Abstract
Background
Neonatal hypoxia-ischemia (HI) remains a major cause of severe brain damage and is often associated with high mortality and lifelong disability. Immature brains are extremely sensitive to hypoxia-ischemia, shown as prolonged mitochondrial neuronal death. Sodium pyruvate (SP), a substrate of the tricarboxylic acid cycle and an extracellular antioxidant, has been considered as a potential treatment for hypoxic-ischemic encephalopathy (HIE), but its effects have not been evaluated in appropriate animal models for hypoxic-ischemic encephalopathy (HIE).
Methods
This investigation employed primary cortical neuron cultures derived from neonatal rats subjected to oxygen and glucose deprivation (OGD) and a well-established neonatal rat hypoxia-ischemia model.
Results
HI caused brain tissue loss and impaired sensorimotor function and spatial memory while SP significantly reduced brain damage and improved neurological performance. These neuroprotective effects of SP are likely the result of improved cerebral metabolism as demonstrated by maintaining ATP levels and preventing an increase in intracellular reactive oxygen species (ROS) levels. SP treatment also decreased levels of Bax, a death signal for immature neurons, blocked caspases-3 activation, and activated a key survival signaling kinase, Akt, both in vitro and in vivo.
Conclusion
SP protected neonatal brain from hypoxic-ischemic injury through maintaining cerebral metabolism and mitochondrial function.
Introduction
Cerebral hypoxia-ischemia (HI) remains a leading cause of severe brain damage that occurs in 0.1-0.2% of term or near-term infants, among whom approximately 20% die and up to 40% of the survivors often suffer devastating disabilities, such as cerebral palsy, mental retardation, and epilepsy1. Because of high mortality and poor prognosis, hypoxic-ischemic damage in neonatal brains continues to be a major medical problem2. Unfortunately, despite our increasing understanding of the mechanisms of neuronal injury, no effective therapy is currently available to mitigate brain damage and improve the prognosis of these newborn patients3.
Following HI, immature neurons die over days to week by a variety of mechanisms and the extent depends on the severity of the insult and the maturation state of the animal4,5. The mechanisms underlying the delayed neuronal death are not completely understood, but likely include energy failure, rapid increase in intracellular reactive oxygen species (ROS), calcium influx, trophic factors deprivation and alteration of the Bcl-2 family of proteins6-8. One mechanism pertaining to the death of immature neurons is the accumulation of Bax, which is highly expressed in the immature brains, to mitochondria where it cleaves pro-caspase-3 resulting in the activation of caspase 35,6.
In recent years, sodium pyruvate (SP) has demonstrated neuroprotective activity in a variety of brain injury models9-15. As a substrate for the tricarboxylic acid cycle (TCA) cycle, SP acts as a free radical scavenger16,17 and increases the Bcl-2/Bax ratio under oxidative stress18. Since oxidative stress is a major inducer of neuronal death following HI, SP is considered a potential candidate to protect immature neurons following hypoxia-ischemia encephalopathy (HIE). Recent studies also demonstrated that ethyl pyruvate, a stable lipophilic ester derivative of pyruvate, reduced hypoxic-ischemic injury to neonatal brain via its anti-cell death and anti-inflammatory mechanism19. Whether SP provides similar neuroprotection against hypoxic-ischemic injury is currently unknown.
This investigation intended to evaluate the effects of SP in protecting neurons from hypoxic-ischemic injury using corresponding in vitro and in vivo models. The evaluation included the time course of neuronal injury following oxygen glucose deprivation (OGD) and the extent of brain injury after HI in the presence or absence of SP. Long-term behavior development was also compared between neonatal rats who received vehicle or SP treatment. Finally, we explored potential mechanisms of SP’s effects in the same OGD and neonatal HI model. Overall, this investigation established the therapeutic benefits and limitations of SP in the treatment of HIE.
Results
Characterization of SP’s neuroprotective effects in vitro and in vivo
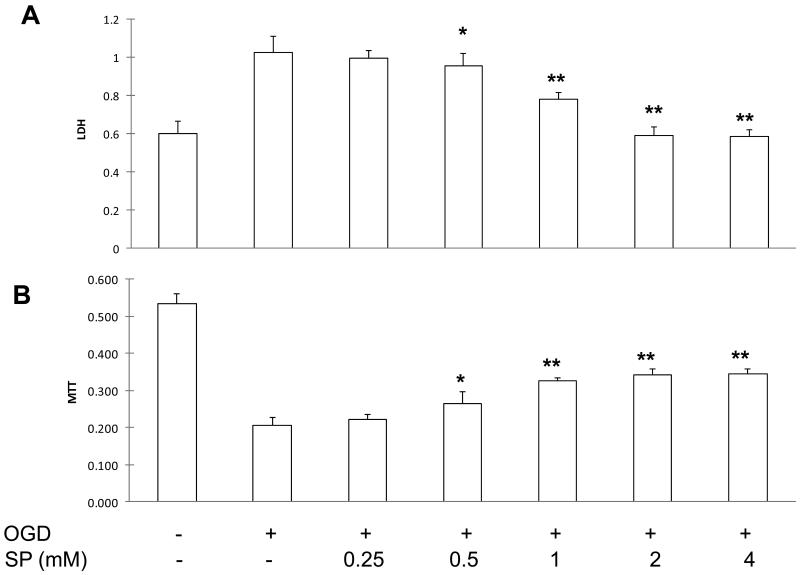
To evaluate the neuroprotective effects of SP, we first determined its effective dose in protecting primary neurons under OGD. At 24 h of reoxygenation, LDH release nearly doubled (no treatment, Figure 1A) and MTT levels were less than 40% of the control levels (Figure 1B). As the concentration of SP increased in the culture media, LDH levels were decreased in the culture media (Figure 1A) and MTT levels were increased (Figure 1B), both dose-dependently, indicating a decrease in neuronal injury and an increase in neuronal viability. Because the best SP neuroprotective effects were seen at 2 mM, we decided to use this concentration in the following in vitro studies.
Figure 1.
SP promoted neuronal survival under OGD. SP reduced OGD induced neuronal injury dose-dependently, as demonstrated by reduced LDH levels in culture media (A) and increased MTT levels in cell lysates (B) at 24 hours of oxygenation. * p <0.05 and ** p <0.01, compared with OGD alone (n=6).
