Abstract
Aims/hypothesis
There is evidence that plasma homocysteine augments vein graft failure and that it augments both micro- and macro-angiopathy in patients with diabetes mellitus. It is therefore suggested that homocysteine may augment vein graft thickening, a major cause of vein graft failure, in diabetic patients, as well as impairing adaptive growth of a new vasa vasorum, possibly through overproduction of superoxide. In order to test these proposals, the effect of folic acid administration, which lowers plasma homocysteine, on vein graft thickening and microvessel density was studied in pigs used as a model of diabetes.
Methods
Non-ketotic hyperglycaemia was induced in Landrace pigs by intravenous injection of streptozotocin, and folic acid was fed daily for 1 month. Vein grafts were excised and the thickness of the neointima and media and microvessel density were assessed by planimetry and superoxide formation.
Results
Plasma total homocysteine was significantly reduced by folic acid in both control and diabetic pigs, whereas glucose was unchanged. Compared with controls, diabetic pigs showed increased neointimal thickness and superoxide formation and decreased adventitial microvessel density. Folic acid reduced neointimal thickness and superoxide formation and augmented microvessel density in diabetic but not in control pigs.
Conclusions
Folic acid administration reduces neointimal thickening, augments vasa vasorum neoformation and reduces oxidative stress in saphenous vein grafts from diabetic pigs. Folic acid may therefore be particularly effective in reducing vein graft failure in diabetic patients.
Keywords: Folic acid, Neointima, Oxidative stress, Type 2 diabetes, Vein graft
Introduction
An autologous saphenous vein continues to be the most widely used conduit for coronary artery bypass graft (CABG) surgery [1] and infrainguinal bypass surgery for reconstruction of lower limb arteries [2]. However, as many as 50% of vein grafts fail within 10 years after the procedure [1, 2]. Vein graft failure involves the replication of medial vascular smooth muscle cells (VSMCs), which migrate across the internal elastic lamina and colonise the intima, where they continue to proliferate to form a new layer of cells called the neointima [1–3]. Superimposed atherogenesis ultimately increases the risk of late graft failure [1–3].
Risk factors for accelerated vein graft failure include diabetes mellitus [4] and hyperhomocysteinaemia [5, 6]. Following CABG there is a significant and sustained (for up to 6 weeks) increase in the plasma concentration of homocysteine [7]. In turn, homocysteine augments vasculopathy in blood vessels from diabetic animals [8]. These effects include the augmentation of oxidative stress, negation of nitric oxide bioactivity, increased VSMC replication and impairment of neovascularisation [8, 9], all of which are associated with an increased risk of vein graft disease [10, 11]. It follows that the sustained increase in plasma homocysteine after CABG may render diabetic patients even more susceptible to vein graft failure.
Therapeutically, plasma homocysteine levels are readily managed by the administration of folic acid, which lowers homocysteine by as much as 30% [12]. Other studies have shown that folic acid reduces coronary restenosis following transluminal percutaneous balloon angioplasty in man [13, 14]. Effects of folic acid on vein graft thickening have not been studied. In diabetic animal models, however, administration of folic acid (0.2 mg kg−1day−1) has been shown to reverse intravascular oxidative stress [15, 16]. The latter study demonstrated that the main impact of experimental type 2 diabetes on vascular oxidative stress was at the medial level, which had a knock-on effect of reducing endothelial nitric oxide formation through its reaction with superoxide released from the medium [16]. In terms of vein graft pathology, the medium plays an axiomatic role, since VSMCs of medial origin are the progenitors of the neointima, as outlined above.
It is suggested, therefore, that folic acid may be particularly effective in reducing vein graft thickening in the diabetic patient by a reduction of intra-graft oxidative stress. In order to test this hypothesis, pigs were rendered diabetic with intravenous injections of streptozotocin [17] and interposition grafting of the saphenous vein into the carotid artery was performed. It is accepted that streptozotocin-induced type 2 diabetes has its limitations, which include toxicity to tissues other than beta islet cells [18–20]. However, streptozotocin still elicits the cardinal indices of type 2 diabetes in the pig that render this model suitable for this investigation, namely, hyperglycaemia, minimal ketonuria, an increase in triacylglycerol and an increase in oxidative stress [17–20]. It was deemed necessary to undertake this preclinical study prior to a clinical trial, since we need to establish whether type 2 diabetes augments neointima formation in vein grafts and whether folate elicits any ameliorative effects, in order to obtain proof of concept. To optimise clinical trial design, it is important to confirm that intravascular oxidative stress plays an axiomatic role, which in turn will aid in making appropriate sampling and measurements in study design.
Thus, folic acid was administered daily at a dose considered to be maximally effective in man (i.e. 0.2 mg kg−1day−1) [12] and which has proved effective in reducing medial oxidative stress in other animal models of type 2 diabetes [15, 16]. After 1 month, grafts were excised and neointimal thickness and replicating cells assessed. As mentioned, neoformation of a vasa vasorum is an important facet of graft adaptation since impairment of re-establishment of the vasa vasorum may render the graft hypoxic, which in turn would promote graft pathology. Intra-adventitial microvessel density was therefore also assessed. Since there is strong evidence that oxidative stress mediates the pathogenic interaction between type 2 diabetes and homocysteine, superoxide formation was measured in graft tissues. Finally, to determine whether folic acid and its active metabolite, 5-methyltetrahydrofolate (5MTHF), have direct effects on oxidative stress, the effect of homocysteine on superoxide formation in saphenous veins from pigs used as a model of diabetes, ex vivo, was also studied.
Methods
Induction of diabetes and surgical procedure
Studies were performed using Landrace pigs weighing 30 kg (Animal Services Unit, University of Bristol, Bristol, UK). Pigs received care in accordance with the Home Office Guidance on the operation of the Animals (Scientific Procedures) Act 1986, published by HMSO, London, UK. The investigation conformed with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). In order to induce hyperosmolar non-ketotic diabetes mellitus, streptozotocin (Sigma, Poole, UK) dissolved in normal saline (60 mg/kg) was injected slowly (over 30 min) into the ear vein on three consecutive days [17]. The development of diabetes was monitored by testing urine for glucose with Multistix (Sigma, Poole, UK) indicators for urinary glucose. Once diabetes was confirmed, pigs were kept in pens for 1 week prior to surgery.
