Abstract
Background
There is an association between adiposity and asthma prevalence, but the relationship to asthma control is unclear.
Objectives
To understand the relationships among adiposity, gender, and asthma control in inner-city adolescents with asthma.
Methods
We prospectively followed 368 adolescents with moderate to severe asthma (ages 12–20 years) living in 10 urban areas for one year. Asthma symptoms and exacerbations were recorded, and pulmonary function and exhaled nitric oxide were measured every 6 weeks. Adiposity measures (BMI, DEXA scans) were made, and blood was collected for allergy markers, adiponectin, leptin, TNF-α, IL-6 and CRP.
Results
More than 60% of females and 50% of males were above the 85th percentile of BMI-for-age. Higher BMI was associated with more symptom days (R= 0.18, P<0.01) and exacerbations (R=0.18, P=0.06) among females only. Adiponectin was inversely related to asthma symptoms (R=− 0.18, P<0.05) and exacerbations (R=− 0.20, P<0.05) and positively with FEV1/FVC (R=0.15, P<0.05) in males only, independent of body size. There was no relationship between adiposity or adipokines and total IgE, blood eosinophils and exhaled nitric oxide. DEXA provided little additional value in relating adiposity to asthma outcome in this population of adolescents.
Conclusion
Adiposity is associated with poorer asthma control in females. Adiponectin is associated with improved asthma control in males.
Keywords: Obesity, Asthma, Adipokines, Leptin, Adiponectin
Introduction
In the past several decades, obesity has increased dramatically, especially among low-socioeconomic minority populations.1–3 Epidemiologic studies have shown that overweight serves as a risk factor for the development of asthma. The plausibility of a causal relationship is supported by prospective studies in both adults and children, suggesting that overweight and obesity can precede onset of asthma,4–6 and studies in adults demonstrating improvement in asthma symptoms with weight loss.7, 8 In light of a growing literature showing an association between obesity and asthma, detailed study of children with asthma living in these areas may shed light on this relationship.
In contrast to the consistent evidence linking obesity to the incidence and prevalence of asthma, studies on the association between asthma control and obesity have been less consistent.9–13 Additionally, many of the studies which focused on obesity and asthma morbidity lacked detailed phenotypic characterization of asthma and have not carefully assessed control of asthma symptoms using a standardized, guidelines based approach.14–18 Even fewer studies have evaluated the interaction of gender and obesity on asthma morbidity.15, 18 This interaction is of importance in view of the variable effect that gender has had in studies of the relationship between obesity and asthma.19, 20
Several hypotheses to explain the asthma-obesity association have been proposed, including the effects of obesity on chest wall mechanics, low-grade-systemic inflammation, and changes in serum concentrations of adipose tissue-derived proteins (adipokines).20 Adipose tissue of the obese expresses increased amounts of pro-inflammatory proteins such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and C-reactive protein (CRP). These cytokines may modify airway inflammation,21 increase airway contractility,22 and modify the response to glucocorticoids.23 The adipokine, leptin, increases with increased adiposity and has pro-inflammatory effects.24 In contrast another adipokine, adiponectin, decreases with increasing adiposity and has anti-inflammatory properties.25 Notably, adipokines may mediate the association between obesity and asthma in a gender-specific fashion.26, 27
The variability of the gender influence on the effect of adiposity on asthma may be due to the use of body mass index (BMI) as a measure of adiposity. BMI does not differentiate between lean body mass and fat mass;28 therefore for a given BMI value, females have a higher proportion of body fat than males.29 It is reasonable to conjecture that the stronger association between BMI and asthma seen in females could be due to the fact that the relationship between BMI and body fat is gender-dependent. 30–36 The use of a direct measure to characterize adiposity, such as dual energy X-ray absorptiometry (DEXA), is needed to resolve this issue.
As part of a larger randomized trial examining the utility of measuring exhaled nitric oxide to improve asthma control in adolescents residing in inner-city neighborhoods (the Asthma Control Evaluation or ACE study),37 we designed a prospective study to examine the effect of weight and body fat on asthma morbidity as measured by symptom report and exacerbations. In this sample of inner-city adolescents with careful guideline-based management of asthma, we evaluated the gender-specific association between asthma morbidity and adiposity using traditional BMI, as well as DEXA scan measures of body fat. To further investigate the mechanisms involved in the relationship of adiposity measures and gender, we also assessed inflammatory biomarkers and adipokines. Some of the results of this study have been previously reported in the form of an abstract.38
Methods
Study Design and Population
The Asthma Adiposity Study included 368 adolescents 12–20 years of age, enrolled from among ACE study participants in 10 major urban areas of the United States. All appropriate institutional review boards approved this study. Written informed consent and assent were obtained. The ACE study was a randomized, double-blind, parallel-group trial with a 3-week run-in to characterize participants, establish treatment, and evaluate adherence.37 The main objective of ACE was to determine if the addition of exhaled nitric oxide (eNO) to a guideline-based approach to selecting therapy improved asthma outcomes over guideline-based therapy alone. At the recruitment, randomization, and follow-up visits, symptoms, rescue medication use, pulmonary function, and adherence were used to assign a medication regimen based upon standardized treatment guidelines across sites. In the intervention group, eNO levels were additionally considered when assigning medications. There were 6 follow-up visits approximately 2 months apart for 1 year. Treatment recommendations were derived from protocol-defined treatment steps based on asthma control and adherence.37 With each step-up in treatment, the intensity of the controller medication regimen increased as reflected by either an increase in inhaled corticosteroid dose or the addition of a leukotriene modifier or long-acting beta agonist.
Asthma Morbidity Outcomes
The primary morbidity outcomes were maximum symptom days and exacerbations during the followup period.37 Maximum symptom days recalled over the previous 2 weeks were assessed every 6–8 weeks and averaged over the 46-week treatment period post-randomization. Exacerbations over the previous 2 months were determined at every visit and summed over the 46-week treatment period. Maximum symptom days were defined as the largest value among number of days with wheezing, chest tightness or cough; number of nights of sleep disturbance; and number of days when activities were affected. An asthma exacerbation was defined as a hospitalization, unscheduled visit, or prednisone course for asthma during the treatment period. The Asthma Control Test (ACT) was administered at each visit and the score averaged over the 46-week treatment period following randomization. A score equal to or less than 19 on the ACT suggests that asthma is not well-controlled.
