Abstract
Recent research detailing the intrinsic functional organization of the brain provides a unique and useful framework to gain a better understanding of the neural bases of Major Depressive Disorder (MDD). In this review, we first present a brief history of neuroimaging research that has increased our understanding of the functional macro-architecture of the brain. From this macro-architectural perspective, we examine the extant body of functional neuroimaging research assessing MDD with a specific emphasis on the contributions of default-mode, executive, and salience networks in this debilitating disorder. Next, we describe recent investigations conducted in our laboratory in which we explicitly adopt a neural-systems perspective in examining the relations among these networks in MDD. Finally, we offer directions for future research that we believe will facilitate the development of more detailed and integrative models of neural dysfunction in depression.
Keywords: Major Depressive Disorder, neural systems, intrinsic functional organization, default-mode network, salience network, executive network, rumination
Introduction
Major Depressive Disorder (MDD) is among the most prevalent of all psychiatric disorders and is associated with enormous personal and societal costs (2009). Recent estimates indicate that almost 20% of the American population, or more than 30 million adults, will experience a clinically significant episode of depression during their lifetime. MDD is also a recurrent disorder: three-quarters of depressed persons have more than one depressive episode, and almost two-thirds of people who have ever had been clinically depressed will be in an episode in any given year over the rest of their lives (Boland & Keller, 2009). At its core, MDD is arguably a disorder of emotion regulation; in addition, however, investigators have consistently documented the occurrence of repetitive, perseverative, negative thinking in depression, as well as biases in attention to and memory for negative information that appear to underlie the increased responsivity to negative stimuli in MDD (see Gotlib & Joormann, 2010, for a review of this literature).
Over the past two decades, investigators have examined patterns of neural activation that are associated with these emotional and cognitive aspects of MDD. Thus, researchers have assessed neural responding in depressed individuals to a variety of positively and negatively valenced emotional stimuli and to cognitive challenges, and have documented anomalous responding in MDD in a range of frontal, temporal, cerebellar, and subcortical regions (see Fitzgerald, Laird, Maller, & Daskalakis, 2008, for a review of this literature). While this work has been important in advancing our understanding of neural aspects of MDD, we still lack a cogent, comprehensive, and therapeutically useful model of brain function and dysfunction in this disorder. In this context, it is important to note that massive interconnectivity among neural ensembles in the brain ensures that neural events seldom occur in isolation; consequently, attempting to understand depression from a neural-network perspective may yield an incremental advancement to existing neural models of MDD. Although investigators engaged in neuroimaging in depression have long noted the potential benefits of exploring MDD at the neural-systems level (e.g., Drevets et al., 1992), it is only recently that brain imaging acquisition and analysis techniques, as well as our understanding of the architecture of the brain, have advanced sufficiently to make network-level explorations and conceptualizations of MDD feasible.
By far, the majority of neuroimaging studies of MDD use paradigms that involve affective or cognitive tasks. Certainly, the results of these studies have informed network-level conceptualizations of depression, such as the formulation that altered cortico-limbic connectivity is a central feature of MDD (Siegle, Thompson, Carter, Steinhauer, & Thase, 2007). It is important to recognize, however, that the brain regions and networks that underlie responses to affective and cognitive challenges in the laboratory may not be the same regions that are involved in the generation or maintenance of spontaneously occurring affect or rumination in MDD. Moreover, given that depression is fundamentally a disorder of mood, it is likely that neural processes that occur over the course of minutes or hours, as opposed to seconds, are more relevant to our understanding of MDD. Increasingly, therefore, researchers are investigating neural functioning in MDD over relatively long periods in the absence of externally presented tasks or stimuli. Investigators conducting these ‘resting-state’ studies typically use either functional magnetic resonance imaging (fMRI) or positron emission tomography (PET) to examine brain activity or connectivity during rest while participants are not performing specific tasks. Importantly, these studies have identified a set of dissociable intrinsic functional networks that exhibit correlated activity across brain regions. We propose that investigating MDD from the perspective afforded by these functionally unified networks can yield a more elegant and useful neural conceptualization of depression; indeed, investigators are increasingly studying these networks in the context of MDD (e.g., Sheline et al., 2009). In this review we focus on these intrinsic networks, the normal cognitive and affective functions with which these networks are associated, and the dysfunction in these networks that may be related to MDD.
Evolving Conceptions of the Intrinsic Functional Organization of the Brain
An early discovery from fMRI investigations of the brain was that functionally homogeneous neural regions showed correlated activity even when they were not actively prompted. Biswal and colleagues (1995) first demonstrated this in an fMRI study of motor regions. Using the activation maps derived from a bilateral finger-tapping task, Biswal et al. planted a region-of-interest ‘seed’ in left motor cortex and queried the time courses of all other voxels during rest to identify other regions in which activation was correlated with activity in this seed region. This analysis showed that, in addition to nearby voxels, the contralateral motor cortex and midline cortical regions were also correlated with the seeded left motor cortex. Thus, in this resting-state analysis, it was the correlation of the time courses, rather than the magnitude of a response, that determined network connectivity. The functional connectivity map that resulted from this analysis was spatially similar to the map of regions that were activated by the bilateral finger-tapping task. Biswal et al. interpreted their data as reflecting actual physiological neural connectivity, rather than connectivity in response to an anticipated or imagined motor task.
Subsequent research has expanded on Biswal et al.’s (1995) conceptualization of an implicit functional organization in the brain. In a meta-analysis of PET studies of visual processing in humans, Shulman et al. (1997) investigated regions in which metabolic activity decreased during an active task. These regions were found to include the posterior cingulate cortex (PCC), bilateral parietal cortex, medial prefrontal cortex (mPFC), and medial temporal lobe (MTL) regions. These decreases in activity, which showed remarkably little spatial variation despite a wide range of extrinsic tasks, prompted investigators to begin to study a consistent, spatially organized baseline state of metabolic functioning. Perhaps most notably, Raichle and his colleagues (2001) examined metabolic demands during eyes-closed rest and described a ‘default-mode’ pattern of metabolic activity in the brain that included PCC, bilateral parietal cortex, and mPFC. By using PET to examine blood flow and oxygen consumption, Raichle et al. were able to determine an absolute level of metabolic activity during rest, rather than a relative level of activity during the transition from rest to activity. Thus, the “default-mode network” (DMN) identified by Raichle et al. reflects an ongoing metabolic demand of these regions, a physiological baseline rather than the relative baseline of BOLD fMRI signals (Ralchle & Snyder, 2007).
