explore novel strategies that might overcome these shortfalls of PDE5 inhibitors.
The therapeutic effect of PDE5 inhibitors might be mediated in part through a reduction of intrapenile oxidative stress [5,6], in particular increased superoxide production, which is now considered central to the aetiology of ED [5,6]. Superoxide reacts with NO to form reactive nitrogen species which reduces the bioavailability of NO and therefore reduces the ‘NO drive’ [7]. A major inducible intravascular source of superoxide in CSM cells (CSMCs) appears to be NADPH oxidase (NOX) [6–9]. In turn, NO inhibits the activity and expression of NOX in CSMCs and vascular SMCs (VSMCs) [10–12] providing endogenous protection against oxidative stress. In turn, sildenafil inhibits NOX expression and activity through augmentation of the NO-cGMP-protein kinase (PK)-G signalling axis [8,9,11,13,14]. Furthermore, superoxide derived from NOX up-regulates PDE5 expression, an effect inhibited by sildenafil through an a priori inhibition of NOX [14]. It was therefore suggested that the therapeutic benefit of PDE5 inhibitors might be mediated, in part, through inhibition of up-regulation of NOX coupled to PDE5 up-regulation [14]. It has long been established that the hydrolysis of cGMP is increased in experimental models of risk factors ED [15–17].
It was recently reported that cavernosal tissue produces another bioactive gas, hydrogen sulphide (H2S) that relaxes CSM [18,19]. H2S has similar properties to NO, including a vasorelaxant effect, which is partially mediated by a functional endothelium, depends on extracellular calcium entry, but is independent of the activation of the cGMP pathway [6]. We also showed that H2S donors, as with NO, inhibit the expression and activity of NOX in human VSMCs through a cAMP-PKA-dependent pathway [20]. Previous studies showed that the NO-donating derivative of sildenafil is far more potent in inhibiting NOX expression and activity [9,11]. It follows therefore that H2S-donating sildenafil might be a more effective drug than sildenafil alone, particularly in those patients who do not respond to therapy. Such a compound (ACS6) has been developed by CTG Pharma SRL (Milan, Italy; Fig. 1). The therapeutic tenet of this drug class is that the H2S donors would exert the additional beneficial effects of the parent drug (i.e. sildenafil) alone. Indeed, ACS6 has been shown to inhibit NOX expression and activity in porcine endothelial cells [21].
FIG. 1.
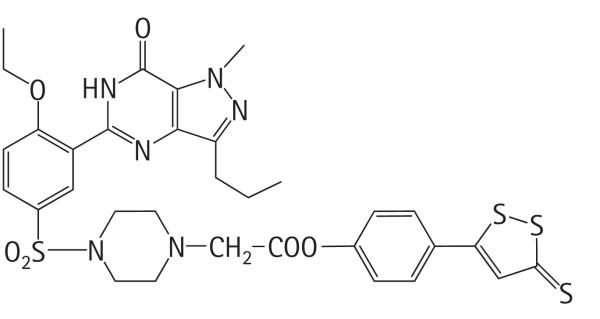
The chemical structure of ACS6.
Thus, to explore the therapeutic potential of ACS6 in treating ED, its effects on the relaxation of isolated cavernosal tissue, NOX expression and activity, superoxide formation and PDE5 expression in isolated CSMCs derived from rabbits was investigated. NOX activity was induced by incubating cells with TNFα, thromboxane A2 (TXA2) analogue, U46619 and 8-isoprostane F2α (8-IPF2α). Oxidative stress augments the formation of these eicosanoids whereas the protective eicosanoid, prostacyclin (PGI2) is reduced by superoxide [22,23]. These changes have long been associated with ED [24,25]. In turn, U46619 and 8-IPF2α up-regulates NOX, whereas PGI2 blocks the up-regulation of NOX [22,23]. In all studies, ACS6 was compared with the H2S-generating chemical, sodium hydrosulphide hydrate (NaHS) and sildenafil citrate alone. To consolidate the study, the in vivo effect of ACS6 and sildenafil citrate on indices of oxidative stress was also studied in rats in which oxidative stress was induced with buthionine sulphoximine (BSO) and cavernosal cGMP, cAMP, glutathionine and plasma TXA2 and 8-IPF2α, measured after 7 days.
MATERIALS AND METHODS
The principles of laboratory care were adhered to according to USA National Institutes of Health and UK Home Office Animal Care regulations. Ethical approval for the study had been obtained from the local Committee at the University of Bristol. Male New Zealand white rabbits (3 kg) were killed by i.v. injection with pentobarbitone (100 mg/kg) via the lateral ear vein. The penis was removed and corpus cavernosum dissected from the tunica vaginalis, placed in cold Dulbecco’s Modified Eagle Medium (DMEM, Gibco BRL Life Technologies Ltd, Paisley, Scotland, UK). The following experiments were then carried out.
ORGAN-BATH EXPERIMENTS
Strips of corpus cavernosal tissue (8 mm by 2 mm) were mounted in organ baths for isometric tension studies [26–28]. The strips were mounted vertically in 20 mL chambers, equipped with two parallel platinum electrodes, containing Krebs’ Ringer bicarbonate buffer (KRB) of the following composition (mM, in distilled water): 119 NaCl, 4.7 KCl, 1.17 MgSO4.7H2O, 1.18 KH2PO4, 2.5 NaHCO3, 2.5 CaCl2, 5 glucose, maintained at 37 °C by a thermoregulated circuit. Tissues were suspended between two tissue bearers, one in fixed position and the other attached to a force-displacement transducer, and data recorded on disc. The KRB buffer was gassed with a mixture of 95% O2/5% CO2 maintained at pH 7.4. An initial tension of 2 g was applied to the suspended tissue strips. All strips were equilibrated for 1 h with frequent changes of KRB buffer. After equilibration, tissues were pre-contracted with phenylephrine (100 μM) and the direct effect on relaxation of NaHS, sildenafil citrate or ACS6 was assessed. The chemical structure and derivitization from sildenafil of ACS6 (4-[[3-(4,7-dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo[4,3d]pyrimidin-5-yl)-4-ethoxyphenyl]sulphonyl]-1-piperazinacetic acid 4-(3-thioxo-3H-1,2-dithiol-5-yl)phenyl ester) is shown in Fig. 1.