Procedures
At each study visit, pulmonary function tests were performed according to ATS guidelines39 and over-read for quality control. Skin testing was performed by the puncture method on the volar surface of the forearm using a Multi-Test II device (Lincoln Diagnostics; Decatur, IL). Serum total IgE was measured with the UniCap System (Phadia; Uppsala, Sweden). FENO was measured (flow rate 50 ml/s) with a rapid-response chemiluminescent analyzer (NIOX™ System, Aerocrine, Sweden) following American Thoracic Society guidelines.40
Height and Weight Measurement
At baseline, subjects were weighed twice to the nearest 0.1 kg using a digital or balance scale that was calibrated daily. Height was measured three times to the nearest 0.1 cm with a stadiometer (Holtain, Ltd, Harpendem model #602VR) that was calibrated daily. Weight and height measurements were averaged, and BMI was calculated.
Body Composition Assessments
DEXA scans were completed for 300 participants across 9 sites. Participants who weighed more than 136.4 kg (300 lbs) were not eligible for a DEXA scan (n=4). There was no significant difference in BMI between participants with DEXA measurements and those without. Total body fat, fat-free mass, and lean soft tissue and bone mineral content [(BMC), kilograms] were assessed.
Adipokines and Biomarkers
Serum leptin, adiponectin, and CRP were measured by Northwest Lipid Research Laboratories (Seattle, Washington). Leptin and adiponectin were measured using a radioimmunoassay (Linco Research, Inc.). CRP was measured immunochemically. Serum IL-6 and TNF-α were measured by ELISA assay (R&D Systems, Minneapolis, MN), and total serum eosinophil counts were performed by local clinical laboratories. Only IL-6 had a number (n=56) of undetectable results. These were assigned a value of half the lower limit of detection.
Statistical Analyses
Partial Pearson’s correlations were calculated for the entire study population to measure the strength of the relationships between baseline adiposity measures and outcomes during the year of follow-up, while controlling for the effects of site, race and age. Effect modification by gender was examined, and a large number of interactions were significant (especially those related to asthma status and DEXA percent body fat); thus, analyses were stratified by gender. Data for FENO, total IgE, blood eosinophils, CRP, IL-6 and TNF-α were log-transformed to better approximate a normal distribution before analysis. A p-value of < 0.05 was considered statistically significant. All statistical analyses were performed using SAS statistical software version 9.2 (SAS Institute Inc; Cary, NC) and the R system for statistical computing version 2.10.0. (R. Development Core Team).
Results
The average age of the Asthma Adiposity Study participants was 14.2 yr (±2.0) and slightly more than half were male (54.1%). Nearly two-thirds were African American (62.5%), and the remainder were predominantly Hispanic (22.6%). Family income levels were low, with approximately 50% having incomes below $15,000 per year. Since most research investigations have found effects of obesity to differ between males and females, the initial characteristics table (Table 1) presents all variables by gender. The population is highly atopic, with 4 to 5 positive skin tests on average (from a panel of 14); males had a higher number of positive skin tests (p<0.001) and total IgE (p=0.002). At recruitment, males had slightly worse lung function and higher exhaled nitric oxide than females (median of 40.6 versus 22.2 ppb; p <0.001). Asthma symptoms, however, were significantly greater among females as compared to males for symptom days (6.1 per two weeks versus 4.9; p=0.002) and ACT score (17.2 versus 19.2; p<0.001). This same pattern of worse lung function and higher FENO for males, but worse asthma symptoms for females, persisted throughout the one-year follow up period.
Table 1.
Characteristics of the 368 Asthma Adiposity Study participants by gender
| Characteristics | Females (n=169) | Males (n=199) | P† |
|---|---|---|---|
| Age at recruitment (yr) | 14.5 ± 2.1 | 14.0 ± 1.9 | 0.02 |
| Race/ethnic group (%) | |||
| African American | 62.7 | 62.3 | 0.99 |
| Hispanic | 22.5 | 22.6 | |
| Other or mixed | 14.8 | 15.1 | |
| Caretaker completed high school (%) | 75.0 | 80.1 | 0.27 |
| Household income <$15,000 (%) | 48.4 | 51.1 | 0.62 |
| ≥ 1 household member employed (%) | 78.1 | 85.9 | 0.05 |
| Number of Positive Skin Tests (of 14 performed) | 4.1 ± 3.2 | 5.3 ± 3.2 | <0.001 |
| Total IgE (kU/L) | 178 (62 – 567) | 315 (120 – 723) | 0.002 |
| Blood eosinophils (per μl) | 168 (84 – 301) | 268 (180 – 437) | <0.001 |
| Asthma status, pulmonary function and utilization at recruitment ‡ | |||
| Maximum symptom days (no. of days/last 2 wks) | 6.1 ± 4.5 | 4.9 ± 4.3 | 0.002 |
| ACT® score in the last month | 17.2 ± 4.3 | 19.2 ± 3.7 | <0.001 |
| FEV1 (% of predicted value) | 93.7 ± 16.5 | 91.3 ± 16.6 | 0.18 |
| FEV1/FVC | 79.2 ± 10.1 | 77.3 ± 9.1 | 0.03 |
| FENO (ppb) | 22.2 (11.8 – 52.3) | 40.6 (17.8 – 72.0) | <0.001 |
| ≥ 1 Exacerbation (%) | 72.8 | 75.4 | 0.57 |
| Asthma status, pulmonary function and utilization during 1 year follow-up | |||
| Maximum symptom days (no. of days/last 2 wks) | 2.1 ± 1.5 | 1.8 ± 1.7 | 0.01 |
| ACT® score in the last month | 21.5 ± 2.3 | 22.1 ± 2.1 | 0.01 |
| FEV1 (% of predicted value) | 97.2 ± 14.8 | 93.9 ± 14.9 | 0.03 |
| FEV1/FVC | 81.3 ± 8.0 | 79.3 ± 8.1 | 0.01 |
| FENO (ppb) | 21.6 (11.0 – 45.6) | 33.6 (18.5 – 59.8) | <0.001 |
| ≥ 1 Exacerbation (%) | 38.5 | 41.2 | 0.59 |
Plus–minus values are means ±SD. Interquartile ranges are included in parentheses with median values.
P-value for Chi-square (categorical variables) or Wilcoxon test (continuous variables) for the differences between females and males.
Maximum symptom days are defined as the largest value among the following variables reported over the prior 2 weeks: (1) number of days with wheezing, chest tightness or cough; (2) number of nights of sleep disturbance; and (3) number of days when activities were affected. ACT® denotes Asthma Control Test® FEV1 forced expiratory volume in one second, FVC forced vital capacity, FENO fractional exhaled nitric oxide. An asthma exacerbation was defined as a hospitalization, unscheduled visit (including emergency department visits), or prednisone course for asthma.