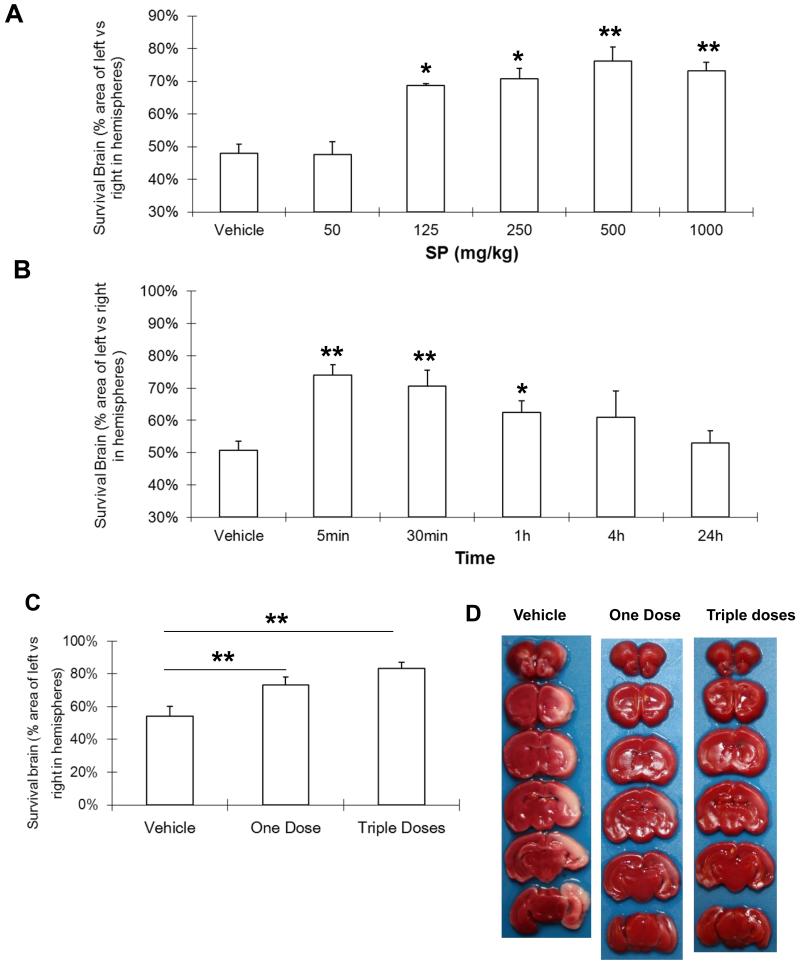
We then investigated SP’s neuroprotective effects in vivo using a well-established neonatal rat HI model20. At 48 h of recovery, SP at concentrations ranging from 125 to 1000 mg/kg markedly reduced hypoxic-ischemic injury to the immature brain (Figure 2A) and the maximal protection was achieved at 500 mg/kg, which increased the surviving brain vol from 48.1% ± 2.7% in the vehicle-treated group to 76.2% ±4.2%. Over 24 h following HI, significant neuroprotection was observed when SP was given within the first h of recovery, with the best protection if SP was administered at 5 min (Figure 2B). In addition, no significant difference was observed between one dose of SP (5 min post HI) and triple doses of SP (Figure 2C & 2D). Therefore, we chose the single SP dose (500 mg/kg) administered intraperitoneally at 5 min post-HI in all subsequent in vivo experiments.
Figure 2.
SP reduced hypoxic-ischemic injury to neonatal rate brain. A. Dose response for SP given 30 min after HI. Vehicle (n=5); SP-50: 50 mg/kg, n=3; SP-125: 125 mg/kg, n=4; SP-250: 250 mg/kg, n=9; SP-500: 500 mg/kg (n=8); SP-1000: 1000 mg/kg (n=8). B. Therapeutic window for SP (500 mg/Kg). Vehicle (n=5) was administered at 5 min, SP was administered at 5 min (n=8), 30 min (n=6), 1 h (n=6), 4 h (n=6), and 24 h (n=9). C. Dosing frequency for SP (500 mg/kg/time). There was no significant difference between one dose (5 min, n=11) and triple dose (5, 65 and 125min, n=11) treated groups. Vehicle (n=8). D. Representative coronal brain sections of vehicle, one dose and triple dose treated group. * p <0.05, ** p <0.01, compared with vehicle treated group.
SP reduced hypoxic-ischemic brain damage
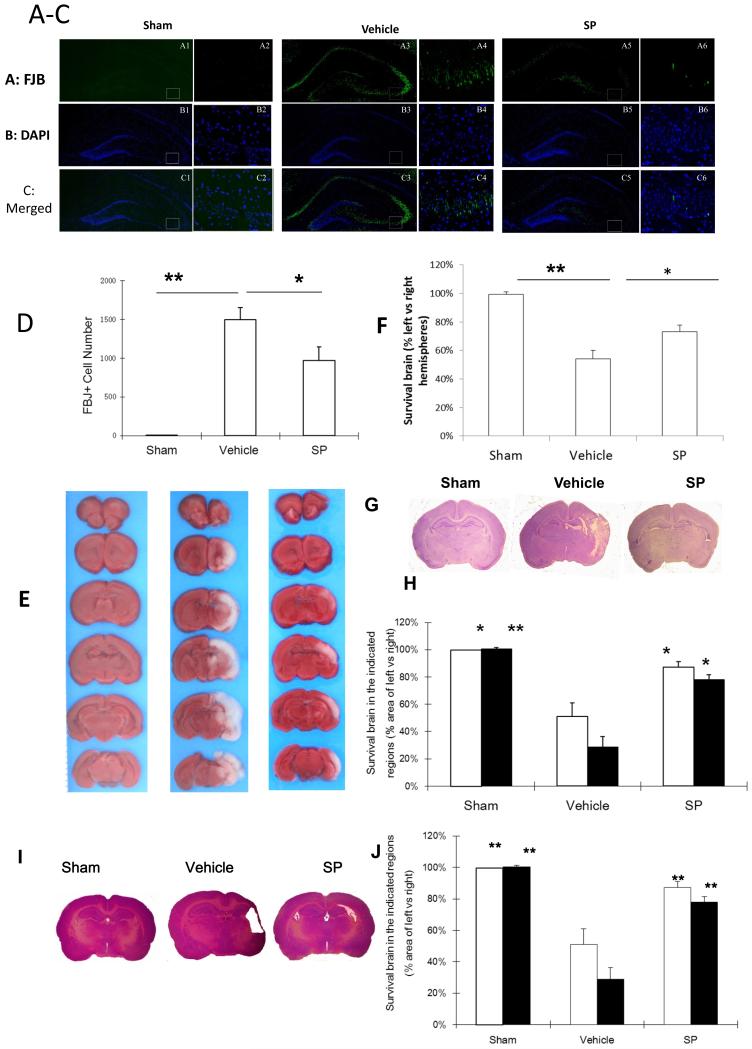
To determine if SP’s effects are due to the reduction in neuronal damage, we labeled injured neurons with FJB at 24 h of recovery (Figure 3A-C). FJB-positive neurons were degenerating neurons and displayed distinct apoptotic nuclei with either condensed or fragmented morphology. Compared to the vehicle-treated group, FJB-positive neurons in the left/ipsilateral cortex at 24 h post-HI were significantly reduced by SP treatment (Figure 3D).
Figure 3.