The effect of these drugs on carbachol-induced (0.01–10 μM) relaxation was also evaluated. Relaxation responses were expressed as the percentage inhibition of phenylephrine-induced contraction. Finally, the protective effects of ACS6 was assessed by incubating intact cavernosal tissue with interleukin 1β (IL-1β; R and D Systems; Abingdon, UK) at 10 ng/mL with or without ACS6 for 12 h, and then assessing relaxation in response to carbachol as described above.
CELL CULTURE
The corpora cavernosa were dissected from the surrounding tunica albuginea, cut into 2 mm2 segments, and placed in DMEM with 10% fetal calf serum (both Gibco Chemical Co). CSMCs were subsequently incubated at 37 °C in 95% air/5% CO2 as previously described [6]. When confluent, CSMCs were quiesced in low-glucose DMEM (containing 100 units/mL penicillin and 100 μg/mL streptomycin; Gibco Chemical Co). After 72 h of quiescence the following experiments were undertaken.
SUPEROXIDE FORMATION
Superoxide release by CSMCs was measured by detecting ferri-cytochrome c reduction [11,14]. CSMCs were incubated with the TNFα (10 ng/mL; R and D Systems), stable analogue of TXA2 (U46619) or 8-IPF2α (Sigma Chemical Co., Poole, Dorset, UK) with or with no NaHS or ACS6 (10 pM/μM) for 16 h at 37 °C in a 95% air-5% CO2 incubator. U46619 or 8-IPF2α have both been shown to augment NOX expression. After incubation the cells were washed three times with DMEM to remove drugs. The washed cells were equilibrated in DMEM without phenol red for 10 min at 37 °C in a 95% air-5% CO2 incubator (Heraeus, Hera Cell, Kandro Laboratory Products, Germany); 20 μM horse heart cytochrome c (or partly acetylated cytochrome c) with or without 500 U/mL copper-zinc superoxide dismutase (SOD) was added and the cells incubated at 37 °C in a 95% air-5% CO2 incubator for 1 h. The reaction medium was removed and reduction of cytochrome c determined at 550 nm in an Anthos Lucy 1 spectrometer (Laboratory-tech International, Ringmer, East Sussex, UK) and converted to μmoles of superoxide using ΔE550 nm = 21.1 mM−1cm−1 as the extinction coefficient. The reduction of cytochrome c that was inhibitable with SOD reflected actual superoxide release. Cells were then washed with PBS, lysed with 0.1% v/v Triton-X100 and total protein content measured using BCA-protein assay kit (Pierce, Rockford, Illinois, USA). Data are expressed as μmol of superoxide/mg protein/h. The role of cAMP and cGMP were investigated by incubating cells with ACS6 and the PKA inhibitor, H89 or the PKG inhibitor, DT-3 peptide [13], both from Calbiochem (Nottingham, UK).
p47phox AND PDE5 EXPRESSION
CSMCs were incubated with the TNFα (10 ng/mL), U46619 (10 ng/mL), or 8-IPF2α (10 ng/mL) with or with no NaHS or ACS6 (10 pM/μM) for 16 h at 37 °C in a 95% air-5% CO2 incubator. After incubation the cells were washed three times with DMEM to remove drugs. After adding protein lysis buffer (Tris buffer, 100 mM, pH 6.8) containing 1% glycerol and 1% SDS, the cell lysates were at stored at −20 °C. The protein content of the lysates was quantified using the MicroBCA kit (Pierce). Samples of equal protein concentration were loaded to 10% Trisglycine SDS gel, as well as rainbow markers (14–220 kDa, Amersham International, UK) to assess molecular weight, and electophoresed. After transfer to Hybond-C nitrocellulose-pure membrane (Amersham International) the membranes were incubated with the primary antibodies to PDE5 (rabbit; 1:1000) or p47phox (rabbit; 1:500; Cell Signalling Technology, UK) in 5% BSA (Sigma Chemical Co)/0.1% sodium azide at 5 °C with constant agitation, for 16 h. After incubation with the secondary antibody, goat antirabbit antibody conjugated with horseradish peroxidase (1:5000, Dako, Cambridgeshire, UK) for 1 h the blots were developed using enhanced chemiluminescence (Amersham International) on X-ray film, and bands scanned using Quantity One Apple Software.
IN VIVO STUDIES
Male Wistar rats (Charles River Italia, Calco, LC, Italy; 180–200 g) were used; they were housed in a conditioned environment (22 ± 1 °C, 55 ± 5% relative humidity, 12 h light/12 h dark cycle) and were given free access to food and tap water. The investigation conformed with the Guide for the Care and Use of Laboratory Animals (USA NIH Publication no. 85–23, revised 1996). Oxidative stress was induced in the rats by addition of BSO, a glutathionine-synthase inhibitor, to the drinking water (30 mmol/L/day) for 7 days. The control group (vehicle) was provided with regular tap water. ACS6 (5.5 mg/kg/day) and sildenafil (5 mg/kg/day), alone or combined with BSO, were given orally by gavage once daily for 7 days. Rats were killed, the penises excised, cavernosal tissue prepared and blood collected from the cava vein into heparinized tubes with indomethacin (100 μmol/L)-rinsed tubes. Plasma was prepared by centrifugation at 2000g for 15 min at 4 °C. Plasma levels of TXB2 (stable spontaneous hydrolysate of TXA2 [29,30]) and total 8-IPF2α (free plus esterified in lipoproteins) were measured by ELISA kits (Cayman Chemical Co. Ann Arbor, MI, USA).
Cavernosal cGMP and cAMP concentrations were measured by specific ELISA kits (Cayman Chemical Co.) [31]. Frozen tissues samples in liquid nitrogen were ground to a fine powder in a stainless-steel mortar. Once the liquid nitrogen had evaporated, the frozen tissues were weighed and homogenized in 10 volumes of 0.1 M HCl to stop the actions of PDEs. Centrifugation was at 10 000g for 15 min at 4 °C, and the supernatant was collected and frozen at −80 °C until assayed.