Investigators have begun to examine factors that may explain the functional connectivity of the brain regions that comprise the DMN. One likely candidate is monosynaptic white-matter connectivity among these regions; another potential factor is common connectivity with a third structure. In testing these possibilities, Greicius and colleagues (2009) conducted a study examining both functional and structural connectivity by using blood oxygenation level-dependent (BOLD) signal and diffusion tensor imaging (DTI), respectively, to visualize connectivity. To obtain seed regions for DTI, Greicius et al. used probabilistic independent component analysis (ICA) to analyze fMRI data. ICA decomposes the whole-brain data into independent spatiotemporal components, including the DMN (Beckmann, DeLuca, Devlin, & Smith, 2005). Using DMN regions, including the PCC, mPFC, and MTL, as seed regions, Greicius et al. identified white-matter tracts that directly connected PCC to mPFC and PCC to MTL, but not to structures outside the DMN. Thus, the temporal correlation of activity in these regions at rest is likely due, at least in part, to tracts that link DMN regions directly.
In an important extension of formulations of macro-architectural organization of the brain, Fox and colleagues (2005) posited that components of the so-called intrinsic functional organization of the brain — the DMN, described above, and the task positive network (TPN; a network of structures that increase in activation during performance of attention-demanding tasks) — have a competitive, anti-correlated relation with each other. Fox et al. noted that, during performance of cognitive tasks, structures comprising the TPN (dorsolateral prefrontal, lateral parietal, and anterior insular cortices) were characterized robustly by increases in activation, whereas structures comprising the DMN showed reliable decreases in activity. Importantly, Fox et al. documented this same negative relation between DMN and TPN during resting-state fMRI scans: fluctuations in activation in one network were associated with inverse activation fluctuations in the other network.
Working to develop a better characterization of the organization of the TPN, Seeley et al. (2007) used resting-state fMRI functional connectivity analysis to demonstrate that the TPN is actually composed of two dissociable networks: an executive network (EN) and a salience network (SN). In specifying the functions of these networks and further demonstrating their dissociability, Seeley et al. showed that the extent of recruitment of nodes in the EN, comprising dorsolateral prefrontal and lateral parietal cortices, correlated positively and selectively with executive task performance, whereas connectivity in the SN, comprising the anterior insula, amygdala, and dorsal anterior cingulate cortex (ACC), correlated positively with ratings of state anxiety.
Conceptualizing Depression from a Neural Network Perspective
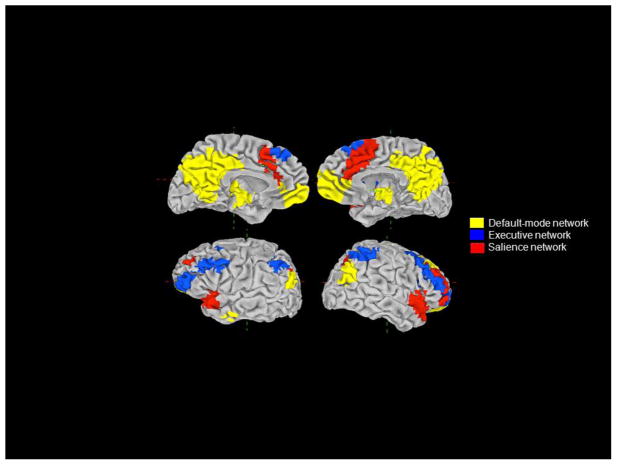
In the following sections we focus on the roles of the DMN, EN, and SN in MDD (see Figure 1 for a whole-brain map showing the loci of each network). It is important to note that these networks are derived from imaging studies of healthy participants; consequently, they encompass normal functional networks based on anatomic connectivity, rather than a network based on interconnected regions that have been implicated in MDD. Yet, we think that these three networks are particularly relevant to understanding the neural bases of MDD in part because their putative functions map well onto significant and specific aspects of depressive symptomatology, including rumination (DMN), emotional disinhibition (EN), and emotional overreactivity (SN). Investigators are increasingly examining how dysfunction in these typical, or “normal,” networks is associated with symptoms of depression, and we review this growing body of evidence below.
Figure 1.
Intrinsic functional networks of primary interest in the study of depression.
The default-mode network in depression
Given the conceptual fit between the self-reflective processes supported by the DMN and self-directed patterns of ruminative thought that play a pivotal role in the maintenance of MDD (Nolen-Hoeksema, Morrow, & Fredrickson, 1993), clinical neuroscientists have made a considerable effort to understand the role of the DMN in MDD. Consistent with researchers’ expectations concerning DMN function in MDD, Sheline et al. (2009) found that depressed persons did not demonstrate the typical pattern of deactivation in several components of the DMN during presumably self-relevant active and passive processing of negative stimuli. In a subsequent study assessing more directly the neural substrates of ruminative responding in depression, (Cooney, Joormann, Eugene, Dennis, & Gotlib, 2010) found increased activation in several DMN structures, including the MPFC, posterior cingulate cortex, and parahippocampus during ruminative self-focus in MDD.
In addition to demonstrations of abnormal DMN responding in MDD, researchers have also found that depressed and nondepressed individuals differ in the spatial extent of the DMN. Specifically, using ICA, which permits investigators to identify different intrinsic brain networks by their unique neural signatures, Greicius and colleagues (2007) found greater contributions to the DMN in depressed than in nondepressed participants from the thalamus and ventral ACC, a structure implicated reliably in self-generated feelings of sadness (Damasio et al., 2000; Mayberg et al., 1999). Further, Greicius et al. found that the extent of recruitment of the ventral ACC was positively correlated with the duration of participants’ current depressive episodes in the MDD group. In a subsequent study, we (Hamilton, Chen, Thomason, Johnson, & Gotlib, 2011) used Granger causality analysis to investigate patterns of neural cause and effect among components of the DMN in depression. We found that activation in the hippocampus, a primary regulator of the hypothalamic-pituitary-adrenal stress response, initiates apparently maladaptive over-responding in the ventral ACC in depressed individuals. In addition, we found mutually excitatory activation between the ventral ACC and the mPFC that was, itself, positively correlated with levels of maladaptive, depressive rumination in MDD.
