Abstract
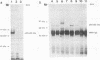
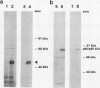
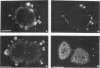
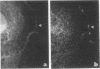
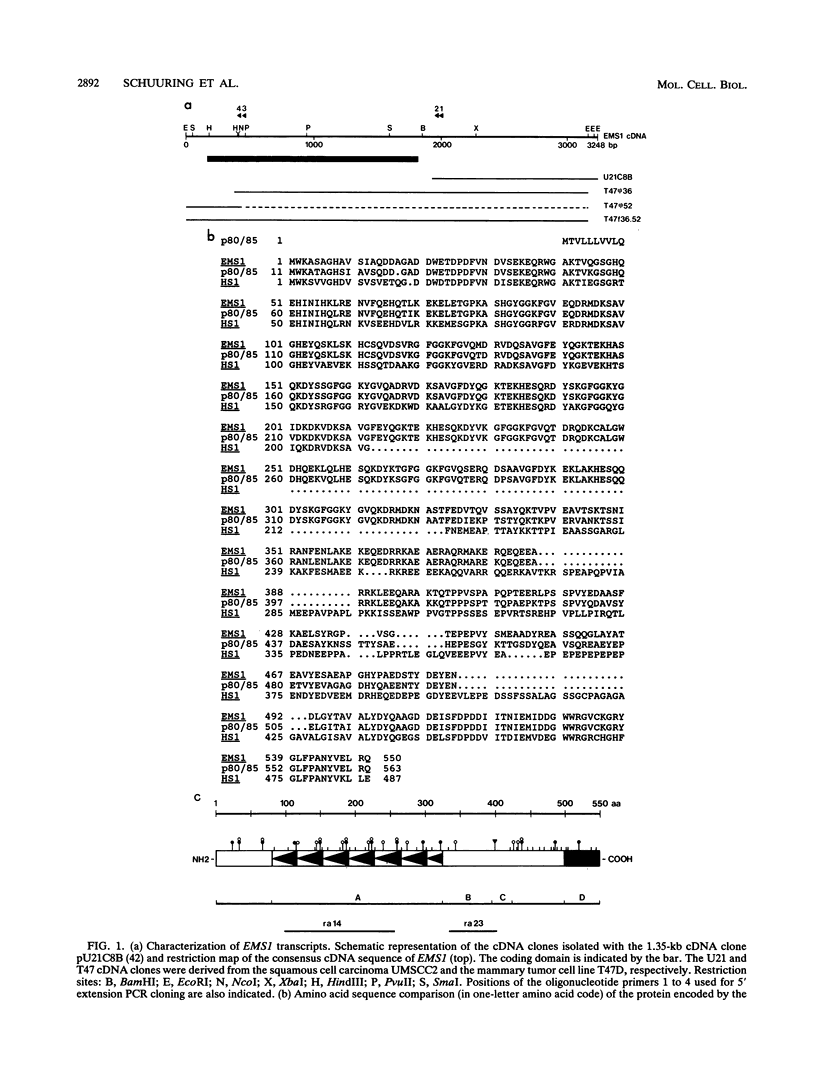
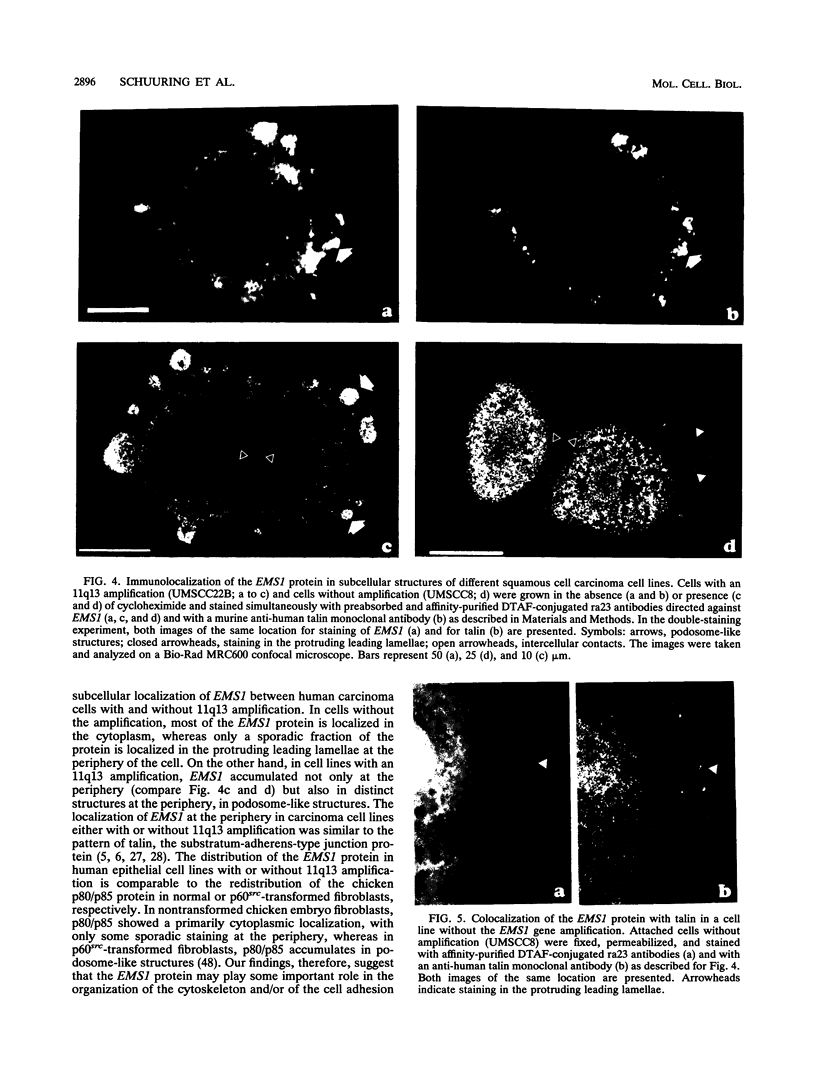
We have previously identified two genes (EMS1 and PRAD1/cyclin D1) in the chromosome 11q13 region that are frequently coamplified and overexpressed in human breast cancer and in squamous cell carcinomas of the head and neck (E. Schuuring, E. Verhoeven, W.J. Mooi, and R.J.A.M. Michalides, Oncogene 7:355-361, 1992). We now report on the characterization of the 80/85-kDa protein that is encoded by the EMS1 gene. Amino acid sequence comparison shows a high homology (85%) to a chicken protein that was recently identified as a substrate for the src oncogene (H. Wu, A.B. Reynolds, S.B. Kanner, R.R. Vines, and J.T. Parsons, Mol. Cell. Biol. 11:5113-5124, 1991). Immunocytochemistry reveals that in epithelial cells, the human EMS1 protein is localized mainly in the cytoplasm and, to a very low extent, in protruding leading lamellae of the cell. However, in carcinoma cells that constitutively overexpress the protein as a result of amplification of the EMS1 gene, the protein, except in cytoplasm, accumulates in the podosome-like adherens junctions associated with the cell-substratum contact sites. The protein was not found in intercellular adherens junctions. Our findings, and the previously reported observations in src-transformed chicken embryo fibroblasts, suggest that the EMS1 protein is involved in regulating the interactions between components of adherens-type junctions. Since amplification of the 11q13 region has been associated with an enhanced invasive potential of these tumors, overexpression and concomitant accumulation of the EMS1 protein in the cell-substratum contact sites might, therefore, contribute to the invasive potential of these tumor cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adnane J., Gaudray P., Simon M. P., Simony-Lafontaine J., Jeanteur P., Theillet C. Proto-oncogene amplification and human breast tumor phenotype. Oncogene. 1989 Nov;4(11):1389–1395. [PubMed] [Google Scholar]
- Borg A., Sigurdsson H., Clark G. M., Fernö M., Fuqua S. A., Olsson H., Killander D., McGurie W. L. Association of INT2/HST1 coamplification in primary breast cancer with hormone-dependent phenotype and poor prognosis. Br J Cancer. 1991 Jan;63(1):136–142. doi: 10.1038/bjc.1991.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressan G. M., Stanley K. K. pUEX, a bacterial expression vector related to pEX with universal host specificity. Nucleic Acids Res. 1987 Dec 10;15(23):10056–10056. doi: 10.1093/nar/15.23.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Connell L. Talin: a cytoskeletal component concentrated in adhesion plaques and other sites of actin-membrane interaction. Cell Motil. 