To measure glutathionine, before homogenization the tissues were mixed 1:1.5 (w/v) with cold PBS. After centrifugation (10 000g for 15 min at 4 °C) the supernatant was collected and stored on ice. The supernatant was deproteinated and frozen at −80 °C until assayed. For the plasma glutathionine assay, 1 mL of plasma was combined with 1 mL of metaphosphoric acid to prevent the ‘ex vivo’ oxidation, incubated for 5 min at room temperature, and then centrifuged at 2000g for 10 min. The supernatant was deproteinated and frozen at −80 °C until assayed. Protein was determined by Bio-Rad method. Total glutathionine levels in tissues and plasma were measured using the Cayman assay kit (780001; Cayman Chemical Co) using a carefully optimized enzymatic recycling method, using glutathionine reductase (absorbance at 405 nm).
Data were expressed as the mean (SEM) of six separate studies. Student’s paired t-test was used to compare effects with the control values. Multiple-group comparisons were made using repeated-measures ANOVA, and significance was indicated at P < 0.05.
RESULTS
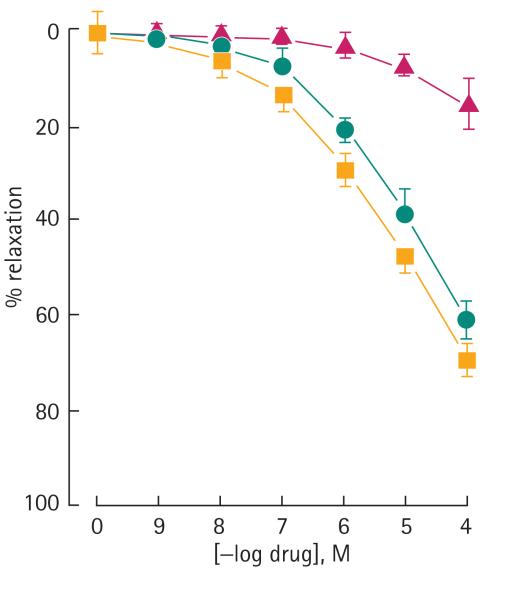
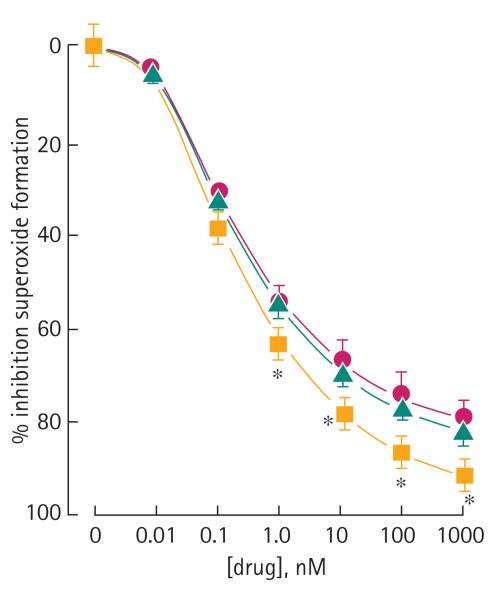
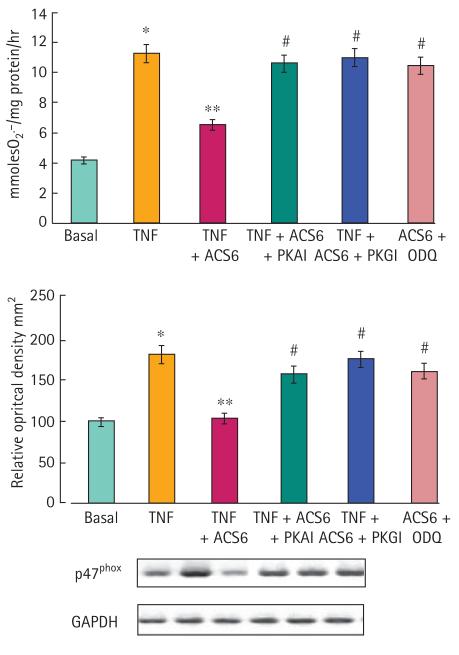
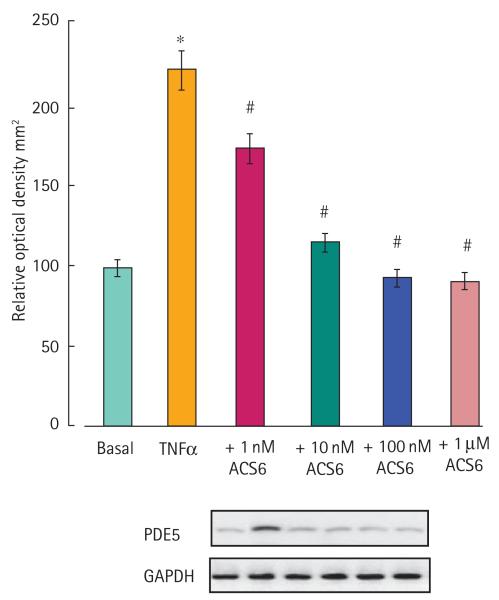
ACS6 and sildenafil citrate elicited a dose-dependent relaxation of isolated rabbit cavernosal strips (Fig. 2). NaHS alone, at least up to 100 μM, had no effect on relaxation (Fig. 2). ACS6 was more potent than NaHS alone but equipotent with sildenafil citrate alone. ACS6 at 1 μM and 10 μM also augmented relaxation elicited with carbachol whereas 100 nM ACS6 had no significant effect (Fig. 3). ACS6, sildenafil citrate and NaHS elicited a dose-dependent inhibition of superoxide formation induced with TNFα after a 16-h incubation of CSMCs (Fig. 4). There was a similar effect when cells were incubated with U46619 and 8-IPF2α (Table 1). ACS6 inhibited p47phox expression after a 16-h incubation with TNFα (Fig. 5). The inhibitory action of ACS6 on both superoxide formation and p47phox after a 16-h incubation with TNFα was blocked by both the PKA and the PKG inhibitors (Fig. 6 and Table 2). By contrast, the inhibitory effect of sildenafil citrate alone on the formation of superoxide and protein expression was reversed by PKG inhibition but not PKA inhibition (Table 2).