To further examine the role of the DMN in MDD, we pooled data from fMRI studies published through January 2011 in which investigators had conducted standard, whole-brain analyses comparing the neural responses of depressed and healthy persons to positive stimuli (e.g., happy faces, rewards, favorite music) and/or negative stimuli (e.g., sad words, punishments, sad pictures). We then calculated the proportion of reported differences that were in the DMN, based on maps from Greicius (2003), as a function of group and valence of stimuli. In addition, we conducted a similar analysis using data comparing depressed to control groups with respect to resting-state regional cerebral blood flow (rCBF) as measured with PET or single photon emission computed tomography (SPECT). These analyses incorporated data from 24 fMRI (Abler, Erk, Herwig, & Walter, 2007; Bar et al., 2007; Canli et al., 2004; Cooney et al., 2010; Frodl et al., 2009; Fu et al., 2007; Fu et al., 2004; Fu et al., 2008; Gotlib et al., 2005; Herwig et al., 2010; Keedwell, Andrew, Williams, Brammer, & Phillips, 2005; Knutson, Bhanji, Cooney, Atlas, & Gotlib, 2008; Kumari et al., 2003; Lawrence et al., 2004; Mitterschiffthaler et al., 2003; Mitterschiffthaler et al., 2008; Osuch et al., 2009; Pizzagalli et al., 2009; Scheuerecker et al., 2010; Strigo, Simmons, Matthews, Craig, & Paulus, 2008; Surguladze et al., 2005; Townsend et al., 2010; Wang et al., 2008; Yang, 2004) and 14 PET/SPECT studies (Aihara et al., 2007; Bench et al., 1992; Brody, Saxena, Mandelkern et al., 2001; Drevets et al., 1992; Germain et al., 2007; Kennedy et al., 2001; Kimbrell et al., 2002; Kohn et al., 2007; Krausz et al., 2007; Mayberg et al., 2005; Perico et al., 2005; Saxena et al., 2001; Skaf et al., 2002; Videbech et al., 2001); see Table 1. As we present in Figure 2, under conditions of task responding, depressed participants show less DMN activation than do healthy participants in response to both negative and positive stimuli. Importantly, however, this pattern is reversed for resting-state rCBF studies, with a greater proportion of incidences of DMN activation noted in MDD. This pattern of findings suggests that neural substrates of self-relational processing in MDD are not activated as much by conditions of affective probes as by allowing spontaneous thought. In addition, and reflecting the challenges faced by studies that rely on eliciting ruminative responses which, by definition, are unwanted, (Johnson, Nolen-Hoeksema, Mitchell, & Levin, 2009) found increased DMN activation in MDD only during non-self-referential thinking; they found, further, that the degree of DMN activation during non-self-referential thinking was associated with individual differences in trait rumination.
Table 1.
Studies measuring regional cerebral bloodflow differences (blue) and response to affective challenge (orange) in depressed and healthy persons.
| Included rCBF Studies | Method |
|---|---|
| Aihara et al. (2007) | PET - [18F]-FDG |
| Bench et al. (1992) | PET - [15O]-H2O |
| Brody et al. (2001) | PET - [18F]-FDG |
| Drevets et al. (1992) | PET - [15O]-H2O |
| Germain et al. (2007) | PET - [18F]-FDG |
| Kennedy et al. (2001) | PET - [18F]-FDG |
| Kimbrell et al. (2002) | PET - [18F]-FDG |
| Kohn et al. (2007) | SPECT - 99mTc-ECD |
| Krausz et al. (2007) | SPECT - 99mTc-ECD |
| Mayberg et al. (2005) | PET - [15O]-H2O |
| Perico et al. (2005) | SPECT - 99mTc-ECD |
| Saxena et al. (2001) | PET - [18F]-FDG |
| Skaf et al. (2002) | SPECT - 99mTc-ECD |
| Videbech et al. (2001) | PET - [15O]-H2O |
| Included fMRI Studies | Affective Challenge |
|---|---|
| Abler et al. (2007) | Anticipation and viewing of negative, neutral, and positive pictures |
| Bar et al. (2007) | Experiencing variable intensity thermal pain |
| Canli et al. (2004) | Performing lexical decision task for neutral, happy, sad, and threat words |
| Cooney et al. (2010) | Thinking about personal traits, abstract ideas, and physical objects |
| Frodl et al (2009) | Emotion (explicit) or gender (implicit) matching of negative faces to target; shape matchinq control |
| Fu et al. (2004) | Gender identification of variable intensity sad faces |
| Fu et al. (2007) | Gender identification of variable intensity happy faces |
| Fu et al. (2008) | Gender identification of variable intensity sad faces |
| Gotlib et al. (2005) | Viewing of happy, sad and neutral faces |
| Herwig et al. (2010) | Viewing of negative and neutral pictures |
| Keedwell et al. (2005) | Remembering happy, sad, and neutral experiences |
| Knutson et al. (2008) | Anticipation and receipt of monetary gains, losses, and neutral outcomes |
| Kumari et al. (2003) | Viewing positive, negative and neutral picture-caption pairs |
| Lawrence et al. (2004) | Viewing high and low intensity happy, sad, and fear faces; neutral-face control |
| Mitterschiffthaler et al. (2003) | Viewing positive and neutral images |
| Mitterschiffthaler et al. (2008) | Identifying colors of sad and neutral words |
| Osuch et al. (2009) | Listening to endorsed favorite and neutral music |
| Pizzagalli et al. (2009) | Anticipation and receipt of monetary gains, losses, and neutral outcomes |
| Scheuerecker et al. (2010) | Emotion (explicit) or gender (implicit) matching of negative faces to target; shape matching control |
| Strigo et al. (2008) | Anticipation and receipt of painful and non-painful thermal stimuli |
| Surguladze et al. (2005) | Viewing of variable intensity happy and sad faces |
| Townsend et al. (2010) | Matching of negative faces to emotion face or word target; shape matching control |
| Wang et al. (2008) | Oddball target detection with sad or neutral picture as background |
| Yang (2004) | Viewing erotic and neutral film clips |
Figure 2.