SP promoted long-term neuronal survival following HI. A: The distribution of FJB+ neurons (green) in ipsilateral cortex (10 ×, A1, A3, A5; 20 ×, A2, A4 A6 from each corresponding square). B: The cell nuclei in the same sections in panel A were stained with DAPI (blue). C: Merged images from corresponding panels A and B. D. Quantification of FJB+ in the ipsilateral hippocampus. n=3-6 per group. E. Representative coronal brain sections from at 2 week old rats. F. Quantification of surviving cortical or hippocampal tissues (sham, n=3; vehicle, n=8; SP, n=8) G. Representative coronal crysel violet stained sections from 14 day-old rats treated with sham (n=3), vehicle (n=5) or SP (n=9). H. Quantification of surviving cortical or hippocampal tissues compared with vehicle treated group. Bar graph: Cortex: White; Hippocampus: Black. I. Representative coronal brain sections from at 2 month old rats (sham, n=3; vehicle, n=8; SP, n=8). J. Quantification of surviving cortical (white) or hippocampal (black) tissues. * p < 0.05, ** p < 0.01, compared to vehicle.
We also analyzed the neuroprotective effects of SP on brain morphology at 7 days (Figure 3E & 3F), two week (Figure 3G & 3H) and 7 week (Figure 3I & 3J) of recovery following HI. At 7 days of recovery, SP treatment increased the surviving brain tissues from 45.3 ± 11.9% to 80 ± 5.5% (cortex) and 39.2 ± 8.7% to 67.3 ± 9% (hippocampus). At 7 week of recovery, SP treatment increased the surviving brain tissues from 51 ± 9.9% to 87.2 ± 3.9% (cortex) and 28.7 ± 7.4% to 77.9% ± 3.6% (hippocampus). Therefore, SP’s neuroprotective effects against hypoxic-ischemic damage to immature brains were sustained.
SP improved long-term behavior development
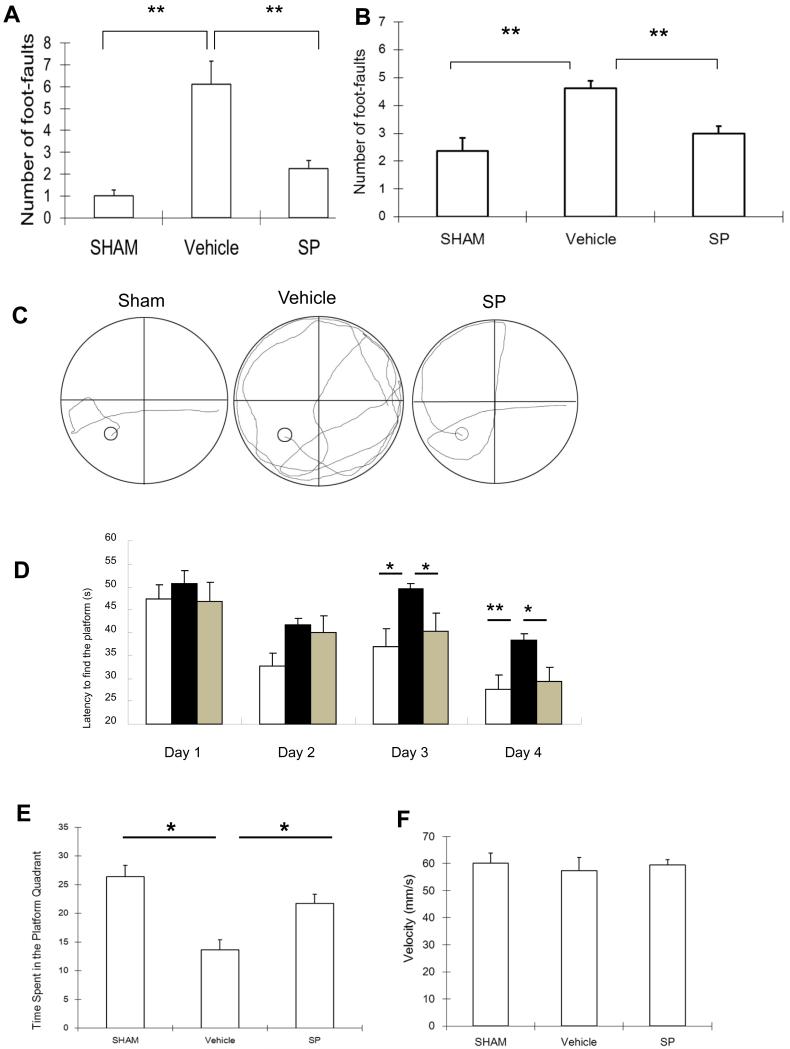
Rats subjected to HI showed a significant impairment of sensorimotor behavior when evaluated by the foot-fault test at 3 week of recovery. When the total number of foot-faults per 50 steps was recorded within 5 min, rats in the vehicle-treated group showed significantly increased foot-faults compared with the sham group (right forelimb, Figure 4A; right hind limb, Figure 4B). SP treatment resulted in a significant reduction in both right forelimb and hind limb foot-faults.
Figure 4.
SP improved the performance of foot-faults and Morris water maze following HI. Foot-faults test results at 3 weeks of recovery on P28 rats. Number of foot-faults of right forelimbs (A) or right hind limbs (B) per 50 steps were counted within 5 min. n=8 per group. C. Water maze was performed at 7 week of recovery. Representative computer tracing of one swimming trail for each treatment was shown. D. The latency to find the hidden platform was significantly higher in vehicle-treated rats than SP-treated rats. Bar graph: Sham: white; Vehicle: black; SP: gray. E. Vehicle-treated rats spent significantly less time than the vehicle-treated controls. F. No difference in the velocity in the probe trial among these three groups. n=8 per group. Mean±SEM. * p < 0.05; ** p < 0.01.
The Morris water maze test was performed at 8 week of recovery. In a spatial maze test, the latency in the vehicle-treated group was significantly increased, whereas SP treatment significantly reduced the latency time (Figure 4C & 4D). There was also no significant difference in the latency among groups in the cued maze test (data not shown). The probe trial was conducted following the spatial maze training and percent time spent in the target quadrant was recorded (Figure 4F). The time spent in the target quadrant was reduced by about 50% in the vehicle-treated group (sham vs. vehicle = 26.4 ± 2 vs. 13.7 ± 1.7). SP treatment significantly increased time spent in the platform (21.7 ± 1.6) (Figure 4E). There was no significant difference in velocity among groups in the water maze test (Figure 4F).