FIG. 2.
The effect of NaHS (triangle), ACS6 (square) and sildenafil citrate (circle) on relaxation of the rabbit corpus cavernosum pre-contracted with phenylephrine. Data are the mean (SEM)of six experiments.
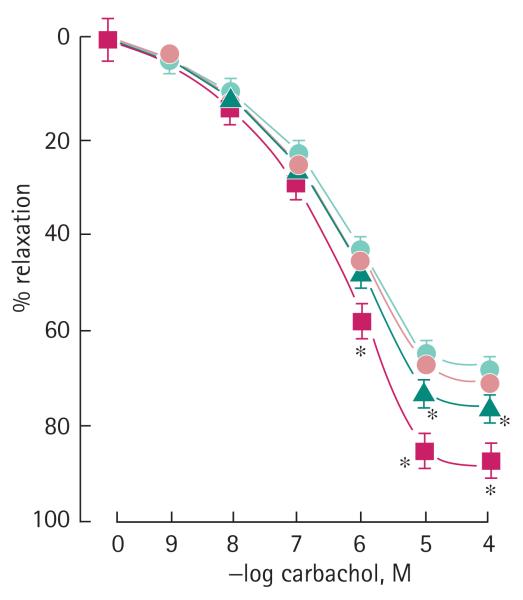
FIG. 3.
The effect of ACS6 on relaxation of the rabbit corpus cavernosum elicited with carbachol. Control, +100 nM ACS6 (circle), 1 μM ACS6 (triangle); +10 μM ACS6 (square). Data are the mean (SEM) of six experiments. *P < 0.05 comparing drug treated with control.
FIG. 4.
Effect of NaHS (triangle), ACS6 (square) and sildenafil citrate (circle) on TNFα-stimulated superoxide formation by CSMC after a 16-h incubation. Data are the mean (SEM) of six experiments, *P < 0.05 comparing ACS6 with sildenafil and #P < 0.05 comparing ACS6 with NaHS.
TABLE 1.
The effect of ACS6 on superoxide formation (μmol/mg protein/min) elicited by 10 nM 8-IPF2α and 10 nM TXA2 analogue, U46619 by CSMC after a 16-h incubation
| ACS6, nM | 8-IPF2α | U46619 |
|---|---|---|
| 0 | 8.0 (0.9) | 7.8 (0.7) |
| 0.01 | 7.8 (1.0) | 7.6 (0.7) |
| 0.1 | 5.3 (0.36)* | 5.1 (0.6)* |
| 1 | 3.3 (0.26)* | 3.0 (0.3)* |
| 10 | 2.3 (0.26)* | 2.0 (0.2)* |
| 100 | 0.4 (0.06)* | 0.1 (0.01)* |
P < 0.05, significantly inhibited.
FIG. 5.
The effect of inhibitors of PKA (PKAI, H89) and PKG (PKGI, DT3 peptide) activity on 1 μM NaHS-inhibited superoxide formation and p47phox expression in CSMCs after a 16-h co-incubation of ACS6 with TNFα + PKA inhibitor or PKG inhibitor. For western analysis, representative Western blots of p47phox and glyceraldehyde phosphate dehydrogenase (GAPDH) expression are shown beneath these data. Data are the mean (SEM) of six experiments. *P < 0.001, basal vs TNFα; **P < 0.001, TNFα vs TNFα + ACS6; #P < 0.001, TNFα + ACS6 vs TNFα + NaHS + inhibitor.
FIG. 6.
The effect of ACS6 on PDE5 expression induced with TNFα. Data are expressed as a percentage of the optical density relative to controls (% OD), the control being 100%. Representative Western blots of PDE5 and GAPDH expression are shown beneath these data. Data are the mean (SEM) of six experiments. *P < 0.01 significantly greater than control; #P < 0.01 and ##P < 0.05 significantly inhibited.
TABLE 2.
The effect of inhibitors of PKA (H89) and PKG (DT3 peptide) or ODQ on 1 μM NaHS-inhibited superoxide formation (mmol/mg protein/min), p47phox expression (% relative optical density), 1 μM sildenafil-inhibited superoxide formation and p47phox expression in CSMCs after a 16-h co-incubation of ACS6 + H89 or DT3 + 10 nM 8-IPF2α or 10 nM TXA2 analogue, U46619, and sildenafil + H89 or DT3 and/or + 10 nM TNFα, 10 nM 8-IPF2α or 10 nM U46619
| ACS6 or sildenafil |
||||||
|---|---|---|---|---|---|---|
| Variable | Basal | Stimulated | only | + PKAI | + PKGI | + ODQ |
| ACS6 | ||||||
| Superoxide | ||||||
| 8-IPF2α | 4 (0.36) | 12 (1)* | 5 (0.5) | 10 (0.9)† | 11.8 (0.4)† | 9.4 (0.5)† |
| U46619 | 4 (0.36) | 13 (2)* | 5 (0.6) | 11 (0.8)† | 10.9 (0.3)† | 9.2 (0.5)† |
| p47phox | ||||||
| 8-IPF2α | 100 | 175 (17)* | 102 (11) | 180 (9)† | 171 (12)† | 152 (15)† |
| U46619 | 100 | 180 (19)* | 101 (10) | 170 (18)† | 165 (19)† | 168 (22)† |
| Sildenafil | ||||||
| Superoxide | ||||||
| TNFα | 4 (0.36) | 15 (1)* | 4 (0.5) | 6 (0.6) | 11.8 (0.4)† | 9.4 (0.5)† |
| 8-IP | 4 (0.36) | 12 (1)* | 6 (0.5) | 7 (0.7) | 13 (0.5)† | 10.4 (0.8)† |
| U46619 | 4 (0.36) | 13 (2)* | 7 (0.6) | 8 (0.8) | 11 (0.3)† | 12.2 (1.5)† |
| p47phox | ||||||
| TNFα | 100 | 210 (19)* | 101 (10) | 110 (18) | 185 (19)† | 168 (22)† |
| 8-IP | 100 | 175 (17)* | 102 (11) | 108 (12) | 171 (12)† | 152 (15)† |
| U46619 | 100 | 180 (19)* | 105 (10) | 120 (18) | 165 (19)† | 168 (22)† |
P < 0.001, basal vs stimulated;
P < 0.001, stimulated + ACS6 vs stimulated + ACS6 + inhibitor.