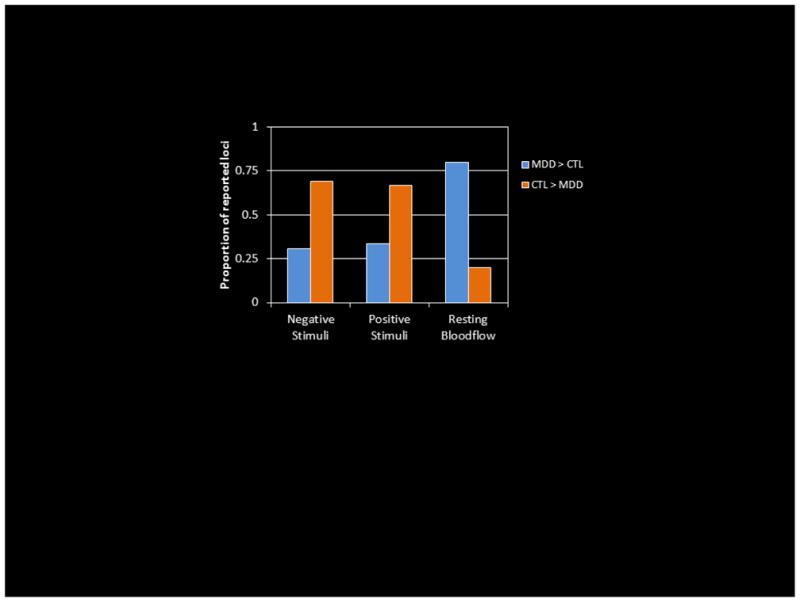
Proportions of reported group-difference loci falling in the default-mode network in functional neuroimaging studies of depression.
The executive network in depression
The foundational role of dysregulated emotional responding in MDD has motivated several investigations of structures that subserve cognitive control in MDD; these investigations have focused most prominently on DLPFC dysfunction. In general, these investigations have found decreased DLPFC functioning in MDD both at rest (e.g., Bench et al., 1992; Mayberg et al., 2005) and in response to negative stimuli (e.g., Pizzagalli et al., 2009; Strigo et al., 2008) (see, however, Frodl et al., (2009). Interestingly, attenuated DLPFC responding in MDD to positive stimuli has been reported much less frequently, suggesting that DLPFC under-responding in MDD is specific to negative stimuli. While this body of findings with respect to DLPFC dysfunction in MDD is intriguing, it leaves unaddressed important questions regarding both the role of DLPFC dysfunction in the pathophysiology of MDD and the relation of DLPFC deactivation at rest to DLPFC under-responding in depression.
Given that most functional neuroimaging studies of MDD are conducted with individuals who are in a depressive episode during scanning, it is not possible to determine from their results whether functional neural anomalies are necessary for depressive pathology, or alternatively, whether aberrations arise as a consequence of having developed depression. In this context, our understanding of the role of DLPFC under-activation in MDD has benefitted from work examining the effects of using transcranial magnetic stimulation (TMS), a technique that involves rapidly oscillating magnetic fields to modulate regional neural response on the cortical surface, to exogenously increase DLPFC activation in depressed persons. Consistent with the formulation that DLPFC under-activation plays a critical role in depressive pathology, meta-analyses have reported intermediate (O’Reardon et al., 2007) to strong (Gross, Nakamura, Pascual-Leone, & Fregni, 2007) effects of using TMS to increase DLPFC activation in order to ameliorate depressive symptoms.
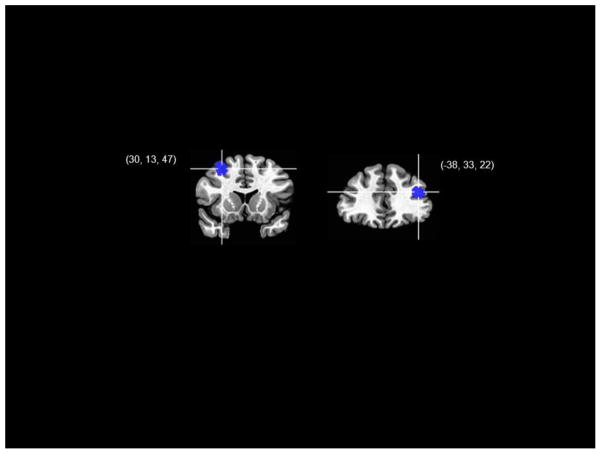
Cognitive theories of MDD posit a cyclical relation between negative affective responding and depressed mood, in which negative affective biases promote depressed mood which, in turn, exacerbates negative emotional responding, which further increases negative biases. In this context, we might ask a similar question at the neural level regarding the relation between DLPFC under-response to negative stimuli and decreased DLPFC activation at rest in MDD. More specifically, is the part of the DLPFC that is most reliably under-active at rest the same region that is under-responsive to negative stimuli? To begin to address this question, we projected data from fMRI investigations of neural response to negative stimuli in MDD and resting-state rCBF studies of MDD onto a common template and assessed the degree of overlap between findings from response and resting-state studies (see our description of this meta-analysis in the previous section for more details). Interestingly, the loci of the most reliable neural response and resting-state DLPFC findings in MDD did not overlap; in fact, as we present in Figure 3, while both regions fell within the boundaries of the EN as specified by Seeley (2007), the part of the DLPFC that shows reliable under-responding to negative stimuli in MDD is contralateral and posterior relative to the part of the DLPFC that is under-activated at rest in depression. These findings support a formulation in which tonic (resting state) and phasic (affective response) neural functional anomalies in MDD are undergirded by separate components of a functionally coherent network. In developing a more precise neural model of MDD, it is critical in future that investigators examining the EN in this disorder focus on the distinct functions supported by these disparate regions of the DLPFC.
Figure 3.
Loci of reliable between-groups differences in the executive network for studies of resting blood flow (right) and neural response to negative stimuli (left) in depression.