1983;3(5-6):405–417. doi: 10.1002/cm.970030509. [DOI] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- David-Pfeuty T., Singer S. J. Altered distributions of the cytoskeletal proteins vinculin and alpha-actinin in cultured fibroblasts transformed by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6687–6691. doi: 10.1073/pnas.77.11.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantl V., Richards M. A., Smith R., Lammie G. A., Johnstone G., Allen D., Gregory W., Peters G., Dickson C., Barnes D. M. Gene amplification on chromosome band 11q13 and oestrogen receptor status in breast cancer. Eur J Cancer. 1990 Apr;26(4):423–429. doi: 10.1016/0277-5379(90)90009-i. [DOI] [PubMed] [Google Scholar]
- Guan J. L., Shalloway D. Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature. 1992 Aug 20;358(6388):690–692. doi: 10.1038/358690a0. [DOI] [PubMed] [Google Scholar]
- Kanner S. B., Reynolds A. B., Vines R. R., Parsons J. T. Monoclonal antibodies to individual tyrosine-phosphorylated protein substrates of oncogene-encoded tyrosine kinases. Proc Natl Acad Sci U S A. 1990 May;87(9):3328–3332. doi: 10.1073/pnas.87.9.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa Y., Ueda M., Ando N., Shinozawa Y., Shimizu N., Abe O. Significance of int-2/hst-1 coamplification as a prognostic factor in patients with esophageal squamous carcinoma. Cancer Res. 1991 Mar 1;51(5):1504–1508. [PubMed] [Google Scholar]
- Kitamura D., Kaneko H., Miyagoe Y., Ariyasu T., Watanabe T. Isolation and characterization of a novel human gene expressed specifically in the cells of hematopoietic lineage. Nucleic Acids Res. 1989 Nov 25;17(22):9367–9379. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lammie G. A., Fantl V., Smith R., Schuuring E., Brookes S., Michalides R., Dickson C., Arnold A., Peters G. D11S287, a putative oncogene on chromosome 11q13, is amplified and expressed in squamous cell and mammary carcinomas and linked to BCL-1. Oncogene. 1991 Mar;6(3):439–444. [PubMed] [Google Scholar]
- Lammie G. A., Peters G. Chromosome 11q13 abnormalities in human cancer. Cancer Cells. 1991 Nov;3(11):413–420. [PubMed] [Google Scholar]
- Lees J. A., Buchkovich K. J., Marshak D. R., Anderson C. W., Harlow E. The retinoblastoma protein is phosphorylated on multiple sites by human cdc2. EMBO J. 1991 Dec;10(13):4279–4290. doi: 10.1002/j.1460-2075.1991.tb05006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew D. J., Dulić V., Reed S. I. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell. 1991 Sep 20;66(6):1197–1206. doi: 10.1016/0092-8674(91)90042-w. [DOI] [PubMed] [Google Scholar]
- Ligtenberg M. J., Vos H. L., Gennissen A. M., Hilkens J. Episialin, a carcinoma-associated mucin, is generated by a polymorphic gene encoding splice variants with alternative amino termini. J Biol Chem. 1990 Apr 5;265(10):5573–5578. [PubMed] [Google Scholar]
- Liscia D. S., Merlo G. R., Garrett C., French D., Mariani-Costantini R., Callahan R. Expression of int-2 mRNA in human tumors amplified at the int-2 locus. Oncogene. 1989 Oct;4(10):1219–1224. [PubMed] [Google Scholar]