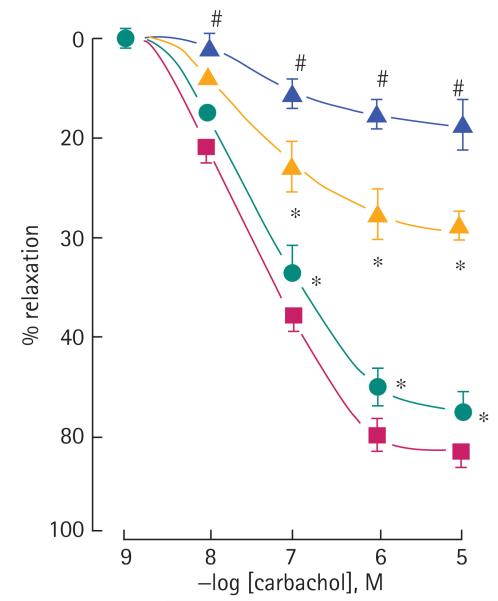
Incubation of intact cavernosal segments with ACS6 for 16 h reversed the inhibitory effect of IL-1β on carbachol-stimulated relaxation (Fig. 7). As we established that IL-1β up-regulates NADPH oxidase expression, which generates superoxide, which in turn negates NO-mediated relaxation, these data confirm that ACS6 blocks the expression of NADPH oxidase and/or the generation of vascular superoxide.
FIG. 7.
Effect of ACS6 (100 nM, blue triangle) and (1 μM, green circle) on reduced NO-dependent relaxation of rabbit corpus cavernosum elicited by incubation with 10 ng/mL IL-1β for 12 h (orange triangle). Untreated control (red square). Data are the mean (SEM) of six experiments. #P < 0.01 controls vs IL-1β treated; *P < 0.01, IL-1β vs IL-1β + ACS6.
In rats treated with BSO, the plasma TXB2 and 8-IPF2α concentrations were higher than in controls, and were significantly reduced by the administration of both ACS6 and sildenafil citrate (Table 3). Cavernosal cAMP, cGMP and glutathionine concentrations were significantly reduced in rats treated with BSO (Table 3), all of which were significantly increased by the administration of both ACS6 and sildenafil citrate (Table 3).
TABLE 3.
The effect of administration of ACS6 (5.5 mg/kg/day) or sildenafil citrate (5 mg/kg/day) on plasma TXA2 and 8-IPF2α, corpus cavernosal cGMP, cAMP and glutathionine concentrations in rats in which oxidative stress and hypertension was induced by administration of BSO
| Plasma, pg/mL |
Cavernosal, pmol/mg protein |
||||
|---|---|---|---|---|---|
| Treatment | TXA2 | 8-IPF2α | cGMP | cAMP | Glutathionine, μmol/mg protein |
| Vehicle | 72.6 (7) | 119 (9) | 1.2 (0.14) | 60 (6) | 25 (2) |
| ACS6 | 70.5 (6) | 90 (6) | 2.5 (0.19) | 133 (9) | 26 (3) |
| Sildenafil | 65.1 (5) | 102 (11) | 2.9 (0.25) | 144 (10) | 28 (2) |
| BSO | 201 (13)* | 332 (27)* | 0.5 (0.04)* | 36 (4)* | 9 (0.6)* |
| BSO + ACS6 | 146 (16)† | 153 (11)† | 1.4 (0.24)† | 86 (6)† | 17 (0.8)† |
| BSO + sildenafil | 128 (15)† | 189 (13)† | 1.1 (0.15)† | 91 (7)† | 15 (0.7)† |
P < 0.05 untreated controls vs BSO treated;
P < 0.05 ACS6 or sildenafil in BSO-treated vs BSO controls.
DISCUSSION
The present study first showed that ACS6 was equipotent with sildenafil citrate in relaxing cavernosal tissue in vitro. NaHS alone had little effect, at least up to 100 μM, indicating that the effect of ACS6 on relaxation is mediated by the sildenafil moiety of the drug, rather than the H2S-donating moiety. ACS6 also augmented relaxation in response to carbachol. It is concluded that the H2S moiety does not confer a greater potency than sildenafil citrate on CSM relaxation. However, this component of the study showed that chemical derivitization of sildenafil with a H2S-donating moiety does not diminish its capacity to relax CSM. This is consistent with a previous report that ACS6 inhibited the hydrolysis of cGMP to the same extent as sildenafil citrate, and that ACS6 releases a significant amount of H2S [21]. ACS6 releases H2S to the same extent as NaHS [21]. Notably, previous studies showed that systemic administration of NaHS to primates and rats promotes erection, in vivo [18,19]. This indicates that H2S might have a proerectile effect upstream of the CSM level, i.e. at pudendal artery, neural innervation, ganglionic or CNS level; this warrants further study.
By contrast, ACS6, sildenafil citrate and NaHS were all potent inhibitors of superoxide formation induced by TNFα, U46619 and 8-IPF2α, all of which are known to induce NADPH oxidase expression and superoxide formation in both CSMCs and arterial SMCs [8,11,14]. ACS6 also blocked the up-regulation of p47phox, a subunit of the NOX complex. These effects of ACS6 were greater than with NaHS alone or with sildenafil alone at equivalent concentrations, confirming that ACS6 has a dual effect, i.e. both the H2S-donating moiety and the PDE5 inhibitory moiety. As superoxide negates the bioactivity of NO, it is suggested that ACS6 confers potentially beneficial effects by inhibiting NADPH oxidase up-regulation. This is consolidated by the observation that the effects of ACS6 were blocked by both PKA and PKG inhibitors. The reversal of the effects of ACS6 with PKA inhibition first indicates that the H2S moiety of the drug is eliciting its effects through activation of adenylyl cyclase and/or activation of PKA. Indeed, it has been established that H2S inhibits NADPH oxidase expression via a PKA-dependent (but not PKG-dependent) mechanism [20].