The salience network in depression
The putative role of the SN in being aware of, and orienting and responding to, biologically relevant stimuli suggests a clear mapping of the functions of this network to negative response biases in MDD. Indeed, heightened response in the amygdala, anterior insula, and dorsal ACC has been documented in MDD across a wide range of negative conditions. For example, investigators have found greater activation in depressed than in nondepressed persons in the amygdala during anticipation of aversive pictures (Abler et al., 2007) and of receiving thermal pain (Strigo et al., 2008), in the anterior insula in anticipation of monetary loss (Pizzagalli et al., 2009) and of receiving thermal pain (Strigo et al., 2008), and in the dorsal ACC during viewing of sad faces (Fu et al., 2004) and words (Mitterschiffthaler et al., 2008). It is noteworthy that, consistent with its putative role in vigilance and threat detection (Whalen, 2007), the amygdala has been implicated in over-response during anticipation of noxious stimuli in depression. In contrast, the insula has been found to be over-active in MDD during the receipt, as opposed to the anticipation, of negative stimuli, a finding consistent with formulations that this structure subserves emotional awareness (Craig, 2009). Importantly, and reflecting network-level disturbance of the SN in MDD, the amygdala and insula have been found to be simultaneously over-responsive to affective challenge in depression (Suslow et al., 2010). The fact the few investigators have found SN over-response to positive stimuli in MDD suggests that, as in the EN, heightened SN response in MDD is specific to negative stimuli.
An important difference between dysfunction in the SN and dysfunction in the DMN and EN in MDD is that whereas all primary nodes of the SN are affected in MDD, individual components of the EN and DMN (primarily the parietal components) have not been found to exhibit aberrant activation in MDD. The relative coherence of the SN response in MDD warrants additional inquiry, specifically with respect whether a common neurobiological mechanism might promote heightened SN response in this disorder. We propose that one such mechanism involves functional anomalies in the pulvinar nucleus of the thalamus in MDD. We offer this proposal because several investigators have reported heightened baseline activation of the pulvinar in PET studies of MDD (Aihara et al., 2007; Brody, Saxena, Stoessel et al., 2001; Drevets & Raichle, 1992; Germain et al., 2007; Saxena et al., 2001), and because of two important properties of the pulvinar. First, the pulvinar has been shown to have monosynaptic connectivity with primary components of the SN, including projections to the amygdala (Jones & Burton, 1976), as well as bidirectional connectivity with the insula and dorsal ACC (Mufson & Mesulam, 1984; Pessoa & Adolphs, 2010). Second, the pulvinar has been found to play a central role in emotion attention, that is, in allocating attentional resources to biologically important stimuli. This role is suggested by evidence that the pulvinar is necessary for feature binding (Ward, Danziger, Owen, & Rafal, 2002), which is a key function of attention in integrating the contributions of distinct cell ensembles that code for different perceptual features (Treisman, 1999). This formulation is supported by the results of a recent fMRI study examining pulvinar responding to stimuli that were either affective or neutral, and either detected or undetected (Padmala, Lim, & Pessoa, 2010). Consistent with the formulation described above, Padmala et al. found pulvinar response only to detected affective stimuli. Given this role of the pulvinar nucleus in emotional attention and awareness, in addition to its connectivity with the amygdala, insula, and dorsal ACC, we propose that increased baseline pulvinar activation acts to potentiate SN response to negative stimuli in MDD.
New Directions: Cross-Network Interactions in Depression
Contemporary neural models of MDD, such as Mayberg’s (1997) reciprocal limbic-cortical dysregulation model, emphasize dysfunctional interactions between neural networks, as opposed to uni-structural or uni-network functional anomalies, as critical in contributing to depressive pathology. Given this emphasis, as well as the putative functions of the default-mode and task-positive networks in supporting passive and self-reflective, and active and externally focused processes, respectively, we investigated the relation between DMN and TPN, and their association with maladaptive and adaptive styles of rumination, in MDD (Hamilton, Furman et al., 2011). Specifically, we used a unique metric to estimate DMN dominance over TPN: the number of fMRI acquisitions for which DMN activity exceeded TPN activity during a resting-state fMRI scan. We then calculated correlations between our metric of DMN dominance over TPN and depressive (maladaptive) and reflective (adaptive) subscales of the Ruminative Responses Scale (Nolen-Hoeksema et al., 1993). We found in depressed, but not in healthy, individuals that greater DMN dominance was associated with higher levels of depressive rumination and lower levels of reflective rumination. In a follow-up analysis, we examined the relations in activation between the right anterior insula, a component of the SN, and TPN and DMN. The right anterior insula has been implicated causally in switching between modes of relative DMN and TPN dominance (Sridharan, Levitin, & Menon, 2008) and in interoceptive error detection (Paulus & Stein, 2006). In this analysis, we estimated response of the right anterior insula at the time of initiations of ascent in DMN and in TPN activity in depressed and healthy individuals. Whereas in the depressed participants we found increased right insula activation at the onset of increases in TPN activation, in the healthy control participants we found increased insula response at the onset of increases in DMN activity. These findings are consistent with the formulation that the DMN supports the representation of negative, self-related information in depression, and that the right insula, when prompted by heightened levels of DMN activity in MDD, initiates potentially adaptive engagement of the TPN.
Importantly, such multi-network conceptualizations of psychopathology as this increasingly are being presented and utilized in clinical neuroscience (Menon, 2011). Supporting this approach, Dannlowski (2009) and others have described anomalous functional connectivity between the amygdala and the DLPFC in MDD. Given that these two structures are implicated in the functioning of the SN and EN networks, respectively, altered connectivity between these structures may reflect disrupted interactions between the SN and EN networks. Sheline and colleagues (2010) recently presented data relevant to understanding how, at a neural level, there might be simultaneous dysfunction in the default mode, salience, and executive networks in MDD. Using resting-state fMRI, Sheline et al. found a dorsomedial prefrontal region (which they call ‘the dorsal nexus’) that showed abnormally elevated connectivity to SN, DMN, and EN networks in depressed individuals. Such integrative work as this will be instrumental in achieving more elegant and therapeutically useful neural-network models of MDD.