Body mass index and percent body fat measured by DEXA are presented in Table 2, along with adipokines and biomarkers related to obesity. Males were taller than females on average, but weight was similar. Both BMI and percent body fat were significantly higher for females. This inner-city population of adolescents with asthma was considerably heavier than the U.S. population,3 with distributions of BMI for females showing 61.5% above the 85th percentile of BMI-for-age and 36.7% above the 95th percentile. More than half the males (52.3%) were above the 85th percentile and 33.7% were heavier than the 95th percentile of BMI-for-age (Figure 1). Serum levels of adipokines and weight-related biomarkers (CRP and IL-6) were higher for females than males, but TNF-α did not differ (Table 2).
Table 2.
Baseline weight status of the Asthma Adiposity Study participants by gender
| Characteristic | Females | Males | P† |
|---|---|---|---|
| Adiposity Measurements | n=169 | n=199 | |
| Height (cm) | 159.9 ± 7.0 | 166.1 ± 10.6 | <0.001 |
| Height Percentile | 49.3 ± 27.5 | 56.0 ± 30.2 | 0.024 |
| Weight (kg) | 70.6 ± 21.3 | 70.0 ± 22.9 | 0.61 |
| Weight Percentile | 79.7 ± 24.1 | 74.3 ± 27.5 | 0.24 |
| BMI (kg/m2) | 27.4 ± 7.1 | 25.0 ± 6.5 | <0.001 |
| BMI Percentile | 82.0 ± 22.3 | 75.6 ± 25.9 | 0.055 |
| Percent body fat‡ | 35.4 ± 8.9 | 21.9 ± 10.2 | <0.001 |
| Adipokines and Biomarkers | n=165 | n=195 | |
| Adiponectin (mcg/mL) | 13.9 ± 6.0 | 11.9 ± 4.5 | 0.003 |
| Leptin (ng/mL) | 20.7 ± 12.0 | 8.6 ± 9.5 | <0.001 |
| C-reactive protein (mg/dL) | 0.09 (0.03 – 0.21) | 0.05 (0.02 – 0.14) | 0.005 |
| Interleukin-6 (pg/mL) | 0.93 (0.40 – 2.10) | 0.68 (0.30 – 1.64) | 0.02 |
| Tumor necrosis factor-α (pg/mL) | 2.00 (1.37 – 3.49) | 1.74 (1.27 – 2.78) | 0.24 |
Plus–minus values are means ±SD. Interquartile ranges are included in parentheses with median values. BMI denotes body mass index, DEXA dual energy X-ray absorptiometry.
P-value for Chi-square (categorical variables) or Wilcoxon test (continuous variables) for the differences between females and males.
The number with DEXA measurements is 136 for females and 164 for males.
Figure 1.
Distribution of BMI among females and males in the Asthma Adiposity Study.
The correlations of BMI and DEXA percent body fat with asthma symptoms, atopy and biomarkers are shown separately for males and females in Table 3. Among females, but not males, higher levels of symptoms were associated with higher BMI and even more strongly associated with a higher percent body fat. Both males and females exhibited a reduced FEV1/FVC ratio with higher BMI and body fat. Levels of leptin, CRP, and IL-6 all increased with increased adiposity, and levels of adiponectin were reduced. Levels of TNF-alpha were not related to adiposity. Females above the 85th percentile of age-adjusted BMI had 0.56 more symptom days per two weeks (95% CI: 0.10–1.01) than females of normal weight and were nearly 2½ times more likely to have an exacerbation (OR=2.49, 95% CI: 1.25–5.14). Figure 2 shows this marked difference in the relationship between percent body fat and asthma symptoms and exacerbations for females as compared to males (interaction p-values < 0.01 and <0.05 respectively). Trunk to peripheral fat ratio, measured by DEXA, was not associated with symptoms or exacerbations (data not shown). In models sequentially examining BMI and percent body fat, percent body fat did not add to the percent of variance explained for maximum symptom days or exacerbations once BMI was accounted for (data not shown). We were prevented from examining pulmonary function outcomes in this manner due to collinearity.
Table 3.
Correlations of asthma status, adipokines and biomarkers with BMI and percent body fat (DEXA) by gender
| BMI | % Body Fat (DEXA) | |||
|---|---|---|---|---|
| Characteristic | Females | Males | Females | Males |
| Asthma Status | ||||
| Maximum Symptom Days | 0.18 * | 0.00 | 0.27 ** | − 0.04 |
| Asthma Control Test | − 0.11 | − 0.04 | − 0.22 ** | 0.02 |
| Any Exacerbations | 0.18 | 0.13 | 0.35 *** | 0.11 |
| Lung Function, Atopy and FENO | ||||
| FEV1 (% of predicted value) | 0.10 | 0.18 * | 0.02 | − 0.02 |
| FEV1/FVC | − 0.26 *** | − 0.18 * | − 0.13 | − 0.20 ** |
| Total IgE (kU/L) | 0.03 | 0.03 | − 0.08 | 0.00 |
| Blood eosinophils (per μl) | 0.14 | − 0.03 | 0.08 | − 0.02 |
| FENO (ppb) | − 0.05 | − 0.08 | − 0.10 | − 0.08 |
| Adipokines and Biomarkers | ||||
| Adiponectin (mcg/mL) | − 0.41 *** | − 0.22 ** | − 0.31 *** | − 0.27 *** |
| Leptin (ng/mL) | 0.70 *** | 0.82 *** | 0.73 *** | 0.87 *** |
| C-reactive protein (mg/dL) | 0.64 *** | 0.58 *** | 0.64 *** | 0.58 *** |
| Interleukin-6 (pg/mL) | 0.22 ** | 0.20 ** | 0.18 * | 0.18 * |
| Tumor necrosis factor-α (pg/mL) | − 0.11 | 0.01 | − 0.10 | 0.03 |
Values are Pearson's correlation coefficients except for any exacerbation where a polyserial correlation was calculated. All values are adjusted for site, race, age and group assignment. P-value for test of difference between females and males
<0.05
<0.01
<0.001
Figure 2.
Relationships between percent body fat (DEXA) and asthma outcomes by gender. Shows relationship of percent body fat with maximum symptom days (Top row) and with exacerbations (Bottom row). All models are adjusted for site, race, age and group assignment (black lines) with 95% confidence intervals (light gray areas).