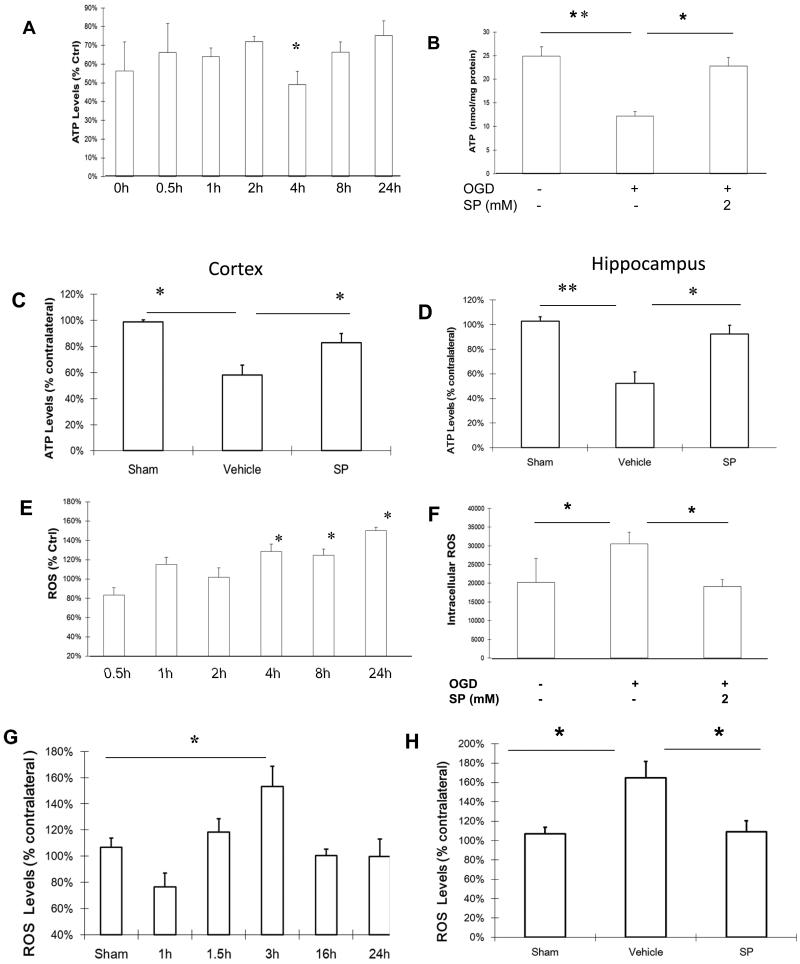
SP suppressed ROS production and maintained ATP production
Upon reoxygenation following OGD, neuronal ATP levels were below the control levels (100%), reaching the lowest levels (50% of controls) at about 4 h and gradually recovered, but did not reach the control levels by 24 h of recovery (Figure 5A). Providing SP in the culture media following reoxygenation elevated ATP levels in cultured neurons (Figure 5B). According to the time course of ROS increase following reoxygenation, the ATP levels in cortical tissue were measured at 3 h and 16 h of recovery. As shown in Figure 5C (3 h) and 5D (16 h), ATP levels in the cortical tissue were significantly reduced at both time points compared to the corresponding vehicle group and SP treatment prevented ATP depletion.
Figure 5.
SP suppressed the increase of intracellular ROS levels following OGD and HI. A.ATP levels in cell lysates over 24 h after OGD. B. At 4 h after OGD, SP maintained ATP levels at control levels (n=3). Following HI, ATP levels were reduced in cortical (C) and hippocampal (D) tissues in the vehicle group, but were maintained in the SP treated group. E. Change of intracellular ROS levels over 24 h after OGD. F. At 24 h following OGD, intracellular ROS levels were significantly increased, but remained at control levels for SP treated neurons (n=6). G. Tissue ROS levels after HI over 24 h, with the highest level at 3h. H. At 3 h following HI, tissue ROS levels remain at control (sham) levels in the SP treatment group. ROS levels in the ipsilateral cortex were expressed as a percentage of ROS levels in the corresponding contralateral cortex. * p < 0.05, ** p < 0.01. n=3-6 in each group.
To investigate potential mechanisms of SP’s neuroprotective effects, we first measured intracellular ROS levels in neurons following OGD with or without SP treatment. As shown in Figure 5E, after OGD, the intracellular ROS levels increased gradually in a time-dependent manner from about one h following reoxygenation and reached their peaks (150% of control) at 24 h. SP treatment at the beginning of the reoxygenation prevented this increase as measured at 24 h (Figure 5F). Following HI, the tissue ROS levels also increased, but with a different time course. As shown in Figure 5G, HI caused a rapid and significant increase in ROS levels in the ipsilateral cortex, peaking at 3 h and decreasing to the level of contralateral side by 16 h of recovery. If SP (500 mg/kg) was administered at 5 min following hypoxia, tissue ROS levels remained the same as those from sham animals when evaluated at 3 h of recovery (Figure 5H).
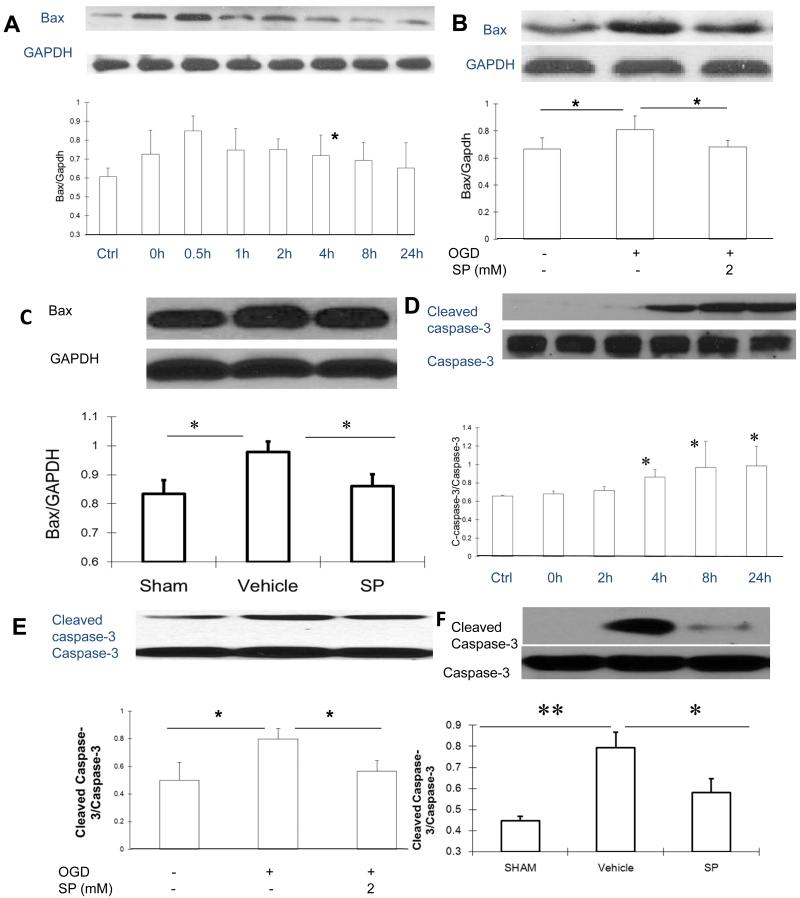
SP reduced Bax levels and caspases-3 activation
Because the accumulation of Bax at the mitochondria is considered a critical event to trigger the death of immature neurons following hypoxic-ischemic injury6, we measured the levels of Bax following reoxygenation after OGD. The levels of Bax immediately increased upon reoxygenation, reached a peak level at 0.5 h, and gradually declined afterwards and returned to control levels at 24 h (Figure 6A). This rapid increase of Bax levels was not seen at 0.5 h if SP was added at the reoxygenation (Figure 6B). At 3 h following HI to neonatal rat brain, we found that Bax was increased in the vehicle group, but remained at control (sham treated) levels if SP was administered (5 min, 500 mg/kg, Figure 6C).