Future Directions
Our review of functional neuroimaging studies of MDD indicates that understanding depression from the perspective afforded by research examining the intrinsic functional organization of the brain could represent a significant contribution to neural theory of this disorder. Our understanding of resting-state networks, both within and between individuals, has advanced significantly over the past decade. With this increased knowledge, however, we have also had to recognize and acknowledge important methodological issues (Cole, Pathak, & Schneider, 2010). While methods used to identify resting-state networks have often involved seed regions or individual-level ICA, these analyses generally do not take into account small but significant variation across individuals in DMN and other networks. Researchers have also identified significant variability in the spatiotemporal characteristics of these networks (Chang & Glover, 2010); indeed, networks like the DMN may actually consist of smaller sub-networks that underlie different aspects of cognitive functions (Leech, Kamourieh, Beckmann, & Sharp, 2011). Despite these caveats, however, careful application of group-level analyses will likely lead to stronger, more accurate, and more systematic characterizations of network dysfunction not only in MDD, but in other psychiatric disorders as well.
While network-level conceptions of neural dysfunction in MDD provide an elegant, integrative framework through which to understand this disorder, they also raise important questions. Are there factors that might unite and integrate the network-level findings we have presented here in the context of a more cohesive framework? For example, we identified robustly increased SN response and decreased EN response to negative stimuli in MDD. While this pattern of findings suggests both over-response and under-regulation of emotional systems in depression, it is not clear what neural factors might unite these findings. A potential mechanism for integrating these apparently disparate effects is the documented decrease in the availability of striatal dopamine in MDD (Meyer et al., 2006) along cortico-striatal-thalamic pathways that connect ventral to dorsal cortical processing regions. This is but one possible mechanism; future research that uses multimodal imaging methods, including combined fMRI and PET, will be important in elucidating the neural and molecular links among different brain networks that are disrupted in major depression.
Acknowledgments
Preparation of this paper was facilitated by NIMH Grants MH59259, MH74849, and MH080683 awarded to Ian H. Gotlib.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abler B, Erk S, Herwig U, Walter H. Anticipation of aversive stimuli activates extended amygdala in unipolar depression. Journal of Psychiatric Research. 2007;41(6):511–522. doi: 10.1016/j.jpsychires.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Aihara M, Ida I, Yuuki N, Oshima A, Kurnano H, Takahashi K, et al. HPA axis dysfunction in unmedicated major depressive disorder and its normalization by pharmacotherapy correlates with alteration of neural activity in prefrontal cortex and limbic/paralimbic regions. Psychiatry Research-Neuroimaging. 2007;155(3):245–256. doi: 10.1016/j.pscychresns.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Bar KJ, Wagner G, Koschke M, Boettger S, Boettger MK, Schlosser R, et al. Increased prefrontal activation during pain perception in major depression. Biological Psychiatry. 2007;62:1281–1287. doi: 10.1016/j.biopsych.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philosophical Transactions of the Royal Society B-Biological Sciences. 2005;360(1457):1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bench CJ, Friston KJ, Brown RG, Scott LC, Frackowiak RSJ, Dolan RJ. The Anatomy of Melancholia - Focal Abnormalities of Cerebral Blood-Flow in Major Depression. Psychological Medicine. 1992;22(3):607–615. doi: 10.1017/s003329170003806x. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Boland RJ, Keller MB. Course and outcome of depression. In: Gotlib IH, Hammen CL, editors. Handbook of Depression. New York: Guilford Press; 2009. [Google Scholar]
- Brody AL, Saxena S, Mandelkern MA, Fairbanks LA, Ho ML, Baxter LR. Brain metabolic changes associated with symptom factor improvement in major depressive disorder. Biological Psychiatry. 2001;50(3):171–178. doi: 10.1016/s0006-3223(01)01117-9. [DOI] [PubMed] [Google Scholar]
- Brody AL, Saxena S, Stoessel P, Gillies LA, Fairbanks LA, Alborzian S, et al. Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy - Preliminary findings. Archives of General Psychiatry. 2001;58(7):631–640. doi: 10.1001/archpsyc.58.7.631. [DOI] [PubMed] [Google Scholar]
- Canli T, Sivers H, Thomason ME, Whitfield-Gabrieli S, Gabrieli JDE, Gotlib IH. Brain activation to emotional words in depressed vs healthy subjects. Neuroreport. 2004;15(17):2585–2588. doi: 10.1097/00001756-200412030-00005. [DOI] [PubMed] [Google Scholar]
- Chang C, Glover GH. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage. 2010;50(1):81–98. doi: 10.1016/j.neuroimage.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Pathak S, Schneider W. Identifying the brain’s most globally connected regions. Neuroimage. 2010;49(4):3132–3148. doi: 10.1016/j.neuroimage.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Cooney RE, Joormann J, Eugene F, Dennis EL, Gotlib IH. Neural correlates of rumination in depression. Cognitive Affective & Behavioral Neuroscience. 2010;10(4):470–478. doi: 10.3758/CABN.10.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel - now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LLB, Parvizi J, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience. 2000;3(10):1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Konrad C, Domschke K, Bauer J, Kugel H, et al. Reduced amygdala-prefrontal coupling in major depression: association with MAOA genotype and illness severity. International Journal of Neuropsychopharmacology. 2009;12(1):11–22. doi: 10.1017/S1461145708008973. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Raichle ME. Neuroanatomical Circuits in Depression - Implications for Treatment Mechanisms. Psychopharmacology Bulletin. 1992;28(3):261–274. [PubMed] [Google Scholar]
- Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A Functional Anatomical Study of Unipolar Depression. Journal of Neuroscience. 1992;12(9):3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Human Brain Mapping. 2008;29(6):683–695. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Scheuerecker J, Albrecht J, Kleemann AM, Muller-Schunk S, Koutsouleris N, et al. Neuronal correlates of emotional processing in patients with major depression. World Journal of Biological Psychiatry. 2009;10(3):202–208. doi: 10.1080/15622970701624603. [DOI] [PubMed] [Google Scholar]