Immediately prior to surgery but before the onset of anaesthesia, blood was taken from the ear vein, plasma was prepared and concentrations of glucose, albumin and homocysteine and erythrocyte folic acid were measured as previously described [7]. All animals underwent interposition grafting of the saphenous vein into the carotid artery [21–23]. Anaesthesia was induced with ketamine (30 mg) and atropine (0.6 mg) administered intramuscularly. After tracheal intubation, anaesthesia was maintained with halothane and oxygen and animals were allowed to ventilate spontaneously throughout. Heparin sodium (1 mg/kg) was administered intravenously and a single dose of 250 mg benzyl penicillin was administered intramuscularly prior to skin incision. A longitudinal incision was made on the outer aspect of the hindlimb. Approximately 10 cm of the vein was then dissected free of surrounding tissue. Care was taken not to remove the adventitia. All side branches were secured with a 6–0 Prolene ligature (Ethicon, Somerville, NJ, USA). The vein was removed from the animal, rinsed in iso-osmotic sodium chloride solution (9 g/l) containing 2 IU/ml heparin and 50μg/ml glyceryl trinitrate, and stored in the same solution at room temperature (23°C) until needed.
A longitudinal neck incision was made just medial to the sternomastoid muscle and the common carotid artery was carefully dissected from the internal jugular vein and vagus nerve within the carotid sheath. A 3 cm segment of the common carotid artery was isolated between vascular clamps and excised, bevelling the cut ends obliquely to 45°. The saphenous vein was cut to the appropriate length, reversed and similarly bevelled, and an end-to-end anastomosis of the vein to the common carotid artery was carried out using a continuous 7–0 Prolene suture. Animals were extubated and, when in a satisfactory condition, returned to their pens and fed a normal chow diet.
Folic acid (Sigma) was fed to one group of control and another group of diabetic pigs once daily for 4 weeks. Folic acid (0.1 mg kg−1day−1) was incorporated into small amounts of mashed potatoes in order to ensure complete ingestion of the vitamin. Folic acid treatment was commenced 1 day before surgery.
After 1 month the vein graft was removed, including 1 cm segments of the proximal and distal carotid arteries, pressure-fixed ex vivo at 100 mmHg using Carson’s fixative and postfixed in the same solution for approximately 24 h before being processed for wax embedding and histology. Blood samples were taken from the ear vein. Blood samples were anticoagulated with EDTA (final concentration, 5 mmol/l) and centrifuged at 2,000 g for 10 min at 4°C. Plasma was aspirated and glucose and triacylglycerol concentrations were measured at the Chemical Pathology Laboratory at Bristol Royal Infirmary. Homocysteine was measured using reverse-phase high-pressure liquid chromatography with fluorescence detection and erythrocyte folic acid concentration was measured using a commercial immunoassay kit (ICN Pharmaceuticals, Basingstoke, UK).
Histology
Histology of vein grafts was carried out as described previously [24, 25]. Sections were dewaxed, rehydrated and stained with haematoxylin and eosin or Miller’s elastic van Gieson stain. For proliferating cell nuclear antigen (PCNA), sections were dewaxed, rehydrated and treated with hydrogen peroxide in methanol to remove endogenous peroxidase and the following staining was carried out. Sections were microwaved in citrate buffer, quenched in 1 in 3 horse serum in Tris-buffered saline, and then incubated with PCNA antibody diluted 1 in 100 overnight at 4°C. Sections were washed and then treated with 1 in 400 biotinylated goat anti-mouse antibody followed by streptavidin–biotinylated horseradish peroxidase detection solution. Visualisation was achieved using 3,3′-diaminobenzidine. After counterstaining with diluted haematoxylin and eosin, sections were dehydrated and mounted.
Vessel wall dimensions were measured by computer-aided planimetry using an Olympus BH-2 microscope (Olympus UK, Southend on Sea, UK) with a colour video camera head (TK-870E; JVC, London, UK) coupled to a Microscale TM/TC image analysis system (Digithurst, Royston, UK). The area enclosed by the endothelium and the internal elastic lamina defined the intima and the area between the internal and external elastic lamina defined the media. Lumen, intima and media perimeters and areas were computed using the lumen boundary and internal and external elastic lamina as delimiters and mean values were calculated for all sections from the same graft. Average intima and media thickness was derived from the area and perimeter data for five sections from each graft, assuming that the sections consisted of circular profiles. This was a valid assumption because the tissues were fixed at normal perfusion pressure. Microvessels in the adventitia (the new vasa vasorum) were stained with lectin, which delineates the endothelium, and counted as described above.
Measurement of superoxide formation
The reduction of ferricytochrome c method was used to measure O2·− [26–29]. Vein graft samples were cut into approximately 1 mm segments, washed and equilibrated in DMEM without phenol and horseradish cytochrome c (Sigma) with or without superoxide dismutase 500 U/ml and then incubated at 37°C in a 95% air–5% CO2 incubator for 1 h and the optical density of the reaction medium was measured by spectrophotometry [26–29]. The possible source of O2·− was determined by co-incubating with 100 μmol/l apocynin (an NADPH oxidase inhibitor [Sigma]), 100 μmol/l diphenyliodonium (an NADPH oxidase inhibitor [Sigma]), 100 μmol/l rotenone (inhibitor of mitochondrial respiration [Sigma]), 100 μmol/l allopurinol (xanthine oxidase inhibitor [Sigma]), 10 μmol/l aspirin (cyclooxygenase inhibitor [Sigma]) and 1 mol/l, l-nitro-arginine methyl ester (L-NAME; nitric oxide synthase inhibitor [Sigma]).
In vitro effect of homocysteine, folate and 5-methyl tetrahydrofolate on superoxide formation
To determine whether folic acid and its active metabolite, 5MTHF, may directly influence superoxide formation in diabetic vascular tissue, saphenous veins were harvested from control diabetic pigs (not treated with folate) 4 weeks after implantation, adventitia were removed and veins were cut into 2 mm rings. These were washed and incubated with increasing concentrations of homocysteine with or without folic acid or 5MTHF, and superoxide generation was assessed as described above.