Figure 6.
SP suppressed Bax increase and inhibited caspases activation following OGD and HI. A. Changes of Bax levels over 24 h following OGD. B. At 0.5 h, Bax levels remained at control levels in SP treated cells. C. Bax levels in cortical tissues derived from sham (n=4), vehicle treated (n=12) or SP treated (n=9) animals at 3 h following OGD. D. Levels of cleaved caspase-3 were increased following OGD, reaching a peak level at 24 h. E. Levels of cleaved caspase-3 at 24 h following OGD with or without SP treatment (n=3). F. Levels of cleaved and total caspases 3 at 24 h of recovery. Sham (n=7), vehicle treated (n=9) and SP treated (n=8). * p < 0.05, ** p < 0.01
Next, we characterized the time course of caspases-3 activation (cleaved caspase-3/caspase-3), a distinguished sign of neuronal apoptosis. As shown in Figure 6D, the levels of cleaved caspases-3 started to increase at 2 h, reached a peak level at 8 h, and remained elevated at 24 h. Adding SP at the onset of reoxygenation prevented the caspase-3 activation when evaluated 24 h later (Figure 6E). We chose 24 h of recovery to examine the level of activated caspase-3, because 24 h was previously reported as the peak of caspases-3 activation following hypoxic-ischemic injury to neonatal rat brain19. As shown in Figure 6F, compared to the sham group, the levels of cleaved caspase-3 were dramatically increased in the vehicle group but were significantly lower when rats received SP treatment.
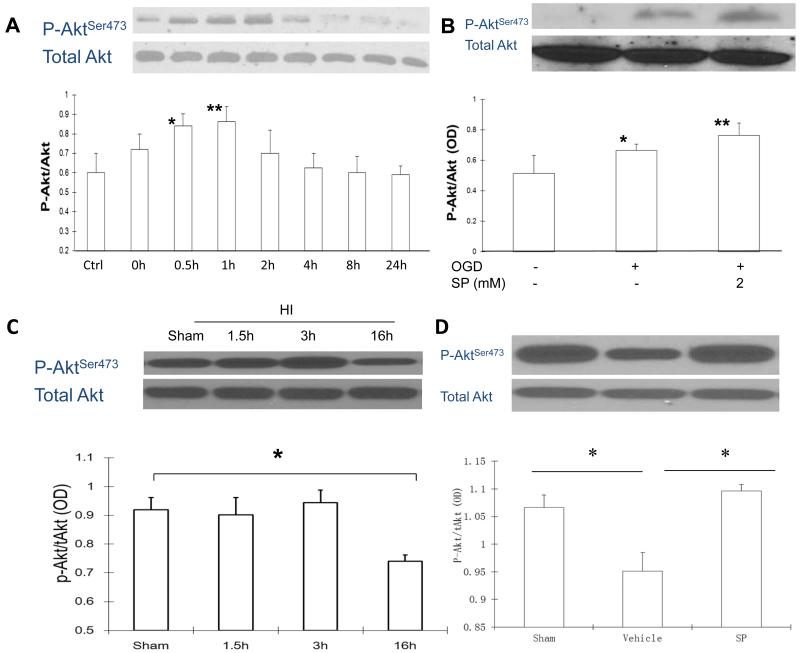
SP increased PI3K/Akt survival signaling
One major upstream inhibitor of Bax activity is the activation of the PI3/Akt signal pathway 21,22. Following OGD, the levels of P-AktSer473 increased above the control levels, reached the maximum level at 1 h and gradually declined after 2 h, while no significant difference was observed for the levels of total Akt (Figure 7A). Since the maximum increase of P-Akt Ser473 occurs at 1 h after reoxygenation, we chose this time point to investigate the effects of SP on Akt activity. As shown in Figure 7B, levels of P-Akt Ser473 were further increased when SP was given at the onset of reoxygenation, suggesting that SP may promote neuronal survival by activating the PI3K/Akt survival signal pathway which may inhibit Bax activation. This hypothesis was also supported by the results from in vivo studies. As shown in Figure 7C, the levels of P-Akt Ser473 were significant decreased in tissue lysates at 16 h after HI, but were maintained/recovered at levels above that of sham animals, if SP was administered after HI (Figure 7D).
Figure 7.
SP activated the Akt survival signal pathway following OGD and HI. A. Levels of total and Aktserine473 over 24 h following OGD. B. Levels of total and Aktserine473 at 1 h following OGD (n=3). C. Levels of total and P-AktSer473 in cortical tissues at 1.5, 3 and 16 h derived from sham HI brains (n = 3 per group). D. Levels of total and P-AktSer473 in cortical tissues derived from sham, vehicle or SP (500 mg/kg, 5 min) treated animals (n= 4-6 per group). * p < 0.05, ** p < 0.01.
Discussion
Following hypoxic-ischemic injury to immature brains, neurons continue to die over days and week5. Preventing the delayed neuronal death has been a focus for developing new therapeutic strategies. For the first time, this study demonstrated that SP not only reduced neuronal death resulting from neonatal hypoxic-ischemic injury, but also improved neurobehavior development, evaluated up to 2 month of recovery. Consistent results derived from corresponding in vitro and in vivo models suggest that SP’s neuroprotective effects are likely the result of improving cerebral energy metabolism and blocking the initiation of the neuronal death program.
SP has been considered a candidate for treating hypoxic-ischemic brain injury mainly because it is a metabolic substrate11 and an antioxidant17,18 that readily passes the blood-brain barrier14. SP also protects cultured primary neurons against 1-methyl-4-phenylpyridinium, 6-hydroxydopamine, hydrogen peroxide17,23, and OGD, as demonstrated in this study. At 24 h after reoxygenation, the presence of SP resulted in a decrease in LDH release (an indicator of neuronal injury) and an increase in MTT levels, both in a dose-dependent manner, supporting its function in promoting neuronal survival under nutritional deprivation.
SP’s neuroprotective effects were previously tested in several animal models. For example, it reduced zinc induced neurotoxicity during transient forebrain ischemia12, reduced traumatic brain injury10,11,13 and insulin-induced hypoglycemic brain injury14. As a potential candidate for treating hypoxic-ischemic brain injury in newborns, the current investigation systematically evaluated the effects of SP using an established neonatal rat HI model20. We observed that a single dose (125 to 1000 mg/kg) of intraperitoneal SP injection dramatically reduced hypoxic-ischemic injury to the immature brain, with optimal effects at 500mg/kg, which is consistent with a rat model of middle cerebral artery occlusion (MCAO)24. Despite these beneficial effects, previous9 and current studies indicate a narrow therapeutic window for SP, which may limit its future clinical use. The therapeutic window of SP for the MCAO model is limited to 30 min24. We found that a single dose of SP at 5 min after HI was the most efficacious but it gradually lost its neuroprotective effects if administered after one h of recovery.