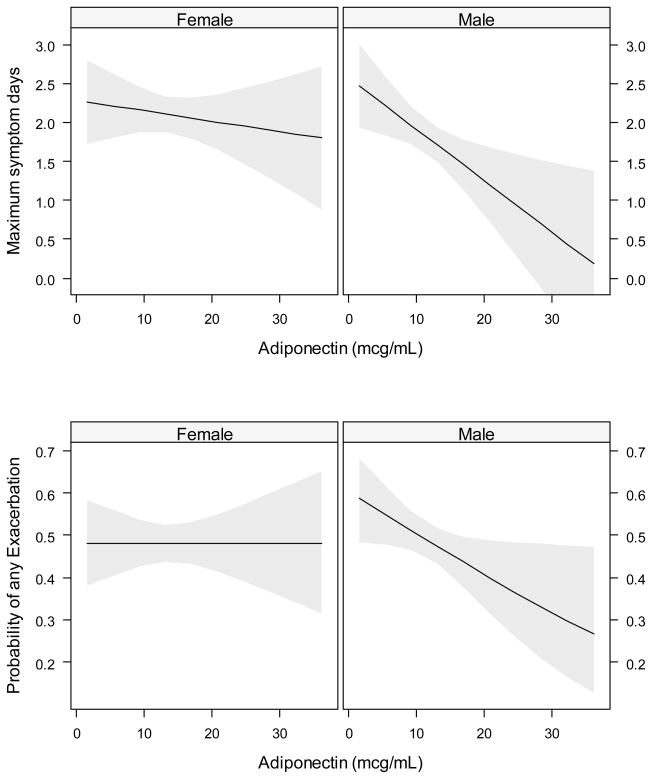
The relationship of adiponectin and leptin to asthma morbidity for both males and females is shown in Table 4. For males, adiponectin levels were inversely related to asthma symptoms and exacerbations (Figure 3, both interaction p-values < 0.10), and were positively associated with ACT scores and FEV1/FVC. Leptin levels were positively related to asthma symptoms in females. The effect of adiponectin was maintained in models adjusting for BMI (data not shown). We were unable to control the leptin models for BMI due to substantial collinearity. There was no association seen between CRP or IL-6 and maximum symptom days or exacerbations.
Table 4.
Correlations of asthma status with adipokines
| Adiponectin | Leptin | |||
|---|---|---|---|---|
| Characteristic | Females | Males | Females | Males |
| Asthma Status | ||||
| Maximum Symptom Days | − 0.06 | − 0.18 * | 0.16 * | − 0.08 |
| Asthma Control Test | 0.00 | 0.19 ** | − 0.13 | 0.03 |
| Any Exacerbations? | − 0.00 | − 0.20 * | 0.11 | 0.11 |
| Lung Function, Atopy and FENO | ||||
| FEV1 (% of predicted value) | − 0.04 | − 0.04 | 0.02 | 0.03 |
| FEV1/FVC | 0.14 | 0.15 * | − 0.12 | − 0.13 |
| Total IgE (kU/L) | − 0.04 | 0.07 | − 0.05 | − 0.02 |
| Blood eosinophils (per μl) | − 0.14 | − 0.05 | 0.09 | − 0.02 |
| FENO (ppb) | − 0.01 | 0.04 | − 0.11 | − 0.07 |
Values are Pearson's correlation coefficients except for any exacerbation where a polyserial correlation was calculated. All values are adjusted for site, race, age and group assignment. P-value for test of difference between females and males
<0.05
<0.01
<0.001
Figure 3.
Relationships between adiponectin and asthma outcomes by gender. Shows relationship of adiponectin with maximum symptom days (Top row) and with exacerbations (Bottom row). All models adjusted for site, race, age and group assignment (black lines) with 95% confidence intervals (light gray areas).
Discussion
In this study of inner-city adolescents with asthma, we have provided an extensive analysis of the relationship between obesity and asthma morbidity using a prospective, well-characterized and closely-monitored cohort. Our major finding is that, as in the case between asthma prevalence and obesity,19, 20 gender has substantial influence on the relationship of adiposity to asthma morbidity. In female adolescents only, increased BMI and body fat were associated with worse asthma control, more asthma exacerbations, and lower FEV1/FVC. In males, we report another novel finding, a protective role of serum adiponectin on the same parameters, independent of adiposity. Notably, these effects were observed despite good adherence to guideline-based management.37 Our study is also the first to provide information on the relationship between adiposity measures other than BMI and asthma morbidity and to examine the relationship of serum adipokines to asthma control and measures of airway inflammation.
Adiponectin is produced exclusively by adipocytes; however, circulating levels decrease with increasing adiposity.25 This protein has anti-inflammatory properties.41–43 It acts on macrophages and monocytes to inhibit production of pro-inflammatory cytokines and to augment IL-10 and IL-1 receptor antagonist expression.44 Despite higher serum levels of adiponectin in females, the protective effect was seen in males only. The sex-specific effect of adiponectin was not explained by the ratio of leptin to adiponectin, which is significantly different in males and females. A possible explanation may be that adiponectin receptors are down regulated with increased adiposity in adolescent females. In a study evaluating asthma prevalence, adiponectin was found to be protective against current asthma prevalence in pre-menopausal women independent of BMI.27 The reported protective effects of adiponectin in human studies are consistent with mouse studies where exogenous administration of adiponectin resulted in an almost complete suppression of allergen-induced airway hyper-reactivity, airway inflammation, and TH2 cytokine expression in the lung.45 In contrast, leptin was associated with poorer asthma control, but an independent effect could not be demonstrated because of the high correlation between adiposity measures and leptin. The role of adiponectin in asthma warrants further study and may have implications for therapy.
Adipocytes and macrophages that infiltrate adipose tissue are important sources of inflammatory cytokines.46 It has been hypothesized that high levels of pro-inflammatory molecules released from adipose tissue into the systemic circulation could contribute to airway inflammation and increase asthma severity.47 However, Sutherland et al. found that although systemic and airway inflammation were present in obesity and asthma, there was no evidence of interaction between the two.48 In our study, the markers of systemic inflammation generally increased with increasing BMI or percent body fat in both genders with females having higher levels than males. However, the markers did not correlate with asthma morbidity. In the absence of bronchial lavage to directly measure cytokines, we cannot determine the association of adiposity with these markers of airway inflammation. However, we demonstrated no correlation of adiposity and serum leptin with FENO. This supports the possibility that adipokines may be related to asthma by a mechanism unrelated to inflammation.