With regard to the sildenafil moiety of ACS6, we previously showed that sildenafil citrate inhibits the formation of superoxide and gp91phox expression through an augmentation of cGMP levels and of PKG activation but not of PKA activation [13]. NO, via a cGMP-PKG-dependent pathway, inhibits the formation of superoxide and gp91phox expression in pulmonary artery endothelial cells [10]. As sildenafil is a PDE5 inhibitor and PDE5 hydrolyses cGMP to inactive GMP [2], it was concluded that the inhibitory effect of sildenafil on NOX expression is mediated by augmentation of the of NO-PKG axis. Thus, the blockade of effects of ACS6 by the PKG inhibitor indicates the sildenafil moiety of ACS6 also coming into play in the present system. It has also been shown that ACS6 retains its PDE5 inhibitory capacity (i.e. hydrolysis of cGMP) despite having been derivitized chemically.
The present study also showed that ACS6 had a potent effect on the up-regulation of PDE5 itself in response to TNFα, U46619 and 8-IPF2α. It was previously reported that PDE5 is up-regulated in CSMCs in response to diverse risk factors for ED, including nicotine, TNFα and homocysteine, which in turn is mediated through an a priori up-regulation of NADPH oxidase expression and activity [11,14]. This effect is mediated in part by activation of Rho kinase by superoxide derived from NOX [22]. We also showed that sildenafil itself inhibits the up-regulation of PDE5, which is mediated by augmentation of the NO-PKG axis [11,14]. In the present study, incubation of intact cavernosal tissue for 16 h with IL-1β elicited a marked inhibition of NO-dependent relaxation in response to carbachol. This effect was blocked by ACS6 at concentrations below those that induce relaxation. IL-1β has been shown to up-regulate NADPH oxidase and negate NO in endothelial cells [14]. As ACS6 inhibits NADPH oxidase expression and activity, these data confirm that over the longer term ACS6 provides a protective effect against oxidative stress and the subsequent effect of up-regulation of PDE5 activity.
The in vivo studies in which oxidative stress was induced with BSO in rats confirm that ACS6 inhibits intracavernosal oxidative stress. BSO elicited a decrease in GMP, cAMP and glutathionine levels in cavernosal tissue, that were restored to control levels by the administration of both sildenafil citrate and ACS6. In rats treated with BSO, plasma TXB2 and 8-IPF2α concentrations were higher than in controls, and were significantly reduced by the administration of both ACS6 and sildenafil citrate. It was shown that increased NADPH oxidase activity increases the formation of both TXB2 and 8-IPF2α and a reduction in PGI2 formation [23], which is consistent with these in vivo data. It is also intriguing that plasma TXB2 might reflect an inhibition of platelet function, as platelets are the principal source of this eicosanoid [5].
An important point from the present study is that the concentration at which ACS6, NaHS and sildenafil inhibited protein expression was far lower than those that elicit relaxation. From a therapeutic perspective therefore, we suggest that the potential of ACS6 lies not only in an acute effect on erection but also on up-regulation of proteins that would result in ED through repeated administration over the longer term (i.e. NADPH oxidase and PDE5). In support of this proposal, there is a growing body of evidence that long-term administration of PDE5 inhibitors reduces ED in experimental animal models [32–34]. In turn, several studies have now shown that pharmacological prevention of intravascular and intracavernosal oxidative stress reduces ED in models of hyperhomocysteinaemia, diabetes mellitus and hypercholesterolaemia [35–37]. It is reasonable to suggest therefore that PDE5 inhibitors might act in the long term, at least in part, through a reduction of oxidative stress.
To summarize, ACS6 elicited a range of effects in common with sildenafil citrate that renders the drug potentially useful for treating ED. The H2S moiety does not confer greater additional potency as a dilator. However, it is important that the primary effect of sildenafil (PDE5 inhibitory capacity) is maintained despite derivitizing the drug with an H2S-donating moiety. By contrast, ACS6 was a more potent inhibitor of NOX and PDE5 up-regulation induced with a range of risk factors for ED. Thus, ACS6 has protective effects that prevent the formation of superoxide and linked up-regulation of PDE5, both of which are associated with ED. Further studies are required to explore the therapeutic potential of ACS6.
Abbreviations
- ACS6
H2S-donating derivative of sildenafil
- BSO
buthionine sulphoximine
- (C)(V)SMCs
cavernosal (vascular) smooth muscle cells
- ED
erectile dysfunction
- IL-1β
interleukin 1β
- 8-IPF2α
8-isoprostane F2α
- NaHS
sodium hydrosulphide
- NOX
NADPH oxidase
- PDE5
type 5 phosphodiesterase
- NO
nitric oxide
- PGI2
prostacyclin
- PK
protein kinase
- TXA2
thromboxane A2
- DMEM
Dulbecco’s Modified Eagle Medium
- KRB
Krebs’ Ringer bicarbonate
- SOD
superoxide dismutase
Footnotes
CONFLICT OF INTEREST P. Del Soldato is a shareholder of CTG Pharma, Milan, Italy. This company has patents on reagents used in this study. A. Sparatore received a grant from CTG Pharma. Source of Funding: British Heart Foundation.