SP’s effects on long-term behavior development have never been evaluated. As revealed by the foot-fault test, HI impaired sensorimotor function, which may be related to the loss of cortical tissue that controls motor movements25,26. This assumption is supported by the improved performance of foot-fault test when SP treatment reduced the loss of cortical tissue. Spatial learning is known to be associated with intact hippocampal functions27. Results from the Morris water maze showed that neonatal rats suffering hypoxic-ischemic injury displayed severe place navigation deficit in adulthood. Although previous studies28,29 suggested that motor impairment could affect the performance of the water maze test, our results showed no difference in the swimming speed among sham, vehicle, and SP treated groups. Therefore, learning impairments in water maze were independent of motor function, as suggested previously30. On the other hand, SP treatment alleviated the place navigation deficit. This effect most likely results from the reduced number of injured hippocampal neurons at 24 h as well as from the reduction in hippocampal tissue loss at two month of recovery.
The above long-term beneficial effects of SP may be due to its ability to improve cerebral energy metabolism. Oxygen and glucose deprivation in vitro (OGD) and in vivo (HI) both caused rapid decreases in ATP levels, suggesting an energy failure from nutrient deprivation. This energy failure was prevented by supplying SP to culture media at reoxygenation or to neonatal rats immediately following HI. Shortly after OGD, intracellular ROS levels, a measure of oxidative stress, start to increase and continue to rise for the entire testing period (24 h). Following HI to neonatal brain, tissue ROS levels initially fell at the beginning of reoxygenation, possibly due to the decrease in ATP, but rapidly increased to about 150% of controls at 3 h of reoxygenation and, then, decreased. The different time course of ROS levels in vivo is likely due to the supply of nutrients and removal of toxic waste with the reperfusion. Despite this decrease, neurons continued to die without intervention as demonstrated at 24 h (FBJ labeling) and two month of recovery. In either case, SP was able to maintain ATP levels from falling and ROS levels from increasing and, more importantly, reduced neuronal death and improved behavior recovery.
To understand the mechanisms underlying SP’s neuroprotective effects, we evaluated its effects on several key pathways involved in the death of immature neurons using the same corresponding in vitro and in vivo models for hypoxic-ischemic neuronal injury. In recent years, mitochondria has been identified as the converging point for death signals, especially for immature neurons31. Bax is one of the pro-apoptotic BCL-2 family members. In the absence of death signal, ectopic expression of Bax triggers cytochrome-c release from mitochondria32-34, leading to caspase activation and the induction of apoptosis. Transient ischemia to rat brain induced a rapid translocation of Bax from cytosol to mitochondria where it heterodimerizes with mitochondrial membrane permeabilization-related proteins adenine nucleotide translocator (ANT), forming mitochondrial membrane permeability35. Starting around 2 h after OGD, Bax levels rapidly increased (0.5 h), preceding the persistent activation of caspase-3 (4 h). An increase in Bax levels and caspases-3 activation was also observed in brain tissue following HI. In either case, SP treatment prevented the increase in Bax levels following OGD in neurons and following HI in brain tissue. SP likely inhibited Bax partially through activating or maintaining the levels of the key survival signaling kinase, Akt22, as shown in the current study.
In conclusion, the current study demonstrated that SP promoted neuronal survival under oxygen and glucose deprivation and reduced hypoxic-ischemic injury to neonatal rat brains. SP not only reduced the number of injured neurons in early phase, but also improved behavior development at two month of recovery. This effect is likely the result of improved cerebral energy metabolism and reduction of Bax expression and caspases activation. Therefore, SP may be used as a potential treatment for neonatal hypoxic-ischemic brain injury if given within the first hour.
Methods and Materials
Primary cortical neuron culture
The cortices were collected from newborn Sprague-Dawley rats as described36. After removing the meninges, the cortical tissue was minced in DMEM medium and then incubated in 0.25% trypsin (Invitrogen, New York, NY) and DNAse (Sigma, St. Louis, MO) for 15 min at 37°C to produce a single cell suspension. After centrifugation, the cells were resuspended in neurobasal (NB, Invitrogen, Grand Island, NY) supplemented with 2% B-27 (Invitrogen 17504-044), 0.5 mM glutamine (Invitrogen), 100U/ml penicillin and 100ug/ml streptomycin and then were plated into poly-L-lysine-coated (Sigma) dishes. The culture medium containing b-fibroblast growth factor (FGF, 5 ng/ml, Sigma) was changed at 24 h and 4 days in vitro (DIV).
Oxygen Glucose Deprivation
At 7 DIV, primary cortical neurons were gently washed and changed to glucose free DMEM (Invitrogen) before being placed into a humidified chamber gassed with 95% N2/5% CO2. The control cells were changed to DMEM with glucose media (Invitrogen) and remained under 5% CO2, 21% O2 and 37°C. After 2.5 h, cells were removed from the hypoxic chamber to regular incubator after changing to NB media and treated with SP.
Neuronal injury was evaluated by levels of lactate dehydrogenase (LDH) in culture media (LDH release) and 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) levels in cell lysate (cell viability) as described37.
Neonatal HI model and treatment
The animal studies were approved by the Indiana University School of Medicine Institutional Animal Use and Care Committee. All rats were grouped randomly and (Harlan Laboratories, Indianapolis, IN) housed under a 12-h light and 12-h dark cycle with food and water ad libitum. Briefly, 7-day-old Sprague–Dawley rat pups (eight per litter, weighing 13-18g) were anaesthetized with a mixture of isoflurane (3~4% for induction and 2% for maintenance) and 30% oxygen/70% N2. The left carotid artery was exposed and permanently ligated with 6-0 surgical silk. The wound was then sutured with 4-0 surgical silk. After a 2-h recovery, the pups were placed in 2-L airtight and watertight glass flasks, containing 8% oxygen and 92% nitrogen, submerged in a 37.0°C water bath. After 2.5 h, the pups were then returned to their dams after SP or vehicle was injected (i.p). The sham group underwent the same operation except for the carotid artery ligation and hypoxia.
2, 3, 5-triphenyltetrazolium chloride monohydrate (TTC) Staining
To confirm survival vol, rat brains were removed and sectioned coronally into six 2-mm slices in a Rodent Brain Matrix (Zivic Miller, Pittsburgh, PA). Slices were incubated in TTC (1%, Sigma) at 37°C for 10 min and fixed in 10% buffered formalin. The survival area on photographed brain sections was measured using Image J and calculated as the ratio of the TTC-stained area of ipsilateral to the area of contralateral (non-ischemic).