The relationship between obesity and asthma severity as classified in published asthma guidelines is controversial. Obesity was associated with severity as classified by Global Initiative for Asthma guidelines among adults in the National Asthma Survey in the USA,17 but not in the European Community Respiratory Health Survey49 or a Canadian sample of asthmatics.11 The impact of increasing body weight on asthma control or response to therapy is not consistently reported in the literature. Elevated BMI has been associated with increased symptoms or health care use for asthma attacks among 3 to 5 year olds in Head Start,50 inner-city children 4 to 9 years old,9 and adults in the National Asthma Survey.17 In the Childhood Asthma Management Program cohort there was no correlation between BMI and markers of asthma control. However, these children were pre-pubertal, and median BMI was much lower than in our study.51 A cross-sectional study of urban adults with asthma found no differences in asthma control as measured by 4 validated asthma control questionnaires with changes in BMI.52 In a recent study comparing obese and non-obese adult asthmatics presenting to emergency rooms, the severity of the asthma exacerbations were found to be similar.12 Responsiveness to asthma treatment shows a similar ambiguity. Secondary analyses performed on participants 18 years of age and older from 5 clinical trials found obese asthmatics (BMI >=40) have a lower response to inhaled corticosteroids (ICS) or ICS combined with a long acting beta agonist.53 In a study of 18 to 50 year olds seeking treatment in an ER, heavier patients (BMI >=25) had more residual wheezing and a higher hospitalization rate.54 A review of the hospitalization course for asthmatics 2 to 18 years of age admitted to an ICU found obese (BMI >=95th %) had longer treatment and hospital stays despite similar severity at presentation.55 In contrast, for inner-city asthmatics 2 to 18 years of age participating in the Breathmobile project, obesity (BMI >=95th %) had no impact on the ability to achieve or maintain asthma control.56 Failure to adequately measure or standardize therapy limits the interpretability of these studies. The design of the ACE study included guidelines-based standardized treatment for all participants and therefore addressed these limitations of previous studies.
Reported differences between males and females in the effect of body size on asthma have been called into question due to methodological and statistical concerns about the studies.57, 58 Our findings demonstrate that in females, asthma control, asthma exacerbations and FEV1/FVC are adversely affected by increasing BMI and percent body fat. The increased morbidity and subsequent treatment found in obese females was not associated with increased atopy or FENO.. Our results are consistent with studies in adults.48, 59 This raises two potential explanations, namely, that increased asthma morbidity is a result of a mechanism other than atopy and inflammation, or that there is a difference in the perception of asthma symptoms among obese females.
Other studies have suggested a difference in perception and reporting of symptoms among obese asthmatics to account for the increased asthma severity. Asthmatic children ages 8 to 12 years who were obese or overweight as defined by BMI reported greater limitation of physical activity than their normal weight peers, yet they had similar maximum aerobic power and levels of habitual activity.60 Despite similar levels of severity, obese asthmatics reported lower quality of life scores.11 Secondary analyses from clinical trial participants found obese asthmatics report a lower response to placebo.61 Further supporting the possibility of perception differences, bronchial reactivity to methacholine has been found to be equivalent51, 62 or less63 among obese asthmatics when compared to lower weight subjects. These studies do not find that this difference in perception is limited to females, and therefore they do not provide an explanation for the gender differences found in this study.
Among children and adolescents, previous studies have reported that an increase in BMI is accompanied by a higher FEV1 and FVC but a lower FEV1/FVC in both non-asthmatics64, 65 and asthmatics.51 This effect may reflect the failure of airway size to increase proportionately to lung volume. The impact of increasing body size on pulmonary function seen in this study is consistent with the previous literature.
The question that arises from previous studies that examined the relationship between asthma and obesity is whether BMI, the most commonly used index of adiposity, is an adequate measure. A similar BMI represents a differing level of percent body fat depending on the gender, race, or sexual maturity of the child\adolescent.66 Our study provides a unique opportunity to examine the relationship between BMI and DEXA measurements of overweight and body fat. The measures were highly correlated in both males and females. While BMI alone has limitations in assessing the true degree of adiposity of an individual child,29 more sophisticated measures provided little additional value in studies relating adiposity to asthma outcomes in African-American and Hispanic adolescents.
The strength of this study is that it combines multiple measures of adiposity with adipokines and prospectively evaluates asthma outcome. By limiting our study to adolescents, it addresses the criticism that conflicting gender effects found in obesity studies do not take into account overlapping periods of development.58 The effects of BMI in females and adiponectin in males on asthma control were seen despite optimal guideline-based management.
Our study has a number of limitations. We measured circulating, but not airway, measures of adipokines and cytokines. It is possible that levels in bronchoalveolar lavage would give additional information on the relationship between asthma morbidity and adiposity. Secondly, the improved control of asthma over the 42-week study period would tend to minimize the differences between obese and non-obese asthmatics. Finally, despite the design of the ACE study, with its uniform comprehensive follow-up, data collection, and standardized treatment, it does not eliminate the possibility that perceptional differences in symptom reporting influenced our results.
In summary, our data support a gender-specific role for adipokines produced by adipose tissue in asthma morbidity as measured by symptoms, pulmonary function, and exacerbations but not reflected in other indicators of airway inflammation. Asthma outcome is adversely affected by adiposity in females but not males; whereas for males, but not for females, adiponectin has a protective effect independent of adiposity.
The implications of this study are highlighted by the high prevalence of those at risk for overweight in this inner-city population of predominantly black and Hispanic adolescents. Future therapeutic approaches for asthma are more likely to succeed if gender and body size are taken into account. Further investigations into the roles of adipokines in asthma morbidity may have particular relevance to the high risk inner city populations.