Data analysis and statistics
Data were collated and analysed using Microsoft Excel and non-parametric statistical analysis was carried out using an Intercooled Stata 8 statistics package (Stata Corporation, College Station, TX, USA). Bartlett’s test for equality of variance (a necessary assumption for one-way analysis of variance) was significant, indicating that non-parametric methods of analysis were required for the morphometric appraisal of vein grafts and PCNA. Thus, values are expressed as median and 25th and 75th interquartile ranges and graphically as box and whisker plots. The Mann–Whitney U test was then used to test the statistical significance of differences between control and treated groups. To test whether drug effects were dose-dependent, Kendall’s tau (τ)-b test was used as a measure of correlation for ordinal categorical data, which takes ties into account. For in vitro data, which were shown to be parametric, Student’s t test and ANOVA were used.
Results
The starting weights in both the control and diabetic pig groups were similar at the beginning of the study and no significant changes in weight occurred 4 weeks later (Table 1). Four weeks after commencement of the study, there were no significant differences in weight between any of the groups (Table 1). Plasma glucose and triacylglycerol concentrations at day 0 and 4 weeks were markedly higher in the diabetic group compared with the control group (Table 1). Plasma homocysteine concentrations were not significantly different between controls and diabetic pigs after 1 month (Table 1). Following administration of folic acid to both control and diabetic groups, however, plasma total homocysteine was significantly reduced in the folic acid groups (Table 1). Folic acid was significantly elevated in all animals whose diet was supplemented with folic acid (Table 1).
Table 1.
Body weights and plasma concentrations of glucose, triacylglycerol, total homocysteine and erythrocyte folate at day 0 and 4 weeks after implantation of vein grafts in pigs
| Group | Body weight (kg) | Glucose (mmol/l) | Triacylglycerol (mmol/l) | Homocysteine (μmol/l) | Folate (mg/l) |
|---|---|---|---|---|---|
| Control, day 0 | 30 (27–31) | 4.2 (2.97–4.46) | 0.23 (0.2–0.25) | 8.9 (7.1–9.3) | 320 (220–440) |
| Control, 4 weeks | 31.5 (31–33) | 4.4 (3.4–5.1) | 0.22 (0.2–0.23) | 8.8 (7.4–9.8) | 440 (290–530) |
| Diabetic, day 0 | 29.5 (29–31) | 28 (19.4–34)* | 0.6 (0.5–0.7)* | 9.2 (7.1–10.6) | 290 (320–640) |
| Diabetic, 4 weeks | 33 (32–34) | 26 (13.9–33)* | 0.9 (0.8–1.6)* | 9.0 (7.1–10.1) | 330 (220–540) |
| Control, folate, day 0 | 34 (31–37) | 4.6 (2.9–4.4) | 0.22 (0.2–0.24) | 8.8 (7.1–9.3) | 790 (440–980)† |
| Control, folate, 4 weeks | 33 (32–35) | 4.5 (3.4–5.1) | 0.21 (0.2–0.23) | 6.7 (6.4–7.4)† | 990 (540–1200)† |
| Diabetic, folate, day 0 | 32 (31.5–32.5) | 26 (11.4–44)* | 0.6 (0.5–0.7)* | 8.9 (7.4–10.4) | 670 (330–870)† |
| Diabetic, folate, 4 weeks | 30 (29–31) | 23 (19.9–30)* | 0.8 (0.7–1.4)* | 5.9 (5.5–7.2)† | 880 (700–1030)† |
Data are median (range); n = 6 animals
p<0.05, controls (CON) vs diabetic (D) animals