Foot-Fault test
The foot-fault test was performed at 21days of recovery25. Rats were placed on an elevated stainless steel wire (diameter 0.4 cm) grid (1 m above the floor with 3 cm2 holes). Each pup was placed on the grid and the number of foot faults was counted out of 50 steps for forelimbs or hind limbs. A foot fault was defined as when the animal misplaced a forelimb or hind limb and the paw fell between the grid bars.
Morris water maze test
Morris water maze tests were performed at 7 week of recovery as described30. The water maze consisted of a pool (6 feet in diameter and 2 feet deep) filled with opaque water and a platform (2 cm below the water’s surface) that rats could step on to escape the water. For each daily four trials (4 days), the rats were placed in the pool facing the wall and initiated randomly from each of the four start locations (north, south, northwest, southeast). A maximum of 60 s was allowed for each rat to find the hidden platform. At the end of the learning trials, a probe trial and a cued trial were performed. All the activities were recorded and the animals’ swimming paths were measured for quantification of latency and swimming speed by the Video Tracking System (CleverSys Inc, Reston, VA).
Fluoro-Jade B (FJB) Staining for Injured Neurons
At 24 h, 7 day or 2 month of recovery, the animals were anesthetized with halothane and then perfused transcardially with 0.9% saline, followed by 4% paraformaldehyde. After post-fixation and cryoprotection in 30% sucrose, the brains were sectioned (30 μm), stained with the cresyl violet, and quantified for brain tissue loss as described38.
For FJB staining, coronal sections (20 μm) were incubated in 0.06% potassium permanganate for 20 min and incubated in a 0.0004% solution of FJB (Histo-Chem Inc., Jefferson, AK) for 20 min and then 0.0004% DAPI for 5 min (Sigma). Sections were then rinsed, air dried, cleared in xylene (2–5 min) and mounted with DPX (Sigma)39. The number of FJB-positive neurons in 3 sections was determined in the ipsilateral hippocampus that suffered hypoxic-ischemic injury.
Intracellular ROS and ATP
For ROS measurement, cells were incubated in 2′, 7′-dichlorodihydro-fluorescein diacetate (DCFH-DA, 50 μM, Sigma) and washed to remove excessive DCFH-DA. For in vivo studies, the ipsilateral and contralateral cortexes were homogenized in RIPA buffer (Invitrogen) on ice and centrifuged at 12,000g at 4°C for 5 min. The supernatant was incubated with DCFH-DA for 30 min at 37°C. In both cases, the DCF fluorescence was measured in a microplate reader with excitation at 485 nm and emission at 535 nm and normalized by protein concentration.
Levels of ATP were measured using a commercial kit (molecular probes, Invitrogen). On 7 DIV, cultured cells were washed, scraped and further lysed by freezing and thawing. After removing the precipitates, ATP levels in the supernatants were determined following the manufacturer’s instructions. For ATP determination in vivo, the ipsilateral and contralateral cortexes were homogenized in lysis buffer (10 mM Tris pH 7.5, 0.1 M NaCl, 1 mM EDTA, 0.01% Triton X-100). ATP content was determined in the supernatants and normalized by protein concentration.
Antibody Used for Western Immunoblot
Anti-Bax antibody (1:1000; Cell Signal Technology, Boston, MA); anti-caspase-3 and anti-cleaved caspase-3 detection (rabbit monoclonal antibody, 1:1000; Cell Signal Technology); anti-total Akt or anti-P-Akt (S473) (rabbit monoclonal antibody, 1:1000; Cell Signal Technology); anti-GAPDH (rabbit monoclonal antibody, 1:1000; Cell Signal Technology). The secondary antibody was anti-rabbit IgG, HRP-linked antibody (1:2500; Cell Signal Technology).
Statistical analysis
Data are presented as mean±SEM. Statistical differences between more than two groups were analyzed by using a one-way ANOVA followed by the Tukey multiple comparison procedure. A p value below 0.05 was considered statistically significant.
Acknowledgments
Statement of financial support: This work is supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (to W-H Lee; grant 1R01HD059979) and the Indiana Spinal Cord Brain Injury Fund.
Footnotes
Rui Pan and Zhihui Rong contributed equally as the first authors.
References
- 1.Graham EM, Ruis KA, Hartman AL, Northington FJ, Fox HE. A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am J Obstet Gynecol. 2008;199:587–95. doi: 10.1016/j.ajog.2008.06.094. [DOI] [PubMed] [Google Scholar]
- 2.Verklan MT. The chilling details: hypoxic-ischemic encephalopathy. J Perinat Neonatal Nurs. 2009;23:59–68. doi: 10.1097/01.JPN.0000346221.48202.7e. quiz 9-70. [DOI] [PubMed] [Google Scholar]
- 3.Perlman JM. Intervention strategies for neonatal hypoxic-ischemic cerebral injury. Clin Ther. 2006;28:1353–65. doi: 10.1016/j.clinthera.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Geddes R, Vannucci RC, Vannucci SJ. Delayed cerebral atrophy following moderate hypoxia-ischemia in the immature rat. Dev Neurosci. 2001;23:180–5. doi: 10.1159/000046140. [DOI] [PubMed] [Google Scholar]
- 5.Northington FJ, Chavez-Valdez R, Martin LJ. Neuronal cell death in neonatal hypoxia-ischemia. Ann Neurol. 2011;69:743–58. doi: 10.1002/ana.22419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagberg H, Mallard C, Rousset CI, Xiaoyang W. Apoptotic mechanisms in the immature brain: involvement of mitochondria. J Child Neurol. 2009;24:1141–6. doi: 10.1177/0883073809338212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351:1985–95. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- 8.Blomgren K, Hagberg H. Free radicals, mitochondria, and hypoxia-ischemia in the developing brain. Free Radic Biol Med. 2006;40:388–97. doi: 10.1016/j.freeradbiomed.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 9.Martin A, Rojas S, Perez-Asensio F, Planas AM. Transient benefits but lack of protection by sodium pyruvate after 2-hour middle cerebral artery occlusion in the rat. Brain Res. 2009;1272:45–51. doi: 10.1016/j.brainres.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 10.Moro N, Sutton RL. Beneficial effects of sodium or ethyl pyruvate after traumatic brain injury in the rat. Exp Neurol. 2010;225:391–401. doi: 10.1016/j.expneurol.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukushima M, Lee SM, Moro N, Hovda DA, Sutton RL. Metabolic and histologic effects of sodium pyruvate treatment in the rat after cortical contusion injury. J Neurotrauma. 2009;26:1095–110. doi: 10.1089/neu.2008.0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JY, Kim YH, Koh JY. Protection by pyruvate against transient forebrain ischemia in rats. J Neurosci. 2001;21:RC171. doi: 10.1523/JNEUROSCI.21-20-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moro N, Ghavim SS, Hovda DA, Sutton RL. Delayed sodium pyruvate treatment improves working memory following experimental traumatic brain injury. Neurosci Lett. 2011;491:158–62. doi: 10.1016/j.neulet.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou D, Qian J, Chang H, Xi B, Sun RP. Pyruvate administered to newborn rats with insulin-induced hypoglycemic brain injury reduces neuronal death and cognitive impairment. Eur J Pediatr. 2011 doi: 10.1007/s00431-011-1489-3. [DOI] [PubMed] [Google Scholar]