Acknowledgments
The Asthma Control Evaluation was a collaboration of the following institutions and investigators (principal investigators are indicated by asterisks): Johns Hopkins University, Baltimore, MD – P Eggleston*, E Matsui, R Wood, K Callahan, M Mensa, L Campbell, R Merrill, P Huffman, D Bunce, H Bradly; Boston University School of Medicine, Boston, MA – G O’Connor*, S Steinbach, N Kozlowski; Children’s Memorial Hospital, Chicago, IL – J Pongracic*, R Kumar, J Kim, R Story, A Donnell, S Desai, A Murthy, S Boudreau-Romano, K Koridek, T Kearney, S Pohlman, J Milam, H Negron, I Flexas; Case Western Reserve University School of Medicine, Cleveland, OH – C Kercsmar*, J Chmiel, M Hart, T Myers, Tracy Dillard, Jackie Juricka, C Kane, V Lockhart-Blue, M Rogers, K Ross, P Vavrek; UT Southwestern Medical Center at Dallas, TX – R Gruchalla*, V Gan, W Neaville, N Gorham, J Teeple, I Dougherty, T George; National Jewish Health, Denver, CO – S Szefler*, A Liu*, J Henley, M Anderson (Denver Health Medical Center), C Campos, P Pinedo, L Soto, M Gleason, R Covar, J Spahn, K Breese, K Patterson, M White, D Sundstrom, H Leo, N Jain, B Song, K Carel, L Stewart, B Macomber, C Mjaanes, A Schiltz, R Harbeck; Mount Sinai School of Medicine, New York, NY – M Kattan*, H Sampson, C Lamm, M Pierce, A Ting, E Sembrano, L Peters, A Valones, M Duarte, Y Fernandez-Pau, P Yaniv, R Castro, M Mishoe, Y Kucuk; Washington University School of Medicine, St Louis, MO – G Bloomberg*, R Strunk, L Bacharier, T Oliver-Welker; The University of Arizona College of Medicine, Tucson, AZ – W Morgan*, M Brown, T Guilbert, F Martinez, E Morales, K Otsuka, M Celaya, D Castellanos, S Ehteshami, M Fierro, G Garcia, J Goodwin, W Hall, Y Meza, J Priefert, J Rennspies, G Terrazas, M Vasquez, R Weese; Children’s National Medical Center, Washington, DC – S Teach*, K Stone, D Quint, A. Newcomer, S. Staples, J. Schmidt, E. Dunbar, R. Chirumamilla; Statistical and Clinical Coordinating Center, Rho, Inc, Chapel Hill, NC – H Mitchell*, G David, A Calatroni, M Curry, M Walter, J Wildfire, A Hodges, R Budrevich, B Shaw, R Bailey, G Allen; Scientific Coordination and Administrative Center, Madison, WI – W Busse*, C Sorkness, R Kelley, P Heinritz, G Crisafi; National Institute of Allergy and Infectious Diseases, Bethesda, MD – P Gergen, E Smartt, M Smolskis, M Fenton.
The study also gratefully acknowledges receiving donated product from GlaxoSmithKline (study drugs) and Lincoln Diagnostics, Inc. (skin testing materials).
Funding
This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under Contracts number NO1-AI-25496 and NO1-JAI-25482, and from the National Center for Research Resources, National Institutes of Health, under grants RR00052, M01RR00533, M01RR00071, 5UL1RR024992-02, and 5M01RR020359-04.
Abbreviations
- ACE
Asthma Control Evaluation
- ACT
Asthma Control Test
- BMI
Body mass index ACC
- CRP
C-reactive protein (mg/dL)
- DEXA
Dual energy X-ray absorptiometry
- FENO
Fraction of exhaled nitric oxide in parts per billion (ppb)
- FEV1
Forced expiratory volume in one second
- FEV1/FVC
Ratio of FEV1 and forced vital capacity
- ICS
Inhaled corticosteroid
- IgE
Immunoglobulin E
- IL-6
Interleukin-6 (pg/mL)
- kU/L
Kilo units/liter
- kUA/L
Kilo units (allergen-specific)/liter
- Mg
Milligram
- mL
Milliliter
- mm
Millimeter
- ng
Nanogram
- TNF-α
Tumor necrosis factor-α (pg/mL)
- WBC
White blood cells
- wt/vol
Weight/volume
Footnotes
Clinical Implications: In an urban adolescent population with moderate to severe asthma, there is a dose-response relationship of adiposity to symptom days and exacerbations in females.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Meyer Kattan, College of Physicians and Surgeons, Columbia University, New York, NY
Rajesh Kumar, Children’s Memorial Hospital, Chicago, IL
Gordon R. Bloomberg, Washington University in St. Louis, St. Louis, MO
Herman E. Mitchell, Rho Federal Systems Division, Inc., Chapel Hill, NC
Agustin Calatroni, Rho Federal Systems Division, Inc., Chapel Hill, NC
Peter J. Gergen, National Institute of Allergy and Infectious Diseases, Bethesda, MD
Carolyn M. Kercsmar, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH
Cynthia M. Visness, Rho Federal Systems Division, Inc., Chapel Hill, NC
Elizabeth C. Matsui, Johns Hopkins University School of Medicine, Baltimore, MD
Suzanne F. Steinbach, Boston University School of Medicine, Boston, MA
Stanley J. Szefler, National Jewish Health and University of Colorado Health Science, Denver, CO
Christine A. Sorkness, University of Wisconsin School of Medicine and Public Health, Madison, WI.
Wayne J. Morgan, University of Arizona College of Medicine, Tucson, AZ
Stephen J. Teach, Children’s National Medical Center, Washington, DC
Vanthaya N. Gan, UT Southwestern Medical Center, Dallas, TX
References
- 1.Miech RA, Kumanyika SK, Stettler N, Link BG, Phelan JC, Chang VW. Trends in the association of poverty with overweight among US adolescents, 1971–2004. Jama. 2006;295:2385–93. doi: 10.1001/jama.295.20.2385. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. JAMA. 2008;299:2401–5. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 4.Camargo CA, Jr, Weiss ST, Zhang S, Willett WC, Speizer FE. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med. 1999;159:2582–8. doi: 10.1001/archinte.159.21.2582. [DOI] [PubMed] [Google Scholar]
- 5.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175:661–6. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gold DR, Damokosh AI, Dockery DW, Berkey CS. Body-mass index as a predictor of incident asthma in a prospective cohort of children. Pediatr Pulmonol. 2003;36:514–21. doi: 10.1002/ppul.10376. [DOI] [PubMed] [Google Scholar]
- 7.Maniscalco M, Zedda A, Faraone S, Cerbone MR, Cristiano S, Giardiello C, et al. Weight loss and asthma control in severely obese asthmatic females. Respir Med. 2008;102:102–8. doi: 10.1016/j.rmed.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 8.Stenius-Aarniala B, Poussa T, Kvarnstrom J, Gronlund EL, Ylikahri M, Mustajoki P. Immediate and long term effects of weight reduction in obese people with asthma: randomised controlled study. British Medical Journal. 2000;320:827–32. doi: 10.1136/bmj.320.7238.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belamarich PF, Luder E, Kattan M, Mitchell H, Islam S, Lynn H, et al. Do obese inner-city children with asthma have more symptoms than nonobese children with asthma? Pediatrics. 2000;106:1436–41. doi: 10.1542/peds.106.6.1436. [DOI] [PubMed] [Google Scholar]