p<0.05, animals treated with folate (F) vs untreated controls or diabetic animals
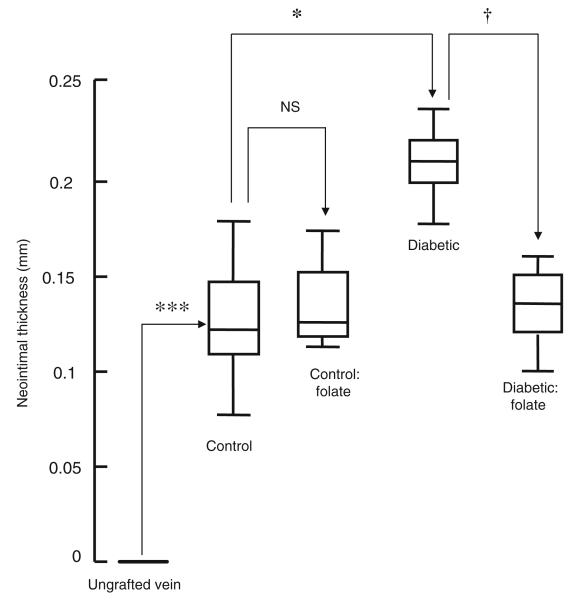
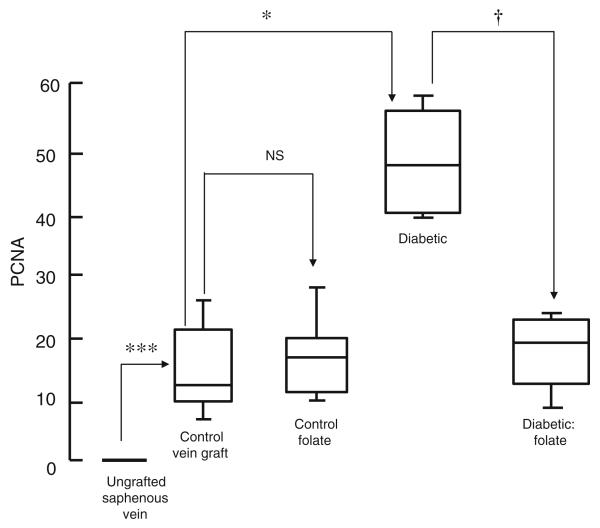
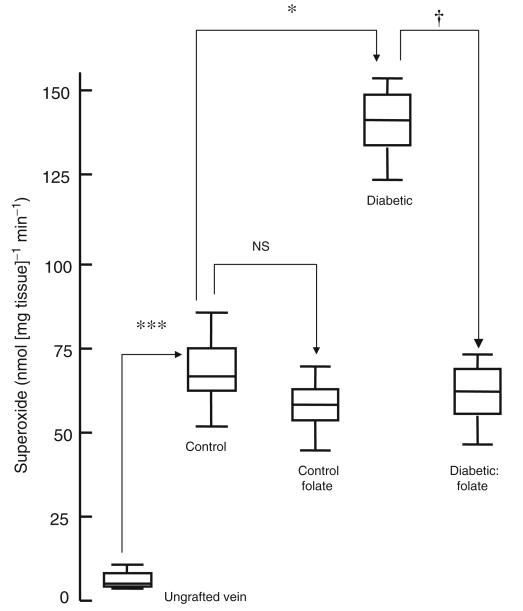
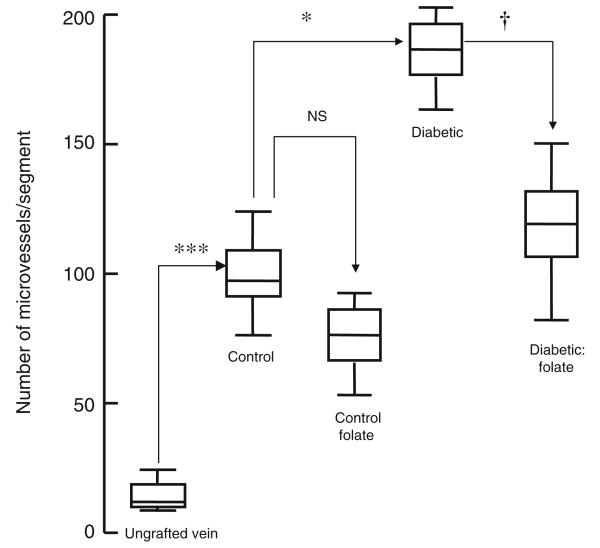
All grafts were patent 4 weeks after implantation. There was a significant increase in lumen area, neointimal thickness and area, medial thickness and area and lumen area in vein grafts from untreated animals compared with ungrafted saphenous veins (Figs 1 and 2; Table 2). Neointimal thickness and PCNA count were increased in vein grafts from diabetic pigs compared with controls, an effect reversed by administration of folic acid (Figs 2 and 3). Microvessel density was reduced in vein grafts from diabetic pigs compared with controls, an effect reversed by administration of folic acid (Figs 1 and 4). Superoxide formation was increased in vein grafts from diabetic pigs compared with controls, an effect reversed by the administration of folic acid (Fig. 5).
Fig. 1.
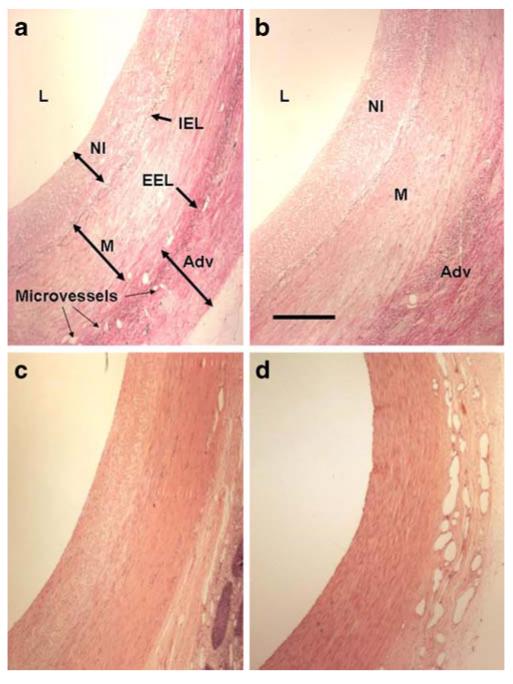
Representative photomicrographs of haematoxylin–eosinstained porcine vein grafts 1 month after implantation. a Control, untreated; b diabetic, untreated; c control, treated with folic acid; d diabetic, treated with folic acid. Adv, adventitia; EEL, external elastic lamina; IEL, internal elastic lamina; L, lumen; M, media; NI, neointima. Scale bar, 100 μm
Fig. 2.
Planimeteric analysis of neointimal thickness of vein grafts from diabetic and non-diabetic pigs treated orally with folic acid once daily for 1 month prior to explantation. Data are expressed as median and interquartile range, n=6. Statistical significance was determined using the Mann–Whitney test with Bonferroni adjustment and the tau-b test. ***p<0.001 (ungrafted vein vs vein graft at 1 month); *p<0.05 (control vs diabetic vein graft at 1 month); †p<0.05 (diabetic vein graft vs diabetic vein graft+folic acid at 1 month)
Table 2.
Medial thickness and lumen areas of vein grafts in control and diabetic pigs, with and without folate and in ungrafted fixed saphenous vein 4 weeks after implantation
| Group | Medial thickness (mm) |
Lumen area (mm3) |
|---|---|---|
| Saphenous vein, day 0 | 0.11 ( 0.09–0.13) | 2.1 (2.1–2.3) |
| Control, 4 weeks | 0.33 (0.29–0.41)** | 8 (6–12)** |
| Control, folate, 4 weeks | 0.31 (0.26–0.44) | 11 (7–11) |
| Diabetes, 4 weeks | 0.42 (0.34–0.5) | 8 (4–11) |
| Diabetes, folate, 4 weeks | 0.38 (0.26–0.44) | 10 (6–14) |
Data are median (range); n=6 animals
p<0.01 vein graft at 4 weeks vs ungrafted saphenous vein at day 0
Fig. 3.
PCNA count (index of proliferating cells) in medial/neointimal region of vein grafts from diabetic and non-diabetic pigs treated orally with folic acid once daily for 1 month prior to explantation. Data are expressed as median and interquartile range, n=6. Statistical significance was determined using the Mann–Whitney test with Bonferroni adjustment and the tau-b test. ***p<0.001 (ungrafted vein vs vein graft at 1 month); *p<0.05 (control vs diabetic vein graft at 1 month); †p<0.05 (diabetic vein graft vs diabetic vein graft+folic acid at 1 month)
Fig. 4.