- Marchisio P. C., Cirillo D., Teti A., Zambonin-Zallone A., Tarone G. Rous sarcoma virus-transformed fibroblasts and cells of monocytic origin display a peculiar dot-like organization of cytoskeletal proteins involved in microfilament-membrane interactions. Exp Cell Res. 1987 Mar;169(1):202–214. doi: 10.1016/0014-4827(87)90238-2. [DOI] [PubMed] [Google Scholar]
- Matsushime H., Ewen M. E., Strom D. K., Kato J. Y., Hanks S. K., Roussel M. F., Sherr C. J. Identification and properties of an atypical catalytic subunit (p34PSK-J3/cdk4) for mammalian D type G1 cyclins. Cell. 1992 Oct 16;71(2):323–334. doi: 10.1016/0092-8674(92)90360-o. [DOI] [PubMed] [Google Scholar]
- Matsushime H., Roussel M. F., Ashmun R. A., Sherr C. J. Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell. 1991 May 17;65(4):701–713. doi: 10.1016/0092-8674(91)90101-4. [DOI] [PubMed] [Google Scholar]
- Moreno S., Nurse P. Substrates for p34cdc2: in vivo veritas? Cell. 1990 May 18;61(4):549–551. doi: 10.1016/0092-8674(90)90463-o. [DOI] [PubMed] [Google Scholar]
- Motokura T., Bloom T., Kim H. G., Jüppner H., Ruderman J. V., Kronenberg H. M., Arnold A. A novel cyclin encoded by a bcl1-linked candidate oncogene. Nature. 1991 Apr 11;350(6318):512–515. doi: 10.1038/350512a0. [DOI] [PubMed] [Google Scholar]
- Nermut M. V., Eason P., Hirst E. M., Kellie S. Cell/substratum adhesions in RSV-transformed rat fibroblasts. Exp Cell Res. 1991 Apr;193(2):382–397. doi: 10.1016/0014-4827(91)90111-7. [DOI] [PubMed] [Google Scholar]
- Pasquale E. B., Maher P. A., Singer S. J. Talin is phosphorylated on tyrosine in chicken embryo fibroblasts transformed by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5507–5511. doi: 10.1073/pnas.83.15.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Gish G. D. SH2 and SH3 domains: from structure to function. Cell. 1992 Oct 30;71(3):359–362. doi: 10.1016/0092-8674(92)90504-6. [DOI] [PubMed] [Google Scholar]
- Pawson T. Non-catalytic domains of cytoplasmic protein-tyrosine kinases: regulatory elements in signal transduction. Oncogene. 1988 Nov;3(5):491–495. [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinnerthaler G., Geiger B., Small J. V. Contact formation during fibroblast locomotion: involvement of membrane ruffles and microtubules. J Cell Biol. 1988 Mar;106(3):747–760. doi: 10.1083/jcb.106.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrschneider L. R. Adhesion plaques of Rous sarcoma virus-transformed cells contain the src gene product. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3514–3518. doi: 10.1073/pnas.77.6.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrschneider L. R., Najita L. M. Detection of the v-abl gene product at cell-substratum contact sites in Abelson murine leukemia virus-transformed fibroblasts. J Virol. 1984 Aug;51(2):547–552. doi: 10.1128/jvi.51.2.547-552.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg C. L., Wong E., Petty E. M., Bale A. E., Tsujimoto Y., Harris N. L., Arnold A. PRAD1, a candidate BCL1 oncogene: mapping and expression in centrocytic lymphoma. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9638–9642. doi: 10.1073/pnas.88.21.9638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M. D., Borgman C. A., Cobb B. S., Vines R. R., Reynolds A. B., Parsons J. T. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuuring E., Verhoeven E., Mooi W. J., Michalides R. J. Identification and cloning of two overexpressed genes, U21B31/PRAD1 and EMS1, within the amplified chromosome 11q13 region in human carcinomas. Oncogene. 1992 Feb;7(2):355–361. [PubMed] [Google Scholar]
- Schuuring E., Verhoeven E., van Tinteren H., Peterse J. L., Nunnink B., Thunnissen F. B., Devilee P., Cornelisse C. J., van de Vijver M. J., Mooi W. J. Amplification of genes within the chromosome 11q13 region is indicative of poor prognosis in patients with operable breast cancer. Cancer Res. 1992 Oct 1;52(19):5229–5234. [PubMed] [Google Scholar]
- Tarone G., Cirillo D., Giancotti F. G., Comoglio P. M., Marchisio P. C. Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp Cell Res. 1985 Jul;159(1):141–157. doi: 10.1016/s0014-4827(85)80044-6. [DOI] [PubMed] [Google Scholar]
- Tsuda H., Hirohashi S., Shimosato Y., Hirota T., Tsugane S., Yamamoto H., Miyajima N., Toyoshima K., Yamamoto T., Yokota J. Correlation between long-term survival in breast cancer patients and amplification of two putative oncogene-coamplification units: hst-1/int-2 and c-erbB-2/ear-1. Cancer Res. 1989 Jun 1;49(11):3104–3108. [PubMed] [Google Scholar]
- Tsukita S., Oishi K., Akiyama T., Yamanashi Y., Yamamoto T., Tsukita S. Specific proto-oncogenic tyrosine kinases of src family are enriched in cell-to-cell adherens junctions where the level of tyrosine phosphorylation is elevated. J Cell Biol. 1991 May;113(4):867–879. doi: 10.1083/jcb.113.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers D. A., Harvey R. C., Faust J. B., Melnyk O., Carey K., Meeker T. C. Characterization of a candidate bcl-1 gene. Mol Cell Biol. 1991 Oct;11(10):4846–4853. doi: 10.1128/mcb.11.10.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Reynolds A. B., Kanner S. B., Vines R. R., Parsons J. T. Identification and characterization of a novel cytoskeleton-associated pp60src substrate. Mol Cell Biol. 1991 Oct;11(10):5113–5124. doi: 10.1128/mcb.11.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Connolly T., Futcher B., Beach D. Human D-type cyclin. Cell. 1991 May 17;65(4):691–699. doi: 10.1016/0092-8674(91)90100-d. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Zhang H., Beach D. D type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell. 1992 Oct 30;71(3):505–514. doi: 10.1016/0092-8674(92)90518-h. [DOI] [PubMed] [Google Scholar]
- Zachary I., Rozengurt E. Focal adhesion kinase (p125FAK): a point of convergence in the action of neuropeptides, integrins, and oncogenes. Cell. 1992 Dec 11;71(6):891–894. doi: 10.1016/0092-8674(92)90385-p. [DOI] [PubMed] [Google Scholar]