- 10.Brenner JS, Kelly CS, Wenger AD, Brich SM, Morrow AL. Asthma and obesity in adolescents: is there an association? J Asthma. 2001;38:509–15. doi: 10.1081/jas-100105872. [DOI] [PubMed] [Google Scholar]
- 11.Lavoie KL, Bacon SL, Labrecque M, Cartier A, Ditto B. Higher BMI is associated with worse asthma control and quality of life but not asthma severity. Respir Med. 2006;100:648–57. doi: 10.1016/j.rmed.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Thomson CC, Clark S, Camargo CA., Jr Body mass index and asthma severity among adults presenting to the emergency department. Chest. 2003;124:795–802. doi: 10.1378/chest.124.3.795. [DOI] [PubMed] [Google Scholar]
- 13.Mosen DM, Schatz M, Magid DJ, Camargo CA., Jr The relationship between obesity and asthma severity and control in adults. J Allergy Clin Immunol. 2008;122:507–11. e6. doi: 10.1016/j.jaci.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 14.Akerman MJ, Calacanis CM, Madsen MK. Relationship between asthma severity and obesity. J Asthma. 2004;41:521–6. doi: 10.1081/jas-120037651. [DOI] [PubMed] [Google Scholar]
- 15.Lavoie KL, Cartier A, Labrecque M, Bacon SL, Lemiere C, Malo JL, et al. Are psychiatric disorders associated with worse asthma control and quality of life in asthma patients? Respir Med. 2005;99:1249–57. doi: 10.1016/j.rmed.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Saint-Pierre P, Bourdin A, Chanez P, Daures JP, Godard P. Are overweight asthmatics more difficult to control? Allergy. 2006;61:79–84. doi: 10.1111/j.1398-9995.2005.00953.x. [DOI] [PubMed] [Google Scholar]
- 17.Taylor B, Mannino D, Brown C, Crocker D, Twum-Baah N, Holguin F. Body mass index and asthma severity in the National Asthma Survey. Thorax. 2008;63:14–20. doi: 10.1136/thx.2007.082784. [DOI] [PubMed] [Google Scholar]
- 18.Varraso R, Siroux V, Maccario J, Pin I, Kauffmann F. Asthma severity is associated with body mass index and early menarche in women. Am J Respir Crit Care Med. 2005;171:334–9. doi: 10.1164/rccm.200405-674OC. [DOI] [PubMed] [Google Scholar]
- 19.Appleton SL, Wilson DH, Tucker G, Ruffin RE, Taylor AW, Adams RJ. Sex differences in asthma morbidity associated with obesity in a representative population sample. J Allergy Clin Immunol. 2008;121:1285–7. e1. doi: 10.1016/j.jaci.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 20.Beuther DA, Weiss ST, Sutherland ER. Obesity and asthma. Am J Respir Crit Care Med. 2006;174:112–9. doi: 10.1164/rccm.200602-231PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doganci A, Sauer K, Karwot R, Finotto S. Pathological role of IL-6 in the experimental allergic bronchial asthma in mice. Clin Rev Allergy Immunol. 2005;28:257–70. doi: 10.1385/CRIAI:28:3:257. [DOI] [PubMed] [Google Scholar]
- 22.Thomas PS, Heywood G. Effects of inhaled tumour necrosis factor alpha in subjects with mild asthma. Thorax. 2002;57:774–8. doi: 10.1136/thorax.57.9.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutherland ER, Goleva E, Strand M, Beuther DA, Leung DY. Body mass and glucocorticoid response in asthma. Am J Respir Crit Care Med. 2008;178:682–7. doi: 10.1164/rccm.200801-076OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenbaum M, Nicolson M, Hirsch J, Heymsfield SB, Gallagher D, Chu F, et al. Effects of gender, body composition, and menopause on plasma concentrations of leptin. J Clin Endocrinol Metab. 1996;81:3424–7. doi: 10.1210/jcem.81.9.8784109. [DOI] [PubMed] [Google Scholar]
- 25.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 26.Nagel G, Koenig W, Rapp K, Wabitsch M, Zoellner I, Weiland SK. Associations of adipokines with asthma, rhinoconjunctivitis, and eczema in German schoolchildren. Pediatr Allergy Immunol. 2008 doi: 10.1111/j.1399-3038.2008.00740.x. [DOI] [PubMed] [Google Scholar]
- 27.Sood A, Cui X, Qualls C, Beckett WS, Gross MD, Steffes MW, et al. Association between asthma and serum adiponectin concentration in women. Thorax. 2008;63:877–82. doi: 10.1136/thx.2007.090803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snijder MB, van Dam RM, Visser M, Seidell JC. What aspects of body fat are particularly hazardous and how do we measure them? Int J Epidemiol. 2006;35:83–92. doi: 10.1093/ije/dyi253. [DOI] [PubMed] [Google Scholar]
- 29.Ellis KJ, Abrams SA, Wong WW. Monitoring childhood obesity: assessment of the weight/height index. Am J Epidemiol. 1999;150:939–46. doi: 10.1093/oxfordjournals.aje.a010102. [DOI] [PubMed] [Google Scholar]
- 30.Castro-Rodriguez JA, Holberg CJ, Morgan WJ, Wright AL, Martinez FD. Increased incidence of asthmalike symptoms in girls who become overweight or obese during the school years. Am J Respir Crit Care Med. 2001;163:1344–9. doi: 10.1164/ajrccm.163.6.2006140. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Dales R, Tang M, Krewski D. Obesity may increase the incidence of asthma in women but not in men: longitudinal observations from the Canadian National Population Health Surveys. Am J Epidemiol. 2002;155:191–7. doi: 10.1093/aje/155.3.191. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Rennie D, Cormier Y, Dosman J. Sex specificity of asthma associated with objectively measured body mass index and waist circumference: the Humboldt study. Chest. 2005;128:3048–54. doi: 10.1378/chest.128.4.3048. [DOI] [PubMed] [Google Scholar]
- 33.Hancox RJ, Milne BJ, Poulton R, Taylor DR, Greene JM, McLachlan CR, et al. Sex differences in the relation between body mass index and asthma and atopy in a birth cohort. Am J Respir Crit Care Med. 2005;171:440–5. doi: 10.1164/rccm.200405-623OC. [DOI] [PubMed] [Google Scholar]
- 34.Hancox RJ, Milne BJ, Taylor DR, Greene JM, Cowan JO, Flannery EM, et al. Relationship between socioeconomic status and asthma: a longitudinal cohort study. Thorax. 2004;59:376–80. doi: 10.1136/thx.2003.010363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loerbroks A, Apfelbacher CJ, Amelang M, Sturmer T. Obesity and adult asthma: potential effect modification by gender, but not by hay fever. Ann Epidemiol. 2008;18:283–9. doi: 10.1016/j.annepidem.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Luder E, Ehrlich RI, Lou WY, Melnik TA, Kattan M. Body mass index and the risk of asthma in adults. Respir Med. 2004;98:29–37. doi: 10.1016/j.rmed.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Szefler SJ, Mitchell H, Sorkness CA, Gergen PJ, O'Connor GT, Morgan WJ, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet. 2008;372:1065–72. doi: 10.1016/S0140-6736(08)61448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kattan M, Kumar R, Calatroni A. Adiponectin modulates asthma control in inner-city asthmatic adolescents. American Journal of Respiratory and Critical Care Medicine. 2009;179:A5442. [Google Scholar]