Adventitial microvessel (neo-vasa vasorum) count in vein grafts from diabetic and non-diabetic pigs treated orally with folic acid once daily for 1 month prior to explantation. Data are expressed as median and interquartile range, n=6. Statistical significance was determined using a Mann–Whitney test with Bonferroni adjustment and the tau-b test. ***p<0.001 (ungrafted vein vs vein graft at 1 month); *p<0.05 (control vs diabetic vein graft at 1 month); †p< 0.05 (diabetic vein graft vs diabetic vein graft+folic acid at 1 month)
Fig. 5.
Superoxide formation in vein grafts from diabetic and non-diabetic pigs treated orally with folic acid once daily for 1 month prior to explantation. Data are expressed as median and interquartile range, n=6. Statistical significance was determined using the Mann–Whitney test with Bonferroni adjustment and the tau-b test. ***p<0.001 (ungrafted vein at day zero vs vein graft at 1 month); *p<0.05 (control vs diabetic vein graft at 1 month); †p<0.05 (diabetic vein graft vs diabetic vein graft+folic acid at 1 month)
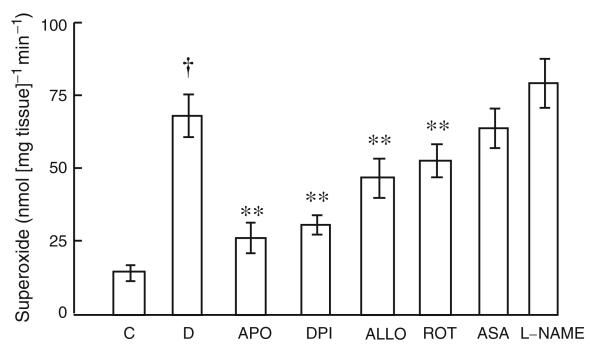
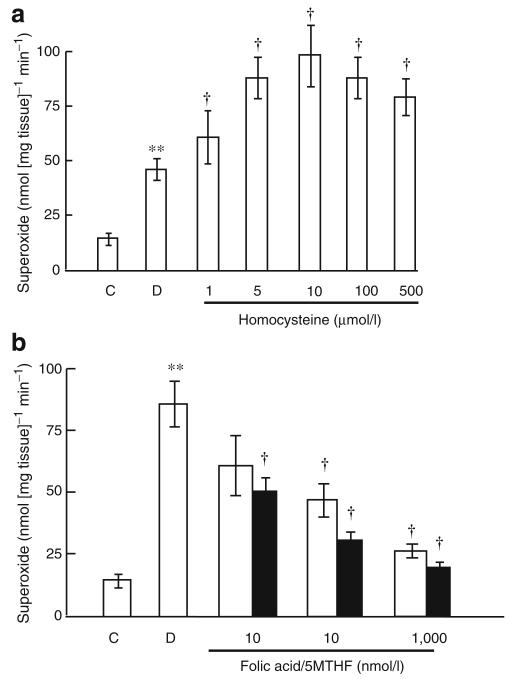
Superoxide formation ex vivo was increased in vein graft tissue from diabetic pigs compared with controls (Fig. 6), an effect inhibited by apocynin, diphenyliodonium, rotenone and allopurinol but not by aspirin or L-NAME (Fig. 6). Ex vivo, homocysteine augmented superoxide formation by isolated saphenous veins, an effect inhibited by both folic acid and 5MTHF (Fig. 7)
Fig. 6.
Superoxide formation in vein grafts from diabetic and non-diabetic pigs and effect of apocynin (APO; an NADPH oxidase inhibitor), diphenyliodonium (DPI, an NADPH oxidase inhibitor), rotenone (ROT; mitochondrial respiration inhibitor), allopurinol (ALLO; xanthine oxidase inhibitor), aspirin (ASA; cyclo-oxygenase inhibitor) and L-NAME (nitric oxide synthase inhibitor). Data are expressed as mean±SEM, n=6. †p<0.0001, control vs diabetic tissue; **p<0.01, comparing inhibitory effect of drugs with untreated diabetic tissue. C, control; D, diabetic
Fig. 7.
Ex vivo effect of (a) homocysteine alone and (b) homocysteine±folic acid (white bars) or 5MTHF (black bars) on acute superoxide formation by isolated porcine diabetic saphenous vein tissue incubated for 1 h. Data are expressed as mean±SEM, n=6. **p<0.01, control vs diabetic tissue; †p<0.01 comparing inhibitory effect of drugs with untreated diabetic tissue. C, control; D, diabetic
Discussion
The present study demonstrates, first, that streptozotocin-induced diabetes mellitus in the pig elicits an increase in neointimal thickness in saphenous vein grafts compared with non-diabetic animals, which has not previously been reported in this species. The PCNA-positive cell count was also markedly augmented by diabetes mellitus, indicating that neointimal thickening was due to increased VSMC replication. This is consistent with previous studies in other species (rabbit, rat and mouse), in which diabetes mellitus also markedly augmented neointimal thickening in vein grafts [19].
In the present study, administration of folic acid elicited inhibition of neointimal thickening in vein grafts from diabetic but not control pigs, even though there was a significant reduction in plasma homocysteine in both control and diabetic groups. Folic acid, in both groups, had no effect on plasma glucose, indicating that these effects were not due to alterations of this variable. These data also indicate that homocysteine may be particularly vasculopathic in diabetic patients undergoing CABG. Previous studies support this proposition. For example, it has been shown that homocysteine, at concentrations between 10 and 100 μmol/l, augments superoxide formation in vascular tissue from diabetic rabbits [8]. In turn, overproduction of superoxide has been implicated in neointima formation in vein grafts [10, 11]. By contrast, even at concentrations as high as 1 mmol/l, homocysteine had no effect on these variables in non-diabetic controls [10, 11]. This indicates that a reduction in homocysteine may not elicit a beneficial effect in non-diabetic persons but may do so specifically in the diabetic patient. Why the vasculature of diabetic patients should be susceptible to oxidative attack by homocysteine has not been elucidated. However, it has been demonstrated that copper homeostasis is markedly altered in diabetes; in particular, copper is dissociated from its binding sites by reactive oxygen species [30]. In turn, homocysteine reacts with copper to augment vasculopathy, including an impairment of angiogenesis [9]. In support of this proposal, chelation of copper with oral penicillamine also inhibits neointima formation in porcine saphenous vein grafts [31].