REFERENCES
- 1.Sullivan ME, Thompson CS, Dashwood MR, et al. Nitric oxide and erection: is erectile dysfunction another manifestation of vascular disease? Cardiovasc Res. 1999;43:6580–65. doi: 10.1016/s0008-6363(99)00135-2. [DOI] [PubMed] [Google Scholar]
- 2.Jeremy JY, Ballard S, Naylor A, Miller MAW, Angelini GD. Effect of sildenafil (VIAGRA™), a specific inhibitor of cGMP phosphodiesterase on cAMP and cGMP formation by the rabbit corpus cavernosa, in vitro. Br J Urol. 1997;79:958–63. doi: 10.1046/j.1464-410x.1997.00206.x. [DOI] [PubMed] [Google Scholar]
- 3.Moreland RB, Goldstein I, Kim NN, Traish A. Sildenafil citrate, a selective phosphodiesterase type 5 Inhibitor. Trends Endocinol Metab. 1999;10:97–104. doi: 10.1016/s1043-2760(98)00127-1. [DOI] [PubMed] [Google Scholar]
- 4.Lau DH, Kommu S, Mumtaz FH, Morgan RJ, Thompson CS, Mikhailidis DP. The management of phosphodiesterase-5 (PDE5) inhibitor failure. Curr Vasc Pharmacol. 2006;4:89–93. doi: 10.2174/157016106776359871. [DOI] [PubMed] [Google Scholar]
- 5.Jeremy JY, Angelini GD, Khan M, et al. Platelets, oxidant stress and erectile dysfunction: an hypothesis. Cardiovasc Res. 2000;46:50–4. doi: 10.1016/s0008-6363(00)00009-2. [DOI] [PubMed] [Google Scholar]
- 6.Jeremy JY, Jones RA, Koupparis AJ, Hotston M, Persad R, Shukla N. Reactive oxygen species and erectile dysfunction: possible role of NADPH oxidase. Int J Impot Res. 2007;19:265–80. doi: 10.1038/sj.ijir.3901523. [DOI] [PubMed] [Google Scholar]
- 7.Jeremy JY, Rowe D, Emsley AM, Newby AC. Nitric oxide and vascular smooth muscle cell proliferation. Cardiovasc Res. 1999;43:580–94. doi: 10.1016/s0008-6363(99)00171-6. [DOI] [PubMed] [Google Scholar]
- 8.Koupparis AJ, Jeremy JY, Muzaffar S, Angelini GD, Shukla N. Sildenafil inhibits the formation of superoxide and the expression of gp47phox NADPH oxidase induced by the thromboxane A2 mimetic, U46619, in corpus cavernosal smooth muscle cells. BJU Int. 2005;96:423–7. doi: 10.1111/j.1464-410X.2005.05643.x. [DOI] [PubMed] [Google Scholar]
- 9.Shukla N, Jones R, Persad R, Angelini GD, Jeremy JY. Effect of sildenafil citrate and a nitric oxide donating sildenafil derivative, NCX 911, on cavernosal relaxation and superoxide formation in hypercholesterolaemic rabbits. Eur Pharmacol. 2005;517:224–31. doi: 10.1016/j.ejphar.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Muzaffar S, Shukla N, Angelini GD, Jeremy JY. Nitroaspirins and SIN-1, but not aspirin, inhibit the expression of endotoxin- and cytokine- induced NADPH oxidase in vascular smooth muscle cells from pig pulmonary arteries. Circulation. 2004;110:1140–7. doi: 10.1161/01.CIR.0000139851.50067.E4. [DOI] [PubMed] [Google Scholar]
- 11.Hotston M, Jeremy JY, Bloor J, et al. Homocysteine and copper interact to promote superoxide formation and type 5 phosphodiesterase expression: inhibition with sildenafil (Viagra™) Asian J Androl. 2008;10:905–13. doi: 10.1111/j.1745-7262.2008.00380.x. [DOI] [PubMed] [Google Scholar]
- 12.Muzaffar S, Jeremy JY, Angelini GD, Stuart Smith K, Shukla N. The role of the endothelium and nitric oxide synthases in modulating superoxide formation induced by endotoxin and cytokines in porcine pulmonary arteries. Thorax. 2003;58:598–604. doi: 10.1136/thorax.58.7.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muzaffar S, Shukla N, Angelini GD, Jeremy JY. Sildenafil citrate and sildenafil nitrate are potent inhibitors of superoxide formation and gp91phox expression in porcine pulmonary artery endothelial cells. Br J Pharmacol. 2005;146:109–17. doi: 10.1038/sj.bjp.0706305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotston M, Jeremy JY, Koupparis A, Persad N, Angelini GD, Shukla N. Sildenafil inhibits the up-regulation of phosphodiesterase type 5 elicited with nicotine and tumour necrosis factor-α in cavernosal vascular smooth muscle cells: mediation by superoxide. BJU Int. 2007;99:612–8. doi: 10.1111/j.1464-410X.2006.06618.x. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan M, Angelini GD, Mikhailidis DP, Morgan RJ, Thompson CS, Jeremy JY. Differential changes of prostaglandin and cyclic nucleotide formation in the corpus cavernosum of the diabetic rabbit. Br J Urol. 1998;82:578–84. [PubMed] [Google Scholar]
- 16.Miller MAW, Thompson CS, Morgan RJ, Mikhailidis DP, Jeremy JY. Hydrolysis of cyclic guanosine monophosphate and cyclic adenosine monophosphate by the penis and aorta of diabetic rat. Br J Urol. 1996;78:252–6. doi: 10.1046/j.1464-410x.1996.06219.x. [DOI] [PubMed] [Google Scholar]
- 17.Miller M, Morgan RJ, Thompson CS, Mikhailidis DP, Jeremy JY. Adenylate and guanylate cyclase activity in the penis and aorta of the diabetic rat. Br J Urol. 1994;74:106–11. doi: 10.1111/j.1464-410x.1994.tb16556.x. [DOI] [PubMed] [Google Scholar]
- 18.Srilatha B, Adaiken PG, Li L, Moore PK. Hydrogen sulphide: a novel endogenous gasotransmitter facilitates erectile function. J Sex Med. 2007;4:1304–11. doi: 10.1111/j.1743-6109.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- 19.Srilatha B, Adaiken PG, Li L, Moore PK. Possible role for the novel gasotransmitter hydrogen sulphide in erectile dysfunction – a pilot study. Eur J Pharmacol. 2006;535:280–2. doi: 10.1016/j.ejphar.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Muzaffar S, Shukla N, Bond M, et al. Exogenous hydrogen sulfide inhibits superoxide formation, NOX-1 expression and Rac (1) activity in human vascular smooth muscle cells. J Vasc Res. 2008;45:521–8. doi: 10.1159/000129686. [DOI] [PubMed] [Google Scholar]