- Fu CHY, Williams SCR, Brammer MJ, Suckling J, Kim J, Cleare AJ, et al. Neural responses to happy facial expressions in major depression following antidepressant treatment. American Journal of Psychiatry. 2007;164(4):599–607. doi: 10.1176/ajp.2007.164.4.599. [DOI] [PubMed] [Google Scholar]
- Fu CHY, Williams SCR, Cleare AJ, Brammer MJ, Walsh ND, Kim J, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment - A prospective, event-related functional magnetic resonance imaging study. Archives of General Psychiatry. 2004;61(9):877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- Fu CHY, Williams SCR, Cleare AJ, Scott J, Mitterschiffthaler MT, Walsh ND, et al. Neural responses to sad facial expressions in major depression following cognitive behavioral therapy. Biological Psychiatry. 2008;64(6):505–512. doi: 10.1016/j.biopsych.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Germain A, Nofzinger EA, Meltzer CC, Wood A, Kupfer DJ, Moore RY, et al. Diurnal variation in regional brain glucose metabolism in depression. Biological Psychiatry. 2007;62(5):438–445. doi: 10.1016/j.biopsych.2006.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Hammen CL. Handbook of depression. New York: Guilford Press; 2009. [Google Scholar]
- Gotlib IH, Joormann J. Cognition and Depression: Current Status and Future Directions. Annual Review of Clinical Psychology. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Sivers H, Gabrieli JDE, Whitfield-Gabrieli S, Goldin P, Minor KL, et al. Subgenual anterior cingulate activation to valenced emotional stimuli in major depression. Neuroreport. 2005;16(16):1731–1734. doi: 10.1097/01.wnr.0000183901.70030.82. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry. 2007;62(5):429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-State Functional Connectivity Reflects Structural Connectivity in the Default Mode Network. Cerebral Cortex. 2009;19(1):72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M, Nakamura L, Pascual-Leone A, Fregni F. Has repetitive transcranial magnetic stimulation (rTMS) treatment for depression improved? A systematic review and meta-analysis comparing the recent vs. the earlier rTMS studies. Acta Psychiatrica Scandinavica. 2007;116(3):165–173. doi: 10.1111/j.1600-0447.2007.01049.x. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Chen G, Thomason ME, Johnson RF, Gotlib IH. Investigating neural primacy in Major Depressive Disorder: Multivariate granger causality analysis of resting-state fMRI time-series data. Molecular Psychiatry. 2011;16:763–772. doi: 10.1038/mp.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in Major Depressive Disorder: Implications for adaptive and maladaptive rumination. Biological Psychiatry. 2011;70(4):327–733. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwig U, Bruhl AB, Kaffenberger T, Baumgartner T, Boeker H, Jancke L. Neural correlates of ‘pessimistic’ attitude in depression. Psychological Medicine. 2010;40(5):789–800. doi: 10.1017/S0033291709991073. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Nolen-Hoeksema S, Mitchell KJ, Levin Y. Medial cortex activity, self-reflection and depression. Social Cognitive and Affective Neuroscience. 2009;4(4):313–327. doi: 10.1093/scan/nsp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG, Burton H. Projection from medial pulvinar to amygdala in primates. Brain Research. 1976;104(1):142–147. doi: 10.1016/0006-8993(76)90654-5. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SCR, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biological Psychiatry. 2005;58(11):843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Evans KR, Kruger S, Mayberg HS, Meyer JH, McCann S, et al. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. American Journal of Psychiatry. 2001;158(6):899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- Kimbrell TA, Ketter TA, George MS, Little JT, Benson BE, Willis MW, et al. Regional cerebral glucose utilization in patients with a range of severities of unipolar depression. Biological Psychiatry. 2002;51(3):237–252. doi: 10.1016/s0006-3223(01)01216-1. [DOI] [PubMed] [Google Scholar]
- Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH. Neural responses to monetary incentives in major depression. Biological Psychiatry. 2008;63(7):686–692. doi: 10.1016/j.biopsych.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn Y, Freedman N, Lester H, Krausz Y, Chisin R, Lerer B, et al. Tc-99m-HMPAO SPECT study of cerebral perfusion after treatment with medication and electroconvulsive therapy in major depression. Journal of Nuclear Medicine. 2007;48(8):1273–1278. doi: 10.2967/jnumed.106.039354. [DOI] [PubMed] [Google Scholar]
- Krausz Y, Freedman N, Lester H, Barkai G, Levin T, Bocher M, et al. Brain SPECT study of common ground between hypothyroidism and depression. International Journal of Neuropsychopharmacology. 2007;10(1):99–106. doi: 10.1017/S1461145706006481. [DOI] [PubMed] [Google Scholar]
- Kumari V, Mitterschiffthaler MT, Teasdale JD, Malhi GS, Brown RG, Giampietro V, et al. Neural abnormalities during cognitive generation of affect in treatment-resistant depression. Biological Psychiatry. 2003;54(8):777–791. doi: 10.1016/s0006-3223(02)01785-7. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biological Psychiatry. 2004;55(6):578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Leech R, Kamourieh S, Beckmann CF, Sharp DJ. Fractionating the Default Mode Network: Distinct Contributions of the Ventral and Dorsal Posterior Cingulate Cortex to Cognitive Control. Journal of Neuroscience. 2011;31(9):3217–3224. doi: 10.1523/JNEUROSCI.5626-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: A proposed model of depression. Journal of Neuropsychiatry and Clinical Neurosciences. 1997;9(3):471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. American Journal of Psychiatry. 1999;156(5):675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends in Cognitive Sciences. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Meyer JH, McNeely HE, Sagrati S, Boovariwala A, Martin K, Verhoeff N, et al. Elevated putamen D-2 receptor binding potential in major depression with motor retardation: An [C-11] raclopride positron emission tomography study. American Journal of Psychiatry. 2006;163(9):1594–1602. doi: 10.1176/ajp.2006.163.9.1594. [DOI] [PubMed] [Google Scholar]
- Mitterschiffthaler MT, Kumari V, Malhi GS, Brown RG, Giampietro VP, Brammer MJ, et al. Neural response to pleasant stimuli in anhedonia: an fMRI study. Neuroreport. 2003;14(2):177–182. doi: 10.1097/00001756-200302100-00003. [DOI] [PubMed] [Google Scholar]