A common denominator pathological event associated independently with diabetes mellitus and hyperhomocysteinaemia is oxidative stress, in particular, superoxide formation [10, 30–34]. Diabetes mellitus is also associated with increased intravascular superoxide formation in vein grafts, a principal source of which is NADPH oxidase [32–34]. Superoxide elicits effects associated with vein graft disease, including the promotion of VSMC replication and neointima formation [10, 11]. The present study confirms that superoxide is markedly increased in vein grafts per se, an effect that is exacerbated by type 2 diabetes. The present study confirms not only that NAPDH oxidase is a major source of superoxide but also that xanthine oxidase and mitochondria contribute, whereas cyclooxygenase and nitric oxide synthase do not. In turn, folic acid administration reversed this effect in diabetic but not non-diabetic vein graft tissue.
Several studies have demonstrated that folic acid administration to man preserves nitric oxide-dependent functions within the vascular endothelium [35–38], an effect ascribed to overproduction of medial superoxide [15, 16]. Homocysteine, at concentrations similar to those in the plasma of pigs and man, enhances superoxide formation in diabetic vascular tissue [16], an effect confirmed by the present ex vivo study of diabetic vein graft tissue [16]. Thus, since folic acid reduces plasma homocysteine, it is reasonable to suggest that the present effect of folic acid in diabetic pigs is due to lowering of this amino acid. An important point of this study is that the present effects are ascribed to an impact at the media–VSMC level rather than the endothelium. Indeed, in the present study superoxide formation was shown to be enhanced in isolated grafts by exogenous homocysteine at circulating concentrations.
However, since lowering of plasma homocysteine by folic acid in control pigs had no effect on neointima formation, this points to a direct effect. Folic acid and/or its metabolite, 5MTHF, directly protect endothelial nitric oxide synthase (eNOS) from uncoupling, independently of homocysteine-lowering capacity [39]. In the present study, folic acid and MTHF directly inhibited superoxide formation at physiological and therapeutic concentrations induced by homocysteine in isolated porcine saphenous veins from diabetic pigs, indicating direct inhibitory effects of both folic acid and 5MTHF on intra-graft oxidative stress, independently of homocysteine.
Another notable finding of the present study was that the number of microvessels in the neoadventitia of vein grafts (neo-vasa vasorum) was markedly reduced in diabetic pigs. The vasa vasorum is a network of microvessels housed in the adventitia that supplies larger conduit vessels with oxygen [3]. Preparation of the saphenous vein for implantation, ipso facto, results in disruption of the integrity of the vasa vasorum, which in turn results in graft hypoxia, further reactive oxygen species (ROS) formation and vein graft pathology. It is now established that restitution of the vasa vasorum (microcirculation) in vein grafts is an important adaptation to arterial conditions. In this context, there is strong evidence that homocysteine is a contributory factor for diabetic microangiopathy [40, 41], including microvascular repair in wound healing [42, 43]. In the present study, diabetes mellitus markedly impaired microvessel density in the adventitia whereas administration of folic acid reversed this effect. This is consistent with the known beneficial effect of folic acid on endothelial nitric oxide formation and the reduction in the over-production of ROS. Since endothelial cells are axiomatic in angiogenesis [42, 43] and nitric oxide mediates angiogenesis [44], it is reasonable to suggest that this impairment is due to the overproduction of ROS and its restitution by folate, due to inhibition of superoxide formation.
To summarise, experimental type 2 diabetes mellitus in the pig resulted in a marked augmentation of neointima formation, impairment of neo-vasa vasorum formation and augmentation of superoxide formation, all of which were reduced by the administration of folic acid. Such was not the case in non-diabetic pigs. Since superoxide promotes neointima formation and impairs angiogenesis and folic acid is known to reduce superoxide formation, it is likely that these effects are due to a reduction of intra-graft oxidative stress. The lack of effect of folic acid in non-diabetic animals indicates that type 2 diabetes renders the graft susceptible to oxidative attack by homocysteine through a hitherto undefined mechanism. From a clinical perspective, therefore, it is reasonable to suggest that administration of folic acid to diabetic patients may prove effective in reducing late vein graft failure. A clinical trial in man to test this hypothesis would therefore seem to be warranted.
Acknowledgements
This work was supported by funding from the David Telling Trust (University of Bristol Health Trust) and by the NIHR Bristol Biomedical Research Unit in Cardiovascular Medicine.
Abbreviations
- CABG
Coronary artery bypass graft surgery
- 5MTHF
5-Methyltetrahydrofolate
- L-NAME
l-Nitro-arginine methyl ester
- PCNA
Proliferating cell nuclear antigen
- ROS
Reactive oxygen species
- VSMC
Vascular smooth muscle cell
Footnotes
Duality of interest The authors declare that there is no duality of interest associated with this manuscript.