- 15.Yi JS, Kim TY, Kyu Kim D, Koh JY. Systemic pyruvate administration markedly reduces infarcts and motor deficits in rat models of transient and permanent focal cerebral ischemia. Neurobiol Dis. 2007;26:94–104. doi: 10.1016/j.nbd.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Desagher S, Glowinski J, Premont J. Pyruvate protects neurons against hydrogen peroxide-induced toxicity. J Neurosci. 1997;17:9060–7. doi: 10.1523/JNEUROSCI.17-23-09060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazzio E, Soliman KF. Pyruvic acid cytoprotection against 1-methyl-4-phenylpyridinium, 6-hydroxydopamine and hydrogen peroxide toxicities in vitro. Neurosci Lett. 2003;337:77–80. doi: 10.1016/s0304-3940(02)01327-7. [DOI] [PubMed] [Google Scholar]
- 18.Lee YJ, Kang IJ, Bunger R, Kang YH. Mechanisms of pyruvate inhibition of oxidant-induced apoptosis in human endothelial cells. Microvasc Res. 2003;66:91–101. doi: 10.1016/s0026-2862(03)00052-9. [DOI] [PubMed] [Google Scholar]
- 19.Shen H, Hu X, Liu C, et al. Ethyl pyruvate protects against hypoxic-ischemic brain injury via anti-cell death and anti-inflammatory mechanisms. Neurobiol Dis. 2010;37:711–22. doi: 10.1016/j.nbd.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rice JE, III, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9:131–41. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- 21.Linseman DA, Phelps RA, Bouchard RJ, et al. Insulin-like growth factor-I blocks Bcl-2 interacting mediator of cell death (Bim) induction and intrinsic death signaling in cerebellar granule neurons. J Neurosci. 2002;22:9287–97. doi: 10.1523/JNEUROSCI.22-21-09287.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuzaki H, Tamatani M, Mitsuda N, et al. Activation of Akt kinase inhibits apoptosis and changes in Bcl-2 and Bax expression induced by nitric oxide in primary hippocampal neurons. Journal of Neurochemistry. 1999;73:2037–46. [PubMed] [Google Scholar]
- 23.Jagtap JC, Chandele A, Chopde BA, Shastry P. Sodium pyruvate protects against H2O2 mediated apoptosis in human neuroblastoma cell line-SK-N-MC. J Chem Neuroanat. 2003;26:109–18. doi: 10.1016/s0891-0618(03)00037-1. [DOI] [PubMed] [Google Scholar]
- 24.Kim JB, Yu YM, Kim SW, Lee JK. Anti-inflammatory mechanism is involved in ethyl pyruvate-mediated efficacious neuroprotection in the postischemic brain. Brain Res. 2005;1060:188–92. doi: 10.1016/j.brainres.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 25.Redell JB, Moore AN, Dash PK. Expression of the prodynorphin gene after experimental brain injury and its role in behavioral dysfunction. Exp Biol Med (Maywood) 2003;228:261–9. doi: 10.1177/153537020322800304. [DOI] [PubMed] [Google Scholar]
- 26.Liu C, Lin N, Wu B, Qiu Y. Neuroprotective effect of memantine combined with topiramate in hypoxic-ischemic brain injury. Brain Res. 2009;1282:173–82. doi: 10.1016/j.brainres.2009.05.071. [DOI] [PubMed] [Google Scholar]
- 27.Amsel A. Hippocampal function in the rat: cognitive mapping or vicarious trial and error? Hippocampus. 1993;3:251–6. doi: 10.1002/hipo.450030302. [DOI] [PubMed] [Google Scholar]
- 28.Chen W, Ma Q, Suzuki H, Hartman R, Tang J, Zhang JH. Osteopontin reduced hypoxia-ischemia neonatal brain injury by suppression of apoptosis in a rat pup model. Stroke. 2011;42:764–9. doi: 10.1161/STROKEAHA.110.599118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumral A, Uysal N, Tugyan K, et al. Erythropoietin improves long-term spatial memory deficits and brain injury following neonatal hypoxia-ischemia in rats. Behav Brain Res. 2004;153:77–86. doi: 10.1016/j.bbr.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–58. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desagher S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10:369–77. doi: 10.1016/s0962-8924(00)01803-1. [DOI] [PubMed] [Google Scholar]
- 32.Pastorino JG, Chen ST, Tafani M, Snyder JW, Farber JL. The overexpression of Bax produces cell death upon induction of the mitochondrial permeability transition. The Journal of Biological Chemistry. 1998;273:7770–5. doi: 10.1074/jbc.273.13.7770. [DOI] [PubMed] [Google Scholar]
- 33.Jurgensmeier JM, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed JC. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci U S A. 1998;95:4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eskes R, Antonsson B, Osen-Sand A, et al. Bax-induced cytochrome C release from mitochondria is independent of the permeability transition pore but highly dependent on Mg2+ ions. J Cell Biol. 1998;143:217–24. doi: 10.1083/jcb.143.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao G, Minami M, Pei W, et al. Intracellular Bax translocation after transient cerebral ischemia: implications for a role of the mitochondrial apoptotic signaling pathway in ischemic neuronal death. J Cereb Blood Flow Metab. 2001;21:321–33. doi: 10.1097/00004647-200104000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Zhong J, Zhao L, Du Y, Wei G, Yao WG, Lee WH. Delayed IGF-1 treatment reduced long-term hypoxia-ischemia-induced brain damage and improved behavior recovery of immature rats. Neurol Res. 2009;31:483–9. doi: 10.1179/174313208X338133. [DOI] [PubMed] [Google Scholar]
- 37.Zhong J, Deng J, Huang S, Yang X, Lee WH. High K+ and IGF-1 protect cerebellar granule neurons via distinct signaling pathways. J Neurosci Res. 2004;75:794–806. doi: 10.1002/jnr.20024. [DOI] [PubMed] [Google Scholar]
- 38.Wei X, Du Z, Zhao L, et al. IFATS collection: The conditioned media of adipose stromal cells protect against hypoxia-ischemia-induced brain damage in neonatal rats. Stem Cells. 2009;27:478–88. doi: 10.1634/stemcells.2008-0333. [DOI] [PubMed] [Google Scholar]
- 39.Shi Q, Zhang P, Zhang J, et al. Adenovirus-mediated brain-derived neurotrophic factor expression regulated by hypoxia response element protects brain from injury of transient middle cerebral artery occlusion in mice. Neurosci Lett. 2009;465:220–5. doi: 10.1016/j.neulet.2009.08.049. [DOI] [PubMed] [Google Scholar]