- Mitterschiffthaler MT, Williams SCR, Walsh ND, Cleare AJ, Donaldson C, Scott J, et al. Neural basis of the emotional Stroop interference effect in major depression. Psychological Medicine. 2008;38(2):247–256. doi: 10.1017/S0033291707001523. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM. Thalamic connections of the insula in the rhesus-monkey and comments on the paralimbic connectivity of the medial pulvinar nucleus. Journal of Comparative Neurology. 1984;227(1):109–120. doi: 10.1002/cne.902270112. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Morrow J, Fredrickson BL. Response styles and the duration of episodes of depressed mood. Journal of Abnormal Psychology. 1993;102(1):20–28. doi: 10.1037//0021-843x.102.1.20. [DOI] [PubMed] [Google Scholar]
- O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: A multisite randomized controlled trial. Biological Psychiatry. 2007;62(11):1208–1216. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Osuch EA, Bluhm RL, Williamson PC, Theberge J, Densmore M, Neufeld RWJ. Brain activation to favorite music in healthy controls and depressed patients. Neuroreport. 2009;20(13):1204–1208. doi: 10.1097/WNR.0b013e32832f4da3. [DOI] [PubMed] [Google Scholar]
- Padmala S, Lim S, Pessoa L. Pulvinar and affective significance: responses track moment-to-moment visibility. Frontiers in Human Neuroscience. 2010:1–9. doi: 10.3389/fnhum.2010.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biological Psychiatry. 2006;60(4):383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Perico CAM, Skaf CR, Yamada A, Duran F, Buchpiguel CA, Castro CC, et al. Relationship between regional cerebral blood flow and separate symptom clusters of major depression: A single photon emission computed tomography study using statistical parametric mapping. Neuroscience Letters. 2005;384(3):265–270. doi: 10.1016/j.neulet.2005.04.088. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Adolphs R. Emotion processing and the amygdala: from a ‘low road’ to ‘many roads’ of evaluating biological significance. Nature Reviews Neuroscience. 2010;11(11):773–782. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, et al. Reduced Caudate and Nucleus Accumbens Response to Rewards in Unmedicated Individuals With Major Depressive Disorder. American Journal of Psychiatry. 2009;166(6):702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralchle ME, Snyder AZ. A default mode of brain function: A brief history of an evolving idea. Neuroimage. 2007;37(4):1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Saxena S, Brody AL, Ho ML, Alborzian S, Ho MK, Maidment KM, et al. Cerebral metabolism in major depression and obsessive-compulsive disorder occurring separately and concurrently. Biological Psychiatry. 2001;50(3):159–170. doi: 10.1016/s0006-3223(01)01123-4. [DOI] [PubMed] [Google Scholar]
- Scheuerecker J, Meisenzahl EM, Koutsouleris N, Roesner M, Schopf V, Linn J, et al. Orbitofrontal volume reductions during emotion recognition in patients with major depression. Journal of Psychiatry & Neuroscience. 2010;35(5):311–320. doi: 10.1503/jpn.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, et al. The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan ZZ, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(24):11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, et al. Common blood flow changes across visual tasks .2. Decreases in cerebral cortex. Journal of Cognitive Neuroscience. 1997;9(5):648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: Related and independent features. Biological Psychiatry. 2007;61(2):198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Skaf CR, Yamada A, Garrido GEJ, Buchpiguel CA, Akamine S, Castro CC, et al. Psychotic symptoms in major depressive disorder are associated with reduced regional cerebral blood flow in the subgenual anterior cingulate cortex: a voxel-based single photon emission computed tomography (SPECT) study. Journal of Affective Disorders. 2002;68(2–3):295–305. doi: 10.1016/s0165-0327(00)00365-7. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigo IA, Simmons AN, Matthews SC, Craig AD, Paulus MP. Association of Major Depressive Disorder With Altered Functional Brain Response During Anticipation and Processing of Heat Pain. Archives of General Psychiatry. 2008;65(11):1275–1284. doi: 10.1001/archpsyc.65.11.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, et al. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biological Psychiatry. 2005;57(3):201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Suslow T, Konrad C, Kugel H, Rumstadt D, Zwitserlood P, Schoning S, et al. Automatic Mood-Congruent Amygdala Responses to Masked Facial Expressions in Major Depression. Biological Psychiatry. 2010;67(2):155–160. doi: 10.1016/j.biopsych.2009.07.023. [DOI] [PubMed] [Google Scholar]
- Townsend JD, Eberhart NK, Bookheimer SY, Eisenberger NI, Foland-Ross LC, Cook IA, et al. fMRI activation in the amygdala and the orbitofrontal cortex in unmedicated subjects with major depressive disorder. Psychiatry Research-Neuroimaging. 2010;183(3):209–217. doi: 10.1016/j.pscychresns.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman A. Solutions to the binding problem: Progress through controversy and convergence. Neuron. 1999;24(1):105–110. doi: 10.1016/s0896-6273(00)80826-0. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B, Pedersen AR, Egander A, Landbo B, Rasmussen NA, et al. The Danish PET/depression project: PET findings in patients with major depression. Psychological Medicine. 2001;31(7):1147–1158. doi: 10.1017/s0033291701004469. [DOI] [PubMed] [Google Scholar]
- Wang LH, Labar KS, Smoski M, Rosenthal MZ, Dolcos F, Lynch TR, et al. Prefrontal mechanisms for executive control over emotional distraction are altered in major depression. Psychiatry Research-Neuroimaging. 2008;163(2):143–155. doi: 10.1016/j.pscychresns.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R, Danziger S, Owen V, Rafal R. Deficits in spatial coding and feature binding following damage to spatiotopic maps in the human pulvinar. Nature Neuroscience. 2002;5(2):99–100. doi: 10.1038/nn794. [DOI] [PubMed] [Google Scholar]
- Whalen PJ. The uncertainty of it all. Trends in Cognitive Sciences. 2007;11(12):499–500. doi: 10.1016/j.tics.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Yang JC. Functional neuroanatomy in depressed patients with sexual dysfunction: Blood oxygenation level dependent functional MR imaging. Korean Journal of Radiology. 2004;5(2):87–95. doi: 10.3348/kjr.2004.5.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]