J. Bloor and N. Shukla are joint first authors.
Contributor Information
J. Bloor, Department of Vascular Surgery, University of Bristol, Bristol, UK
N. Shukla, Bristol Heart Institute, Bristol Royal Infirmary, Marlborough Street, Bristol BS2 8HW, UK
F. C. T. Smith, Department of Vascular Surgery, University of Bristol, Bristol, UK
G. D. Angelini, Bristol Heart Institute, Bristol Royal Infirmary, Marlborough Street, Bristol BS2 8HW, UK
J. Y. Jeremy, Bristol Heart Institute, Bristol Royal Infirmary, Marlborough Street, Bristol BS2 8HW, UK, j.y.jeremy@bristol.ac.uk
References
- 1.Mortwani JG, Topol EJ. Aortocoronary saphenous vein graft disease. Pathogenesis, predisposition and prevention. Circulation. 1998;97:916–931. doi: 10.1161/01.cir.97.9.916. [DOI] [PubMed] [Google Scholar]
- 2.Varty K, Allen KE, Bell PRF, London NJM. Infra-inguinal vein graft stenosis. Br J Surg. 1993;80:825–833. doi: 10.1002/bjs.1800800706. [DOI] [PubMed] [Google Scholar]
- 3.Jeremy JY, Gadsdon P, Shukla N, et al. On the biology of saphenous vein grafts fitted with external synthetic sheaths and stents. Biomaterials. 2007;28:895–908. doi: 10.1016/j.biomaterials.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 4.Jeremy JY, Mehta D, Bryan AJ, Lewis D, Angelini GD. Platelets and saphenous vein graft failure following coronary artery bypass graft surgery. Platelets. 1997;8:295–309. doi: 10.1080/09537109777168. [DOI] [PubMed] [Google Scholar]
- 5.Iwama Y, Mokuno H, Yokoi H. Elevated levels of plasma homocysteine related to saphenous vein graft disease after coronary artery bypass graft surgery. J Cardiol. 1998;32:357–362. [PubMed] [Google Scholar]
- 6.Irvine C, Wilson YG, Currie IC. Hyperhomocysteinaemia is a risk factor for vein graft stenosis. Eur J Vasc Endovasc Surg. 1996;12:304–309. doi: 10.1016/s1078-5884(96)80249-8. [DOI] [PubMed] [Google Scholar]
- 7.Jeremy JY, Shukla N, Angelini GD, et al. Sustained increases in homocysteine, copper and ceruloplasmin following coronary artery bypass grafting. Ann Thorac Surg. 2002;74:1553–1557. doi: 10.1016/s0003-4975(02)03807-9. [DOI] [PubMed] [Google Scholar]
- 8.Shukla N, Thompson CS, Angelini GD, et al. Homocysteine augments the reduction of endothelium dependent relaxation and cGMP formation in diabetic rabbits. Diabetologia. 2002;45:1325–1331. doi: 10.1007/s00125-002-0888-4. [DOI] [PubMed] [Google Scholar]
- 9.Shukla N, Angelini GD, Jeremy JY. Interactive effects of homocysteine and copper on angiogenesis in porcine isolated saphenous vein. Ann Thorac Surg. 2007;84:43–49. doi: 10.1016/j.athoracsur.2007.03.087. [DOI] [PubMed] [Google Scholar]
- 10.Jeremy JY, Yim AP, Wan S, Angelini GD. Oxidative stress, nitric oxide and vascular disease. Cardiovasc Surg. 2002;17:324–327. doi: 10.1111/j.1540-8191.2001.tb01151.x. [DOI] [PubMed] [Google Scholar]
- 11.Jeremy JY, Rowe D, Emsley AM, et al. Nitric oxide and vascular smooth muscle cell proliferation. Cardiovasc Res. 1999;43:658–665. doi: 10.1016/s0008-6363(99)00171-6. [DOI] [PubMed] [Google Scholar]
- 12.Wald DS, Law MR, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ. 2002;325:1202. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schnyder G, Roffi M, Pin R, et al. Decreased rate of coronary restenosis after lowering of plasma homocysteine levels. N Engl J Med. 2001;345:1593–1600. doi: 10.1056/NEJMoa011364. [DOI] [PubMed] [Google Scholar]
- 14.Schnyder G, Roffi M, Pin R, et al. Effect of homocysteine-lowering therapy on restenosis after percutaneous coronary intervention for narrowings in small coronary arteries. Am J Cardiol. 2003;91:1265–1269. doi: 10.1016/s0002-9149(03)00281-9. [DOI] [PubMed] [Google Scholar]
- 15.Shukla N, Hotston M, Persad N, Angelini GD, Jeremy JY. The administration of folic acid improves erectile function and reduces intracavernosal oxidative stress in the diabetic rabbit. Br J Urol Int. 2009;103:98–103. doi: 10.1111/j.1464-410X.2008.07911.x. [DOI] [PubMed] [Google Scholar]
- 16.Shukla N, Greaves NS, Angelini GD, Jeremy JY. Folic acid reduces intravascular oxidative stress in diabetic rabbits. Metabolism. 2008;57:774–781. doi: 10.1016/j.metabol.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 17.Jensen-Waern M, Andersson M, Kruse R, et al. Effects of streptozotocin-induced diabetes in domestic pigs with focus on the amino acid metabolism. Lab Anim. 2009;43:249–254. doi: 10.1258/la.2008.008069. [DOI] [PubMed] [Google Scholar]
- 18.Thompson CS. Animal models of diabetes mellitus: relevance to vascular complications. Curr Pharm Des. 2008;14:309–324. doi: 10.2174/138161208783497679. [DOI] [PubMed] [Google Scholar]
- 19.Jeremy JY, Thomas AC. Animal models for studying neointima formation. Curr Vasc Pharmacol. 2010 doi: 10.2174/157016110790887027. (in press) [DOI] [PubMed] [Google Scholar]
- 20.Koopmans SJ, Mroz Z, Dekker R, et al. Association of insulin resistance with hyperglycemia in streptozotocin-diabetic pigs: effects of metformin at isoenergetic feeding in a type 2-like diabetic pig model. Metabolism. 2006;55:960–971. doi: 10.1016/j.metabol.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Mehta D, George SJ, Jeremy JY, et al. External stenting reduces long-term medial and neointimal thickening and platelet derived growth factor expression in a pig model of arterio-venous bypass grafting. Nat Med. 1984;4:235–239. doi: 10.1038/nm0298-235. [DOI] [PubMed] [Google Scholar]
- 22.Wan S, Yim A, Bulbulia RA, et al. The endothelin 1A receptor antagonist BSF 302146 is a potent inhibitor of porcine vein graft thickening in vivo. J Thorac Cardiovasc Surg. 2004;127:1317–1322. doi: 10.1016/j.jtcvs.2003.06.018. [DOI] [PubMed] [Google Scholar]