- 39.Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 40.American Thoracic Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–30. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 41.Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–6. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 42.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 43.Kobashi C, Urakaze M, Kishida M, Kibayashi E, Kobayashi H, Kihara S, et al. Adiponectin inhibits endothelial synthesis of interleukin-8. Circ Res. 2005;97:1245–52. doi: 10.1161/01.RES.0000194328.57164.36. [DOI] [PubMed] [Google Scholar]
- 44.Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun. 2004;316:924–9. doi: 10.1016/j.bbrc.2004.02.130. [DOI] [PubMed] [Google Scholar]
- 45.Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2006;118:389–95. doi: 10.1016/j.jaci.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 46.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shore SA. Obesity and asthma: lessons from animal models. J Appl Physiol. 2007;102:516–28. doi: 10.1152/japplphysiol.00847.2006. [DOI] [PubMed] [Google Scholar]
- 48.Sutherland TJ, Cowan JO, Young S, Goulding A, Grant AM, Williamson A, et al. The association between obesity and asthma: interactions between systemic and airway inflammation. Am J Respir Crit Care Med. 2008;178:469–75. doi: 10.1164/rccm.200802-301OC. [DOI] [PubMed] [Google Scholar]
- 49.de Marco R, Marcon A, Jarvis D, Accordini S, Almar E, Bugiani M, et al. Prognostic factors of asthma severity: a 9-year international prospective cohort study. J Allergy Clin Immunol. 2006;117:1249–56. doi: 10.1016/j.jaci.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 50.Vargas PA, Perry TT, Robles E, Jo CH, Simpson PM, Magee JM, et al. Relationship of body mass index with asthma indicators in head start children. Ann Allergy Asthma Immunol. 2007;99:22–8. doi: 10.1016/S1081-1206(10)60616-3. [DOI] [PubMed] [Google Scholar]
- 51.Tantisira KG, Litonjua AA, Weiss ST, Fuhlbrigge AL. Association of body mass with pulmonary function in the Childhood Asthma Management Program (CAMP) Thorax. 2003;58:1036–41. doi: 10.1136/thorax.58.12.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clerisme-Beaty EM, Karam S, Rand C, Patino CM, Bilderback A, Riekert KA, et al. Does higher body mass index contribute to worse asthma control in an urban population? J Allergy Clin Immunol. 2009;124:207–12. doi: 10.1016/j.jaci.2009.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boulet LP, Franssen E. Influence of obesity on response to fluticasone with or without salmeterol in moderate asthma. Respir Med. 2007;101:2240–7. doi: 10.1016/j.rmed.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 54.Rodrigo GJ, Plaza V. Body mass index and response to emergency department treatment in adults with severe asthma exacerbations: a prospective cohort study. Chest. 2007;132:1513–9. doi: 10.1378/chest.07-0936. [DOI] [PubMed] [Google Scholar]
- 55.Carroll CL, Bhandari A, Zucker AR, Schramm CM. Childhood obesity increases duration of therapy during severe asthma exacerbations. Pediatr Crit Care Med. 2006;7:527–31. doi: 10.1097/01.PCC.0000243749.14555.E8. [DOI] [PubMed] [Google Scholar]
- 56.Kwong KY, Rhandhawa I, Saxena J, Morphew T, Jones CA. Ability to control persistent asthma in obese versus non-obese children enrolled in an asthma-specific disease management program (breathmobile) J Asthma. 2006;43:661–6. doi: 10.1080/02770900600925270. [DOI] [PubMed] [Google Scholar]
- 57.Chinn S. Obesity and asthma. Paediatr Respir Rev. 2006;7:223–8. doi: 10.1016/j.prrv.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 58.Matricardi PM, Gruber C, Wahn U, Lau S. The asthma-obesity link in childhood: open questions, complex evidence, a few answers only. Clin Exp Allergy. 2007;37:476–84. doi: 10.1111/j.1365-2222.2007.02664.x. [DOI] [PubMed] [Google Scholar]
- 59.McLachlan CR, Poulton R, Car G, Cowan J, Filsell S, Greene JM, et al. Adiposity, asthma, and airway inflammation. J Allergy Clin Immunol. 2007;119:634–9. doi: 10.1016/j.jaci.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 60.Pianosi PT, Davis HS. Determinants of physical fitness in children with asthma. Pediatrics. 2004;113:e225–9. doi: 10.1542/peds.113.3.e225. [DOI] [PubMed] [Google Scholar]
- 61.Peters-Golden M, Swern A, Bird SS, Hustad CM, Grant E, Edelman JM. Influence of body mass index on the response to asthma controller agents. Eur Respir J. 2006;27:495–503. doi: 10.1183/09031936.06.00077205. [DOI] [PubMed] [Google Scholar]
- 62.Mansell AL, Walders N, Wamboldt MZ, Carter R, Steele DW, Devin JA, et al. Effect of body mass index on response to methacholine bronchial provocation in healthy and asthmatic adolescents. Pediatr Pulmonol. 2006;41:434–40. doi: 10.1002/ppul.20368. [DOI] [PubMed] [Google Scholar]
- 63.Bibi H, Shoseyov D, Feigenbaum D, Genis M, Friger M, Peled R, et al. The relationship between asthma and obesity in children: is it real or a case of over diagnosis? J Asthma. 2004;41:403–10. doi: 10.1081/jas-120026097. [DOI] [PubMed] [Google Scholar]
- 64.Pistelli R, Brancato G, Forastiere F, Michelozzi P, Corbo GM, Agabiti N, et al. Population values of lung volumes and flows in children: effect of sex, body mass and respiratory conditions. Eur Respir J. 1992;5:463–70. [PubMed] [Google Scholar]
- 65.Schwartz JD, Katz SA, Fegley RW, Tockman MS. Analysis of spirometric data from a national sample of healthy 6- to 24-year-olds (NHANES II) Am Rev Respir Dis. 1988;138:1405–14. doi: 10.1164/ajrccm/138.6.1405. [DOI] [PubMed] [Google Scholar]
- 66.Daniels SR, Khoury PR, Morrison JA. The utility of body mass index as a measure of body fatness in children and adolescents: differences by race and gender. Pediatrics. 1997;99:804–7. doi: 10.1542/peds.99.6.804. [DOI] [PubMed] [Google Scholar]