- 21.Muzaffar S, Shukla N, Sparatore A, Del Soldato P, Angelini GD, Jeremy JY. H2S-donating sildenafil (ACS6) inhibits superoxide formation and gp91phox expression in arterial endothelial cells: role of protein kinases A and G. Br J Pharmacol. 2008;155:984–94. doi: 10.1038/bjp.2008.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muzaffar S, Shukla N, Bond M, Newby AC, Angelini GD, Jeremy JY. Acute inhibition of superoxide formation and Rac (1) activation by nitric oxide and iloprost in human vascular smooth muscle cells in response to the thromboxane A (2) analogue, U46619. Prostaglandins Leukotr Essent Fatty Acids. 2008;78:247–55. doi: 10.1016/j.plefa.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muzaffar S, Shukla N, Lobo C, Angelini GD, Jeremy JY. Iloprost inhibits NADPH oxidase expression and superoxide release in porcine pulmonary arteries and cells stimulated with thromboxane A2, isoprostane F2α and cytokines. Br J Pharmacol. 2004;141:488–96. doi: 10.1038/sj.bjp.0705626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeremy JY, Mikhailidis DP, Dandona P. Simulating the diabetic environment modifies in vitro prostacyclin synthesis. Diabetes. 1983;32:217–21. doi: 10.2337/diab.32.3.217. [DOI] [PubMed] [Google Scholar]
- 25.Jeremy JY, Mikhailidis DP, Thompson CS, Dandona P. Experimental diabetes mellitus inhibits prostacyclin synthesis by the rat penis: pathological implications. Diabetologia. 1985;28:365–8. doi: 10.1007/BF00283145. [DOI] [PubMed] [Google Scholar]
- 26.Thompson C, Khan M, Mikhailidis DP, Morgan RJ, Angelini GD, Jeremy JY. Effect of sildenafil on relaxation of the corpus cavernosum of the diabetic rabbit. Eur J Pharmacol. 2001;425:57–64. doi: 10.1016/s0014-2999(01)01077-9. [DOI] [PubMed] [Google Scholar]
- 27.Khan MA, Thompson CS, Jeremy JY, Mumtaz FH, Mikhailidis P, Morgan RJ. The effect of superoxide dismutase on nitric oxide-mediated and electrical field-stimulated diabetic rabbit cavernosal smooth muscle relaxation. BJU Int. 2001;87:98–103. doi: 10.1046/j.1464-410x.2001.00965.x. [DOI] [PubMed] [Google Scholar]
- 28.Shukla N, Thompson CS, Angelini GD, et al. Homocysteine enhances impairment of endothelium – dependent relaxation and guanosine cyclic monophosphate formation in aortae from diabetic rabbits. Diabetologia. 2002;45:1325–31. doi: 10.1007/s00125-002-0888-4. [DOI] [PubMed] [Google Scholar]
- 29.Mikhailidis DP, Barradas MA, Jeremy JY. The effect of ethanol on platelet function and vascular prostanoids. Alcohol. 1990;7:171–80. doi: 10.1016/0741-8329(90)90080-v. [DOI] [PubMed] [Google Scholar]
- 30.Gisinger C, Jeremy JY, Speiser P, Mikhailidis D, Dandona P, Schernthaner G. Effect of vitamin E supplementation on platelet thromboxane A2 production in Type 1 diabetic patients: a double blind cross over trial. Diabetes. 1988;37:262–4. doi: 10.2337/diab.37.9.1260. [DOI] [PubMed] [Google Scholar]
- 31.Plane F, Wiley KE, Jeremy JY, Cohen RA, Garland CL. Evidence that different mechanisms underlie smooth muscle relaxation to nitric oxide and nitric oxide donors in the rabbit isolated carotid artery. Br J Pharmacol. 1998;123:1251–358. doi: 10.1038/sj.bjp.0701746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathers MJ, Klotz T, Brandt AS, Roth S, Sommer F. Long-term treatment of erectile dysfunction with a phosphodiesterase-5 inhibitor and dose optimization based on nocturnal penile tumescence. BJU Int. 2008;101:1129–34. doi: 10.1111/j.1464-410X.2007.07376.x. [DOI] [PubMed] [Google Scholar]
- 33.Kovanecz I, Rambhatla A, Ferrini MG, et al. Chronic daily tadalafil prevents the corporal fibrosis and veno-occlusive dysfunction that occurs after cavernosal nerve resection. BJU Int. 2007;101:203–10. doi: 10.1111/j.1464-410X.2007.07223.x. [DOI] [PubMed] [Google Scholar]
- 34.Kovanecz I, Rambhatla A, Ferrini MG, et al. Long-term continuous sildenafil treatment ameliorates corporal veno-occlusive dysfunction (CVOD) induced by cavernosal nerve resection in rats. Int J Impotence Res. 2008;20:201–12. doi: 10.1038/sj.ijir.3901612. [DOI] [PubMed] [Google Scholar]
- 35.Shukla N, Jones R, Persad R, Angelini GD, Jeremy JY. Effect of sildenafil citrate and a nitric oxide donating sildenafil derivative, NCX 911, on cavernosal relaxation and superoxide formation in hypercholesterolaemic rabbits. Eur J Pharmacol. 2005;517:224–31. doi: 10.1016/j.ejphar.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 36.Koupparis AJ, Jeremy JY, Persad N, Angelini GD, Shukla N. Penicillamine administration reverses the inhibitory effect of hyperhomocysteinaemia on endothelium-dependent relaxation in the corpus cavernosum in the rabbit. BJU Int. 2006;98:440–4. doi: 10.1111/j.1464-410X.2006.06212.x. [DOI] [PubMed] [Google Scholar]
- 37.Shukla N, Greaves NS, Angelini GD, Jeremy JY. The administration of folic acid reduces intravascular oxidative stress in diabetic rabbits. Metabolism. 2008;57:774–81. doi: 10.1016/j.metabol.2008.01.017. [DOI] [PubMed] [Google Scholar]