- 23.Wan S, Shukla N, Angelini GD, Yim AP, Johnson JL, Jeremy JY. Nitric oxide-donating aspirin (NCX 4016) inhibits neointimal thickening in a pig model of saphenous vein-carotid artery interposition grafting: a comparison with aspirin and morpholinosydnonimine (SIN-1) J Thorac Cardiovasc Surg. 2007;134:1033–1039. doi: 10.1016/j.jtcvs.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 24.Vijayan V, Shukla N, Johnson JL, et al. Long-term reduction of medial and intimal thickening in porcine saphenous vein grafts with a polyglactin biodegradable external sheath. J Vasc Surg. 2004;40:1011–1019. doi: 10.1016/j.jvs.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 25.Wan S, Arifi AA, Chan MCW, et al. Perivenous application of fibrin glue in porcine saphenous vein–carotid artery interposition grafts: impact on medial thickening. Eur J Cardiothorac Surg. 2006;29:742–746. doi: 10.1016/j.ejcts.2005.12.059. [DOI] [PubMed] [Google Scholar]
- 26.Muzaffar S, Jeremy JY, Angelini GD, Stuart Smith K, Shukla N. The role of the endothelium and nitric oxide synthases in modulating superoxide formation induced by endotoxin and cytokines in porcine pulmonary arteries. Thorax. 2003;58:598–604. doi: 10.1136/thorax.58.7.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muzaffar S, Shukla N, Angelini GD, Jeremy JY. Sildenafil citrate and sildenafil nitrate are potent inhibitors of superoxide formation and gp91phox expression in porcine pulmonary artery endothelial cells. Br J Pharmacol. 2005;146:109–117. doi: 10.1038/sj.bjp.0706305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muzaffar S, Shukla N, Lobo C, Angelini GD, Jeremy JY. Iloprost inhibits NADPH oxidase expression and superoxide release in porcine pulmonary arteries and cells stimulated with thromboxane A2, isoprostane F2α and cytokines. Br J Pharmacol. 2004;141:488–496. doi: 10.1038/sj.bjp.0705626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muzaffar S, Shukla N, Angelini GD, Jeremy JY. Hypoxia simultaneously induces the expression of gp91phox and endothelial nitric oxide synthase which promotes peroxynitrite formation in the pulmonary artery. Thorax. 2005;60:305–313. doi: 10.1136/thx.2003.018796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shukla N, Maher J, Masters J, Angelini GD, Jeremy JY. Does oxidative stress change ceruloplasmin from a protective to a vasculopathic risk factor? Atherosclerosis. 2006;187:238–250. doi: 10.1016/j.atherosclerosis.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 31.Wan S, Yim A, Shukla N, et al. Orally administered penicillamine is a potent inhibitor of neointimal and medial thickening in porcine saphenous vein grafts. J Thorac Cardiovasc Surg. 2007;133:494–500. doi: 10.1016/j.jtcvs.2006.08.071. [DOI] [PubMed] [Google Scholar]
- 32.West EJ, Guzik TJ, Black E, et al. Enhanced superoxide production in experimental venous bypass graft intimal hyperplasia. Arterioscler Thromb Vasc Biol. 2000;21:189–194. doi: 10.1161/01.atv.21.2.189. [DOI] [PubMed] [Google Scholar]
- 33.West NE, Qian H, Guzik TJ, et al. Nitric oxide synthase (nNOS) gene transfer modifies venous bypass graft remodeling: effects on vascular smooth muscle cell differentiation and superoxide production. Circulation. 2001;104:1526–1532. doi: 10.1161/hc3801.095693. [DOI] [PubMed] [Google Scholar]
- 34.Guzik TJ, Mussa S, Gastaldi D, et al. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105:1656–1662. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- 35.Mangoni AA, SherWood RA, Asonganyi B, Swift CG, Thomas S, Jackson SH. Short-term oral folic acid supplementation enhances endothelial function in patients with type 2 diabetes. Am J Hypertens. 2005;218:220–226. doi: 10.1016/j.amjhyper.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 36.Title LM, Ur E, Giddens K, McQueen MJ, Nassar BA. Folic acid improves endothelial dysfunction in type 2 diabetes—an effect independent of homocysteine-lowering. Vasc Med. 2006;11:101–109. doi: 10.1191/1358863x06vm664oa. [DOI] [PubMed] [Google Scholar]
- 37.van Etten RW, de Koning EJ, Verhaar MC, et al. Impaired NO-dependent vasodilation in patients with type II (non-insulin-dependent) diabetes mellitus is restored by acute administration of folate. Diabetologia. 2002;45:1004–1010. doi: 10.1007/s00125-002-0862-1. [DOI] [PubMed] [Google Scholar]
- 38.Tawakol A, Omland T, Gerhard M, Wu JT, Creager MA. Hyperhomocysteinaemia is associated with impaired endothelium-dependent vasodilation in humans. Circulation. 1997;95:1119–1121. doi: 10.1161/01.cir.95.5.1119. [DOI] [PubMed] [Google Scholar]
- 39.Antoniades C, Shirodaria C, Warrick N, et al. 5-Methyltetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels: effects on vascular tetrahydrobiopterin availability and endothelial nitric oxide synthase coupling. Circulation. 2006;114:1193–1120. doi: 10.1161/CIRCULATIONAHA.106.612325. [DOI] [PubMed] [Google Scholar]
- 40.Van Hecke MV, Dekker JM, Nijpels G, et al. Homocysteine, S-adenosylmethionine and S-adenosylhomocysteine are associated with retinal microvascular abnormalities: the Hoorn Study. Clin Sci (Lond) 2008;114:479–487. doi: 10.1042/CS20070275. [DOI] [PubMed] [Google Scholar]
- 41.Brazionis L, Rowley K, Sr, Itsiopoulos C, Harper CA, O’Dea K. Homocysteine and diabetic retinopathy. Diabetes Care. 2008;31:50–56. doi: 10.2337/dc07-0632. [DOI] [PubMed] [Google Scholar]
- 42.Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. Med Res Rev. 2003;23:117–145. doi: 10.1002/med.10024. [DOI] [PubMed] [Google Scholar]
- 43.Brem H, Canic T. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117:1219–1222. doi: 10.1172/JCI32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lugue Contreras D, Vargas Robles H, Romo E, Rios A, Escalante B. The role of nitric oxide in the post-ischemic revascularization process. Pharmacol Ther. 2006;112:553–563. doi: 10.1016/j.pharmthera.2006.05.003. [DOI] [PubMed] [Google Scholar]